Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Acanthamoeba
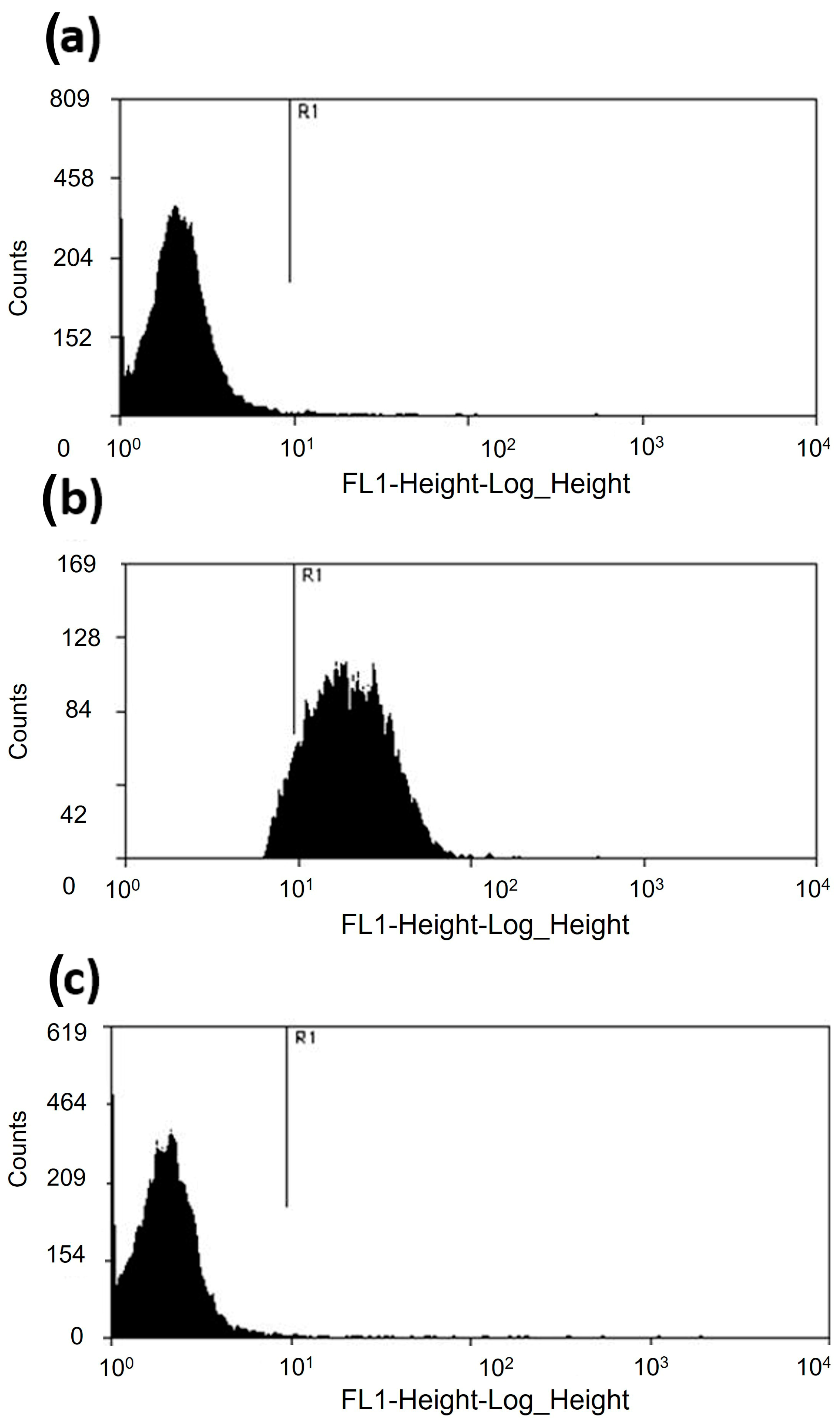
2.2. Viability Assays
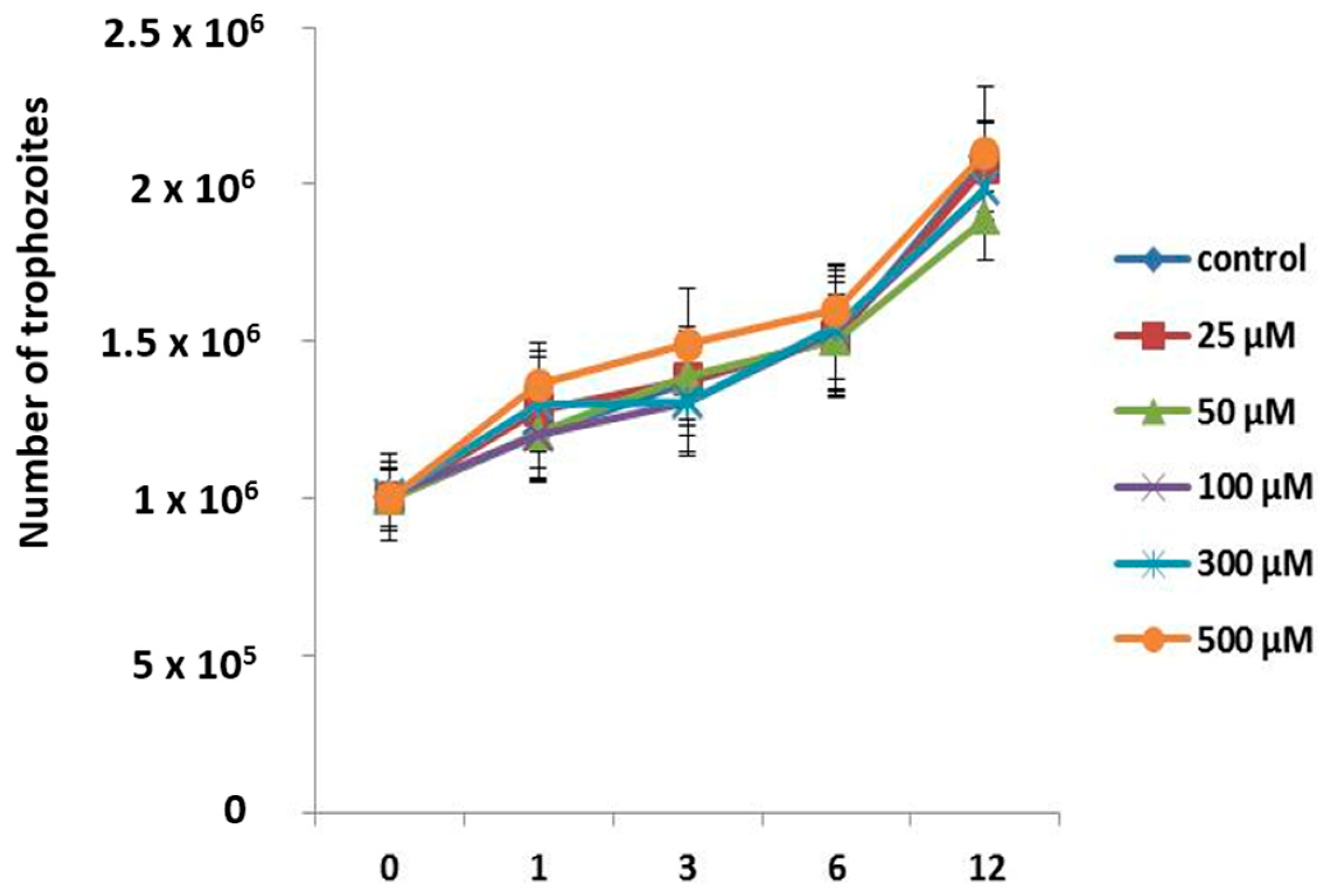
2.3. Growth Curves
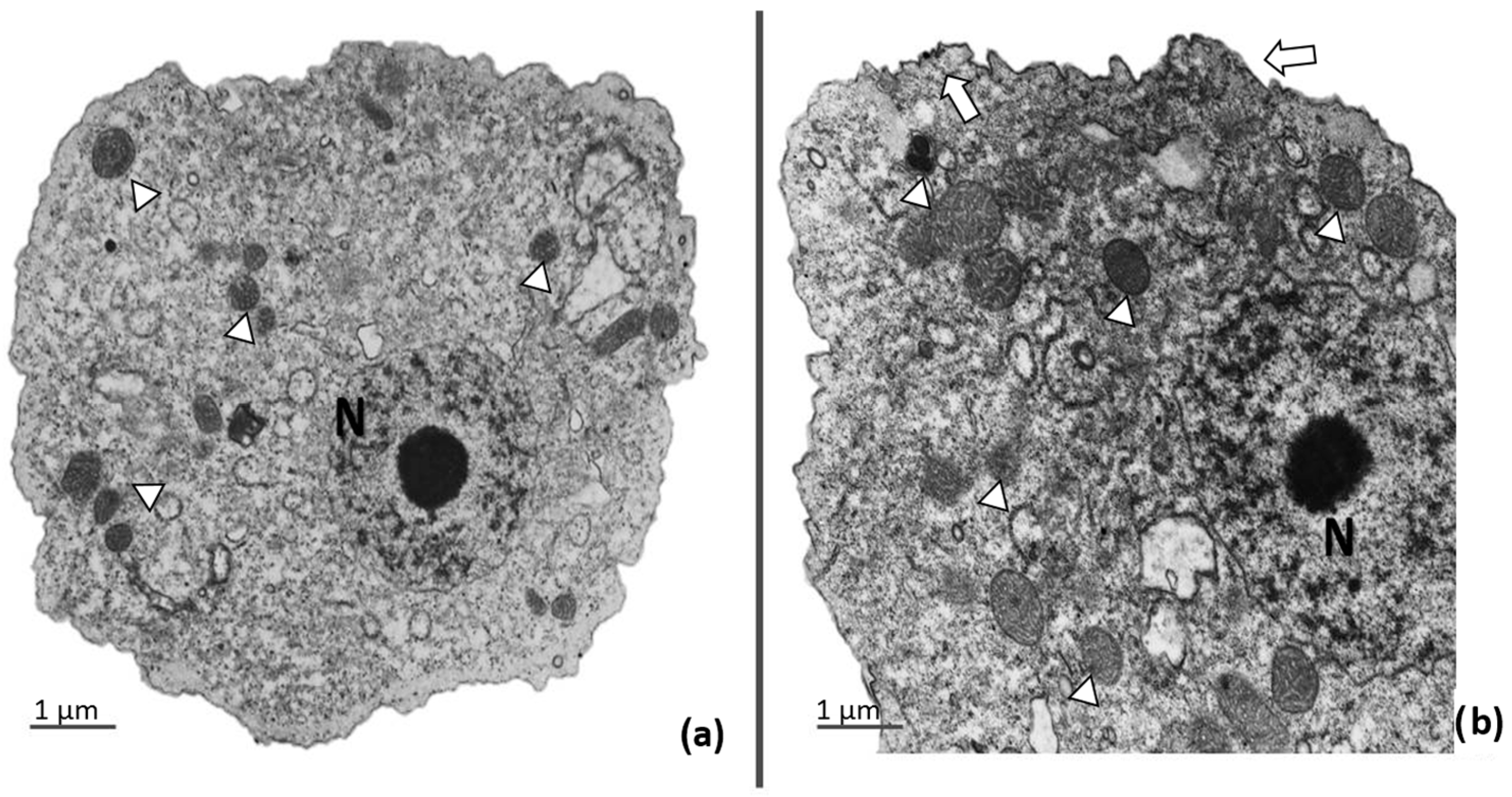
2.4. Transmission Electron Microscopy
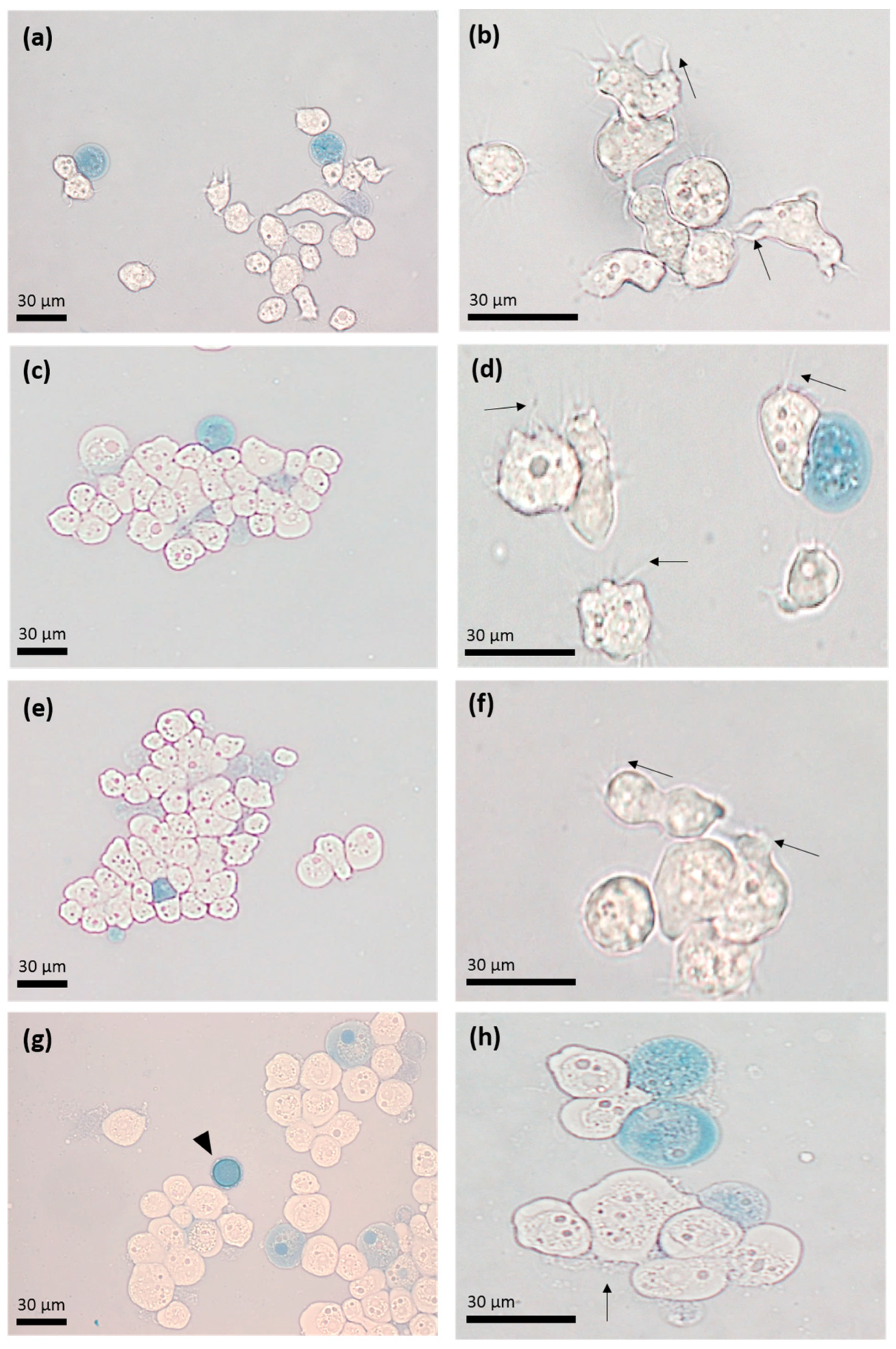
2.5. Cytopathic Effect of A. castellanii
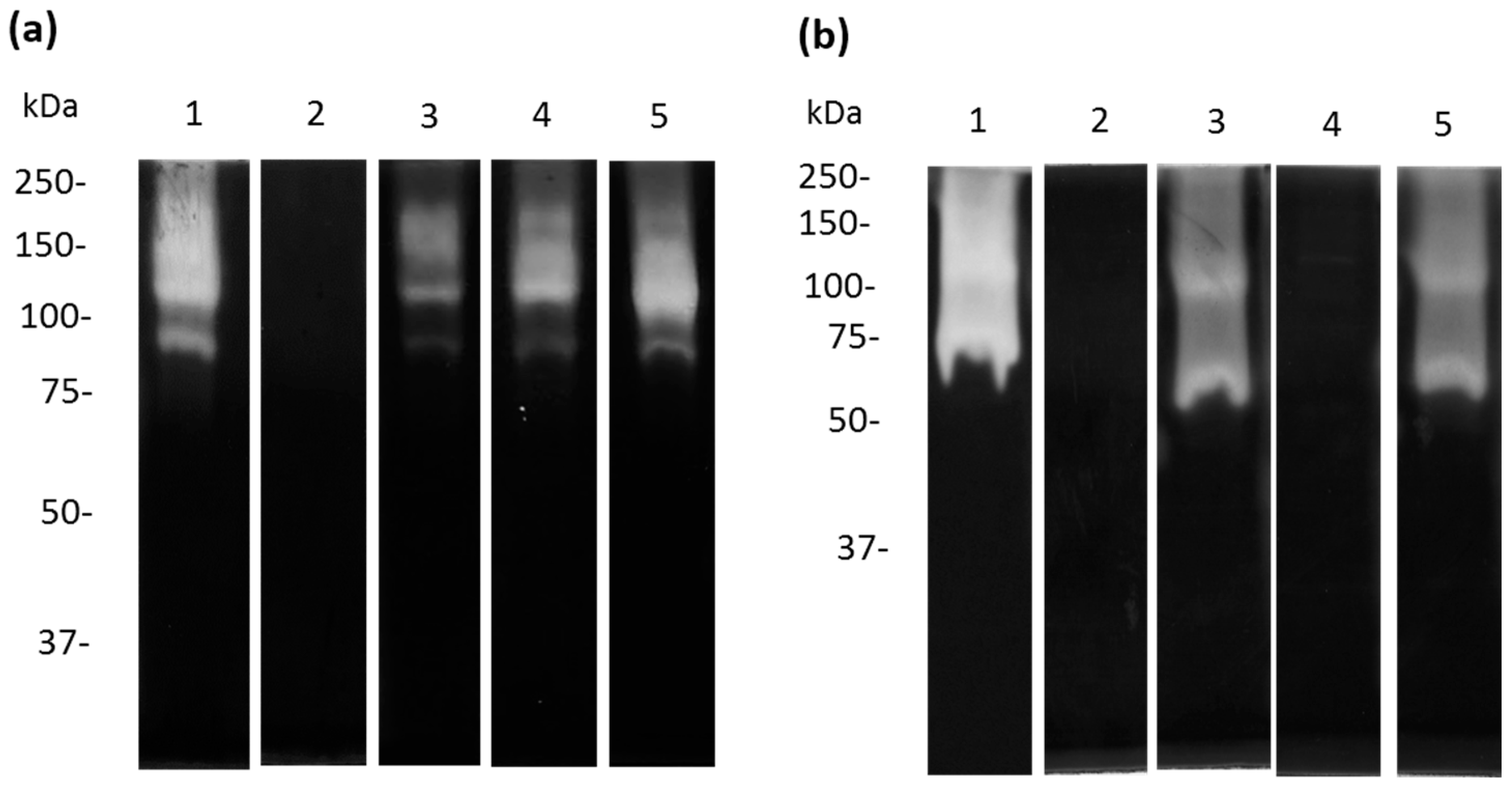
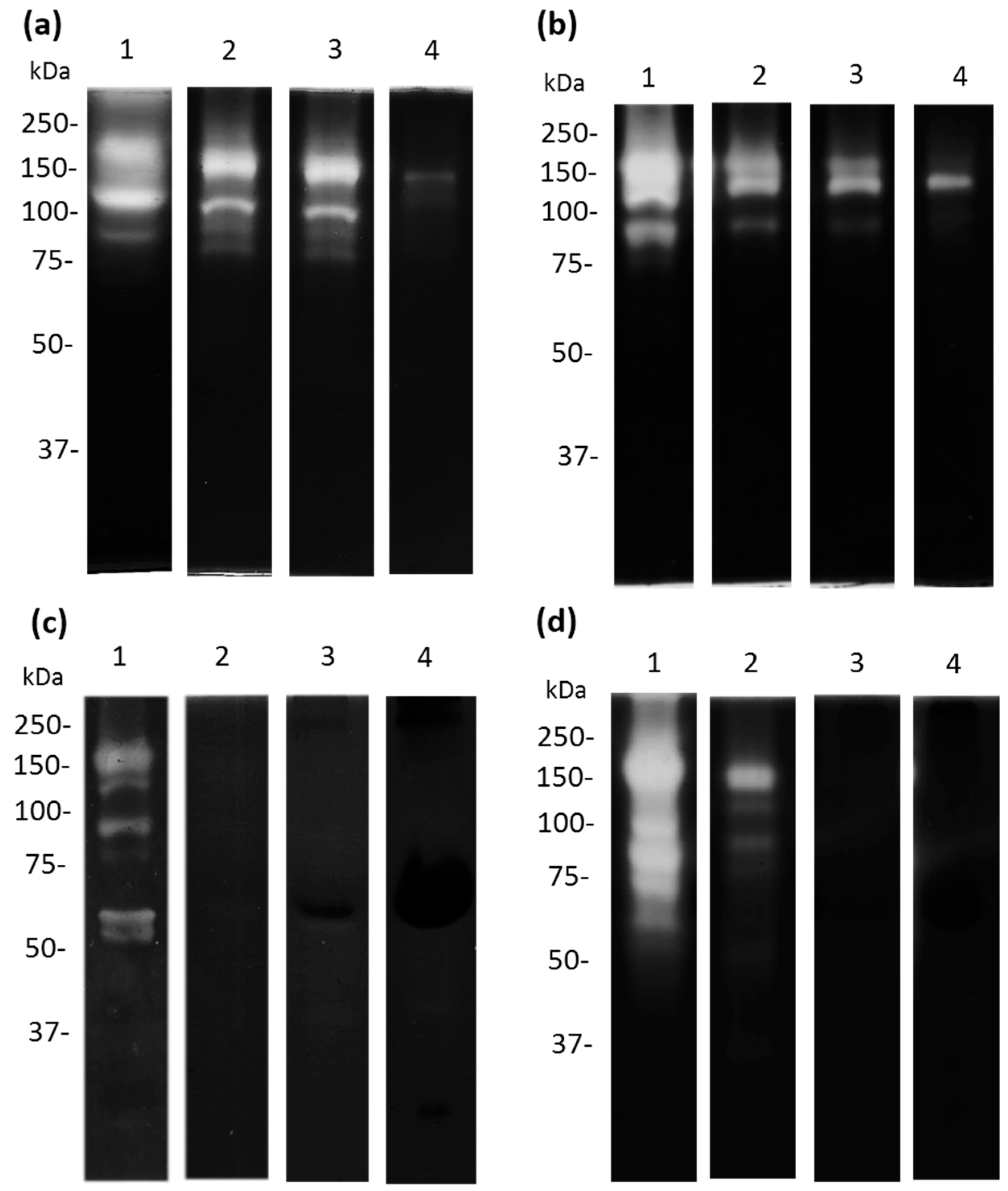
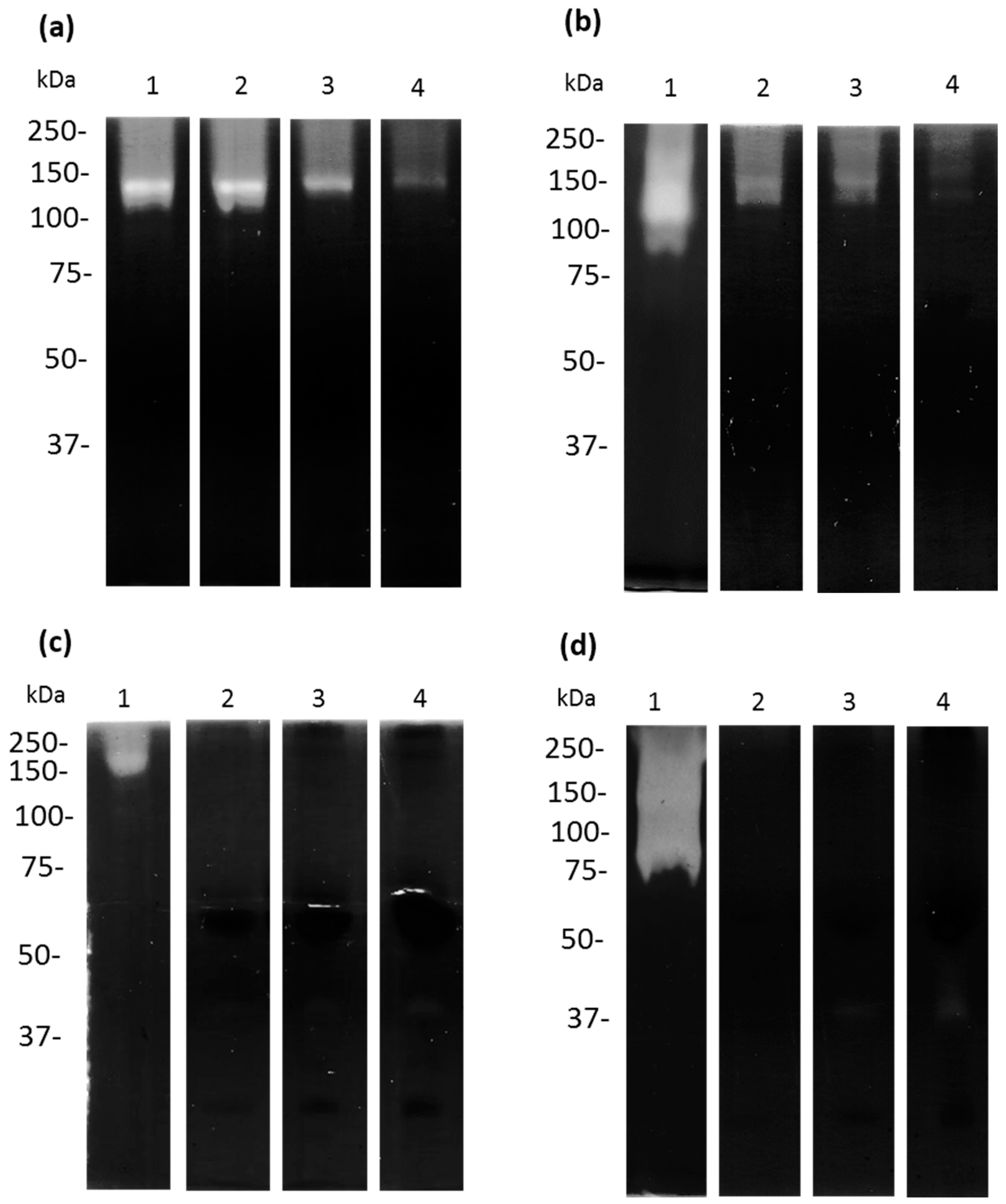
2.6. Zymography Assays
2.7. Protease Inhibitors
3. Results
3.1. Acanthamoeba castellanii Resists the Microbicidal Effect of apo-bLf
3.2. Apo-bLf Does Not Have an Amoebostatic Effect on A. castellanii
3.3. Ultrastructural Analysis of Trophozoites Treated with apo-bLf
3.4. Apo-bLf Inhibits the Cytopathic Effect of A. castellanii
3.5. Cysteine and Serine Proteases of A. castellanii Degrade Human and Bovine apo-Lactoferrin
3.6. Apo-bLf Inhibits the Proteolytic Profile of A. castellanii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visvesvara, G.S. Infections with free-living amebae. Hand. Clin. Neurol. 2013, 114, 153–168. [Google Scholar] [CrossRef]
- Trabelsi, H.; Dendana, F.; Sellami, A.; Sellami, H.; Cheikhrouhou, F.; Neji, S.; Makni, F.; Ayadi, A. Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol. Biol. 2012, 60, 399–405. [Google Scholar] [CrossRef]
- De Jonckheere, J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol. 2011, 11, 1520–1528. [Google Scholar] [CrossRef]
- Rojas-Hernandez, S.; Jarillo-Luna, A.; Rodriguez-Monroy, M.; Moreno-Fierros, L.; Campos-Rodriguez, R. Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parasitol. Res. 2004, 94, 31–36. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G.A. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Microbiol. 2007, 51, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A. Free-living amoebae: Pathogenicity and immunity. Parasite Immunol. 1991, 13, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.S.; Jones, N.S.; Mason, J. The rheology of nasal mucus: A review. Clin. Otolaryngol. Allied. Sci. 1998, 23, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Estrada, G.; Luna-Castro, S.; Pina-Vazquez, C.; Samaniego-Barron, L.; Leon-Sicairos, N.; Serrano-Luna, J.; de la Garza, M. Iron-saturated lactoferrin and pathogenic protozoa: Could this protein be an iron source for their parasitic style of life? Future Microbiol. 2012, 7, 149–164. [Google Scholar] [CrossRef]
- Luna-Castro, S.; Aguilar-Romero, F.; Samaniego-Barron, L.; Godinez-Vargas, D.; de la Garza, M. Effect of bovine apo-lactoferrin on the growth and virulence of Actinobacillus pleuropneumoniae. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2014, 27, 891–903. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Willcox, M.D. Role of lactoferrin in the tear film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef]
- De la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar]
- Avalos-Gomez, C.; Ramirez-Rico, G.; Ruiz-Mazon, L.; Sicairos, N.L.; Serrano-Luna, J.; de la Garza, M. Lactoferrin: An Effective Weapon in the Battle Against Bacterial Infections. Curr. Pharm. Des. 2022, 40, 3243–3260. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawakita, T.; Inaba, T.; Okada, N.; Ito, M.; Shimmura, S.; Watanabe, M.; Shinmura, K.; Tsubota, K. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE 2012, 7, e33148. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef]
- Rainard, P. Bacteriostatic activity of bovine lactoferrin in mastitic milk. Vet. Microbiol. 1987, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.T., 3rd; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Dzitko, K.; Dziadek, B.; Dziadek, J.; Dlugonska, H. Toxoplasma gondii: Inhibition of the intracellular growth by human lactoferrin. Pol. J. Microbiol. 2007, 56, 25–32. [Google Scholar]
- Ikadai, H.; Tanaka, T.; Shibahara, N.; Tanaka, H.; Matsuu, A.; Kudo, N.; Shimazaki, K.; Igarashi, I.; Oyamada, T. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712. [Google Scholar] [CrossRef]
- Leon-Sicairos, N.; Reyes-Lopez, M.; Ordaz-Pichardo, C.; de la Garza, M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem. Cell Biol. 2006, 84, 327–336. [Google Scholar] [CrossRef]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: A randomized controlled trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Campos, M.; Pecho, I.; Prada, A.; McMahon, R.J.; Cleary, T.G. Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46, 1881–1883. [Google Scholar] [CrossRef]
- Waarts, B.L.; Aneke, O.J.; Smit, J.M.; Kimata, K.; Bittman, R.; Meijer, D.K.; Wilschut, J. Antiviral activity of human lactoferrin: Inhibition of alphavirus interaction with heparan sulfate. Virology 2005, 333, 284–292. [Google Scholar] [CrossRef]
- Reyes-Lopez, M.; Ramirez-Rico, G.; Serrano-Luna, J.; de la Garza, M. Activity of Apo-Lactoferrin on Pathogenic Protozoa. Pharmaceutics 2022, 14, 1702. [Google Scholar] [CrossRef]
- Coronado-Velazquez, D.; Silva-Olivares, A.; Castro-Munozledo, F.; Lares-Jimenez, L.F.; Rodriguez-Anaya, L.Z.; Shibayama, M.; Serrano-Luna, J. Acanthamoeba mauritaniensis genotype T4D: An environmental isolate displays pathogenic behavior. Parasitol. Int. 2020, 74, 102002. [Google Scholar] [CrossRef]
- Serrano-Luna, J.; Cervantes-Sandoval, I.; Calderon, J.; Navarro-Garcia, F.; Tsutsumi, V.; Shibayama, M. Protease activities of Acanthamoeba polyphaga and Acanthamoeba castellanii. Can. J. Microbiol. 2006, 52, 16–23. [Google Scholar] [CrossRef]
- Ramirez Rico, G.; Martinez-Castillo, M.; de la Garza, M.; Shibayama, M.; Serrano-Luna, J. Acanthamoeba castellanii Proteases are Capable of Degrading Iron-Binding Proteins as a Possible Mechanism of Pathogenicity. J. Eukaryot. Microbiol. 2015, 62, 614–622. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rico, G.; Drago-Serrano, M.E.; Leon-Sicairos, N.; de la Garza, M. Lactoferrin: A Nutraceutical with Activity against Colorectal Cancer. Front. Pharm. 2022, 13, 855852. [Google Scholar] [CrossRef] [PubMed]
- Zarzosa-Moreno, D.; Avalos-Gomez, C.; Ramirez-Texcalco, L.S.; Torres-Lopez, E.; Ramirez-Mondragon, R.; Hernandez-Ramirez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Aguila, A.; Herrera, A.G.; Morrison, D.; Cosgrove, B.; Perojo, A.; Montesinos, I.; Perez, J.; Sierra, G.; Gemmell, C.G.; Brock, J.H. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol. Med. Microbiol. 2001, 31, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Samaranayake, Y.H.; Samaranayake, L.P.; Nikawa, H. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 1999, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Omata, Y.; Saito, A.; Shimazaki, K.; Igarashi, I.; Suzuki, N. Growth inhibitory effects of bovine lactoferrin to Toxoplasma gondii parasites in murine somatic cells. J. Vet. Med. Sci. 1996, 58, 61–65. [Google Scholar] [CrossRef]
- Alsam, S.; Jeong, S.R.; Dudley, R.; Khan, N.A. Role of human tear fluid in Acanthamoeba interactions with the human corneal epithelial cells. Int. J. Med. Microbiol. 2008, 298, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Suzuki, C.; Wada, H.; Nomachi, M.; Imayasu, M.; Araki-Sasaki, K. Effects of lactoferrin on the viability and the encystment of Acanthamoeba trophozoites. Biochem. Cell Biol. 2017, 95, 48–52. [Google Scholar] [CrossRef]
- Leboffe, L.; Giansanti, F.; Antonini, G. Antifungal and Antiparasitic Activities of Lactoferrin. Anti-Infect. Agents Med. Chem. 2009, 8, 114–127. [Google Scholar] [CrossRef]
- Leon-Sicairos, N.; Reyes-Lopez, M.; Canizalez-Roman, A.; Bermudez-Cruz, R.M.; Serrano-Luna, J.; Arroyo, R.; de la Garza, M. Human hololactoferrin: Endocytosis and use as an iron source by the parasite Entamoeba histolytica. Microbiology 2005, 151, 3859–3871. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Martinez-Castillo, M.; Avalos-Gómez, C.; De la Garza, M. Bovine apo-lactoferrin affects the secretion of proteases in Mannheimia haemolytica A2. Acces Microbiol. 2021, 3, 269. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Noguera-Obenza, M.; Ebel, F.; Guzman, C.A.; Gomez, H.F.; Cleary, T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003, 71, 5149–5155. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Yu, Z.; Wu, S.; Ding, L.; Liu, J. Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-CoV-2. Leb. Wiss Technol. 2022, 154, 112684. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rico, G.; Martinez-Castillo, M.; Cárdenas-Zúñiga, R.; Coronado-Velázquez, D.; Silva-Olivares, A.; De la Garza, M.; Shibayama, M.; Serrano-Luna, J. Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin. Microorganisms 2023, 11, 708. https://doi.org/10.3390/microorganisms11030708
Ramírez-Rico G, Martinez-Castillo M, Cárdenas-Zúñiga R, Coronado-Velázquez D, Silva-Olivares A, De la Garza M, Shibayama M, Serrano-Luna J. Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin. Microorganisms. 2023; 11(3):708. https://doi.org/10.3390/microorganisms11030708
Chicago/Turabian StyleRamírez-Rico, Gerardo, Moises Martinez-Castillo, Roberto Cárdenas-Zúñiga, Daniel Coronado-Velázquez, Angélica Silva-Olivares, Mireya De la Garza, Mineko Shibayama, and Jesús Serrano-Luna. 2023. "Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin" Microorganisms 11, no. 3: 708. https://doi.org/10.3390/microorganisms11030708
APA StyleRamírez-Rico, G., Martinez-Castillo, M., Cárdenas-Zúñiga, R., Coronado-Velázquez, D., Silva-Olivares, A., De la Garza, M., Shibayama, M., & Serrano-Luna, J. (2023). Acanthamoeba castellanii Genotype T4: Inhibition of Proteases Activity and Cytopathic Effect by Bovine Apo-Lactoferrin. Microorganisms, 11(3), 708. https://doi.org/10.3390/microorganisms11030708




