1. Introduction
Acanthamoeba keratitis (AK) is a rare but potentially blinding corneal infection caused by the protozoan
Acanthamoeba, an opportunistic free-living parasite. Contact lens (CL) wear and trauma become the most important risk factors for AK. With the increasing use of contact lenses around the world, the incidence of AK is obviously rising [
1,
2]. Carnt et al. reported 36–65 AK cases per year in the UK from 2010 to 2017, representing a three to five times increase in incidence compared to 2004–2009 (15–23 cases per year) [
3]. Normally, the host immune system is capable of controlling this pathogen, while, under certain circumstances, for instance, wearing soft contact lens or orthokeratology [
4], could render individuals more susceptible to
Acanthamoeba infection. Because AK is frequently misdiagnosed as fungal or viral keratitis, as well as
Acanthamoeba being able to differentiate into cysts inside host tissues, which resist multiple antiseptics, AK is difficult to treat and so may result in blindness and corneal scarring, even in immunocompetent people [
5], as compared to
Acanthamoeba encephalitis, which occurs in immunocompromised patients more often. Understanding the micro change in AK is essential to further explore more interventions for pathogen killing.
Classically, inherent immunity is the main way to kill protozoa. The activated macrophages polarized into the M1 phenotype, which produces nitric oxide synthase, reactive oxygen species, interleukin-1 β (IL-1β), and tumor necrosis factor-alpha (TNF-α) to defend against infection [
6]. Meanwhile, macrophages can also be alternatively polarized into the M2 phenotype, which produces arginase-1 to control excessive inflammation and promote wound healing [
6,
7]. However, the histopathology showed the absence of inflammatory infiltrates in the deep stroma of the cornea with
Acanthamoeba [
8]. Even the zone containing amoebic cysts could be without noticeable inflammation [
9]. Although neutrophils are promptly recruited and visible around
Acanthamoeba trophozoites in the early inflammatory response of innate immunity [
10,
11], the histopathology suggested the disturbed immune response in AK, especially in the late stage, in which
Acanthamoeba are mainly present in the cornea as cysts. RNA sequencing could allow for the deep and efficient probing of the transcriptome, which may provide some clues about host immune reactivity to the
Acanthamoeba. The immune status and the roles of macrophages in the late stage of AK could be furtherly investigated, based on transcriptome sequencing technologies.
Signaling lymphocyte activation molecule (SLAM) family receptors are expressed on different types of hematopoietic cells and play an important role in immune regulation in health and disease. SLAM family-7 (SLAMF7), an immunomodulatory transmembrane receptor that is highly and uniformly expressed, is expressed on macrophages, natural killer cells, and other immune cells [
10]. Previously, SLAMF7 was found to have increased expression on M1 macrophages in a murine model with Pseudomonas aeruginosa keratitis [
11], which, in turn, promoted polarization of M2 through signal transducer and activators of transcription 6 (STAT6) activation and alleviated corneal inflammation [
11]. The checks and balances between the differentiated states of the macrophages may also play a role in
Acanthamoeba infection. However, the polarization states of macrophage, the expression level of SLAMF7, and the transcription factor STAT6 remain unclear in AK.
In this study, we used bulk RNA sequencing to illustrate the mRNA expression and enriched signaling pathways of corneas in AK patients, which may contribute to unveiling the immune condition between Acanthamoeba and host cornea. At the same time, we investigated the expression level of the SLAMF7/STAT6 pathway in the late stage of AK. The activation of SLAMF7/STAT6 signaling may affect immunological competence and tissue repair in AK. These findings may provide evidence and novel targets for the immunotherapy of AK.
2. Materials and Methods
2.1. Patients Enrolled
This study was conducted at the Beijing Tongren Hospital between September 2021 and May 2022 with the approval of the Medical Ethics Committee of Beijing Tongren Hospital (TRECKY2021-024), Beijing, China. The cornea tissues were from three AK patients and three donors from the local eye bank (Beijing Tongren Eye bank, Beijing, China). Because of an ethical problem, only corneal tissue in the advanced or late stage of AK cases could be collected. Based on Jiang’s staging criteria for AK, the inclusion of late-stage AK in this study was as follows: (1) typical clinical manifestations and at least one positive laboratory test (corneal scraping or cultures for Acanthamoeba); (2) presence of a deep stromal ulcer (diameter > 4 mm), a pale or yellowish-white purulent infiltration, and obvious anterior chamber hypopyon. At the same time, these participants with a history of ocular infection, ocular inflammation, ocular trauma or eye surgery, mixed ocular infections, cancer, and chronic infections such as Hepatitis B, C, and HIV were excluded. All participants were informed of the goals of the study and their written informed consent was obtained in accordance with the declaration of Helsinki. Patient information was recorded using a standard protocol, which included demographics, time to diagnosis from symptom onset, risk factors, a history of steroid use, preoperative and postoperative visual acuity of the affected eye, and surgical modality. Risk factors for AK include contact lens wear, ocular trauma, or exposure to potentially contaminated water. For all subjects, slit lamp, and in vivo confocal microscopy (IVCM) images were obtained. Slit lamp images were taken per eye at 10× times magnification using a calibrated Haag-Streit BX900 slit-lamp bio-microscope (Haag Streit AG, Bern, Switzerland). In addition, the confocal microscope HRT3 (Heidelberg Engineering, Heidelberg, Germany) was used for detecting Acanthamoeba trophozoite and cysts, and inflammatory cells with 800 magnifications. Laboratory examinations for AK included corneal scrapings, optical microscopic observation after Giemsa staining, and cultures on non-nutrient agar plates overlaid with Escherichia coli, performed in the Department of Ocular Microbiology at the Beijing Institute of Ophthalmology.
2.2. mRNA Extraction and Sequencing
Three AK patients received corneal transplantation. Half of each patient’s corneal button was used for RNA extraction. For two of the three patients, the other half of the corneal button was used for a histopathology examination. For the third patient, a quarter of the other half of the corneal button was used in Western blot, and the other quarter of that was used in quantitative real-time PCR (qRT-PCR). Total RNA was extracted and purified from cornea tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s procedure. The RNA amount and purity of each sample were quantified using NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA). The RNA integrity was assessed by Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) with RIN number > 7.0 and confirmed by electrophoresis with denaturing agarose gel. Poly-A mRNA was purified from 1 μg total RNA using Dynabeads Oligo (dT)25-61005 (Thermo Fisher, Carlsbad, CA, USA) using two rounds of purification. Then, the poly-A mRNA was fragmented into small pieces using Magnesium RNA Fragmentation Module (NEB, cat. e6150, Ipswich, MA, USA) at 94 °C 5 min. Then, the cleaved RNA fragments were reverse transcribed to create the cDNA by SuperScript™ II Reverse Transcriptase (Invitrogen, cat. 1896649, USA), which were next used to synthesize U-labeled second-stranded DNAs with Escherichia coli DNA polymerase I (NEB, cat.m0209, USA), RNase H (NEB, cat.m0297, USA), and dUTP Solution (Thermo Fisher, cat. R0133, USA). An A-base was then added to the blunt ends of each strand, preparing them for ligation to the indexed adapters. Each adapter contains a T-base overhang for ligating the adapter to the A-tailed fragmented DNA. Single- or dual-index adapters were ligated to the fragments and size selection was performed with AMPureXP beads. After the heat-labile UDG enzyme (NEB, cat.m0280, USA) treatment of the U-labeled second-stranded DNAs, the ligated products were amplified with PCR. The average insert size for the final cDNA library was 300 ± 50 bp. At last, we performed the 2 × 150 bp paired-end sequencing (PE150) on an illumina Novaseq™ 6000.
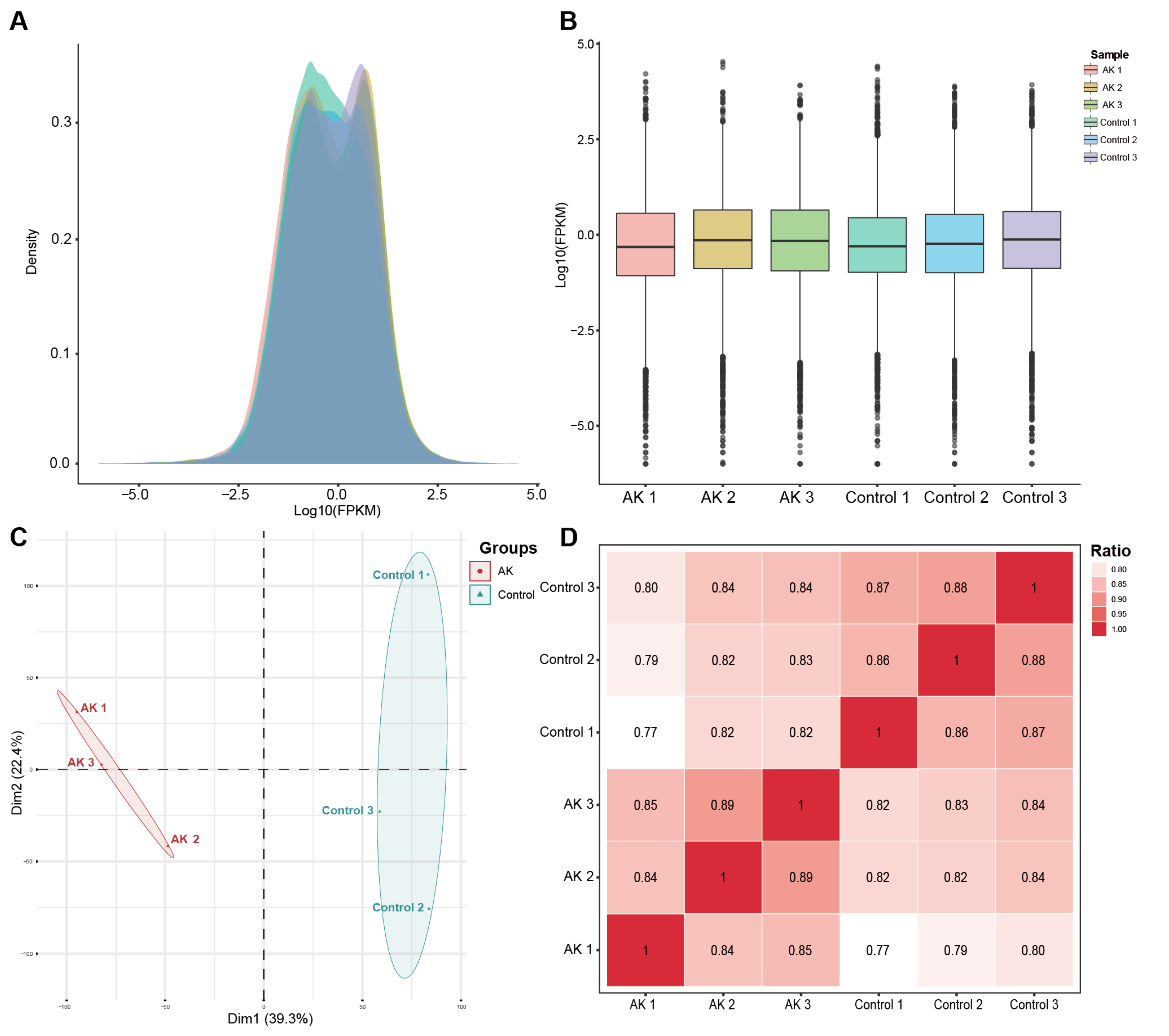
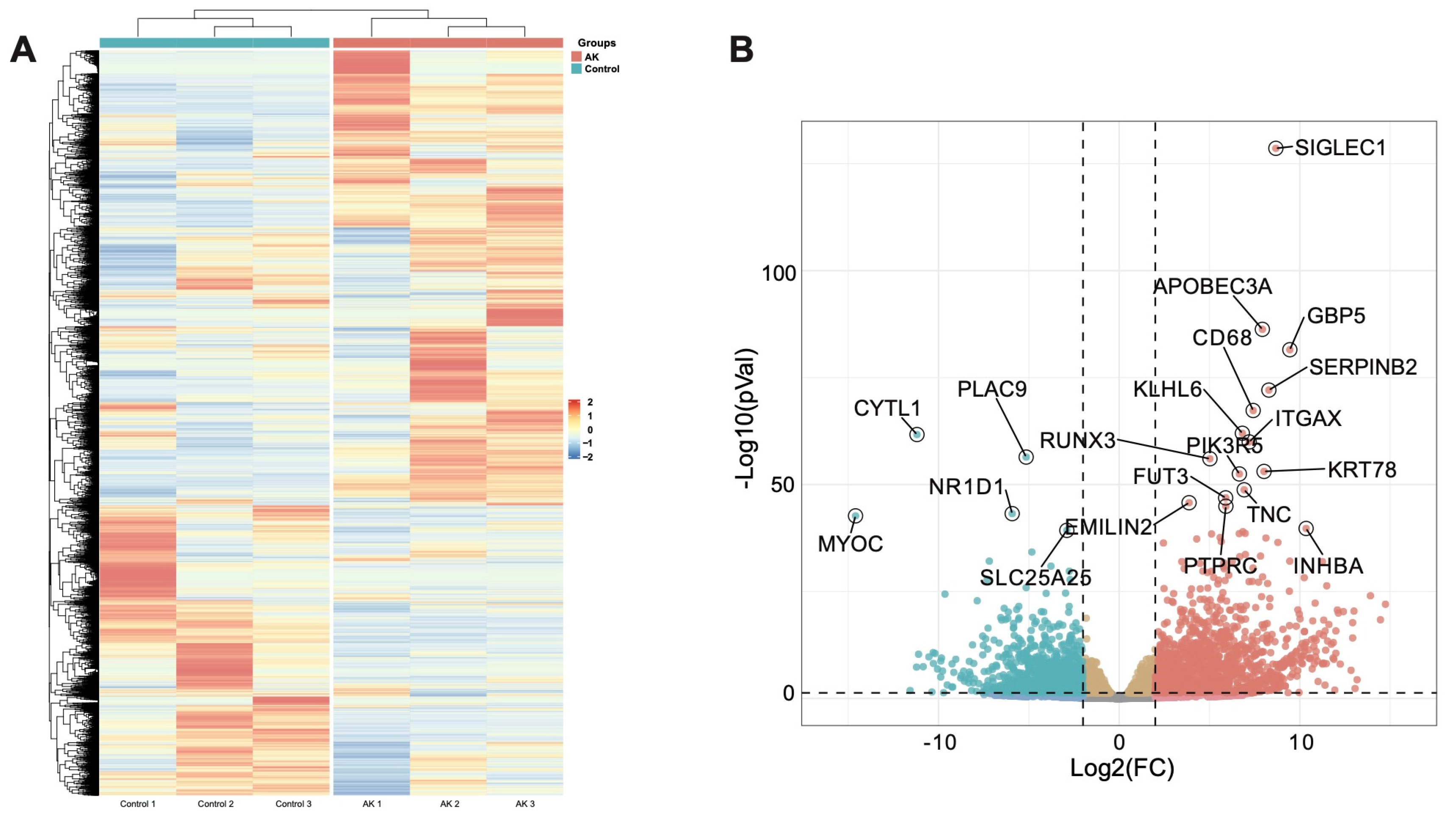
2.3. Differentially Expressed Gene Analysis
Fastp software (
https://github.com/OpenGene/fastp, accessed on 1 May 2022) was used to remove the reads that contained adaptor contamination, low-quality bases, and undetermined bases with default parameters. Then, sequence quality was also verified using fastp. HISAT2 (
https://ccb.jhu.edu/software/hisat2, accessed on 1 May 2022) was used to map reads to the reference genome of Homo sapiens GRCh38. The mapped reads of each sample were assembled using StringTie (
https://ccb.jhu.edu/software/stringtie, accessed on 1 May 2022) with default parameters. Then, all transcriptomes from all samples were merged to reconstruct a comprehensive transcriptome using gffcompare (
https://github.com/gpertea/gffcompare/, accessed on 1 May 2022). After the final transcriptome generation, StringTie was used to determine the expression level for mRNAs by calculating FPKM. The differentially expressed mRNAs were selected with fold change > 4 or fold change < −4 and with
p value < 0.05 by R package DESeq2 (
https://github.com/mikelove/DESeq2, accessed on 10 May 2022).
2.4. Pathway Enrichment Analysis
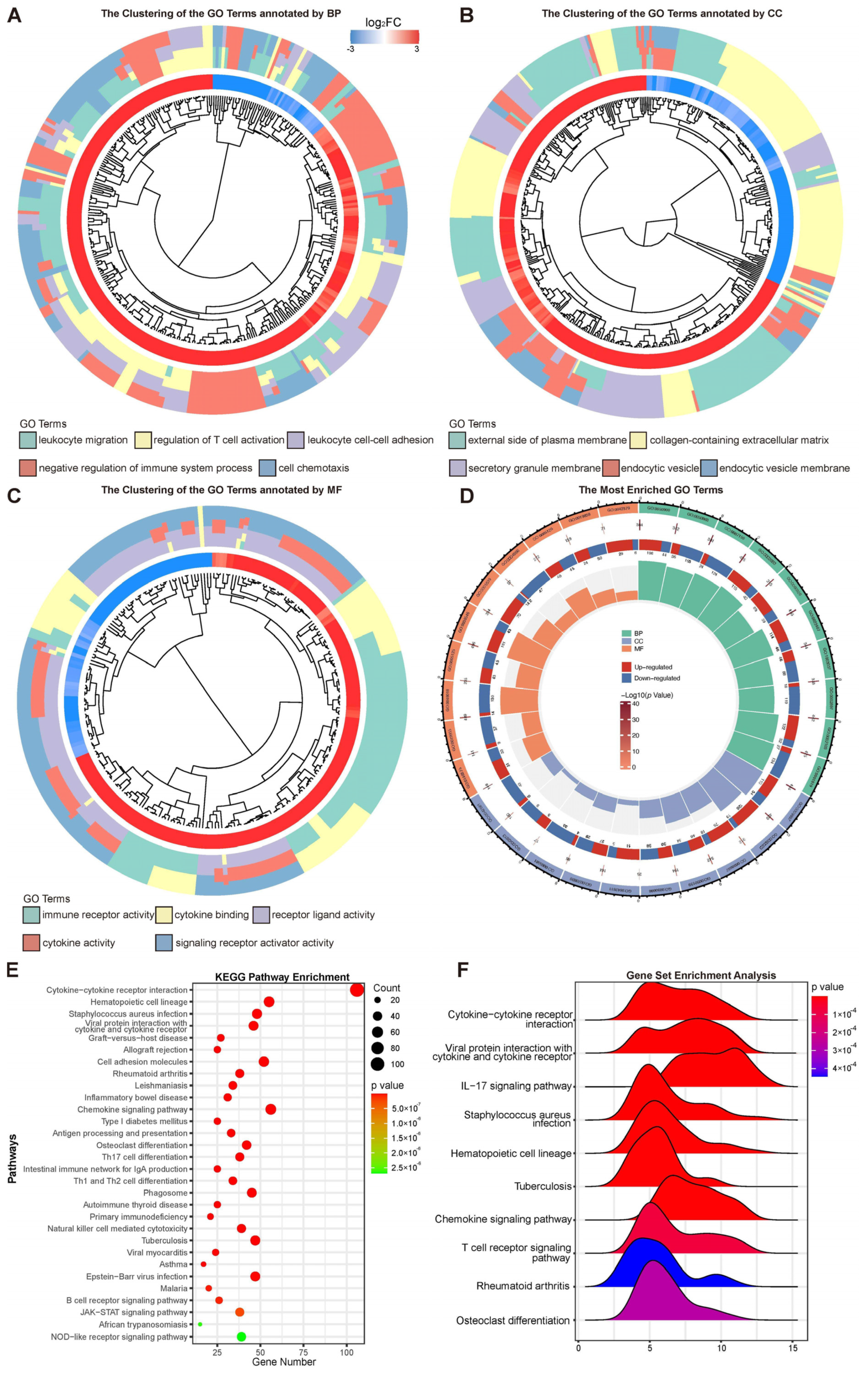
To better understand the function of differentially expressed genes, Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) were performed on the obtained differential genes. Since GO term has three independent aspects of biological process (BP), cellular component (CC), and molecular function (MF), clustering results were obtained based on the semantic similarity of GO terms. Then, KEGG and GO term gene set enrichment analysis (GSEA) was conducted using clusterProfiler. Normalized enrichment score (|NES| > 1), nominal p < 0.05, and false discovery rate (FDR) < 0.25 were considered statistically significant. The raw counts were loaded into R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analysis and the ridge plot was used for data representation.
2.5. Immune Infiltration Analysis
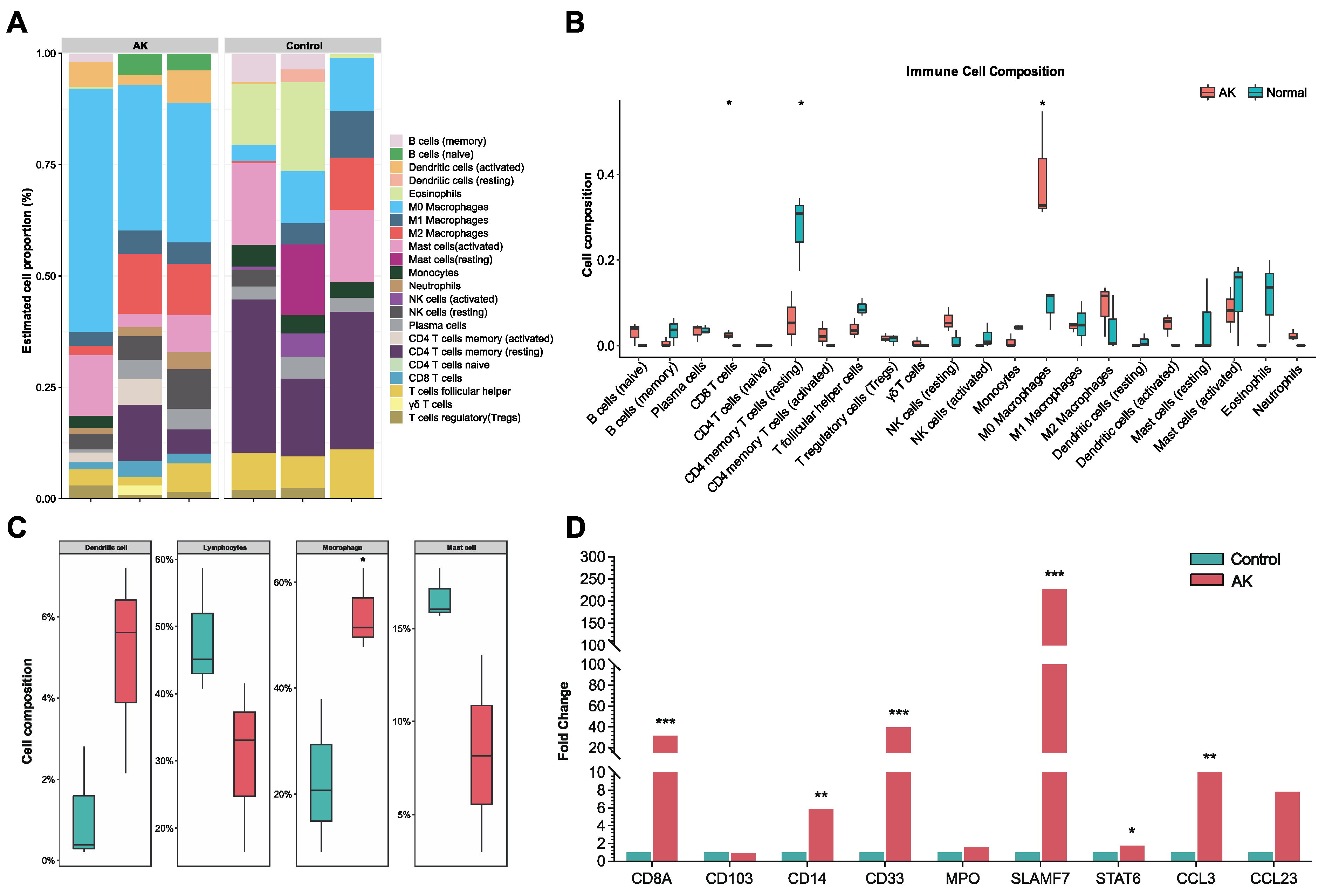
Based on Chen et al. method of CIBERSORT, the RNA content of all tissues was analyzed [
12]. Under LM22 as the signature gene file and permutation = 1000, the 22 immune cell profiles were reconstructed. The sum of all proportions of immune cell fractions was normalized to 1 in each sample and ggplot2 (version 3.3.6;
https://ggplot2.tidyverse.org/, accessed on 1 July 2022) was used to generate a stacked bar plot for better visualization. Further, the 4 aggregated immune cell types, including total lymphocytes, total dendritic cells, total macrophage, and total mast cell were calculated, following the method of Li et al. [
13].
2.6. Histopathology Examinations
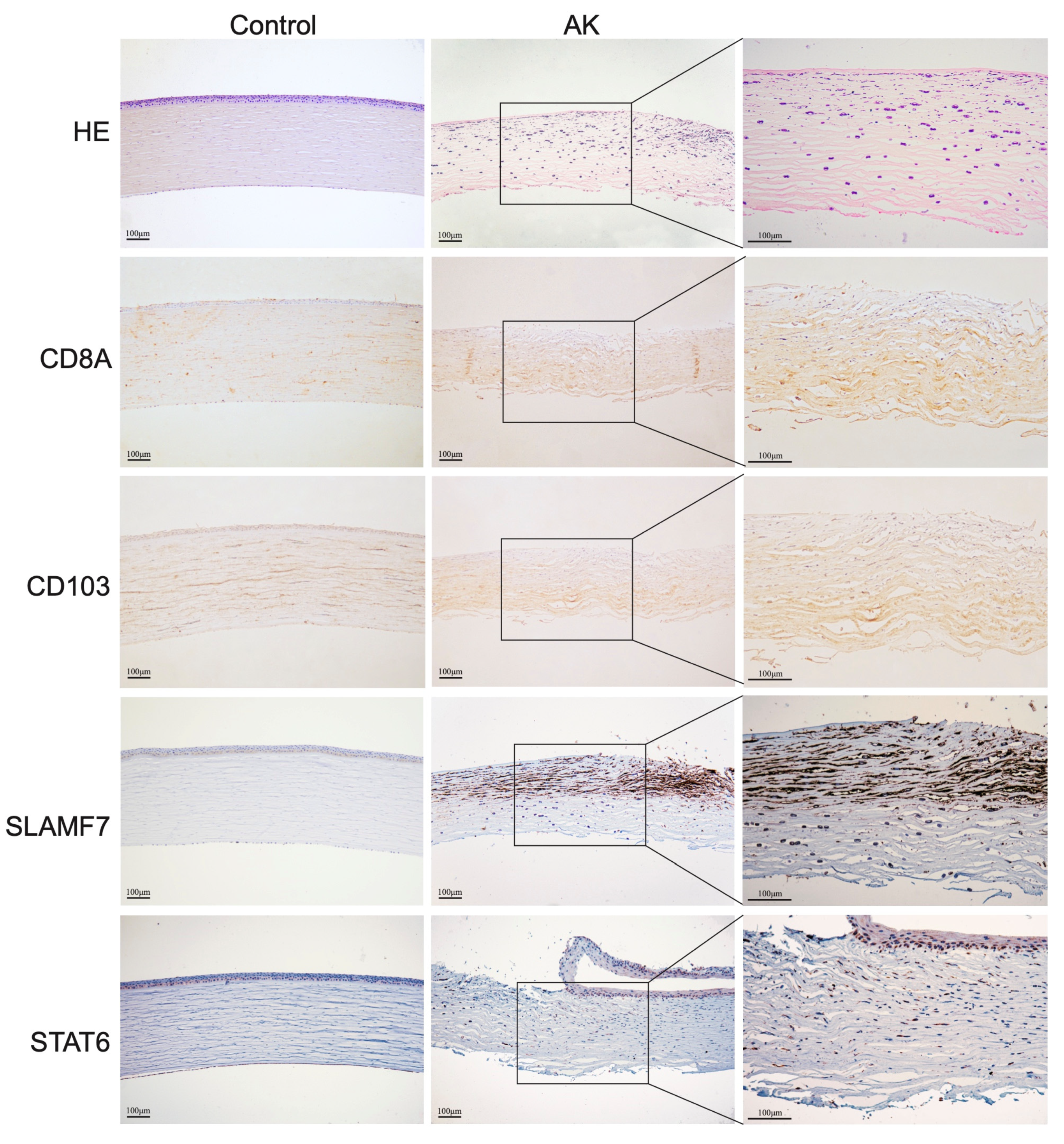
The corneal button was fixed in 4% paraformaldehyde (PFA) overnight and embedded in paraffin. Then, it was sectioned with a thickness of 4 μm, and slices were dewaxed and hydrated. Hematoxylin and eosin (HE) staining was conducted according to routine protocols. The slices for immunohistochemical staining were repaired with EDTA buffer. Endogenous peroxide activity was blocked by incubation of the slides with 3% H2O2 for 20 min. After being permeabilized (0.5% Triton X-100 in PBS) and blocked (10% Normal donkey serum and 0.5% Triton in PBS), the rabbit anti-human CD8a (1:800; Cell Signaling Technology, Danvers, MA, USA, #98941), CD4 (1:1000; Abcam, Boston, MA, USA, #ab183685), SLAMF7 (1:2000; Abcam, #ab32520), and STAT6 antibodies (1:200; Abcam, #ab230945) were applied overnight at 4 °C. The sections were washed and incubated with HRP-conjugated goat anti-rabbit IgG (1: 2000; Abcam, #ab205718) for 1 h at room temperature. They were photographed with a microscope equipped with a digital camera (Olympus BX-51, Olympus, Tokyo, Japan) and the cells with brown-stained cytoplasm were considered positive.
2.7. Western Analysis
Equal amounts of proteins were electrophoresed on 10% PAGE gels and transferred to a polyvinylidene fluoride membrane with a wet transfer system. The membranes were blocked with 5% skim milk for 1 h. After washing with PBS plus 0.05% Tween 20 (PBST), the membrane was incubated with primary antibodies overnight at 4 °C. In addition to the anti-human SLAMF7 and STAT6 antibodies described above, the rabbit anti-human phospho-stat6 antibody (1:1000; Cell Signaling Technology, #9361) and GAPDH antibody (1:2000; Proteintech, Rosemont, IL, USA, #1E6D9) were also used for Western analysis. The following day, the same secondary antibody as described in the aforementioned histopathology examinations was performed for 1 h at room temperature. Membranes were visualized by chemiluminescence using an enhanced chemiluminescence substrate.
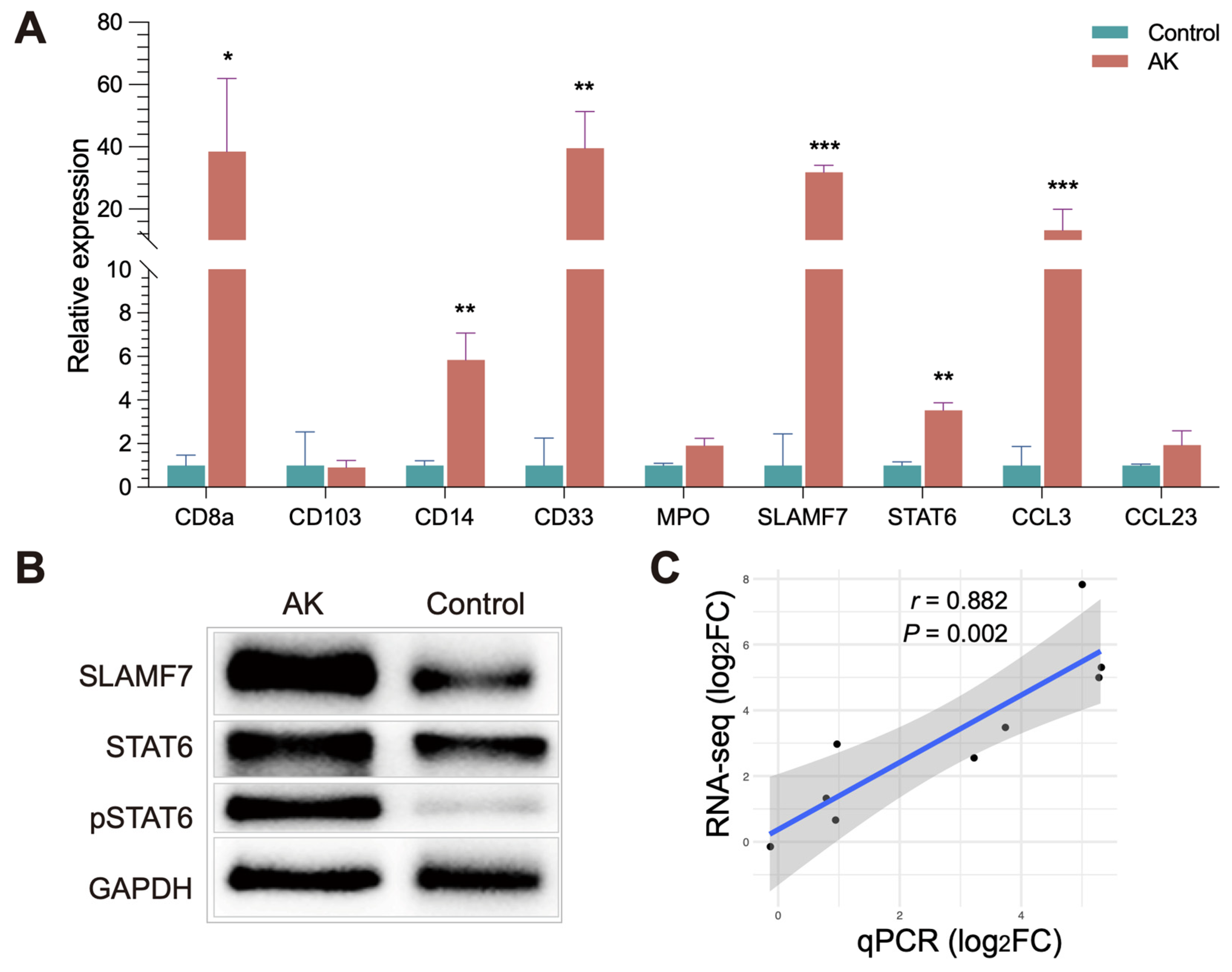
2.8. Quantitative Real-Time PCR
Total RNA of the cornea was extracted using Tissue Total RNA Isolation Kit V2 (Vazyme Medical Technology, Nanjing, China). RNA was reverse transcribed into cDNA using the qPCR RT Master Mix Kit (FSQ-301, Toyobo, Japan). qRT-PCR was performed using the Taq Pro Universal SYBR qPCR Master Mix (Vazyme Medical Technology, Nanjing, China) and conducted on ABI 7500 System. All experiments were repeated at least three times, and
p < 0.05 indicated a statistically significant difference. Relative expression levels were calculated using the comparative threshold cycle (Ct) method. Human β-actin expression acted as an endogenous reference to normalize relative gene expression. Primer sets used in qRT-PCR are listed in
Table S1.
2.9. Statistical Analysis
The data of qRT-PCR are presented as the mean ± standard error of the mean (SEM) according to statistical analysis. Statistical comparisons were analyzed using an unpaired Student’s t-test by graphing software. Correlation between RNA-seq and qRT-PCR was calculated by linear regression and Pearson correlation. p < 0.05 indicated statistical significance.
4. Discussion
AK is a rare disease with unfavorable therapeutic efficacy and dismal prognosis, which could occur in normal individuals [
17]. To date, the process of the immune response in the cornea of AK has been slightly revealed. To our knowledge, this is the first study to make bulk RNA sequencing of corneas in AK patients and analyze the immune state. In the current study, we performed a comprehensive evaluation of the immune landscape of AK and found that only several immune cells (M0 macrophage and CD8 T cells) had a significantly increased infiltration. However, most immune cells lacked significant infiltrate in the cornea of AK patients, compared with completely healthy controls. The findings herein suggested that escape from recognition by immune effector cells might appear in late-stage AK and ultimately lead to an extremely poor prognosis.
Previous studies had suggested this point. The clinicopathologic report found the macrophage and neutrophils presented in regions with cysts, but distribution of CD4 or CD8 T cells did not directly associate with
Acanthamoeba parasites. NK cells were absent in cornea of AK-infected individuals in the report [
18]. Another clinicopathologic study also found the
Acanthamoeba cysts grouped in a zone without noticeable inflammation [
9]. Compared with our study, they mentioned the infiltration of neutrophils, the origin of which is probably from the granulomatous retro-corneal membrane in their sample. Typically, the neutrophils are identified as the first responders to acute infections. In the current study, the samples were taken from the late-stage AK patients and HE staining showed no additional retro-corneal membrane.
The innate immune system is responsible for immediate host defense against pathogens upon infection. Former research in fungal keratitis indicated that inflammatory cytokines, IL-1β, IL-6, TNF- α, as well as Wnt, Hippo, and cGMP-PKG signaling pathways were significantly associated with the disease [
19]. Transcriptome analysis showed dominated innate immune response and lower levels of adaptive immune pathways in both late-stage bacterial and late-stage fungal keratitis [
20]. Meanwhile, in AK, T cells and B cells were not found around
Acanthamoeba-infected cornea regions and serum antibodies are not protective against this infection, as recrudescence also occurs [
18,
21], suggesting that adaptive immune response fails to protect the host and innate immune response is vital for disease resolution. As the depletion of conjunctival macrophages led to exacerbated
Acanthamoeba infection and increased number of neutrophils were found to accumulate in
Acanthamoeba-infected corneas [
10,
22], macrophages and neutrophils were believed to be two elements in the innate immune system important in
Acanthamoeba infection [
23]. Both cells were shown to kill
Acanthamoeba trophozoites in vitro [
24,
25]. In vivo, although cysts are not chemotactic towards macrophages and neutrophils, their lysates can be recognized and, thus, be phagocytosed by macrophages and be killed by neutrophils via myeloperoxidase [
26,
27,
28]. Interestingly, unlike neutrophils, macrophages were shown to maintain at the infection site, demonstrating their role in mounting immune response, as well as tissue repair [
18,
29,
30]. The state of pathway may provide the clue. In our study, lots of immune signaling pathways were activated, such as the leukocyte migration, leukocyte cell–cell adhesion, and immune receptor activity. At the same time, many pathways about immunoregulation were activated to inhibit the immune response, such as the regulation of T cell activation and the negative regulation of the immune system process. Several immune pathways, which most significantly increase in expression, have been identified as being of value in the defense against
Acanthamoeba infection, such as the IL-17 signaling pathway and Th17 cell differentiation [
16,
31]. The cross-talk among immune signaling pathways resulted in the current immune status.
We found part of the landscape of immune condition in human late-stage AK, where dendritic cells and macrophages increased, while lymphocytes decreased. As antigen-presenting cells, dendritic cells are crucial in both innate and acquired immune responses. The increased activated dendritic cells are in line with the concept that microbial molecules could induce the maturation of dendritic cells, while the decreased lymphocytes suggested that signaling pathways between dendritic cells and lymphocytes may be hampered in late-stage AK [
29]. M0 macrophages were significantly increased in the AK group compared to the control. Previous studies have found that
Acanthamoeba can activate proinflammatory M1 macrophage to produce IL-12 and IL-6 via TLR4-MyD88 pathway [
30]. In our study, transcriptome data of late-stage AK showed that the amount of M1 macrophage had no difference between AK and control groups, and M2 macrophages are increased in the AK group. SLAMF7, the regulator associated with M2 macrophages, was significantly increased in the AK group, suggesting its association with M2 polarization. Changes in SLAMF7 and STAT6 in AK patients were also observed by immunohistochemistry.
Figure 6A,B show that SLAMF7 and STAT6 were abundant in AK groups, mainly expressed in epithelial and anterior stromal cells. The activation of SLAMF7/STAT6 signaling pathway may offer direction about where to intervene in AK. The treatment of AK should gradually change from the traditional pharmacological treatment to immunotherapy. Therefore, excavating novel therapeutic targets for AK is significant. The present study provided clues for further study and data source.
Still, some limitations are present in this study. For example, in that lack of corneas from AK patients resulted in the small number of specimens recruited for analysis. Because bulk RNA sequencing is subject to having less accurate analysis to reveal the immune condition, single-cell sequencing should be carried out in the future and the results should be compared to other types of keratitis. On the other hand, although the function of SLAMF7/STAT6 pathway had been validated in P. aeruginosa keratitis and the expression of pathway had been observed in AK, further experimental validation is required to verify the effect and major target of SLAMF7/STAT6 pathway.