Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics
Abstract
1. Introduction
2. Materials and Methods
3. Results
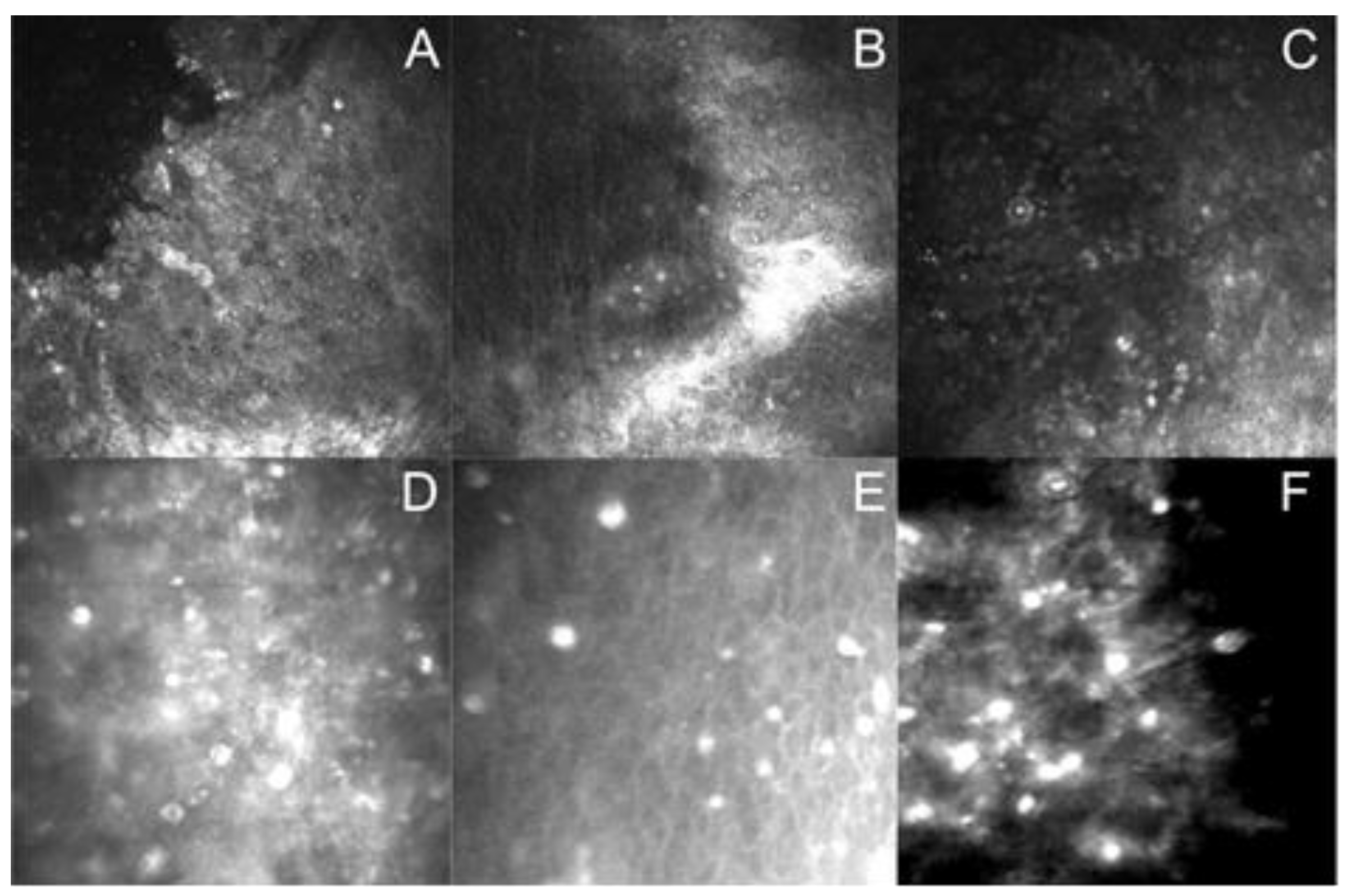
3.1. Laboratory Differential Diagnosis
Assessment of In Vitro Cultivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.J.; Visvesvara, G.S. Free-living, amphizoic and opportunistic amoebas. Brain Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Walochnik, J.; Obwaller, A.; Aspöck, H. Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclnical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 2000, 66, 4408–4413. [Google Scholar] [CrossRef] [PubMed]
- Rohr, U.; Weber, S.; Michel, R.; Selenka, F.; Wilhelm, M. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol. 1998, 64, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef]
- Caumo, K.; Frasson, A.P.; Pens, C.J.; Panatieri, L.F.; Frazzon, A.P.; Rott, M.B. Potentially pathogenic Acanthamoeba in swimming polls: In survey in the southern Brazilian city of Porto Alegre. Ann. Trop. Med. Parasitol. 2009, 103, 477–485. [Google Scholar] [CrossRef]
- Gianinazzi, C.; Schild, M.; Wüthrich, F.; Müller, N.; Schürch, N.; Gottstein, B. Potentially human pathogenic Acanthamoeba isolated from a heated indoor swimming pool in Switzerland. Exp. Parasitol. 2009, 121, 180–186. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef]
- van Hamme, C.; Dumont, M.; Delos, M.; Lachapelle, J.M. Cutaneous acanthamoebiasis in a lung transplant patient. Ann. Dermatol. Vénéréol. 2001, 28, 1237–1240. [Google Scholar]
- Teknos, T.N.; Poulin, M.D.; Laruentano, A.M.; Li, K.K. Acanthamoeba rhinosinusitis: Characterization, diagnosis and treatment. Am. J. Rhinol. Allergy 2000, 14, 387–391. [Google Scholar] [CrossRef]
- Visvesvara, G.S. Amebic meningoencephalitides and keratitis: Challenges in diagnosis and treatment. Curr. Opin. Infect. Dis. 2010, 23, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Naginton, J.; Watson, P.G.; Playfair, T.J.; McGill, J.; Jones, B.R.; Steele, A.D. Amoebic infection of the eye. Lancet 1974, 2, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.B.; McCulley, J.P.; Luckenbach, M.; Gelender, H.; Newton, C.; McDonald, M.B.; Visvesvara, G.S. Acanthamoeba keratitis associated with soft contact lenses. Am. J. Ophthalmol. 1985, 100, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Pathogenesis of Acanthamoeba infections. Microb. Pathog. 2003, 34, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Factors affecting the epidemiology of Acanthamoeba keratitis. Ophthalmic Epidemiol. 2007, 14, 53–60. [Google Scholar] [CrossRef]
- Chomicz, L.; Piekarczyk, J.; Starościak, B.; Fiedor, P.; Piekarczyk, B.; Szubińska, D.; Zawadzki, P.J.; Walski, M. Comparative studies on the occurrence of protozoans, bacteria and fungi in the oral cavity of patients with systemic disorders. Acta Parasitol. 2002, 47, 147–153. [Google Scholar]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Trabelsi, H.; Sellami, A.; Dendena, F.; Sellami, H.; Cheikh-Rouhou, F.; Makni, F.; Dhiaa, S.B.; Ayadi, A. Free-living amoebae (FLA): Morphological and molecular identification of Acanthamoeba in dental unit water. Amibes libres: Identification morphologique et moléculaire d’Acanthamoeba dans l’eau des unités dentaires. Parasite 2010, 17, 67–70. [Google Scholar] [CrossRef]
- Zawadzki, P.J.; Perkowski, K.; Padzik, M.; Mierzwińska-Nastalska, E.; Szaflik, J.P.; Conn, D.B.; Chomicz, L. Examination of oral microbiota diversity in adults and older adults as an approach to prevent spread of risk factors for human infections. BioMed Res Int. 2017, 2017, 8106491. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- Wesołowska, M.; Cisowska, A.; Myjak, P.; Marek, J.; Jurowskaka-Liput, J.; Jakubaszko, J. Acanthamoeba keratitis in contact lens wearers in Poland. Adv. Clin. Exp. Med. 2006, 15, 553–555. [Google Scholar]
- Gatti, S.; Rama, P.; Matuska, S.; Berrilli, F.; Cavallero, A.; Carletti, S.; Bruno, S.; Maserati, R.; Di Cave, D. Isolation and genotyping of Acanthamoeba strains from corneal infections in Italy. J. Med. Microbiol. 2010, 59, 1324–1330. [Google Scholar] [CrossRef]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Conover, C.S.; Chen, S.; McConnell, K.A.; Chang, D.C.; et al. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis. 2009, 15, 1236–1242. [Google Scholar] [CrossRef]
- Duarte, J.L.; Furst, C.; Klisiowicz, D.R.; Klassen, G.; Costa, A.O. Morphological, genotypic, and physiological characterization of Acanthamoeba isolates from keratitis patients and the domestic environment in Vitoria, Espírito Santo, Brazil. Exp. Parasitol. 2013, 135, 9–14. [Google Scholar] [CrossRef]
- Page, M.A.; William, D.M. Acanthamoeba Keratitis: A 12-year experience covering a wide spectrum of presentations, diagnoses, and outcomes. J. Ophthalmol. 2013, 2013, 670242. [Google Scholar] [CrossRef]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, P.; Rao, G.N. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br. J. Ophthalmol. 2000, 84, 1103–1108. [Google Scholar] [CrossRef]
- Syam, P.P.; Narendran, R.; van der Hoek, J. Persistent Acanthamoeba keratitis in a non-contact lens wearer following exposure to bird seed dust. Br. J. Ophthalmol. 2005, 89, 388–389. [Google Scholar] [CrossRef]
- Ertabaklar, H.; Türk, M.; Dayanir, V.; Ertug, S.; Walochnik, J. Acanthamoeba keratitis due to Acanthamoeba genotype T4 in a non-contact lens wearer in Turkey. Parasitol. Res. 2007, 100, 241–246. [Google Scholar] [CrossRef]
- Garg, P.; Kalra, P.; Joseph, J. Non-contact lens related Acanthamoeba keratitis. Indian J. Ophthalmol. 2017, 65, 1079–1086. [Google Scholar] [CrossRef]
- Chomicz, L.; Conn, D.B.; Padzik, M.; Szaflik, J.P.; Walochnik, J.; Zawadzki, P.J.; Pawlowski, W.; Dybicz, M. Emerging threats for human health in Poland: Pathogenic isolates from drug resistant Acanthamoeba keratitis monitored in terms of their in vitro dynamics and temperature adaptability. BioMed Res. Int. 2015, 2015, 231285. [Google Scholar] [CrossRef] [PubMed]
- Walochnik, J.; Picher, O.; Aspöck, C.; Ullmann, M.; Sommer, R.; Aspöck, H. Interactions of “Limax amoebae” and gramnegative bacteria: Experimental studies and review of current problems. Tokai J. Exp. Clin. Med. 1999, 23, 273–278. [Google Scholar]
- Berger, P.; Papazian, L.; Drancourt, M.; Lascolat, B.; Auffray, J.P.; Raoult, D. Amoeba associated microorganisms and diagnosis of nosocomial pneumonia. Emerg. Infect. Dis. 2006, 12, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Couso, H.; Paniagua-Crespo, E.; Ares-Mazás, E. Acanthamoeba as a temporal vehicle of Cryptosporidium. Parasitol. Res. 2007, 100, 1151–1154. [Google Scholar] [CrossRef]
- Thomas, V.; McDonnell, G.; Denyer, S.P.; Maillard, J.-Y. Free-Living Amoebae and their intracellular pathogenic microorganisms: Risks for Water Quality. FEMS Microbiol. Rev. 2010, 34, 231–259. [Google Scholar] [CrossRef]
- Cateau, E.; Verdon, J.; Fernandez, B.; Hechard, Y.; Rodier, M.H. Acanthamoeba sp. promotes the survival and growth of Acinetobacter baumannii. FEMS Microbiol. Lett. 2011, 319, 19–25. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M. Peralta RHS Acanthamoeba spp. as an universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10–15. [Google Scholar] [CrossRef]
- Chomicz, L.; Szaflik, J.P.; Padzik, M.; Izdebska, J. Acanthamoeba keratitis: The emerging vision-threatening corneal disease. In Advances in Common Eye Infections; Rumelt, S., Ed.; IntechOpen: London, UK, 2016; Volume 4, pp. 99–120. [Google Scholar]
- Maycock, N.J.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, treatment, and outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef]
- Shimmura-Tomita, M.; Takano, H.; Kinoshita, N.; Toyoda, F.; Tanaka, Y.; Takagi, R.; Kobayashi, M.; Kakehashi, A. Risk factors and clinical signs of severe Acanthamoeba keratitis. Clin. Ophthalmol. 2018, 12, 2567–2573. [Google Scholar] [CrossRef]
- Tu, E.Y.; Joslin, C.E.; Sugar, J.; Booton, G.C.; Shoff, M.E.; Fuerst, P.A. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea 2008, 27, 764–772. [Google Scholar] [CrossRef]
- Rezaei Kanavi, M.; Naghshgar, N.; Javadi, M.A.; Sadat Hashemi, M. Various confocalscan features of cysts and trophozoites in cases with Acanthamoeba keratitis. Eur. J. Ophthalmol. 2012, 22, 46–50. [Google Scholar] [CrossRef]
- Huang, P.; Tepelus, T.; Vickers, L.A.; Baghdasaryan, E.; Huang, J.; Irvine, J.A.; Hsu, H.Y.; Sadda, S.; Lee, O.L. Quantitative analysis of depth, distribution, and density of cysts in Acanthamoeba keratitis using confocal microscopy. Cornea 2017, 36, 927–932. [Google Scholar] [CrossRef]
- Wei, Z.; Cao, K.; Wang, L.; Baudouin, C.; Labbe, A.; Liang, Q. Corneal changes in Acanthamoeba keratitis at various levels of severity: An in vivo confocal microscopic study. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Varacalli, G.; Di Zazzo, A.; Mori, T.; Dohlman, T.H.; Spelta, S.; Coassin, M.; Bonini, S. Challenges in Acanthamoeba Keratitis: A Review. J. Clin. Med. 2021, 10, 942. [Google Scholar] [CrossRef]
- Juárez, M.M.; Tártara, L.I.; Cid, A.G.; Real, J.P.; Bermúdez, J.M.; Rajal, V.B.; Palma, S.D. Acanthamoeba in the eye, can the parasite hide even more? Latest developments on the disease. Contact Lens Anterior Eye 2018, 41, 245–251. [Google Scholar] [CrossRef]
- Szentmary, N.; Daas, L.; Shi, L.; Lenke Laurik, K.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis—Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The biology of Acanthamoeba keratitis. Exp. Eye Res. 2021, 202, 108365. [Google Scholar] [CrossRef]
- Chomicz, L.; Padzik, M.; Szaflik, J.P.; Nahorski, W.L.; Kryczka, T.; Szaflik, J. Monitoring of in vitro dynamics of Acanthamoeba strains isolated from infected eyes as a useful tool in keratitis management. Exp. Parasitol. 2014, 145, 73–77. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and Pathogenesis, 2nd ed.; Caister Academic Press: Poole, UK, 2015. [Google Scholar]
- Köhsler, M.; Leitsch, D.; Mülle, N.; Walochnik, J. Validation of reference genes for the normalization of RT-qPCR gene expression in Acanthamoeba spp. Sci. Rep. 2020, 10, 10362. [Google Scholar] [CrossRef]
- Baltaza, W.; Padzik, M.; Szaflik, J.P.; Dybicz, M.; Grochowska, A.; Kuryłowicz, A.; Chomicz, L. Comparative examination on selected amphizoic amoebae in terms of their in vitro temperature tolerance—A possible indirect marker of potential pathogenicity of Acanthamoeba strains. Ann. Parasitol. 2018, 64, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, T.; Criado-Fornelio, A.; Martinez, J.; Blanco, M.A.; Fuentes, I.; Perez-Serrano, J. Isolation and molecular characterization of Acanthamoeba from patients with keratitis in Spain. Eur. J. Protistol. 2017, 61, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Solarczyk, P.; Cholewiński, M.; Hadaś, E. Genotypic characterization of amoeba isolated from Acanthamoeba keratitis in Poland. Parasitol. Res. 2015, 114, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Szostakowska, B.; Idzińska, A.; Chomicz, L. The first genotype determination of Acanthamoeba potential threat to human health, isolated from natural water reservoirs in Poland. Parasitol. Res. 2014, 113, 2693–2699. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001, 2, 1903–1911. [Google Scholar] [CrossRef]
- Červa, L.; Novak, K. Amoebic meningoencephalitis: Sixteen fatalities. Science 1968, 160, 92. [Google Scholar] [CrossRef]
- Chomicz, L.; Żebrowska, J.; Piekarczyk, J.; Starościak, B.; Myjak, P.; Walski, M.; Kazimierczuk, Z. In vitro studies on susceptibility of Acanthamoeba castellanii to selected chemical agents. Acta Parasitol. 2005, 50, 25–31. [Google Scholar]
- Page, F.C. A revised classification of the Gymnamoebia (Protozoa: Sarcodina). Zool. J. Linn. Soc. 1976, 58, 61–77. [Google Scholar] [CrossRef]
- Bonini, S.; Di Zazzo, A.; Varacalli, G.; Coassin, M. Acanthamoeba Keratitis: Perspectives for Patients. Curr. Eye Res. 2020, 46, 771–776. [Google Scholar]
- Padzik, M.; Baltaza, W.; Conn, D.B.; Szaflik, J.P.; Chomicz, L. Effect of povidone iodine, chlorhexidine digluconate and toyocamycin on amphizoic amoebic strains, infectious agents of Acanthamoeba keratitis—A growing threat to human health worldwide. Ann. Agric. Environ. Med. 2018, 25, 725–731. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; San Juan Martin, C.; Soliveri de Carranza, J.; Copa-Patino, J.L.; Pérez-Serrano, J. Acanthamoeba castellanii: In vitro UAH-T17c3 trophozoite growth study in different culture media. Parasitol. Res. 2012, 110, 2563–2567. [Google Scholar] [CrossRef]
- Bouten, M.; Elsheikha, H.M. Diagnosis and Management of Acanthamoeba Keratitis: A Continental Approach. Parasitologia 2022, 2, 167–197. [Google Scholar] [CrossRef]

| Corneal Sample | Acanthamoeba Strain | Probable Factors Predisposing to AK | Duration of Symptoms Before Proper Diagnosis | Amoebic Forms Visualized in Contrast Phase Microscopic Slides of: | |
|---|---|---|---|---|---|
| Corneal Scrapings | In vitro Cultures | ||||
| 1f | A. polyphaga | swimming in a lake | 35 days # | cysts and moving trophozoites | cysts and trophozoites |
| 2f | A. castellanii | CL | 30 days | cysts | cysts and trophozoites |
| 3f | A. castellanii | not identified | 24 days | no amoebae detected | a few cysts and trophozoites |
| 4f | A. castellanii | CL | 5 days | no amoebae detected | cysts and trophozoites |
| 5f | A. mauritanensis | CL | 35 days # | cysts | multiple cysts and trophozoites |
| 6f | A. mauritanensis | CL, washing in tap water | 30 days # | cysts and trophozoites | multiple cysts and trophozoites |
| 7f | Acanthamoeba sp. | CL | 7 days | a few cysts detected | no amoebae detected |
| 8m | A. castellanii | CL, swimming in a pool | 26 days # | cysts | cysts and trophozoites |
| 9m | A. castellanii | CL | 38 days # | cysts | cysts and trophozoites |
| 10f | Acanthamoeba sp. | CL, eye injuries | 6 months #; misdiagnosis | cysts | cysts and trophozoites |
| 11f | A. culbertsoni | CL | 8 days # | a few cysts detected | cysts and trophozoites |
| 12f | Acanthamoeba sp. | CL | 10 days # | cysts | cysts and trophozoites |
| 13m | A. castellanii | CL | 26 days # | multiple cysts and trophozoites | cysts and trophozoites |
| 14m | Acanthamoeba sp. | swimming with CL in a pool | 10 days # | several cysts | no amoebae detected |
| 15m | Acanthamoeba sp. | CL | 21 days # | cysts | cysts and trophozoites |
| 16f | Acanthamoeba sp. | CL | 8 days # | cysts | and trophozoites |
| 17m | Acanthamoeba sp. | swimming with CL in a pool | 8 days # | no scrapings | not cultivated |
| 18m | Acanthamoeba sp. | not identified | 6 months #; misdiagnosis | cysts | cysts and trophozoites |
| 19f | Acanthamoeba sp. | showering with CL | 26 days # | cysts | no amoebae developed |
| 20m | Acanthamoeba sp. | CL, swimming in a pool | 8 days # | no scrapings | not cultivated |
| Corneal Sample | Acanthamoeba Strain/Accession No in GenBank | Duration of Cultures Survival | Ability to Intense Multiply in Culture Medium |
|---|---|---|---|
| 1f | A. polyphaga MZ401143 | 35 months, still active | all time of cultivation, still |
| 3f | A. castellanii MZ401144 | 15 days | during 2 cycles of sub-culturing |
| 4f | A. castellanii MZ401145 | 10 days | during 1 cycle of sub-culturing |
| 5f | A. mauritanensis MZ401146 | 25 months, still active | all time of cultivation |
| 8m | A. castellanii MZ401150 | 3 months | during 2 cycles of sub-culturing |
| 9m | A. castellanii MZ401151 | 3.5 months | during 3 cycles of sub-culturing |
| 10f | Acanthamoeba sp. MZ401148 | 6 months, still active | during 10 cycles of sub-culturing |
| 11f | A. culbertsoni MZ401149 | 7 months, still active | all time of cultivation |
| 13m | A. castellanii MZ401152 | 2 months | during 2 cycles of sub-culturing |
| 15m | Acanthamoeba sp. | 4 weeks | during 2 cycles of sub-culturing |
| 16f | Acanthamoeba sp. | 5 weeks | during 2 cycles of sub-culturing |
| 18m | Acanthamoeba sp. | 2.5 months | during 5 cycles of sub-culturing |
| No. | Acanthamoeba Strain | Range of Overall Acanthamoeba Number (×103) Range of Cysts (%) | AVG | SD | Range of Overall Acanthamoeba Number (×103) Range of Cysts (%) | AVG | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 °C | 37 °C | ||||||||||
| 1f | A. polyphaga | 66.75 | 97.50 | 114.60 | 92.95 | 24.25 | 105.00 | 120.80 | 140.65 | 122.15 | 17.86 |
| 1.20 | 2.80 | 3.20 | 2.40 | 1.06 | 2.00 | 3.00 | 3.50 | 2.83 | 0.76 | ||
| 4f | A. castellanii | 12.40 | 15.75 | 28.12 | 18.76 | 8.28 | 10.15 | 14.05 | 15.80 | 13.33 | 2.89 |
| 2.20 | 6.00 | 12.20 | 6.80 | 5.05 | 2.40 | 3.50 | 7.20 | 4.37 | 2.51 | ||
| 5f | A. mauritanensis | 52.24 | 60.55 | 73.40 | 62.06 | 10.66 | 61.30 | 79.30 | 105.50 | 82.03 | 22.23 |
| 5.20 | 7.00 | 8.20 | 6.80 | 1.51 | 4.00 | 6.50 | 8.00 | 6.17 | 2.02 | ||
| 9m | A. castellanii | 24.05 | 45.60 | 62.24 | 43.96 | 19.15 | 46.50 | 62.40 | 80.82 | 63.24 | 17.18 |
| 2.30 | 4.00 | 6.00 | 4.10 | 1.85 | 3.00 | 3.80 | 7.00 | 4.60 | 2.12 | ||
| 11f | A. culbertsoni | 55.50 | 75.20 | 88.00 | 72.90 | 16.37 | 67.00 | 87.78 | 97.80 | 84.19 | 15.71 |
| 1.80 | 3.20 | 5.50 | 3.50 | 1.87 | 3.00 | 4.70 | 5.30 | 4.33 | 1.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomicz, L.; Szaflik, J.P.; Szostakowska, B.; Izdebska, J.; Baltaza, W.; Łazicka-Gałecka, M.; Kuligowska, A.; Machalińska, A.; Zawadzki, P.J.; Szaflik, J. Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics. Microorganisms 2023, 11, 1174. https://doi.org/10.3390/microorganisms11051174
Chomicz L, Szaflik JP, Szostakowska B, Izdebska J, Baltaza W, Łazicka-Gałecka M, Kuligowska A, Machalińska A, Zawadzki PJ, Szaflik J. Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics. Microorganisms. 2023; 11(5):1174. https://doi.org/10.3390/microorganisms11051174
Chicago/Turabian StyleChomicz, Lidia, Jacek P. Szaflik, Beata Szostakowska, Justyna Izdebska, Wanda Baltaza, Monika Łazicka-Gałecka, Agnieszka Kuligowska, Anna Machalińska, Paweł J. Zawadzki, and Jerzy Szaflik. 2023. "Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics" Microorganisms 11, no. 5: 1174. https://doi.org/10.3390/microorganisms11051174
APA StyleChomicz, L., Szaflik, J. P., Szostakowska, B., Izdebska, J., Baltaza, W., Łazicka-Gałecka, M., Kuligowska, A., Machalińska, A., Zawadzki, P. J., & Szaflik, J. (2023). Successive Acanthamoeba Corneal Isolates Identified in Poland Monitored in Terms of In Vitro Dynamics. Microorganisms, 11(5), 1174. https://doi.org/10.3390/microorganisms11051174






