Abstract
Recent studies reported that highly abundant alkane content exists in the ~11,000 m sediment of the Mariana Trench, and a few key alkane-degrading bacteria were identified in the Mariana Trench. At present, most of the studies on microbes for degrading hydrocarbons were performed mainly at atmospheric pressure (0.1 MPa) and room temperature; little is known about which microbes could be enriched with the addition of n-alkanes under in-situ environmental pressure and temperature conditions in the hadal zone. In this study, we conducted microbial enrichments of sediment from the Mariana Trench with short-chain (SCAs, C7–C17) or long-chain (LCAs, C18–C36) n-alkanes and incubated them at 0.1 MPa/100 MPa and 4 °C under aerobic or anaerobic conditions for 150 days. Microbial diversity analysis showed that a higher microbial diversity was observed at 100 MPa than at 0.1 MPa, irrespective of whether SCAs or LCAs were added. Non-metric multidimensional scaling (nMDS) and hierarchical cluster analysis revealed that different microbial clusters were formed according to hydrostatic pressure and oxygen. Significantly different microbial communities were formed according to pressure or oxygen (p < 0.05). For example, Gammaproteobacteria (Thalassolituus) were the most abundant anaerobic n-alkanes-enriched microbes at 0.1 MPa, whereas the microbial communities shifted to dominance by Gammaproteobacteria (Idiomarina, Halomonas, and Methylophaga) and Bacteroidetes (Arenibacter) at 100 MPa. Compared to the anaerobic treatments, Actinobacteria (Microbacterium) and Alphaproteobacteria (Sulfitobacter and Phenylobacterium) were the most abundant groups with the addition of hydrocarbon under aerobic conditions at 100 MPa. Our results revealed that unique n-alkane-enriched microorganisms were present in the deepest sediment of the Mariana Trench, which may imply that extremely high hydrostatic pressure (100 MPa) and oxygen dramatically affected the processes of microbial-mediated alkane utilization.
1. Introduction
Hydrocarbons are ubiquitous in the oceans and are mainly derived from natural seepage and oil spills [1,2,3]. Alkanes, which are divided into n-alkanes, branched-chain alkanes, and cycloalkanes, are major constituents of petroleum [4]. A range of alkanes is produced by plants, green algae, bacteria, and animals [5,6]. In particular, marine cyanobacteria were reported to produce and accumulate hydrocarbons (mainly C15 and C17 alkanes), with a global ocean hydrocarbon production of ∼308–771 million tons annually [2,6].
Microbes from all three domains of life can utilize hydrocarbons as the sole source of carbon and energy [7,8]. A total of at least 175 prokaryotic genera are known to degrade hydrocarbons under aerobic or anaerobic conditions [9]. For example, hydrocarbon-degrading bacteria contained Alcanivorax, Cycloclasticus, Marinobacter, Oleispira, etc.; hydrocarbon-degrading fungi mainly consisted of Aspergillus, Mucor, Fusarium, and Penicilium [5,8,10,11,12,13,14,15]. The degradation mechanism of alkanes is that most alkane-degrading bacteria secrete diverse surfactants that facilitate emulsification and uptake of the alkanes [5,16].
Many environmental factors, such as hydrostatic pressure, oxygen, and hydrocarbon components, affect hydrocarbon biodegradation [17]. A series of accumulating findings revealed that high hydrostatic pressure (HHP) has an important influence on hydrocarbon biodegradation in deep-sea environments [7,18,19,20,21,22,23,24,25,26]. First, high pressure inhibited the growth of hydrocarbon-degrading bacteria [20,22,27,28,29]. For example, the bacterial growth rates and yields for n-hexadecane utilization at in situ temperatures (4 °C) and high pressure (50 MPa) in the deep ocean were much lower as compared to the incubations conducted at 0.1 MPa and 4 °C [20]. The growth and activity of hydrocarbon-degrading Alcanivorax borkumensis were impaired at <10 MPa [27]. The growth of strain Sphingobium yanoikuyae in the degradation of aromatic hydrocarbons was severely inhibited at 8 MPa [22]. The growth of Rhodococcus isolated from deep-sea sediment is impaired at 15 MPa [29]. Second, a 4% decrease in n-alkane biodegradation was observed for every 1 MPa increase [28]. Finally, high pressure is also known to affect the succession of microbial communities in hydrocarbon degradation [19,25,30]. For instance, members of the genus Sulfitobacter were highly abundant in hydrocarbon degradation at 0.1 MPa, whereas Photobacterium dominated the hydrocarbon-degrading microbial communities at 30 MPa [25].
It has been reported that different components of hydrocarbon were primary drivers for selecting well-adapted specific microbial communities [31,32]. Microbial community preferentially utilized oil-derived short-chain and higher-molecular weight alkanes [33,34]. Several studies indicated that Colwellia had the capability to oxidize ethane, propane, and butane [35], Oceanospirillum could degrade cyclohexane [36], and Cycloclasticus could utilize BTEX (benzene, toluene, ethylbenzene, and xylenes) as well as polycyclic aromatic hydrocarbons (PAHs) [9].
Microorganisms could degrade all kinds of hydrocarbons under both aerobic and anaerobic conditions [37,38]. The processes of aerobic hydrocarbon degradation generally occurred throughout the marine water column and oxic surface sediments even in deep waters [39], whereas anaerobic hydrocarbon degradation primarily existed in anoxic bottom sediments and within hydrocarbon seeps [40,41]. Aerobic hydrocarbon degradation requires oxygen as a reactant for alkane activation. The aerobic oxidation of n-alkanes can be catalyzed by different enzymes, including methane monooxygenases [42], cytochrome P450, integral membrane non-heme iron monooxygenases (named AlkB) [5,43], flavin-binding monooxygenase (named AlmA) [44], and a long-chain alkane hydroxylase (named LadA) [45]. The aerobic microorganisms for hydrocarbon degradation mainly contained Bacillus spp., Acinetobacter spp., and Pseudomonas spp. [37]. On the contrary, anaerobic degradation of hydrocarbons has been shown to occur with Fe3+, SO42− and NO3− as electron acceptors [4,46]. The anaerobic degradation rate of hydrocarbons is usually shown to be several orders of magnitude lower, and the related genes and enzymes involved in these pathways are less known [47]. At present, the mainly anaerobic alkane-degraders are affiliated with members of the Proteobacteria (Gamma-, Delta-, and Epsilon-), Firmicutes, and Euryarchaeota [41,48,49]. However, most studies focused on soil, freshwater, shorelines, and surface water contamination at atmospheric pressure; there are few studies involved in anaerobic alkane degradation under high pressure [40,50,51].
The ocean’s hadal zone (depth > 6000 m) is characterized by extreme physical-chemical conditions, including high hydrostatic pressure (HHP, ~110 MPa), low temperature (LT, 1.0–2.5 °C), low dissolved oxygen (156 μM), and lack of sunlight [52,53,54]. All these factors can have a crucial influence on microbial processes and therefore further affect the rate and extent of hydrocarbon biodegradation in the hadal zone [18,19]. The latest findings revealed that abundant n-alkanes were detected in the surface sediments of the Mariana Trench, with total concentrations of n-alkanes ranging from 0.341 to 3.465 μg/g dry weight (dw) [14,55]. These n-alkanes were mainly derived from bacteria or marine algae in the euphotic zone and subsequently exported to the deeper waters and hadal zones of the Mariana Trench [14]. The other relevant studies reported that the abundance and expression of alkane degradation genes, such as those encoding medium-chain alkane 1-monooxygenase (AlkB) and long-chain alkane monooxygenase (AlmA), tended to increase in the bottom waters of the Mariana Trench [14]. However, the key microorganisms truly responsible for n-alkane degradation still unknown in the deepest habitat on earth (e.g., the Mariana Trench, 11,000 m).
Up to now, most investigations on hydrocarbon degradation were conducted at atmospheric pressure (0.1 MPa) [56,57,58,59,60], and only a few studies focused on the effect of high hydrostatic pressure, oxygen, and n-alkane components on alkane enrichments (the highest simulated pressure was up to 60 MPa) [14], and the corresponding results may not apply to the hadal zone of the Mariana Trench. To understand how each of the three factors (pressure, oxygen, and n-alkanes) affected microbial diversity and community composition in processes of aerobic and anaerobic hydrocarbon input under in-situ conditions in MT, simulation investigations in the lab were conducted. Our goals are to (1) compare the differences of microbial communities with hydrocarbon addition at 0.1 or 100 MPa, and evaluate the effect of pressure on microbial communities for hydrocarbon utilization; (2) compare the differences of the microbial groups responsible for aerobic and anaerobic alkane treatments at in situ conditions, and assess the effect of oxygen on microbial communities for hydrocarbon enrichments; (3) compare the differences of microbial communities involved in different alkane (C7–17 or C18–C36) addition, and assess the effect of alkane components on microbial communities for hydrocarbon utilization.
2. Materials and Methods
2.1. Sediments Sampling
Sediment samples were collected from the Challenger Deep of the Mariana Trench (11.329° N, 142.198° E, water depth of 10,898 m) during the cruise from 2019.12 to 2020.1. A box corer (with a base area of 400 cm2 and a height of 25 cm) attached to the Hadal Lander II was used to collect sediment samples from the trench [61]. Briefly, two hours after the lander touched down on the ocean floor, the box corer was slowly lowered into the seafloor until it was about 25 cm below the sediment surface. The box corer was then sealed with a lid, and the lander’s cover was put back on. The sediment samples were quickly resampled using sterile plastic corers after being recovered on board, and they were kept at 4 °C until further analysis.
2.2. Enrichments of n-Alkanes Experiments under High-Pressure
Sediment samples (10,898 m) were enriched using sterile LMO medium (i.e., 26 g NaCl, 5 g MgCl2·6H2O, 1.4 g CaCl2·2H2O, 4 g Na2SO4, 0.3 g NH4Cl, 0.1 g KH2PO4, 0.96 g KCl, 20 mM NaHCO3, and 4 mL of vitamin mixtures dissolved in 1 L ddH2O, pH 7~8) with short-chain n-alkanes (SCAs, C7–C17) or long-chain n-alkanes (LCAs, C18–C36) mixtures as a sole carbon source, and then incubated at 0.1 MPa/100 MPa and 4 °C for about 150 days in the dark.
A total of five incubation treatments were conducted in our study. Firstly, anaerobic enrichment cultures of SCAs or LCAs consisted of 100 g of sediment, 20 mL of sterile LMO medium supplemented with 120 μL of the C7–C17 mixtures (1:1, v/v, 1 mL/L final concentration), or 0.12 g of the C18–C36 mixtures (1:1, w/w, 1 g/L final concentration) at 0.1 MPa or 100 MPa and 4 °C for 7, 18, 30, and 11, 21, 30, 150 days, respectively. All these components were injected into a sterile anaerobic culture bag with the addition of resazurin solution (1 g/L) as a redox indicator. Finally, all the anaerobic culture bags needed to be oxygen-free before incubation. Triplicate incubations were done for all treatments.
On the other hand, aerobic enrichments of SCAs or LCAs comprised 100 g of sediments, 20 mL of LMO medium supplemented with 120 μL of C7–C17 mixture (or 0.12 g of C18–C36 mixture), and 40 mL (25% of the total volume) of oxygenated-saturated FluorinertTM (3MTM Corp., Minneapolis, MN, USA) [62] at 100 MPa and 4 °C for 150 days. All incubation components were placed in a sterile pouch (Kapak SealPak 500, 120 mL) and then sealed with a heat sealer. Triplicate incubations were done for all treatments.
Then, these high-pressure bags were incubated in several cylindrical, stain-less steel pressure vessels (Feiyu Petrochemical Instrument Equipment Inc., Nantong Guilin, China), with a maximum working pressure of 120 MPa. Hydrostatic pressure was created by pumping water using a hand-operated pump.
2.3. DNA Extraction
The genomic DNA from the cultures was extracted with the FastDNA Spin Kit for Soil (MP Biomedical, Solon, OH, USA) according to the manufacturer’s protocols. The obtained total DNA was dissolved in 100 μL DNase-free ddH2O. The DNA concentration was determined by Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA) and stored at −80 °C until further analysis.
2.4. PCR Amplification of 16S rRNA Gene
The genomic DNA of each sample was amplified using the barcoded primers 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′) [63], aiming at the V4 region of the 16S rRNA gene. The 25 μL PCR system included 10 μL Premix TaqTM (Takara, Dalian, China), 0.5 μL of each primer (10 μM), about 10 ng of DNA, and 13 μL DNase-free ddH2O. The PCR was conducted on a thermocycler PCR system (GeneAmp 9700, Applied Biosystems) with the following program: 94 °C for 3 min; 35 cycles of 94 °C for 45 s, 50 °C for 60 s, and 72 °C for 90 s; 72 °C for 10 min. Negative and positive controls were also conducted in each run.
2.5. Illumina Miseq Sequencing and Data Processing
The amplicon samples were pooled in equimolar amounts and sequenced with an Illumina MiSeq platform (Illumina, San Diego, CA, USA) at the Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw sequences were trimmed with cutadapt (sequences with average quality scores lower than 20 were filtered, and primer sequences were cut off) [64]. The trimmed sequences were truncated, denoised, and filtered for chimeras using DADA2 commands. The amplicon sequence variants (ASVs) of 16S rRNA sequences were then classified with the classify-sklearn plugin against the SILVA 138.1 database in QIIME2 [65]. Finally, the mitochondria and unclassified ASVs were removed. These processes produced 25,181–258,284 reads of 55 samples in the ASV table, and the sequences were resampled to the minimum number of sequences (25,181) among all samples.
2.6. Statistical and Ecological Analyses
The non-metric multidimensional scaling (nMDS) and hierarchical cluster analyses were performed using the PRIMER v7 package [66]. Linear discriminant analysis (LDA; http://huttenhower.sph.harvard.edu/galaxy/root?tool_id=PICRUSt_normalize, accessed on 20 Feburary 2023) was used to identify potential biomarkers at the genus level, with an LDA score threshold of 5.0. The phylogenetic tree was constructed using the MEGA v11.0 software [67], and the heatmap was visualized by TBtools software [68].
3. Results
3.1. Analysis of Microbial Diversity
High-throughput sequencing (Illumina MiSeq) of the V4 region of the 16S rRNA gene resulted in up to 3,340,943 high-quality sequences, and a total of 1567 amplicon sequence variants (ASVs) were identified from 55 samples (Tables S1 and S2). The sequencing data were rarified to 25,181 reads per sample based on the lowest number of sequences, and the number of ASVs varied from 17 (SCAs01-30d-1) to 408 (SCAs100-150d-2) (Tables S1 and S2). The Good’s coverage ranged from 99.8% to 100% (Table S2), suggesting that the sequencing depth was reasonable and the diversities of the microbial community were well covered.
Generally, the Shannon index tended to decrease with incubation time at 0.1 MPa, whereas the Shannon index remained nearly constant at 100 MPa (Figure S1 and Table S2).
A higher Shannon diversity index was observed at high-pressure (100 MPa) than that at atmospheric pressure (0.1 MPa) whether it’s short-chain alkanes (SCAs, C7~C17) or long-chain alkanes (LCAs, C18~C36) enrichments. For instance, the value of the Shannon index at 0.1 MPa ranged from 0.8–3.3, whereas the corresponding values were 2.2–3.9 at 100 MPa (Figure S1 and Table S2).
On the other hand, the Shannon index of SCA treatments was always higher than that of LCA treatments, irrespective of pressure and oxygen. For example, the value of the Shannon index in anaerobic SCA treatments (1.3–3.9) was higher than that of LCA treatments (0.8–2.7 a) at 0.1 MPa and 100 MPa. Interestingly, the Shannon index for aerobic SCA enrichments (2.8) was higher than that of LCAs (2.6) at 100 MPa (Figure S1 and Table S2). In addition, comparing anaerobic with aerobic enrichments, the Shannon index of SCAs100-150d (3.6) was much higher than that of SCAs100-150d-ae (2.8). However, much lower diversity was observed at LCAs100-150d (2.3) than that of LCAs-150d- ae (2.6) (Figure S1 and Table S2).
The nMDS analysis was performed to evaluate the similarities of microbial communities according to different pressures, oxygen levels, and n-alkane components. A total of five clusters were formed: the SCA01, LCAs01, SCAs100, LCAs100, and 100 MPa-ae clusters (Figure 1).
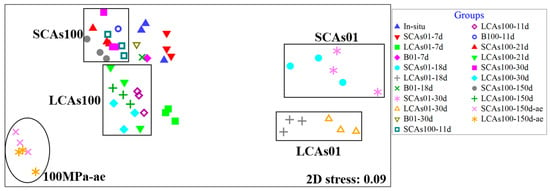
Figure 1.
The non-metric multidimensional scaling (nMDS) analysis of microbial communities for aerobic and anaerobic hydrocarbon incubation at 0.1 and 100 MPa in the sediments of the Mariana Trench. Note: SCAs100, MT sediments added short-chain alkanes incubated anaerobically at 100 MPa; LCAs100, MT sediments added long-chain alkanes incubated anaerobically at 100 MPa; SCAs01, MT sediments added short-chain alkanes incubated anaerobically at 0.1 MPa; LCAs01, MT sediments added long-chain alkanes incubated anaerobically at 0.1 MPa; 100 MPa-ae, MT sediments added short-chain or long-chain alkanes incubated aerobically at 100 MPa.
Hierarchical cluster analysis (Figure S2) also revealed five distinct sample branches according to the hydrostatic pressure: two branches included the sediments incubated with SCAs or LCAs at 0.1 MPa, and another two branches contained the sediments incubated with SCAs or LCAs at 100 MPa. On the other hand, according to oxygen availability, the fifth branch included aerobic SCAs or LCAs-enriched samples at 100 MPa. Notably, the in-situ samples were clustered closely with the SCAs-100 samples.
3.2. Microbial Community Structure
Microbial community analysis (Figure 2) showed that different compositions of the microbial community were identified among the three different incubation treatments (0.1 MPa vs. 100 MPa; anaerobic vs. aerobic; SCAs vs. LCAs) after 150 days of incubation.
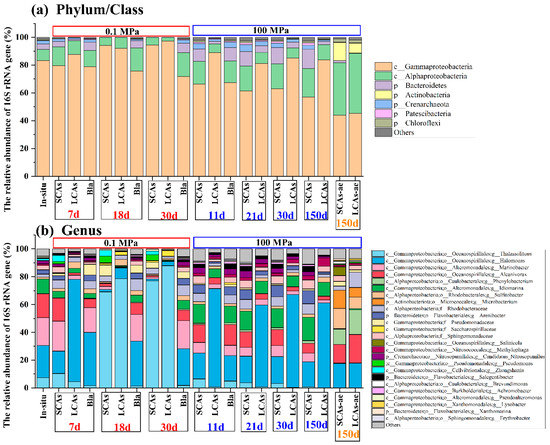
Figure 2.
The most abundant microbial communities in the aerobic and anaerobic hydrocarbon enrichments under 0.1 and 100 MPa conditions at phylum (a) and genus (b) levels, respectively. Note: SCAs, short-chain alkanes; LCAs, long-chain alkanes; SCAs-ae: MT sediments with short-chain alkanes incubated aerobically; LCAs-ae: MT sediments with long-chain alkanes incubated aerobically; Bla, Blank.
Before incubation, the in-situ microbial communities at the phylum/class level were dominated by Gammaproteobacteria (83.2% of the total reads), Alphaproteobacteria (7.9%), Bacteroidetes (4.1%), and Crenarchaeota (2.3%) (Figure 2a). The dominant in-situ microbial genera mainly included Halomonas (23.1%), Marinobacter (20.2%), and Alcanivorax (17.1%) (Figure 2b).
After incubation, the dominant microbial community in anaerobic n-alkane treatments at 0.1 MPa was significantly different from the corresponding treatments at 100 MPa (p < 0.05).
For example, at 0.1 MPa, Gammaproteobacteria tended to increase with incubation time, with the average relative abundance changing from 79.7% (or 87.7%) at 7 d to 94.4% (or 97.2%) at 30 d in anaerobic SCAs (or LCAs) treatments. However, Alphaproteobacteria seemed to decrease with incubation time, with the average relative abundance varying from 13.3% (or 9.7%) at 7 d to 5.5% (or 2.6%) at 30 d in anaerobic SCA (or LCA) treatments. In addition, Bacteroidetes appeared to decrease dramatically in both SCAs and LCAs treatments, with 0.1% of the average relative abundance (Figure 2a).
On the contrary, at 100 MPa, Both Alphaproteobacteria and Bacteroidetes increased with the incubation period in SCAs and LCAs microcosms under anaerobic conditions. The relative abundance of Alphaproteobacteria elevated slowly from 16.3% (and 6.4%) at 11 d to 20.4% (and 10.9%) at 150 d in SCA and LCA enrichments. Similarly, the relative abundance of Bacteroidetes increased from 8.8% (and 1.7%) at 11 d to 13.9% (and 2.2%) at 150 d in SCA and LCA treatments. Nevertheless, the average relative abundance of Gammaproteobacteria decreased gradually from 66.3% (and 88.9%) at 11d to 57.1% (and 83.8%) at 150d under SCAs (and LCAs) incubation (Figure 2a).
On the other hand, the microbial communities for aerobic SCA or LCA enrichments at 100 MPa were obviously different from those of anaerobic treatments. For instance, the microbial communities in the aerobic SCAs or LCAs treatments at 100 MPa mainly consisted of Gammaproteobacteria (44.0% or 45.4%), Alphaproteobacteria (37.7% or 43.0%), and Actinobacteria (13.1% or 7.0%). The relative proportion of Gammaproteobacteria decreased sharply, whereas Alphaproteobacteria and Actinobacteria obviously increased their relative abundance in aerobic treatments compared to the in-situ samples (Figure 2a).
At the genus level, Thalassolituus was the predominant microbial taxon for SCAs (or LCAs) enrichments at 0.1 MPa, with the relative abundance obviously increasing from 10.4% (or 4.5%) at 7 d to 77.1% (or 87.9%) at 30 d (Figure 2b and Figure 3).
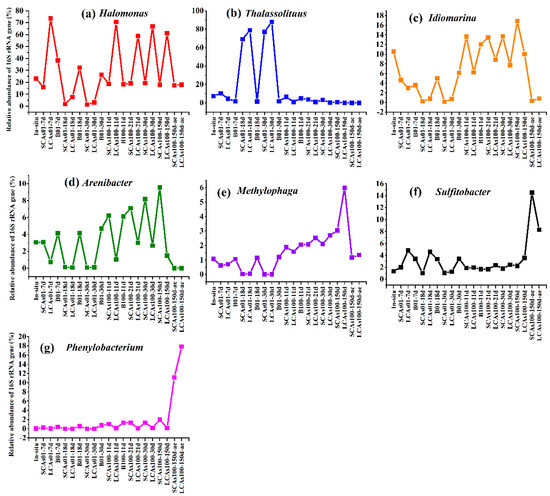
Figure 3.
The top 7 genera in microbial communities in aerobic and anaerobic hydrocarbon enrichments at 0.1 and 100 MPa after incubated for 150 days. (a) The variations of relative abundance in Halomonas genus among different treatments and incubation time; (b) The variations of relative abundance in Thalassolituus genus among different treatments and incubation time; (c) The variations of relative abundance in Idiomarina genus among different treatments and incubation time; (d) The variations of relative abundance in Arenibacter genus among different treatments and incubation time; (e) The variations of relative abundance in Methylophaga genus among different treatments and incubation time; (f) The variations of relative abundance in Sulfitobacter genus among different treatments and incubation time; (g) The variations of relative abundance in Phenylobacterium genus among different treatments and incubation time.
However, at 100 MPa, the dominant microbial genera for anaerobic SCA enrichments rapidly changed into Idiomarina and Arenibacter, with the relative abundance increasing from 13.6% and 6.2% at 11 d to 16.9% and 9.6% at 150 d (Figure 2b and Figure 3). Differently, the microbial communities for anaerobic LCAs100 enrichments were predominated by Halomonas (changed from 70.6% at 11 d to 61.2% at 150 d), Idiomarina (6.2–10.0%), and Methylophaga (1.6–6.0%).
3.3. LDA Analysis
LDA analysis (Figure 4) was used to identify the significantly different microbial indicators (with an LDA score threshold of 5.0, p < 0.05) that were responsible for the dissimilarities between the microbial communities for SCA or LCA enrichments under different hydrostatic pressure or oxygen conditions.
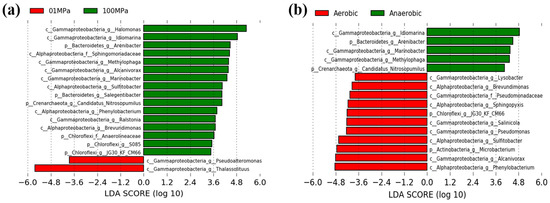
Figure 4.
The linear discriminant analysis (LDA) identified the significantly different indicators between different pressure or oxygen treatments, with LDA scores of 5.0. (a) 0.1 MPa vs. 100 MPa; (b) aerobic vs. anaerobic.
Our results showed that a total of 2 and 16 indicative phylotypes were detected when comparing 0.1 MPa and 100 MPa, respectively (Figure 4a). For instance, two key genera, Thalassolituus (Gammaproteobacteria) and Pseudoalteromonas (Gammaproteobacteria), were responsible for the community differences at 0.1 MPa, whereas 16 dominant taxa, such as Halomonas (Gammaproteobacteria), Idiomarina (Gammaproteobacteria), Arenibacter (Bacteroidetes), and Sphingomonadaceae (Alphaproteobacteria) took charge of the community discrepancy at 100 MPa.
On the other hand, a total of 11 and 5 indicative microorganisms were observed when comparing aerobic and anaerobic conditions (Figure 4b). For example, Phenylobacterium, Alcanivorax, and Microbacterium were the main microbial indicators in aerobic treatments, whereas Idiomarina, Arenibacter, and Marinobacter contributed to the community differences for anaerobic hydrocarbon enrichments.
3.4. Phylogenetic Analysis
At the ASV level, a total of 35 representative ASVs (more than 5% of the relative abundance) were chosen to construct the phylogenetic tree, and the corresponding relative abundances of the ASVs were also shown in the heatmap (Figure 5). The phylogenetic analysis revealed that these dominant microbes belonged to the three phyla Gammaproteobacteria, Alphaproteobacteria, Bacteroidetes, and Actinobacteria.
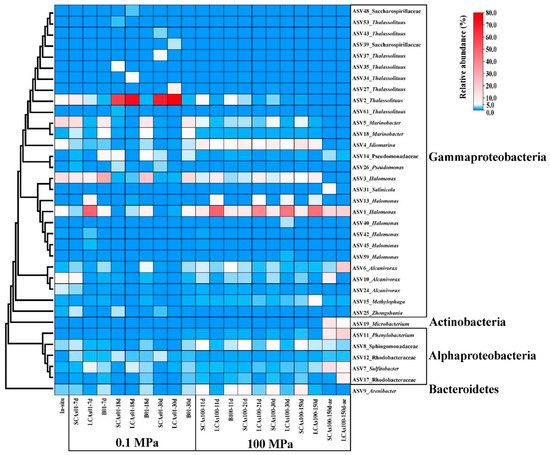
Figure 5.
The phylogenetic tree of the 35 most abundant ASVs and 16 genera and the corresponding relative abundance are listed in the heatmap at the right.
Our results showed that a total of eight ASVs assigned to Thalassolituus were the most abundant taxon and responsible for anaerobic SCA and LCA enrichments at 0.1 MPa, with the relative abundance dramatically reaching 69.0% and 78.0% at 30 d. In contrast, Idiomarina (ASV4) dominated the anaerobic SCAs microcosm at 100 MPa, with the relative abundance increasing from 9.8% at 11 d to 13.4% at 150 d, whereas Halomonas (ASV1) was responsible for the anaerobic LCA enrichments at 100 MPa, with the relative abundance reaching 46.3% at 150 d. In addition, Sulfitobacter (ASV7), Microbacterium (ASV19), and Phenylobacterium (ASV11) were the most important species in the aerobic SCAs incubation at 100 MPa, with the relative abundance reaching 14.5%, 13.0%, and 11.1%, respectively. Alcanivorax (ASV6), Phenylobacterium (ASV11), and Halomonas (ASV1) predominated the aerobic LCA treatments at 100 MPa, with a relative abundance of 20.4%, 17.8%, and 17.6%, respectively (Figure 5).
4. Discussion
4.1. Effect of Hydrostatic Pressure on Microbial Diversity and Community Composition in n-Alkane Enrichment Cultures
High pressure has an important influence on hydrocarbon biodegradation in deep-sea environments [7,19,20,22,23,24,25,26]. For instance, high pressure inhibited the growth of hydrocarbon-degrading bacteria [20,22,24,28,29], inhibited n-alkane biodegradation [28,29], and affected the succession of the microbial communities in the hydrocarbon enrichments [19,25,30].
In this study, microbial diversity for hydrocarbon enrichments at 100 MPa was obviously higher than that at 0.1 MPa (Figure S1 and Table S2), suggesting that the hydrostatic pressure had an important influence on microbial diversity with the addition of n-alkanes. One possible explanation is that the few microorganisms were significantly dominated at 0.1 MPa over time, resulting in a decrease in total bacterial diversity [69]. Luis and colleagues studied the effects of pressure on hydrocarbon-degrading microbial communities in subarctic sediments and also revealed that the Shannon index at 0.1 MPa was lower than that of 30 MPa (Luis et al., 2018). However, another similar investigation reported that the alpha diversities (such as Simpson and Shannon indices) of the sediment communities treated with oil at 10 MPa were lower than those of 0.1 MPa incubations (Noirungsee et al., 2020). In all, the microbial diversity associated with hydrocarbon incubation was mainly dependent on the combined effect of various environmental conditions, including pressure, temperature, oil, dispersant, etc. (Luis et al., 2018; Noirungsee et al., 2020).
The nMDS (Figure 1) and hierarchical cluster analysis (Figure S2) commonly revealed that the microbial community was divided into two distinct clusters, i.e., 0.1 MPa-incubated and 100 MPa-treated groups, according to the hydrostatic pressure, suggesting an obvious shift of n-alkanes-enriched microbial communities influenced by high pressure. These results are consistent with previous similar studies conducted on subarctic sediments (Luis et al., 2018) and sediments from the Gulf of Mexico (Noirungsee et al., 2020). Interestingly, SCAs100 samples were clustered close to the in-situ samples; this is because the in-situ sediments of the Mariana Trench contained much higher amounts of short-chain alkanes (such as C16 and C18) but not long-chain alkanes [14]. Therefore, when SCAs or LCAs were added to the sediment samples, the similar microbial communities in the in-situ sediments grew rapidly in response to the SCA input, whereas different communities developed after the addition of LCAs.
After being incubated for 30 days, Gammaproteobacteria increased their relative abundance with time, and was the most abundant class in microbial communities with addition of SCAs and LCAs at 0.1 MPa (Figure 2), which is consistent with hydrocarbon-contaminated marine environments [12,36,70,71]. Many accumulating studies also demonstrated that Gammaproteobacteria usually increased their abundances in the oil-contaminated microcosms at 0.1 MPa, which had already been described as petroleum degraders [12,35,72,73,74,75,76], This result helps explain the enrichment of these bacteria in our study. However, the relative abundance of Gammaproteobacteria showed a decreasing trend when pressure was elevated to 100 MPa from 0 d to 150 days. These two opposite observations reflected that members of Gammaproteobacteria may be piezosensitive species with increasing pressure. Similar findings were also observed in a recent study investigating the effect of high pressure (22 MPa) on surface-water microbial communities [30].
In contrast, Alphaproteobacteria and Bacteroidetes showed an increase in relative abundance with incubation time (0–150 days) in 100 MPa-treated groups, whereas a decreasing trend was observed in these two phyla with time (0–30 days) at 0.1 MPa incubation (Figure 2). Likewise, Bacteroidetes reached the highest relative abundance in oil-contaminated surface waters when simulating deep-sea conditions, i.e., 22 MPa and 4 °C [30]. Several Bacteroidetes species also dominated hydrocarbon-degrading communities in sediment samples at 30 MPa (Luis et al., 2018).
At the genus and ASV levels, eight ASVs related to Thalassolituus almost dominated the gammaproteobacterial community involved in anaerobic hydrocarbon treatments at 0.1 MPa, with 77.1–87.9% of the average relative abundance (compared to 7.3% of the in-situ samples) (Figure 2, Figure 3, Figure 4 and Figure 5). However, Thalassolituus appeared to disappear (less than 1% of the relative abundance at 150 d) when pressure increased to 100 MPa (Figure 2, Figure 3, Figure 4 and Figure 5), suggesting that this species might be piezo-sensitive and could not resist extremely high pressure, as the abrupt changes in pressure could change bacterial physiology and often lead to cellular lysis [77]. Meanwhile, LDA analysis also showed that Thalassolituus was the most important microbial indicator responsible for community differences at 0.1 MPa (Figure 4). Thalassolituus is a typical marine strain of obligate hydrocarbonoclastic bacteria (OHCB) and grows almost exclusively on aliphatic hydrocarbons [72]. Thalassolituus was the most dominant alkane degrader over a range of chain-length n-alkanes (C10–C32) degradation in the estuary [31] and marine oil pollution environments [78,79]. Previous reports have also shown that Thalassolituus-related species are dominant in crude oil biodegradation, confirming the important role this taxon plays in oil enrichment [11,12,80,81,82,83].
Two important genera, Idiomarina and Arenibacter, increased their abundances to their maximum values (16.9% and 9.6%, respectively) in the anaerobic SCA enrichments at 100 MPa after being incubated for 150 days and seemed to play a major role in these treatments (Figure 2, Figure 3, Figure 4 and Figure 5). Recent relevant studies reported that Idiomarina can degrade polycyclic aromatic hydrocarbons (PAHs), crude oil, diesel oils, hydrocarbons, and biphenyl in various environments [30,84,85,86,87,88,89,90]. On the other hand, previous studies showed that Arenibacter strains were demonstrated to have the potential to degrade crude oil and PAHs, acting by producing biosurfactants or bioemulsifiers to increase the bioavailability of hydrocarbons [91,92,93,94]. However, these studies were only conducted at atmospheric pressure and temperatures of 21–28 °C; the enrichments of Arenibacter with the addition of n-alkanes in the sediments of the Mariana Trench at in-situ pressure and temperature were not reported. Together, our study implied that Idiomarina and Arenibacter may have the potential to utilize a variety of low-molecular-weight alkanes at 100 MPa and supply carbon and energy to meet their growth requirements.
4.2. Effect of Different Hydrocarbon Components on Microbial Diversity and Community Structure
The molecular mass and structure of the hydrocarbons affect the rate and extent of their biodegradation; as the molecular mass, ring number, and alkyl-branching increase, the degradation processes become slower [25,95]. The in-lab simulations of the deep-sea plume demonstrated that 6–13 carbon alkanes were degraded first, with half-lives of 6–7 days [96]. We found that two different components of n-alkanes would influence the composition of the microbial community, and specific microbes may dominate or be outcompeted in the hydrocarbon enrichments [31,32,97].
Our study showed that the different molecular masses of n-alkanes may affect the microbial participants of their enrichments. In our study, the Shannon index of SCA treatments was always higher than that of LCA treatments, irrespective of pressure and oxygen (Figure S1 and Table S2), which suggested that microbes may favor the simple short-chain alkanes but not the long-chain alkanes in any case. On the other hand, compared to SCA treatments, three key gammaproteobacterial genera, including Halomonas, Idiomarina, and Methylophaga, were the predominant taxa for anaerobic LCA enrichments at 100 MPa and obviously increased their relative abundances (61.2%, 10%, and 6.0%, respectively) with the addition of LCAs incubated for about 150 days (Figure 2, Figure 3, Figure 4 and Figure 5). These results may suggest that Halomonas, Idiomarina, and Methylophaga had the special abilities to resist extremely high hydrostatic pressure and low temperature and may have had the related enzymes to degrade and utilize various high-molecular-weight alkanes (C18–C36) at 100 MPa/4 °C under anaerobic conditions.
Many related investigations found that Halomonas was frequently involved in hydrocarbon degradation and produced an amphiphilic exopolymeric substance (EPS) for emulsifying hydrocarbons [75,98,99,100]. Halomonas have been reported to degrade PAHs in marine sediments [57,101,102], hypersaline habitats [87,103], California lake [104], and the Persian Gulf [86]. Halomonas could also degrade complex hydrocarbons such as diesel and crude oil in psychrotrophic habitats, including Antarctica [105] and the oil-contaminated deep ocean [12]. Nevertheless, few reports revealed that the Halomonas genus could utilize hydrocarbon under high hydrostatic pressure conditions [106].
Meanwhile, Methylophaga was strictly aerobic and moderately halophilic and showed an exclusive requirement for C1 sources (methanol, methylamine, and dimethylsulfide) as the sole carbon and energy source [107]. Methylophaga was observed to be involved in the enrichment of high molecular-weight PAHs [108,109], high-molecular-weight hydrocarbons [74,110,111], and crude oil [94].
4.3. Effect of Oxygen Concentration on Microbial Community Structure of n-Alkanes Enrichment Cultures
Oxygen concentrations exert important influences on the biodegradation of n-alkanes. This is due to the fact that aerobic alkane degraders use oxygen as a reactant for alkane molecule activation, whereas anaerobic alkane degraders use nitrate or sulfate as an electron acceptor [5]. The processes of aerobic and anaerobic alkanes degradation were obviously distinct, therefore, the main microbial communities responsible for aerobic and anaerobic hydrocarbon degradation were completely different.
In this study, we found that oxygen concentration significantly affected the microbial community with n-alkane input at 100 MPa/4 °C based on nMDS analysis (Figure 1) and hierarchical cluster analysis (Figure S2). Most importantly, compared to the corresponding anaerobic treatments, Actinobacteria (e.g., Microbacterium) and Alphaproteobacteria (such as Sulfitobacter and Phenylobacterium) were the most abundant phyla (genera) responsible for aerobic hydrocarbon enrichments at 100 MPa (Figure 2, Figure 3, Figure 4 and Figure 5).
At present, most incubation experiments on Actinobacteria are conducted at atmospheric pressure and room temperature. For instance, some actinobacterial isolates and their production peroxidases were demonstrated to play a key role in the processes of crude oil [112] and petroleum hydrocarbon biodegradation [17]. On the other hand, other actinobacterial genera were able to degrade efficiently high molecular weight PAHs [113,114,115], and medium and long chain n-alkanes (C12–C20–C24–C30) [116]. In our study, the relative abundance of Actinobacteria increased from almost 0.0% in in-situ sediments to 7.0–13.1% in aerobic n-alkane treatments at 100 MPa (Figure 2), which suggests that Actinobacteria may adapt well to 100 MPa and may play a critical role in aerobic n-alkane utilization. To our knowledge, this is the first report about members of Actinobacteria possibly involved in hydrocarbon degradation and utilization at extremely high pressure (100 MPa).
Only one genus of Microbacterium, affiliated with the Actinobacteria phylum, reached its maximum abundance of 6.9–13.0% in the aerobic n-alkane incubation at 100 MPa after 150 days (Figure 2, Figure 3, Figure 4 and Figure 5), which implied that Microbacterium had the potential to utilize hydrocarbons, especially short-chain alkanes. A series of related studies also revealed that Microbacterium could degrade hydrocarbons [117,118], crude oil [119], and PAHs [120,121,122,123,124] in various environments, such as hydrocarbon-contaminated soil and estuarine sediments. Nevertheless, these studies were performed at room temperature and did not involve hydrostatic pressure.
The relative abundance of Sulfitobacter increased to 8.2–14.5%, becoming the second abundant taxon in the aerobic n-alkanes incubation at 100 MPa (Figure 2, Figure 3, Figure 4 and Figure 5). This result was consistent with previous studies. For example, Sulfitobacter has been identified as a degrader of crude oil [25,73,125,126,127,128,129], hydrocarbons [130,131], and petroleum [30] in all kinds of environments. In addition, the draft genome sequences of two Sulfitobacter sp. revealed the presence of genes on their genomes involved in aromatic hydrocarbon enrichments [132]. However, only a few reports revealed that Sulfitobacter sp. was the most abundant species when enriching the surface-water samples with crude oil at 22 MPa.
The relative abundance of Phenylobacterium increased to 11.1–17.9%, which became the top 3 dominant genera in the aerobic hydrocarbon enrichments at 100 MPa after 150 days (Figure 2, Figure 3, Figure 4 and Figure 5). A previous study showed that a new strain of Phenylobacterium was isolated and identified as petroleum-hydrocarbon-degrading bacteria from the contaminated soil [133,134,135]. Phenylobacterium is generally capable of degrading PAHs from crude oil-contaminated soils [136,137,138,139]. DNA-SIP technology revealed that Phenylobacterium was active benzo [a] pyrene (BaP) degrader in the BaP-contaminated soils [140]. Our results may reveal that Phenylobacterium increased may have the special abilities to utilize hydrocarbon and supply their growth and respiration requirements.
5. Conclusions
Our study ingeniously simulated how the indigenous sediment microbial communities of the Mariana Trench responded to the SCAs or LCAs input under anaerobic or aerobic conditions at 0.1 MPa/100 MPa and 4 °C after long-period culture. Primarily, hydrostatic pressure and alkane components obviously affected microbial diversity; a higher microbial diversity was observed at 100 MPa than at 0.1 MPa. Meanwhile, microbial diversity in SCA treatments was always higher than that of LCA treatments. Furthermore, hydrostatic pressure and oxygen significantly affect the microbial community in the SCAs and LCAs enrichment cultures. For example, Gammaproteobacteria was the most abundant class for both anaerobic SCAs and LCA enrichments at 0.1 MPa, whereas Alphaproteobacteria and Bacteroidetes increased their relative abundance with anaerobic hydrocarbon input at 100 MPa. At 100 MPa, the most abundant phyla responsible for aerobic SCA and LCA enrichments were Actinobacteria and Alphaproteobacteria. These findings suggested that the sediment microorganisms may be important n-alkane degraders in the Mariana Trench. Our study further revealed the profound influence of pressure, oxygen, and hydrocarbon components on unique alkane-enriched microorganisms in the sediments of the Mariana Trench, especially under in-situ pressure and temperature. Future research may focus on the physiological and metabolic potential of these alkane-related microorganisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11030630/s1, Figure S1: The temporal variations of Shannon index in microbial communities with aerobic and anaerobic n-alkanes enrichments at 0.1 and 100 MPa in the sediments of Mariana Trench; Figure S2: Hierarchical cluster analysis of microbial communities for aerobic and anaerobic hydrocarbon enrichments at 0.1 and 100 MPa in the sediments of Mariana Trench; Table S1: The ASV table of 55 samples in this study; Table S2: The sample ID, the number of reads and ASVs, Shannon and Simpson diversity indices, and value of Coverage in the 55 samples.
Author Contributions
Y.L., S.C. and J.W. designed the experiment, analyzed the data, wrote the article, and revised the manuscript. Y.L. and Z.X. conducted the experimental procedures. J.F., L.Z. and J.W. provided helpful comments and suggestions to improve the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (91951210), by the grant of Laboratory for Marine Biology and Biotechnology (OF2019NO06), Pilot National Laboratory for Marine Science and Technology (Qingdao), and by the National Natural Science Foundation of China (92251303 and 92241303).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
All raw sequence datasets of 16S rRNA genes from this study have been deposited into the NCBI Sequence Read Achieve (SRA) database with accession no. PRJNA930158.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; del Cardayre, S.B. Microbial Biosynthesis of Alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Biller, S.J.; Davey, M.P.; Cotton, C.A.R.; Perez Sepulveda, B.M.; Turchyn, A.V.; Scanlan, D.J.; Smith, A.G.; Chisholm, S.W.; Howe, C.J. Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 13591–13596. [Google Scholar] [CrossRef] [PubMed]
- Love, C.R.; Arrington, E.C.; Gosselin, K.M.; Reddy, C.M.; Van Mooy, B.A.S.; Nelson, R.K.; Valentine, D.L. Microbial production and consumption of hydrocarbons in the global ocean. Nat. Microbiol. 2021, 6, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Widdel, F.; Knittel, K.; Galushko, A. Anaerobic Hydrocarbon-Degrading Microorganisms: An Overview. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1997–2021. [Google Scholar]
- Rojo, F. Degradation of alkanes by bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef]
- Valentine, D.L.; Reddy, C.M. Latent hydrocarbons from cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 13434–13435. [Google Scholar] [CrossRef]
- Grossi, V.; Yakimov, M.M.; Al Ali, B.; Tapilatu, Y.; Cuny, P.; Goutx, M.; La Cono, V.; Giuliano, L.; Tamburini, C. Hydrostatic pressure affects membrane and storage lipid compositions of the piezotolerant hydrocarbon-degrading Marinobacter hydrocarbonoclasticus strain# 5. Environ. Microbiol. 2010, 12, 2020–2033. [Google Scholar]
- Xue, J.; Yu, Y.; Bai, Y.; Wang, L.; Wu, Y. Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: A review. Curr. Microbiol. 2015, 71, 220–228. [Google Scholar] [CrossRef]
- Hazen, T.C.; Prince, R.C.; Mahmoudi, N. Marine oil biodegradation. Environ. Sci. Technol. 2016, 50, 2121–2129. [Google Scholar] [CrossRef]
- Harayama, S.; Kasai, Y.; Hara, A. Microbial communities in oil-contaminated seawater. Curr. Opin. Biotechnol. 2004, 15, 205–214. [Google Scholar] [CrossRef]
- Coulon, F.; McKew, B.A.; Osborn, A.M.; McGenity, T.J.; Timmis, K.N. Effects of temperature and biostimulation on oil-degrading microbial communities in temperate estuarine waters. Environ. Microbiol. 2007, 9, 177–186. [Google Scholar] [CrossRef]
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jansson, J.K.; Probst, A.; Borglin, S.E.; Fortney, J.L. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 2010, 330, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wang, W. Enzymes and genes involved in aerobic alkane degradation. Front. Microbiol. 2013, 4, 116. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Lin, H.; Wang, X.; Li, M.; Liu, Y.; Yu, M.; Zhao, M.; Pedentchouk, N.; Lea-Smith, D.J. Proliferation of hydrocarbon-degrading microbes at the bottom of the Mariana Trench. Microbiome 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Giuliano, L.; Gentile, G.; Crisafi, E.; Chernikova, T.N.; Abraham, W.-R.; Lünsdorf, H.; Timmis, K.N.; Golyshin, P.N. Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int. J. Syst. Evol. Microbiol. 2003, 53, 779–785. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rathore, D.S.; Sheikh, M.; Singh, S.P. Marine Actinobacteria: New Horizons in Bioremediation. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Springer Nature: Singapore, 2021; pp. 425–449. [Google Scholar]
- Margesin, R.; Schinner, F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef]
- Scoma, A.; Yakimov, M.M.; Boon, N. Challenging Oil Bioremediation at Deep-Sea Hydrostatic Pressure. Front. Microbiol. 2016, 7, 1203. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Walker, J.D.; Colwell, R.R. Deep-sea bacteria: Growth and utilization of n-hexadecane at in situ temperature and pressure. Can. J. Microbiol. 1975, 21, 682–687. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Larter, S.R. Biological activity in the deep subsurface and the origin of heavy oil. Nature 2003, 426, 344–352. [Google Scholar] [CrossRef]
- Schedler, M.; Hiessl, R.; Valladares Juárez, A.G.; Gust, G.; Müller, R. Effect of high pressure on hydrocarbon-degrading bacteria. AMB Express 2014, 4, 77. [Google Scholar] [CrossRef]
- Prince, R.C.; Nash, G.W.; Hill, S.J. The biodegradation of crude oil in the deep ocean. Mar. Pollut. Bull. 2016, 111, 354–357. [Google Scholar] [CrossRef]
- Scoma, A.; Boon, N. Osmotic Stress Confers Enhanced Cell Integrity to Hydrostatic Pressure but Impairs Growth in Alcanivorax borkumensis SK2. Front. Microbiol. 2016, 7, 729. [Google Scholar] [CrossRef] [PubMed]
- Marietou, A.; Chastain, R.; Beulig, F.; Scoma, A.; Hazen, T.C.; Bartlett, D.H. The Effect of Hydrostatic Pressure on Enrichments of Hydrocarbon Degrading Microbes from the Gulf of Mexico Following the Deepwater Horizon Oil Spill. Front. Microbiol. 2018, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Hackbusch, S.; Noirungsee, N.; Viamonte, J.; Sun, X.; Bubenheim, P.; Kostka, J.E.; Müller, R.; Liese, A. Influence of pressure and dispersant on oil biodegradation by a newly isolated Rhodococcus strain from deep-sea sediments of the gulf of Mexico. Mar. Pollut. Bull. 2020, 150, 110683. [Google Scholar] [CrossRef] [PubMed]
- Scoma, A.; Barbato, M.; Borin, S.; Daffonchio, D.; Boon, N. An impaired metabolic response to hydrostatic pressure explains Alcanivorax borkumensis recorded distribution in the deep marine water column. Sci. Rep. 2016, 6, 31316. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Lincoln, S.A.; Valladares Juárez, A.G.; Schedler, M.; Macalady, J.L.; Müller, R.; Freeman, K.H. The influence of pressure on crude oil biodegradation in shallow and deep Gulf of Mexico sediments. PLoS ONE 2018, 13, e0199784. [Google Scholar] [CrossRef] [PubMed]
- Hackbusch, S. The Influence of Elevated Pressure and Hydrocarbon Input on the Deep Sea Microbial Community of the Gulf of Mexico; Technische Universität Hamburg: Hamburg, Germany, 2019. [Google Scholar]
- Fasca, H.; de Castilho, L.V.A.; de Castilho, J.F.M.; Pasqualino, I.P.; Alvarez, V.M.; de Azevedo Jurelevicius, D.; Seldin, L. Response of marine bacteria to oil contamination and to high pressure and low temperature deep sea conditions. MicrobiologyOpen 2018, 7, e00550. [Google Scholar] [CrossRef]
- McKew, B.A.; Coulon, F.; Osborn, A.M.; Timmis, K.N.; McGenity, T.J. Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary, UK. Environ. Microbiol. 2007, 9, 165–176. [Google Scholar] [CrossRef]
- Potts, L.D.; Perez Calderon, L.J.; Gontikaki, E.; Keith, L.; Gubry-Rangin, C.; Anderson, J.A.; Witte, U. Effect of spatial origin and hydrocarbon composition on bacterial consortia community structure and hydrocarbon biodegradation rates. FEMS Microbiol. Ecol. 2018, 94, fiy127. [Google Scholar] [CrossRef]
- Valentine, D.L.; Kessler, J.D.; Redmond, M.C.; Mendes, S.D.; Heintz, M.B.; Farwell, C.; Hu, L.; Kinnaman, F.S.; Yvon-Lewis, S.; Du, M.; et al. Propane Respiration Jump-Starts Microbial Response to a Deep Oil Spill. Science 2010, 330, 208–211. [Google Scholar] [CrossRef]
- Kessler, J.D.; Valentine, D.L.; Redmond, M.C.; Du, M.; Chan, E.W.; Mendes, S.D.; Quiroz, E.W.; Villanueva, C.J.; Shusta, S.S.; Werra, L.M.; et al. A Persistent Oxygen Anomaly Reveals the Fate of Spilled Methane in the Deep Gulf of Mexico. Science 2011, 331, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Redmond, M.C.; Valentine, D.L. Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20292–20297. [Google Scholar] [CrossRef]
- Mason, O.U.; Hazen, T.C.; Borglin, S.; Chain, P.S.G.; Dubinsky, E.A.; Fortney, J.L.; Han, J.; Holman, H.-Y.N.; Hultman, J.; Lamendella, R.; et al. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef]
- Head, I.M.; Gray, N.D.; Larter, S.R. Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front. Microbiol. 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Walters, C.C. 19—Biodegradation of oil hydrocarbons and its implications for source identification. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed.; Stout, S.A., Wang, Z., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 869–916. [Google Scholar]
- Prince, R.C.; McFarlin, K.M.; Butler, J.D.; Febbo, E.J.; Wang, F.C.Y.; Nedwed, T.J. The primary biodegradation of dispersed crude oil in the sea. Chemosphere 2013, 90, 521–526. [Google Scholar] [CrossRef]
- Netzer, F.v.; Pilloni, G.; Kleindienst, S.; Krüger, M.; Knittel, K.; Gründger, F.; Lueders, T. Enhanced Gene Detection Assays for Fumarate-Adding Enzymes Allow Uncovering of Anaerobic Hydrocarbon Degraders in Terrestrial and Marine Systems. Appl. Environ. Microbiol. 2013, 79, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Stagars, M.H.; Ruff, S.E.; Amann, R.; Knittel, K. High Diversity of Anaerobic Alkane-Degrading Microbial Communities in Marine Seep Sediments Based on (1-methylalkyl) succinate Synthase Genes. Front. Microbiol. 2016, 6, 1511. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Funhoff, E.G. Alkane hydroxylases involved in microbial alkane degradation. Appl. Microbiol. Biotechnol. 2007, 74, 13–21. [Google Scholar] [CrossRef]
- Austin, R.N.; Groves, J.T. Alkane-oxidizing metalloenzymes in the carbon cycle. Metallomics 2011, 3, 775–787. [Google Scholar] [CrossRef]
- Throne-Holst, M.; Wentzel, A.; Ellingsen, T.E.; Kotlar, H.-K.; Zotchev, S.B. Identification of Novel Genes Involved in Long-Chain n-Alkane Degradation by Acinetobacter sp. Strain DSM 17874. Appl. Environ. Microbiol. 2007, 73, 3327–3332. [Google Scholar] [CrossRef]
- Feng, L.; Wang, W.; Cheng, J.; Ren, Y.; Zhao, G.; Gao, C.; Tang, Y.; Liu, X.; Han, W.; Peng, X.; et al. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc. Natl. Acad. Sci. USA 2007, 104, 5602–5607. [Google Scholar] [CrossRef]
- Foght, J. Anaerobic Biodegradation of Aromatic Hydrocarbons: Pathways and Prospects. Microb. Physiol. 2008, 15, 93–120. [Google Scholar] [CrossRef]
- Heider, J.; Schühle, K. Anaerobic Biodegradation of Hydrocarbons Including Methane; Springer: Berlin/Heidelberg, Germany, 2006; pp. 605–634. [Google Scholar]
- Gray, N.D.; Sherry, A.; Hubert, C.; Dolfing, J.; Head, I.M. Chapter 5—Methanogenic Degradation of Petroleum Hydrocarbons in Subsurface Environments: Remediation, Heavy Oil Formation, and Energy Recovery. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 72, pp. 137–161. [Google Scholar]
- Khelifi, N.; Amin Ali, O.; Roche, P.; Grossi, V.; Brochier-Armanet, C.; Valette, O.; Ollivier, B.; Dolla, A.; Hirschler-Réa, A. Anaerobic oxidation of long-chain n-alkanes by the hyperthermophilic sulfate-reducing archaeon, Archaeoglobus fulgidus. ISME J. 2014, 8, 2153–2166. [Google Scholar] [CrossRef]
- Acosta-González, A.; Rosselló-Móra, R.; Marqués, S. Characterization of the anaerobic microbial community in oil-polluted subtidal sediments: Aromatic biodegradation potential after the Prestige oil spill. Environ. Microbiol. 2013, 15, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Wawrik, B.; Isom, C.; Boling, W.B.; Callaghan, A.V. Interrogation of Chesapeake Bay sediment microbial communities for intrinsic alkane-utilizing potential under anaerobic conditions. FEMS Microbiol Ecol 2015, 91, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.J.; Fujii, T.; Mayor, D.J.; Solan, M.; Priede, I.G. Hadal trenches: The ecology of the deepest places on Earth. Trends Ecol. Evol. 2010, 25, 190–197. [Google Scholar] [CrossRef]
- Nunoura, T.; Takaki, Y.; Hirai, M.; Shimamura, S.; Makabe, A.; Koide, O.; Kikuchi, T.; Miyazaki, J.; Koba, K.; Yoshida, N. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, E1230–E1236. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Wei, Y.; Fang, J. The hadal biosphere: Recent insights and new directions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 155, 11–18. [Google Scholar] [CrossRef]
- Guan, H.; Chen, L.; Luo, M.; Liu, L.; Mao, S.; Ge, H.; Zhang, M.; Fang, J.; Chen, D. Composition and origin of lipid biomarkers in the surface sediments from the southern Challenger Deep, Mariana Trench. Geosci. Front. 2019, 10, 351–360. [Google Scholar] [CrossRef]
- Liu, C.; Shao, Z. Alcanivorax dieselolei sp. nov., a novel alkane-degrading bacterium isolated from sea water and deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2005, 55, 1181–1186. [Google Scholar] [CrossRef]
- Cui, Z.; Lai, Q.; Dong, C.; Shao, Z. Biodiversity of polycyclic aromatic hydrocarbon-degrading bacteria from deep sea sediments of the Middle Atlantic Ridge. Environ. Microbiol. 2008, 10, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lai, Q.; Cui, Z.; Tan, T.; Shao, Z. A pyrene-degrading consortium from deep-sea sediment of the West Pacific and its key member Cycloclasticus sp. P1. Environ. Microbiol. 2008, 10, 1948–1963. [Google Scholar] [CrossRef]
- Tapilatu, Y.; Acquaviva, M.; Guigue, C.; Miralles, G.; Bertrand, J.-C.; Cuny, P. Isolation of alkane-degrading bacteria from deep-sea Mediterranean sediments. Lett. Appl. Microbiol. 2010, 50, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wang, J.; Gu, L.; Zheng, T.; Shao, Z. Alcanivorax marinus sp. nov., isolated from deep-sea water. Int. J. Syst. Evol. Microbiol. 2013, 63, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wei, X.; Song, W.; Wang, L.; Cao, J.; Wu, J.; Thomas, T.; Jin, T.; Wang, Z.; Wei, W.; et al. Novel Chloroflexi genomes from the deepest ocean reveal metabolic strategies for the adaptation to deep-sea habitats. Microbiome 2022, 10, 75. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, M.; Xie, Z.; Ning, D.; Zhou, J.; Yu, X.; Liu, R.; Zhang, L.; Fang, J. Microbial Community Structure and Ecological Networks during Simulation of Diatom Sinking. Microorganisms 2022, 10, 639. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. Msystems 2016, 1, e00009–e00015. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable, and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 852–857. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. Getting started with PRIMER v7. PRIMER-E Plymouth Plymouth Mar. Lab. 2015, 20. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, S.; Grim, S.; Sogin, M.; Bracco, A.; Crespo-Medina, M.; Joye, S.B. Diverse, rare microbial taxa responded to the Deepwater Horizon deep-sea hydrocarbon plume. ISME J. 2016, 10, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Jurelevicius, D.; Alvarez, V.M.; Marques, J.M.; Lima, L.R.F.D.S.; Dias, F.D.A.; Seldin, L. Bacterial Community Response to Petroleum Hydrocarbon Amendments in Freshwater, Marine, and Hypersaline Water-Containing Microcosms. Appl. Environ. Microbiol. 2013, 79, 5927–5935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J. Evaluating bacterial community structures in oil collected from the sea surface and sediment in the northern Gulf of Mexico after the Deepwater Horizon oil spill. MicrobiologyOpen 2013, 2, 492–504. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef]
- Jung, S.W.; Park, J.S.; Kown, O.Y.; Kang, J.-H.; Shim, W.J.; Kim, Y.-O. Effects of crude oil on marine microbial communities in short term outdoor microcosms. J. Microbiol. 2010, 48, 594–600. [Google Scholar] [CrossRef]
- Dubinsky, E.A.; Conrad, M.E.; Chakraborty, R.; Bill, M.; Borglin, S.E.; Hollibaugh, J.T.; Mason, O.U.; Piceno, Y.M.; Reid, F.C.; Stringfellow, W.T.; et al. Succession of Hydrocarbon-Degrading Bacteria in the Aftermath of the Deepwater Horizon Oil Spill in the Gulf of Mexico. Environ. Sci. Technol. 2013, 47, 10860–10867. [Google Scholar] [CrossRef]
- Gutierrez, T.; Singleton, D.R.; Berry, D.; Yang, T.; Aitken, M.D.; Teske, A. Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 2013, 7, 2091–2104. [Google Scholar] [CrossRef]
- Meng, L.; Liu, H.; Bao, M.; Sun, P. Microbial community structure shifts are associated with temperature, dispersants and nutrients in crude oil-contaminated seawaters. Mar. Pollut. Bull. 2016, 111, 203–212. [Google Scholar] [CrossRef]
- Koyama, S.; Kobayashi, H.; Inoue, A.; Miwa, T.; Aizawa, M. Effects of the piezo-tolerance of cultured deep-sea eel cells on survival rates, cell proliferation, and cytoskeletal structures. Extremophiles 2005, 9, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Giuliano, L.; Denaro, R.; Crisafi, E.; Chernikova, T.N.; Abraham, W.-R.; Luensdorf, H.; Timmis, K.N.; Golyshin, P.N. Thalassolituus oleivorans gen. nov., sp. nov., a novel marine bacterium that obligately utilizes hydrocarbons. Int. J. Syst. Evol. Microbiol. 2004, 54, 141–148. [Google Scholar] [CrossRef]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; Golyshin, P.N.; McKew, B.A. Differential Protein Expression during Growth on Medium Versus Long-Chain Alkanes in the Obligate Marine Hydrocarbon-Degrading Bacterium Thalassolituus oleivorans MIL-1. Front. Microbiol. 2018, 9, 3130. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Denaro, R.; Genovese, M.; Cappello, S.; D’Auria, G.; Chernikova, T.N.; Timmis, K.N.; Golyshin, P.N.; Giluliano, L. Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ. Microbiol. 2005, 7, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- McKew, B.A.; Coulon, F.; Yakimov, M.M.; Denaro, R.; Genovese, M.; Smith, C.J.; Osborn, A.M.; Timmis, K.N.; McGenity, T.J. Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ. Microbiol. 2007, 9, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Camilli, R.; Reddy, C.M.; Yoerger, D.R.; Van Mooy, B.A.S.; Jakuba, M.V.; Kinsey, J.C.; McIntyre, C.P.; Sylva, S.P.; Maloney, J.V. Tracking Hydrocarbon Plume Transport and Biodegradation at Deepwater Horizon. Science 2010, 330, 201–204. [Google Scholar] [CrossRef]
- Kryachko, Y.; Dong, X.; Sensen, C.W.; Voordouw, G. Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie Van Leeuwenhoek 2012, 101, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Lai, Q.; Shao, Z. Gene diversity of CYP153A and AlkB alkane hydroxylases in oil-degrading bacteria isolated from the Atlantic Ocean. Environ. Microbiol. 2010, 12, 1230–1242. [Google Scholar] [CrossRef]
- Shao, Z.; Yuan, J.; Lai, Q.; Zheng, T. The Diversity of PAH-degrading bacteria in a deep-sea water column above the Southwest Indian Ridge. Front. Microbiol. 2015, 6, 853. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Boroujeni, N.A. Enrichment and identification of naphthalene-degrading bacteria from the Persian Gulf. Mar. Pollut. Bull. 2016, 107, 59–65. [Google Scholar] [CrossRef]
- Gomes, M.B.; Gonzales-Limache, E.E.; Sousa, S.T.P.; Dellagnezze, B.M.; Sartoratto, A.; Silva, L.C.F.; Gieg, L.M.; Valoni, E.; Souza, R.S.; Torres, A.P.R.; et al. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int. Biodeterior. Biodegrad. 2018, 126, 231–242. [Google Scholar] [CrossRef]
- Nzila, A.; Jung, B.K.; Kim, M.-C.; Ibal, J.C.; Budiyanto, F.; Musa, M.M.; Thukair, A.; Kim, S.-J.; Shin, J.-H. Complete genome sequence of the polycyclic aromatic hydrocarbons biodegrading bacterium Idiomarina piscisalsi strain 10PY1A isolated from oil-contaminated soil. Korean J. Microbiol. 2018, 54, 289–292. [Google Scholar] [CrossRef]
- Fakhrzadegan, I.; Hassanshahian, M.; Askari Hesni, M.; Saadatfar, A. A study of crude oil-degrading bacteria from mangrove forests in the Persian Gulf. Mar. Ecol. 2019, 40, e12544. [Google Scholar] [CrossRef]
- Rizzo, C.; Papale, M.; Lo Giudice, A. Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers. J. Mar. Sci. Eng. 2022, 10, 1135. [Google Scholar] [CrossRef]
- Kwon, K.; Kwon, Y.M.; Kim, S.-J. Aerobic Hydrocarbon-Degrading Bacteroidetes. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; McGenity, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–91. [Google Scholar]
- Gutierrez, T.; Rhodes, G.; Mishamandani, S.; Berry, D.; Whitman, W.B.; Nichols, P.D.; Semple, K.T.; Aitken, M.D. Polycyclic Aromatic Hydrocarbon Degradation of Phytoplankton-Associated Arenibacter spp. and Description of Arenibacter algicola sp. nov., an Aromatic Hydrocarbon-Degrading Bacterium. Appl. Environ. Microbiol. 2014, 80, 618–628. [Google Scholar] [CrossRef]
- Gutierrez, T.; Whitman, W.B.; Huntemann, M.; Copeland, A.; Chen, A.; Kyrpides, N.; Markowitz, V.; Pillay, M.; Ivanova, N.; Mikhailova, N.; et al. Genome Sequence of Arenibacter algicola Strain TG409, a Hydrocarbon-Degrading Bacterium Associated with Marine Eukaryotic Phytoplankton. Genome Announc. 2016, 4, e00765-16. [Google Scholar] [CrossRef]
- Mishamandani, S.; Gutierrez, T.; Berry, D.; Aitken, M.D. Response of the bacterial community associated with a cosmopolitan marine diatom to crude oil shows a preference for the biodegradation of aromatic hydrocarbons. Environ. Microbiol. 2016, 18, 1817–1833. [Google Scholar] [CrossRef]
- Bagby, S.C.; Reddy, C.M.; Aeppli, C.; Fisher, G.B.; Valentine, D.L. Persistence and biodegradation of oil at the ocean floor following Deepwater Horizon. Proc. Natl. Acad. Sci. USA 2017, 114, E9–E18. [Google Scholar] [CrossRef]
- Hu, P.; Dubinsky, E.A.; Probst, A.J.; Wang, J.; Sieber, C.M.K.; Tom, L.M.; Gardinali, P.R.; Banfield, J.F.; Atlas, R.M.; Andersen, G.L. Simulation of Deepwater Horizon oil plume reveals substrate specialization within a complex community of hydrocarbon degraders. Proc. Natl. Acad. Sci. USA 2017, 114, 7432–7437. [Google Scholar] [CrossRef]
- Viggor, S.; Juhanson, J.; Jõesaar, M.; Mitt, M.; Truu, J.; Vedler, E.; Heinaru, A. Dynamic changes in the structure of microbial communities in Baltic Sea coastal seawater microcosms modified by crude oil, shale oil or diesel fuel. Microbiol. Res. 2013, 168, 415–427. [Google Scholar] [CrossRef]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-Degrading Bacteria and the Bacterial Community Response in Gulf of Mexico Beach Sands Impacted by the Deepwater Horizon Oil Spill. Appl. Environ. Microbiol. 2011, 77, 7962–7974. [Google Scholar] [CrossRef] [PubMed]
- Mnif, S.; Chamkha, M.; Sayadi, S. Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions. J. Appl. Microbiol. 2009, 107, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Whitman, W.B.; Huntemann, M.; Copeland, A.; Chen, A.; Kyrpides, N.; Markowitz, V.; Pillay, M.; Ivanova, N.; Mikhailova, N.; et al. Genome Sequence of Halomonas sp. Strain MCTG39a, a Hydrocarbon-Degrading and Exopolymeric Substance-Producing Bacterium. Genome Announc. 2015, 3, e00793-15. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al-Sabahi, J.; Al-Maqrashi, F.; Al-Habsi, A.; Al-Hinai, M. Characterization of hydrocarbon-degrading bacteria isolated from oil-contaminated sediments in the Sultanate of Oman and evaluation of bioaugmentation and biostimulation approaches in microcosm experiments. Int. Biodeterior. Biodegrad. 2014, 89, 58–66. [Google Scholar] [CrossRef]
- Melcher, R.J.; Apitz, S.E.; Hemmingsen, B.B. Impact of Irradiation and Polycyclic Aromatic Hydrocarbon Spiking on Microbial Populations in Marine Sediment for Future Aging and Biodegradability Studies. Appl. Environ. Microbiol. 2002, 68, 2858–2868. [Google Scholar] [CrossRef]
- García, M.T.; Mellado, E.; Ostos, J.C.; Ventosa, A. Halomonas organivorans sp. nov., a moderate halophile able to degrade aromatic compounds. Int. J. Syst. Evol. Microbiol. 2004, 54, 1723–1728. [Google Scholar] [CrossRef]
- Wright, M.H.; Bentley, S.R.; Greene, A.C. Draft Genome Sequence of Halomonas sp. Strain ML-15, a Haloalkaliphilic, Polycyclic Aromatic Hydrocarbon-Degrading Bacterium. Microbiol. Resour. Announc. 2020, 9, e01175-20. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Cesàro, A.; Liut, G.; Baldi, F. An antarctic psychrotrophic bacterium Halomonas sp. ANT-3b, growing on n-hexadecane, produces a new emulsyfying glycolipid. FEMS Microbiol. Ecol. 2005, 53, 157–166. [Google Scholar] [CrossRef]
- Yan, F.; Fang, J.; Cao, J.; Wei, Y.; Liu, R.; Wang, L.; Xie, Z. Halomonas piezotolerans sp. nov., a multiple-stress-tolerant bacterium isolated from a deep-sea sediment sample of the New Britain Trench. Int. J. Syst. Evol. Microbiol. 2020, 70, 2560–2568. [Google Scholar] [CrossRef]
- Janvier, M.; Grimont, P.A.D. The genus Methylophaga, a new line of descent within phylogenetic branch γ of proteobacteria. Res. Microbiol. 1995, 146, 543–550. [Google Scholar] [CrossRef]
- Vila, J.; Nieto, J.M.; Mertens, J.; Springael, D.; Grifoll, M. Microbial community structure of a heavy fuel oil-degrading marine consortium: Linking microbial dynamics with polycyclic aromatic hydrocarbon utilization. FEMS Microbiol. Ecol. 2010, 73, 349–362. [Google Scholar] [CrossRef]
- Muangchinda, C.; Rungsihiranrut, A.; Prombutara, P.; Soonglerdsongpha, S.; Pinyakong, O. 16S metagenomic analysis reveals adaptability of a mixed-PAH-degrading consortium isolated from crude oil-contaminated seawater to changing environmental conditions. J. Hazard. Mater. 2018, 357, 119–127. [Google Scholar] [CrossRef]
- Mishamandani, S.; Gutierrez, T.; Aitken, M. DNA-based stable isotope probing coupled with cultivation methods implicates Methylophaga in hydrocarbon degradation. Front. Microbiol. 2014, 5, 76. [Google Scholar] [CrossRef]
- Gutierrez, T.; Aitken, M.D. Role of methylotrophs in the degradation of hydrocarbons during the Deepwater Horizon oil spill. ISME J. 2014, 8, 2543–2545. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Ehiosun, K.I. Assessment of crude oil degradation efficiency of newly isolated actinobacteria reveals untapped bioremediation potentials. Bioremediat. J. 2016, 20, 133–143. [Google Scholar] [CrossRef]
- Margesin, R.; Moertelmaier, C.; Mair, J. Low-temperature biodegradation of petroleum hydrocarbons (n-alkanes, phenol, anthracene, pyrene) by four actinobacterial strains. Int. Biodeterior. Biodegrad. 2013, 84, 185–191. [Google Scholar] [CrossRef]
- Isaac, P.; Martínez, F.L.; Bourguignon, N.; Sánchez, L.A.; Ferrero, M.A. Improved PAHs removal performance by a defined bacterial consortium of indigenous Pseudomonas and actinobacteria from Patagonia, Argentina. Int. Biodeterior. Biodegrad. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Khurana, S.M.P. Importance of Actinobacteria for Bioremediation. In Plant Biotechnology: Progress in Genomic Era; Khurana, S.M.P., Gaur, R.K., Eds.; Springer: Singapore, 2019; pp. 277–307. [Google Scholar]
- De Pasquale, C.; Palazzolo, E.; Piccolo, L.L.; Quatrini, P. Degradation of long-chain n-alkanes in soil microcosms by two actinobacteria. J. Environ. Sci. Health Part A 2012, 47, 374–381. [Google Scholar] [CrossRef]
- Lumactud, R.; Fulthorpe, R.; Sentchilo, V.; Meer, J.R.v.d. Draft Genome Sequence of Microbacterium foliorum Strain 122 Isolated from a Plant Growing in a Chronically Hydrocarbon-Contaminated Site. Genome Announc. 2017, 5, e00434-17. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Reyes-Peralta, J.; Mendoza-Herrera, A.; Rivera, G.; Bocanegra-García, V. Characterization of a Microbacterium sp. strain isolated from soils contaminated with hydrocarbons in the burgos basin, Mexico. Rev. Int. De Contam. Ambient. 2021, 37. [Google Scholar] [CrossRef]
- Schippers, A.; Bosecker, K.; Spröer, C.; Schumann, P. Microbacterium oleivorans sp. nov. and Microbacterium hydrocarbonoxydans sp. nov., novel crude-oil-degrading Gram-positive bacteria. Int. J. Syst. Evol. Microbiol. 2005, 55, 655–660. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y.; Zhou, L.; Shen, Y.Y. Characterization of Microbacterium sp. F10a and its role in polycyclic aromatic hydrocarbon removal in low-temperature soil. Can. J. Microbiol. 2009, 55, 529–535. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, O.S.; Olatoye, N.O. Biodegradation of anthracene by a novel actinomycete, Microbacterium sp. isolated from tropical hydrocarbon-contaminated soil. World J. Microbiol. Biotechnol. 2014, 30, 335–341. [Google Scholar] [CrossRef]
- Salam, L.; Obayori, O.; Campbell, C.; Ilori, M.; Amund, O. Pyrene biodegradation potentials of an actinomycete, Microbacterium esteraromaticum isolated from tropical hydrocarbon-contaminated soil. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 995–1000. [Google Scholar] [CrossRef]
- Wongbunmak, A.; Khiawjan, S.; Suphantharika, M.; Pongtharangkul, T. BTEX- and naphthalene-degrading bacterium Microbacterium esteraromaticum strain SBS1-7 isolated from estuarine sediment. J. Hazard. Mater. 2017, 339, 82–90. [Google Scholar] [CrossRef]
- Logeshwaran, P.; Subashchandrabose, S.R.; Krishnan, K.; Sivaram, A.K.; Annamalai, P.; Naidu, R.; Megharaj, M. Polycyclic aromatic hydrocarbons biodegradation by fenamiphos degrading Microbacterium esteraromaticum MM1. Environ. Technol. Innov. 2022, 27, 102465. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Lødeng, A.G.G. Microbial Diversity during Biodegradation of Crude Oil in Seawater from the North Sea. Microb. Ecol. 2005, 49, 94–103. [Google Scholar] [CrossRef]
- Størdal, I.F.; Olsen, A.J.; Jenssen, B.M.; Netzer, R.; Hansen, B.H.; Altin, D.; Brakstad, O.G. Concentrations of viable oil-degrading microorganisms are increased in feces from Calanus finmarchicus feeding in petroleum oil dispersions. Mar. Pollut. Bull. 2015, 98, 69–77. [Google Scholar] [CrossRef]
- Liu, J.; Bacosa, H.P.; Liu, Z. Potential Environmental Factors Affecting Oil-Degrading Bacterial Populations in Deep and Surface Waters of the Northern Gulf of Mexico. Front. Microbiol. 2017, 7, 2131. [Google Scholar] [CrossRef]
- Gontikaki, E.; Potts, L.D.; Anderson, J.A.; Witte, U. Hydrocarbon-degrading bacteria in deep-water subarctic sediments (Faroe-Shetland Channel). J. Appl. Microbiol. 2018, 125, 1040–1053. [Google Scholar] [CrossRef]
- Ryther, C.M.; Ortmann, A.C.; Wohlgeschaffen, G.; Robinson, B.J. Temperate Coastal Microbial Communities Rapidly Respond to Low Concentrations of Partially Weathered Diesel. Microb. Ecol. 2022, 84, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Prabagaran, S.R.; Manorama, R.; Delille, D.; Shivaji, S. Predominance of Roseobacter, Sulfitobacter, Glaciecola and Psychrobacter in seawater collected off Ushuaia, Argentina, Sub-Antarctica. FEMS Microbiol. Ecol. 2007, 59, 342–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Yang, L.; Kong, Q.; Zhang, H. Microbial Degradation Mechanisms of Surface Petroleum Contaminated Seawater in a Typical Oil Trading Port. SSRN Electron. J. [CrossRef]
- Mas-Lladó, M.; Piña-Villalonga, J.M.; Brunet-Galmés, I.; Nogales, B.; Bosch, R. Draft Genome Sequences of Two Isolates of the Roseobacter Group, Sulfitobacter sp. Strains 3SOLIMAR09 and 1FIGIMAR09, from Harbors of Mallorca Island (Mediterranean Sea). Genome Announc. 2014, 2, e00350-14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, H.; Wang, Q.; Ge, Y.; Liu, W.; Christie, P. Enrichment of the soil microbial community in the bioremediation of a petroleum-contaminated soil amended with rice straw or sawdust. Chemosphere 2019, 224, 265–271. [Google Scholar] [CrossRef]
- Kuri, M.L.; Kumari, V.; Roy, S. Phenylobacterium Korensee Best Indigenous Petroleum Hydrocarbon Degrading Bacteria Isolated from Contaminated Soil of Bahror, Alwar Region, India. Int. J. Contemp. Res. Rev. 2019, 10, 20203–20211. [Google Scholar] [CrossRef]
- Kuri, M.; Kumari, V.; Roy, S. Biodegradable Capability of the Indigenous Micrococcus sp. Oil Degrading Bacteria Isolated from Oil Contaminated Soil, Motor Workshop Area of Bahror, Alwar, Rajasthan, India. Int. J. Adv. Eng. Nano Technol. 2021, 4, 1–4. [Google Scholar] [CrossRef]
- Yang, S.; Wen, X.; Zhao, L.; Shi, Y.; Jin, H. Crude oil treatment leads to shift of bacterial communities in soils from the deep active layer and upper permafrost along the China-Russia Crude Oil Pipeline route. PLoS ONE 2014, 9, e96552. [Google Scholar] [CrossRef]
- Rodgers-Vieira, E.A.; Zhang, Z.; Adrion, A.C.; Gold, A.; Aitken, M.D. Identification of Anthraquinone-Degrading Bacteria in Soil Contaminated with Polycyclic Aromatic Hydrocarbons. Appl. Environ. Microbiol. 2015, 81, 3775–3781. [Google Scholar] [CrossRef]
- Singleton, D.R.; Adrion, A.C.; Aitken, M.D. Surfactant-induced bacterial community changes correlated with increased polycyclic aromatic hydrocarbon degradation in contaminated soil. Appl. Microbiol. Biotechnol. 2016, 100, 10165–10177. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Uribe, M.L.; Peña-Cabriales, J.J.; Rivera-Cruz, M.D.C.; Délano-Frier, J.P. Native bacteria isolated from weathered petroleum oil-contaminated soils in Tabasco, Mexico, accelerate the degradation petroleum hydrocarbons in saline soil microcosms. Environ. Technol. Innov. 2021, 23, 101781. [Google Scholar] [CrossRef]
- Wang, B.; Teng, Y.; Yao, H.; Christie, P. Detection of functional microorganisms in benzene [a] pyrene-contaminated soils using DNA-SIP technology. J. Hazard. Mater. 2021, 407, 124788. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).