Comparative Molecular and Epidemiological Analyses of Israeli Bluetongue Viruses Serotype 1 and 9 Causing Outbreaks in 2018–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Samples
2.2. Virus Isolation (VI)
2.3. Nucleic Acid Extraction and Pan-BTV Real-Time Polymerase Chain Reaction (RT-PCR)
2.4. Type-Specific RT-PCRs
2.5. Sequencing and Phylogenetic Analyses
3. Results
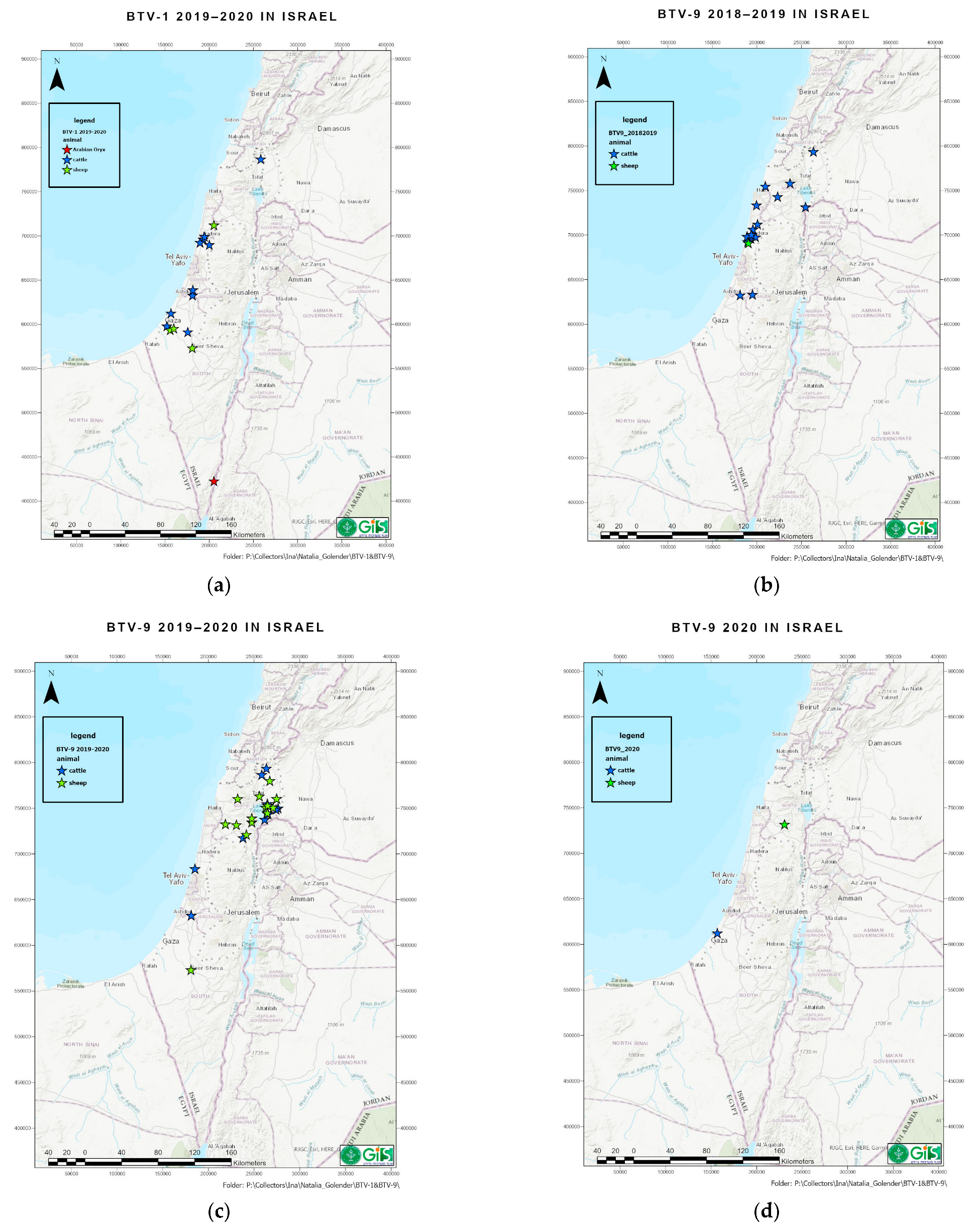
3.1. Clinical Signs in Affected Animals and Geographic Distribution of BTV-1
3.2. Clinical Signs in Affected Animals and Geographic Distribution of BTV-9
3.3. BTV Detection by Pan-BTV RT-qPCR from Field Samples Collected in 2018–2020
3.4. Virus Isolation (VI) from Field Collected in 2018–2020
3.5. Serotype Specific RT-qPCR Result of Tested BTV-Positive Tested in 2018–2020
3.6. Sequencing, Pairwise and BLAST Analyses of BTV-1 and BTV-9
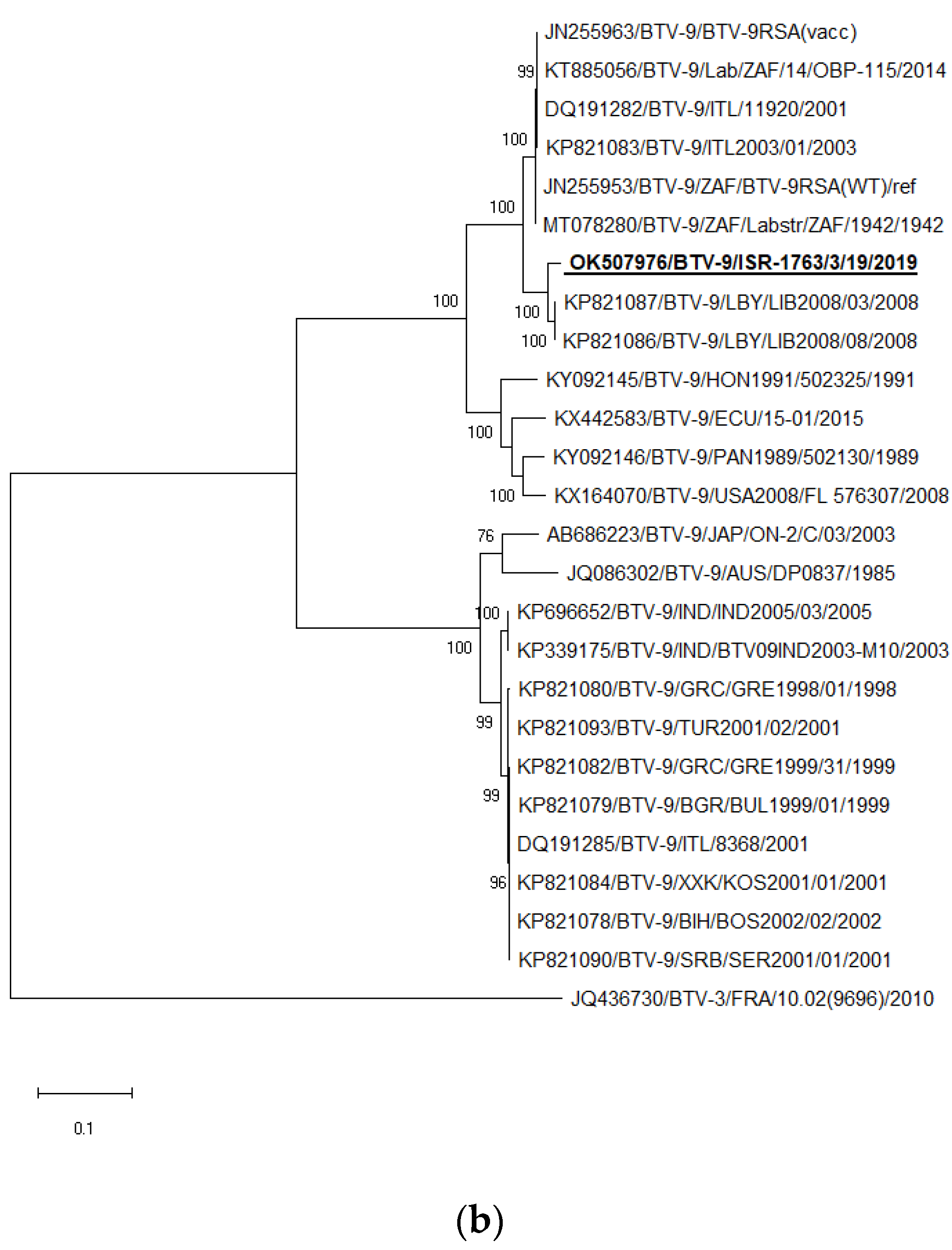
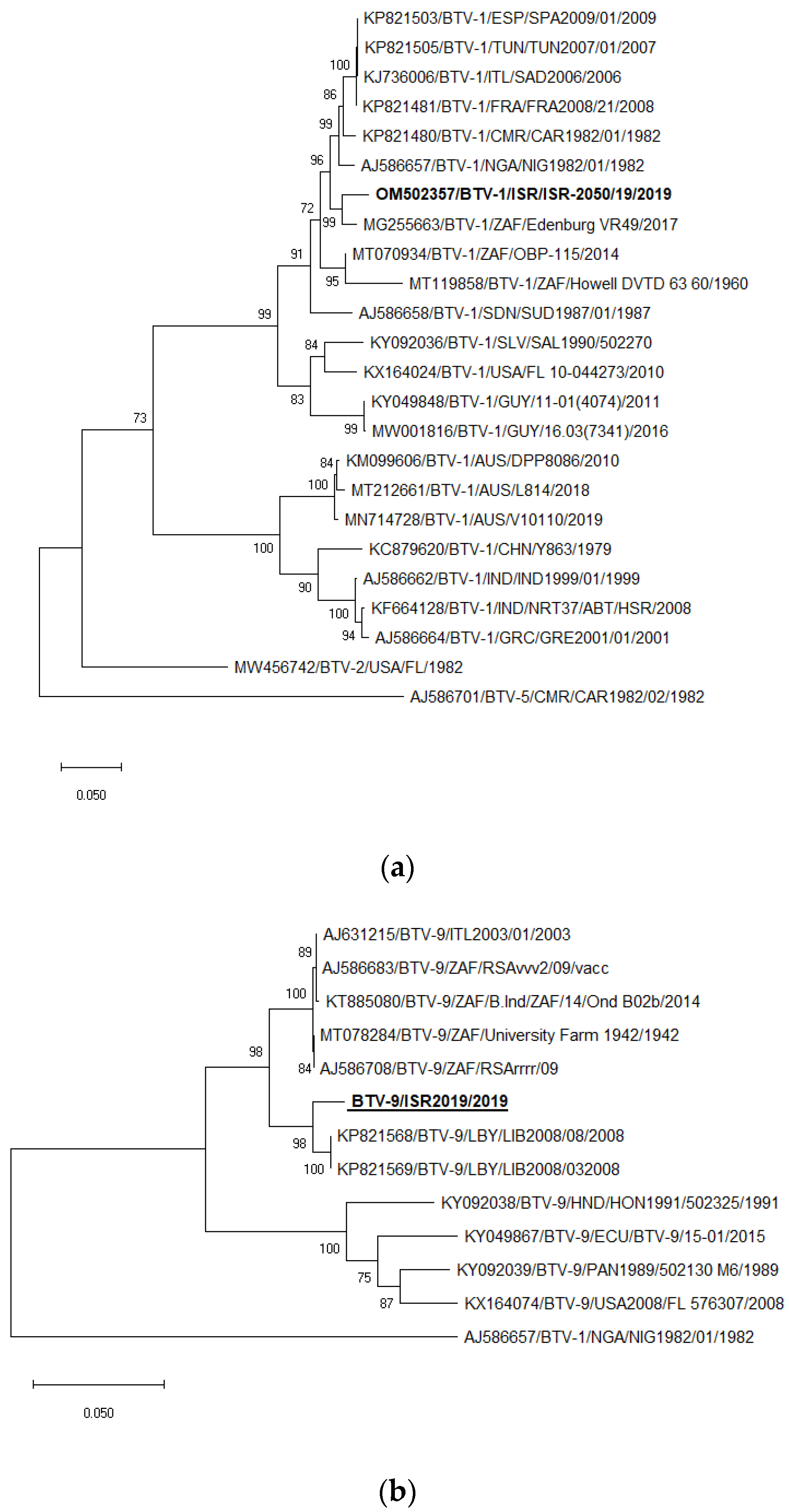
3.7. Phylogenetic Analysis of BTV-1 and BTV-9
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- The OIE Website. Available online: http://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/BLUETONGUE.pdf (accessed on 1 October 2022).
- Menzies, F.D.; McCullough, S.J.; McKeown, I.M.; Forster, J.L.; Jess, S.; Batten, C.; Murchie, A.K.; Gloster, J.; Fallows, J.G.; Pelgrim, W.; et al. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet. Rec. 2008, 163, 203–209. [Google Scholar] [CrossRef]
- Backx, A.; Heutink, R.; van Rooij, E.; van Rijn, P. Transplacental and oral transmission of wild-type bluetongue virus serotype 8 in cattle after experimental infection. Vet. Microbiol. 2009, 138, 235–243. [Google Scholar] [CrossRef]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6, 20627. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Lorusso, A.; Paladini, C.; Migliaccio, P.; Di Gennaro, A.; Di Provvido, A.; Scacchi, A.M.; Monaco, F. Bluetongue serotype 2 and 9 modified live vaccine viruses as causative agents of abortion in livestock: A retrospective analysis in Italy. Transbound. Emerg. Dis. 2014, 61, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Batten, C.; Darpel, K.; Henstock, M.; Fay, P.; Veronesi, E.; Gubbins, S.; Graves, S.; Frost, L.; Oura, C. Evidence for transmission of bluetongue virus serotype 26 through direct contact. PLoS ONE 2014, 9, e96049. [Google Scholar] [CrossRef] [PubMed]
- Bréard, E.; Schulz, C.; Sailleau, C.; Bernelin-Cottet, C.; Viarouge, C.; Vitour, D.; Guillaume, B.; Caignard, G.; Gorlier, A.; Attoui, H.; et al. Bluetongue virus serotype 27: Experimental infection of goats, sheep and cattle with three BTV-27 variants reveal atypical characteristics and likely direct contact transmission BTV-27 between goats. Transbound. Emerg. Dis. 2018, 65, e251–e263. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a second open reading frame in genome segment 10 of bluetongue virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.O.; Beer, M.; Hoffmann, B. Putative Novel Serotypes ’33’ and ’35’ in Clinically Healthy Small Ruminants in Mongolia Expand the Group of Atypical BTV. Viruses 2020, 13, 42. [Google Scholar] [CrossRef]
- Ries, C.; Vögtlin, A.; Hüssy, D.; Jandt, T.; Gobet, H.; Hilbe, M.; Burgener, C.; Schweizer, L.; Häfliger-Speiser, S.; Beer, M.; et al. Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses. 2021, 13, 721. [Google Scholar] [CrossRef]
- Gerdes, G.H. A South African overview of the virus, vectors, surveillance and unique features of bluetongue. Vet. Ital. 2004, 40, 39–42. [Google Scholar] [PubMed]
- Lorusso, A.; Sghaier, S.; Ancora, M.; Marcacci, M.; Di Gennaro, A.; Portanti, O.; Mangone, I.; Teodori, L.; Leone, A.; Camma’, C.; et al. Molecular epidemiology of bluetongue virus serotype 1 circulating in Italy and its connection with northern Africa. Infect. Genet. Evol. 2014, 28, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Biswas, S.K.; Sharma, G.; Saxena, A.; Tewari, N.; Mahajan, S.; Pandey, A.B. Full genome sequencing of the bluetongue virus-1 isolate MKD20/08/Ind from goat in India. Braz. J. Microbiol. 2016, 47, 527–528. [Google Scholar] [CrossRef]
- Saminathan, M.; Singh, K.P.; Khorajiya, J.H.; Dinesh, M.; Vineetha, S.; Maity, M.; Rahman, A.F.; Misri, J.; Malik, Y.S.; Gupta, V.K.; et al. An updated review on bluetongue virus: Epidemiology, pathobiology, and advances in diagnosis and control with special reference to India. Vet. Q. 2020, 40, 258–321. [Google Scholar] [CrossRef]
- Boyle, D.B.; Amos-Ritchie, R.; Broz, I.; Walker, P.J.; Melville, L.; Flanagan, D.; Davis, S.; Hunt, N.; Weir, R. Evolution of bluetongue virus serotype 1 in northern Australia over 30 years. J. Virol. 2014, 88, 13981–13989. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, N.; Xu, Q.; Sun, E.; Qin, Y.; Zhao, J.; Feng, Y.; Wu, D. Complete genomic sequence of bluetongue virus serotype 1 from China. J. Virol. 2012, 86, 1288–1289. [Google Scholar] [CrossRef]
- Hwang, J.M.; Kim, J.G.; Yeh, J.Y. Serological evidence of bluetongue virus infection and serotype distribution in dairy cattle in South Korea. BMC Vet. Res. 2019, 15, 255. [Google Scholar] [CrossRef]
- Viarouge, C.; Lancelot, R.; Rives, G.; Bréard, E.; Miller, M.; Baudrimont, X.; Doceul, V.; Vitour, D.; Zientara, S.; Sailleau, C. Identification of bluetongue virus and epizootic hemorrhagic disease virus serotypes in French Guiana in 2011 and 2012. Vet. Microbiol. 2014, 174, 78–85. [Google Scholar] [CrossRef]
- Puggioni, G.; Pintus, D.; Meloni, G.; Scivoli, R.; Rocchigiani, A.M.; Manunta, D.; Savini, G.; Oggiano, A.; Ligios, C. Persistence of Bluetongue virus serotype 1 virulence in sheep blood refrigerated for 10 years. Vet. Ital. 2018, 54, 349–353. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Arenas-Montes, A.; Lorca-Oró, C.; Pujols, J.; González, M.A.; Napp, S.; Gómez-Guillamón, F.; Zorrilla, I.; Miguel, E.S.; Arenas, A. Role of wild ruminants in the epidemiology of bluetongue virus serotypes 1, 4 and 8 in Spain. Vet. Res. 2011, 42, 88. [Google Scholar] [CrossRef]
- Corbière, F.; Nussbaum, S.; Alzieu, J.P.; Lemaire, M.; Meyer, G.; Foucras, G.; Schelcher, F. Bluetongue virus serotype 1 in wild ruminants, France, 2008–2010. J. Wildl. Dis. 2012, 48, 1047–1051. [Google Scholar] [CrossRef]
- Neidbalski, W. Bluetongue virus in Europe: The current epidemiological situation. Med. Weter. 2022, 78, 109–114. [Google Scholar] [CrossRef]
- El Hage, J.; Lorusso, A.; Carmine, I.; Di Gennaro, A.; Portanti, O.; Olivieri, S.; Casaccia, C.; Pisciella, M.; Teodori, L.; Sghaier, S.; et al. Bluetongue virus in Lebanon. Transbound. Emerg. Dis. 2013, 60, 390–394. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Savini, G.; Spedicato, M.; Monaco, F.; Carmine, I.; Lorusso, A.; Francesco, T.; Mazzei, M.; Forzan, M.; Eldaghayes, I.; et al. Exploiting serological data to understand the epidemiology of bluetongue virus serotypes circulating in Libya. Vet. Med. Sci. 2019, 5, 79–86. [Google Scholar] [CrossRef]
- Maan, S.; Samuel, A.R.; Maan, N.S.; Attoui, H.; Rao, S.; Mertens, P.P. Molecular epidemiology of bluetongue viruses from disease outbreaks in the Mediterranean Basin. Vet. Ital. 2004, 40, 489–496. [Google Scholar]
- Naresh, G.; Putty, K.; Reddy, Y.N.; Jyothi, Y.K. Type-specific seroprevalence of bluetongue in India during 2018 and 2019. Vet. World 2020, 13, 2092–2096. [Google Scholar] [CrossRef]
- Bellis, G.; Bishop, A.; Cameron, A.; Doherty, B.; Ellis, T.; Gard, G.; Johnson, S.; Kirkland, P.; Melville, L.; Muller, M.; et al. The History of Bluetongue, Akabane and Ephemeral Fever Viruses and Their Vectors in Australia 1975–1999; Animal Health Australia: Canberra, Australia, 2001. [Google Scholar]
- Schirtzinger, E.E.; Jasperson, D.C.; Ostlund, E.N.; Johnson, D.J.; Wilson, W.C. Recent US bluetongue virus serotype 3 isolates found outside of Florida indicate evidence of reassortment with co-circulating endemic serotypes. J. Gen. Virol. 2018, 99, 157–168. [Google Scholar] [CrossRef]
- Verdezoto, J.; Breard, E.; Viarouge, C.; Quenault, H.; Lucas, P.; Sailleau, C.; Zientara, S.; Augot, D.; Zapata, S. Novel serotype of bluetongue virus in South America and first report of epizootic haemorrhagic disease virus in Ecuador. Transbound. Emerg. Dis. 2018, 65, 244–247. [Google Scholar] [CrossRef]
- Taylor, W.P.; Mellor, P.S. Distribution of bluetongue virus in Turkey, 1978–1981. Epidemiol. Infect. 1994, 112, 623–633. [Google Scholar] [CrossRef]
- Ertürk, A.; Tatar, N.; Kabakli, O.; Incoglu, S.; Cizmeci, S.G.; Barut, F.M. The current situation of bluetongue in Turkey. Vet. Ital. 2004, 40, 137–140. [Google Scholar]
- Golender, N.; Bumbarov, V.; Eldar, A.; Zamir, L.; Tov, B.E.; Kenigswald, G.; Tiomkin, E. Isolation of Sango viruses from Israeli symptomatic cattle. Int. J. Vet. Sci. Res. 2021, 7, 069–072. [Google Scholar] [CrossRef]
- Golender, N.; Varsano, J.S.; Nissimyan, T.; Tiomkin, E. Identification of novel reassortant Shuni virus strain in clinical cases of Israeli ruminants, 2020-2021. Trop. Med. Infect Dis. 2022, 13, 297. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.; Kovtunenko, A.; David, D.; Guini-Rubinstein, M.; Sol, A.; Beer, M.; Eldar, A.; Wernike, K. Identification and Genetic Characterization of Viral Pathogens in Ruminant Gestation Abnormalities, Israel, 2015-2019. Viruses 2021, 13, 2136. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.Y. Detection of Epizootic Hemorrhagic Disease Virus Serotype 1, Israel. Emerg. Infect. Dis. 2019, 25, 825–827. [Google Scholar] [CrossRef]
- Golender, N.; Khinich, Y.; Gorohov, A.; Abramovitz, I.; Bumbarov, V. Epizootic hemorrhagic disease virus serotype 6 outbreak in Israeli cattle in 2015. J. Vet. Diagn. Investig. 2017, 29, 885–888. [Google Scholar] [CrossRef]
- Shimshony, A. Bluetongue in Israel—A brief historical overview. Vet. Ital. 2004, 40, 116–118. [Google Scholar]
- Golender, N.; Bumbarov, V.; Elda, R.A.; Lorusso, A.; Kenigswald, G.; Varsano, J.S.; David, D.; Schainin, S.; Dagoni, I.; Gur, I.; et al. Bluetongue Serotype 3 in Israel 2013–2018: Clinical Manifestations of the Disease and Molecular Characterization of Israeli Strains. Front. Vet. Sci. 2020, 6, 112. [Google Scholar] [CrossRef]
- Golender, N.; Eldar, A.; Ehrlich, M.; Kenigswald, G.; Shlamovitz, I.; Even-Tov, B.; Zamir, L.; Klement, E.; Bumbarov, V. Genomic Analysis Illustrated a Single Introduction and Evolution of Israeli Bluetongue Serotype 8 Virus Population 2008-2019. Microorganisms 2021, 14, 1955. [Google Scholar] [CrossRef]
- Komarov, A.; Goldsmit, L. A disease similar to Blue Tongue in cattle and sheep in Israel. Ref. Vet. 1951, 8, 96–100. [Google Scholar]
- Wernike, K.; Hoffmann, B.; Beer, M. Simultaneous detection of five notifiable viral diseases of cattle by single-tube multiplex real-time RT-PCR. J. Virol. Methods. 2015, 217, 28–35. [Google Scholar] [CrossRef]
- Lorusso, A.; Sghaier, S.; Di Domenico, M.; Barbria, M.E.; Zaccaria, G.; Megdich, A.; Portanti, O.; Seliman, I.B.; Spedicato, M.; Pizzurro, F.; et al. Analysis of bluetongue serotype 3 spread in Tunisia and discovery of a novel strain related to the bluetongue virus isolated from a commercial sheep pox vaccine. Infect. Genet. Evol. 2018, 59, 63–71. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Belaganahalli, M.N.; Potgieter, A.C.; Kumar, V.; Batra, K.; Wright, I.M.; Kirkland, P.D.; Mertens, P.P. Development and Evaluation of Real Time RT-PCR Assays for Detection and Typing of Bluetongue Virus. PLoS ONE 2016, 11, e0163014. [Google Scholar] [CrossRef]
- Chrzastek, K.; Lee, D.H.; Smith, D.; Sharma, P.; Suarez, D.; Pantin-Jackwood, M.; Kapczynski, D.R. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology 2017, 509, 159–166. [Google Scholar] [CrossRef]
- Ries, C.; Domes, U.; Janowetz, B.; Böttcher, J.; Burkhardt, K.; Miller, T.; Beer, M.; Hoffmann, B. Isolation and Cultivation of a New Isolate of BTV-25 and Presumptive Evidence for a Potential Persistent Infection in Healthy Goats. Viruses 2020, 12, 983. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Xu, G.; Wilson, W.; Mecham, J.; Murphy, K.; Zhou, E.M.; Tabachnick, W. VP7: An attachment protein of bluetongue virus for cellular receptors in Culicoides variipennis. J. Gen. Virol. 1997, 78, 1617–1623. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; van der Spek, A.N.; van Rijn, P.A. Epidemiologic characteristics of bluetongue virus serotype 8 laboratory-confirmed outbreaks in the Netherlands in 2007 and a comparison with the situation in 2006. Prev. Vet. Med. 2009, 92, 1–8. [Google Scholar] [CrossRef]
- Szmaragd, C.; Wilson, A.; Carpenter, S.; Mertens, P.P.; Mellor, P.S.; Gubbins, S. Mortality and case fatality during the recurrence of BTV-8 in northern Europe in 2007. Vet. Rec. 2007, 161, 571–572. [Google Scholar] [CrossRef]
- Machała, M.K.; Brydak, L.B. Grypa w róznych aspektach. Czqsc II--Epidemiologia i nadzór nad grypq oraz profilaktyka [Various sides of influenza. Part II--epidemiology, influenza surveillance and prophylaxis]. Pol. Merkur. Lekarski. 2006, 21, 277–285. [Google Scholar]

| Species | Cattle | Sheep | Goat | Wild Ruminants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year/Organ | w.b. | s/l | a.f | w.b. | s/l | a.f | w.b. | s/l | a.f | w.b. | s/l | a.f | Total | Total VI | Source | |
| 2018 | № of tested samples | 629 | 18 | 53 | 72 | 32 | 198 | 8 | 21 | 14 | 4 | 13 | 3 | 1065 | [35,40] * | |
| № of tested animals | 629 | 18 | 51 | 72 | 32 | 156 | 8 | 21 | 14 | 4 | 13 | 3 | 1021 | |||
| № of pos. samples | 217 | 6 | 1 | 36 | 0 | 6 | 3 | 2 | 0 | 0 | 0 | 0 | 271 | |||
| № of pos. animals | 217 | 6 | 1 | 36 | 0 | 6 | 3 | 2 | 0 | 0 | 0 | 0 | 271 | |||
| № of isolated BTV-2 | 1 | 1 | ||||||||||||||
| № of isolated BTV-3 | 1 | 6 | 1 | 8 | ||||||||||||
| № of isolated BTV-4 | 1 | 9 | 10 | |||||||||||||
| № of isolated BTV-6 | 2 | 2 | ||||||||||||||
| № of isolated BTV-15 | 9 | 9 | ||||||||||||||
| 2019 | № of tested samples | 490 | 45 | 112 | 109 | 51 | 140 | 7 | 5 | 17 | 23 | 26 | 5 | 1030 | [35,40] * | |
| № of tested animals | 490 | 45 | 87 | 109 | 51 | 98 | 7 | 5 | 13 | 23 | 26 | 5 | 959 | |||
| № of pos. samples | 106 | 8 | 1 | 52 | 17 | 9 | 4 | 0 | 0 | 0 | 0 | 0 | 197 | |||
| № of pos. animals | 106 | 8 | 1 | 52 | 17 | 7 | 4 | 0 | 0 | 0 | 0 | 0 | 195 | |||
| № of isolated BTV-1 | 3 | 2 | 5 | |||||||||||||
| № of isolated BTV-3 | 5 | 1 | 2 | 8 | ||||||||||||
| № of isolated BTV-4 | 1 | 6 | 7 | |||||||||||||
| № of isolated BTV-9 | 7 | 17 | 1 | 25 | ||||||||||||
| 2020 | № of tested samples | 560 | 86 | 93 | 155 | 76 | 186 | 2 | 22 | 28 | 3 | 59 | 6 | 1276 | current | |
| № of tested animals | 560 | 86 | 59 | 155 | 72 | 100 | 2 | 20 | 18 | 3 | 51 | 5 | 1131 | study | ||
| № of pos. samples | 169 | 10 | 1 | 72 | 12 | 22 | 0 | 2 | 1 | 0 | 7 | 0 | 296 | |||
| № of pos. animals | 169 | 10 | 1 | 72 | 8 | 18 | 0 | 2 | 1 | 0 | 7 | 0 | 288 | |||
| № of isolated BTV-2 | 1 | 3 | 4 | |||||||||||||
| № of isolated BTV-3 | 1 | 5 | 2 | 8 | ||||||||||||
| № of isolated BTV-4 | 3 | 3 | ||||||||||||||
| № of isolated BTV-6 | 1 | 1 | ||||||||||||||
| № of isolated BTV-12 | 1 | 1 | ||||||||||||||
| total | № of tested samples | 1679 | 149 | 258 | 336 | 159 | 524 | 17 | 48 | 59 | 30 | 98 | 14 | 3371 | current | |
| № of tested animals | 1679 | 149 | 197 | 336 | 155 | 354 | 17 | 46 | 45 | 30 | 90 | 13 | 3111 | study | ||
| № of pos. samples | 492 | 24 | 3 | 160 | 29 | 37 | 7 | 4 | 1 | 0 | 7 | 0 | 764 | |||
| № of pos. animals | 492 | 24 | 3 | 160 | 25 | 31 | 7 | 4 | 1 | 0 | 7 | 0 | 754 | |||
| № of isolated BTV-1 | 3 | 2 | 5 | 5 | ||||||||||||
| № of isolated BTV-2 | 2 | 3 | 5 | 5 | ||||||||||||
| № of isolated BTV-3 | 7 | 1 | 13 | 2 | 1 | 24 | 24 | |||||||||
| № of isolated BTV-4 | 2 | 18 | 20 | 20 | ||||||||||||
| № of isolated BTV-6 | 3 | 3 | 3 | |||||||||||||
| № of isolated BTV-9 | 7 | 17 | 1 | 25 | 25 | |||||||||||
| № of isolated BTV-12 | 1 | 1 | 1 | |||||||||||||
| № of isolated BTV-15 | 9 | 9 | 9 | |||||||||||||
| Cattle | Sheep | Goat | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT-qPCR | w.b. | s/l | a.f | w.b. | s/l | a.f | w.b. | s/l | a.f | Total | ||
| 2018 | BTV-3 | № of tested samples | 155 | 5 | 1 | 31 | 0 | 16 | 3 | 2 | 0 | 213 |
| № of positive | 13 | 2 | 0 | 8 | 0 | 0 | 1 | 1 | 0 | 25 | ||
| % of positive | 8.4 | 33.3 | 0 | 25.8 | 0 | 0 | 33.3 | 50 | 0 | 11.7 | ||
| BTV-4 | № of tested samples | 155 | 5 | 1 | 31 | 0 | 16 | 3 | 2 | 0 | 213 | |
| № of positive | 11 | 2 | 1 | 17 | 0 | 1 | 1 | 0 | 0 | 33 | ||
| % of positive | 7.1 | 33.3 | 100 | 54.8 | 0 | 6.2 | 33.3 | 0 | 0 | 15.4 | ||
| BTV-8 | № of tested samples | 155 | 5 | 1 | 31 | 0 | 16 | 3 | 2 | 0 | 213 | |
| № of positive | 0 | 0 | 0 | 0 | 0 | 1 * | 0 | 0 | 0 | 1 * | ||
| % of positive | 0 | 0 | 0 | 0 | 0 | 6.3 * | 0 | 0 | 0 | 0.5 * | ||
| BTV-9 | № of tested samples | 155 | 6 | 1 | 31 | 0 | 16 | 3 | 2 | 0 | 213 | |
| № of positive | 22 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 24 | ||
| % of positive | 14.2 | 0 | 0 | 0 | 0 | 6.3 | 0 | 0 | 0 | 11.27 | ||
| BTV-15 | № of tested samples | 155 | 6 | 1 | 31 | 0 | 16 | 3 | 2 | 0 | 213 | |
| № of positive | 111 | 3 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 118 | ||
| % of positive | 71.6 | 50 | 0 | 6.4 | 0 | 6.3 | 33.3 | 0 | 0 | 55.4 | ||
| 2019 | BTV-1 | № of tested samples | 101 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 191 |
| № of positive | 13 | 1 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 19 | ||
| % of positive | 12.9 | 37.3 | 0 | 3.9 | 13.3 | 0 | 25 | 0 | 0 | 9.9 | ||
| BTV-3 | № of tested samples | 102 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 192 | |
| № of positive | 19 | 2 | 0 | 16 | 3 | 1 | 0 | 0 | 0 | 41 | ||
| % of positive | 18.6 | 25 | 0 | 30.8 | 20 | 11.1 | 0 | 0 | 0 | 21.4 | ||
| BTV-4 | № of tested samples | 102 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 192 | |
| № of positive | 12 | 3 | 0 | 17 | 2 | 0 | 1 | 0 | 0 | 35 | ||
| % of positive | 11.8 | 37.5 | 0 | 32.7 | 13.3 | 0 | 25 | 0 | 0 | 18.2 | ||
| BTV-8 | № of tested samples | 103 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 193 | |
| № of positive | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | ||
| % of positive | 0.97 | 0 | 0 | 3.9 | 0 | 0 | 0 | 0 | 0 | 1.6 | ||
| BTV-9 | № of tested | 102 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 192 | |
| № of positive | 60 | 1 | 0 | 18 | 9 | 2 | 0 | 0 | 0 | 91 | ||
| % of positive | 58.8 | 12.5 | 0 | 34.6 | 60 | 22.2 | 0 | 0 | 0 | 47.4 | ||
| BTV-15 | № of tested samples | 103 | 8 | 1 | 52 | 15 | 9 | 4 | 0 | 1 | 193 | |
| № of positive | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| % of positive | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.52 | ||
| 2020 | BTV-1 | № of tested samples | 142 | 5 | 1 | 69 | 7 | 11 | 0 | 1 | 0 | 236 |
| № of positive | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | ||
| % of positive | 4.22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.54 | ||
| BTV-3 | № of tested samples | 142 | 5 | 1 | 69 | 7 | 11 | 0 | 1 | 0 | 236 | |
| № of positive | 63 | 3 | 0 | 37 | 6 | 7 | 0 | 1 | 0 | 117 | ||
| % of positive | 43.4 | 60 | 1 | 53.6 | 82.7 | 63.6 | 0 | 100 | 0 | 49.6 | ||
| BTV-4 | № of tested samples | 142 | 5 | 1 | 69 | 7 | 11 | 0 | 1 | 0 | 236 | |
| № of positive | 19 | 1 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 29 | ||
| % of positive | 13.4 | 20 | 0 | 13.04 | 0 | 0 | 0 | 0 | 0 | 12.3 | ||
| BTV-8 | № of tested samples | 142 | 5 | 1 | 69 | 7 | 11 | 0 | 1 | 0 | 236 | |
| № of positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| % of positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BTV-9 | № of tested samples | 142 | 5 | 1 | 69 | 7 | 11 | 0 | 1 | 0 | 236 | |
| № of positive | 10 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 13 | ||
| % of positive | 7.1 | 20 | 100 | 1.5 | 0 | 0 | 0 | 0 | 0 | 5.5 | ||
| Year | Species | Mixed BTV serotypes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, 3 | 1, 3, 4 | 1, 3, 9 | 1, 8 | 1, 9 | 2, 3 | 2, 4 | 3, 4 | 3, 6 | 3, 9 | 3, 15 | 4, 9 | 4, 15 | 4, EHDV | UT, EHDV | ||
| 2018 | cattle | 1 | 4 | 2 | 1 | 3 | ||||||||||
| 2019 | cattle | 1 | 3 | 1 | 1 | 2 | ||||||||||
| sheep | 2 | 1 | 2 | 1 | ||||||||||||
| 2020 | cattle | 1 | 1 | 1 | 1 | 5 | 2 | 1 | 3 | |||||||
| Arabian Oryx | 1 | |||||||||||||||
| Segment | Identity (%) | Accession Number/Serotype/Strain/Year | Country of Isolation |
|---|---|---|---|
| 1 | 96.86 | MT879211/BTV-4/GRE2014/08/2014 | Greece |
| 2 | 98.00 | KP821022/BTV-1/SUD1987/01/1987 | Sudan |
| 3 | 98.12 | MN200304/BTV-3/ISR-2210/18/2018 | Israel |
| 4 | 98.53 | MF124295/BTV-3/TUN2016/Zarzis/2016 | Tunisia |
| 5 | 98.40 | MH383093/BTV-6/ISR-2095/3/17/2017 | Israel |
| 6 | 96.67 | MG255663/BTV-1/VR49_2017/2017 | South Africa |
| 7 | 98.16 | KP821612/BTV-1/LIB2007/06/2007 | Libya |
| 8 | 97.00 | MG255457/BTV-5/01012015/2015 | South Africa |
| 9 | 98.66 | MF124300/BTV-3/TUN2016/Zarzis/2016 | Tunisia |
| 10 | 99.15 | MG255548/BTV-3/VR11_2017/2017 | South Africa |
| Segment | Identity (%) | Accession Number/Serotype/Strain/Year | Country of Isolation |
|---|---|---|---|
| 1 | 99.59 | MG344990/BTV-3/ISR-2153/16/2016 | Israel |
| 2 | 99.82 | KP821086/BTV-9/LIB2008/08/2008 | Libya |
| 3 | 99.19 | MH383091/BTV-6/ISR-2095/3/17/2017 | Israel |
| 4 | 98.47 | MF124295/BTV-3/TUN2016/Zarzis/2016 | Tunisia |
| 5 | 99.51 | ON087703/BTV/ISR-2237/18/2018 | Israel |
| 6 | 98.14 | KP821568/BTV-9/LIB2008/08/2008 | Libya |
| 7 | 99.80 | MK893198/BTV-4/ISR-1899/13/2013 | Israel |
| 8 | 99.90 | MK893195/BTV-4/ISR-1779/3/17/2017 | Israel |
| 9 | 96.53 | MG255454/BTV-5/01012015/2015 | South Africa |
| 10 | 98.21 | KP196612/BTV/BT 57/08/2008 | South Africa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golender, N.; Klement, E.; Kovtunenko, A.; Even-Tov, B.; Zamir, L.; Tiomkin, E.; Kenigswald, G.; Hoffmann, B. Comparative Molecular and Epidemiological Analyses of Israeli Bluetongue Viruses Serotype 1 and 9 Causing Outbreaks in 2018–2020. Microorganisms 2023, 11, 366. https://doi.org/10.3390/microorganisms11020366
Golender N, Klement E, Kovtunenko A, Even-Tov B, Zamir L, Tiomkin E, Kenigswald G, Hoffmann B. Comparative Molecular and Epidemiological Analyses of Israeli Bluetongue Viruses Serotype 1 and 9 Causing Outbreaks in 2018–2020. Microorganisms. 2023; 11(2):366. https://doi.org/10.3390/microorganisms11020366
Chicago/Turabian StyleGolender, Natalia, Eyal Klement, Anita Kovtunenko, Boris Even-Tov, Lior Zamir, Eitan Tiomkin, Gabriel Kenigswald, and Bernd Hoffmann. 2023. "Comparative Molecular and Epidemiological Analyses of Israeli Bluetongue Viruses Serotype 1 and 9 Causing Outbreaks in 2018–2020" Microorganisms 11, no. 2: 366. https://doi.org/10.3390/microorganisms11020366
APA StyleGolender, N., Klement, E., Kovtunenko, A., Even-Tov, B., Zamir, L., Tiomkin, E., Kenigswald, G., & Hoffmann, B. (2023). Comparative Molecular and Epidemiological Analyses of Israeli Bluetongue Viruses Serotype 1 and 9 Causing Outbreaks in 2018–2020. Microorganisms, 11(2), 366. https://doi.org/10.3390/microorganisms11020366







