Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Forest Litter Sampling and Fermentation Process
2.2. Physico-Chemical Analyses
2.3. DNA Extraction and Quantification
2.4. Quantification of Microbial Communities by qPCR
2.5. Molecular Profiling of Microbial Communities
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characteristics
3.2. Microbial Abundances
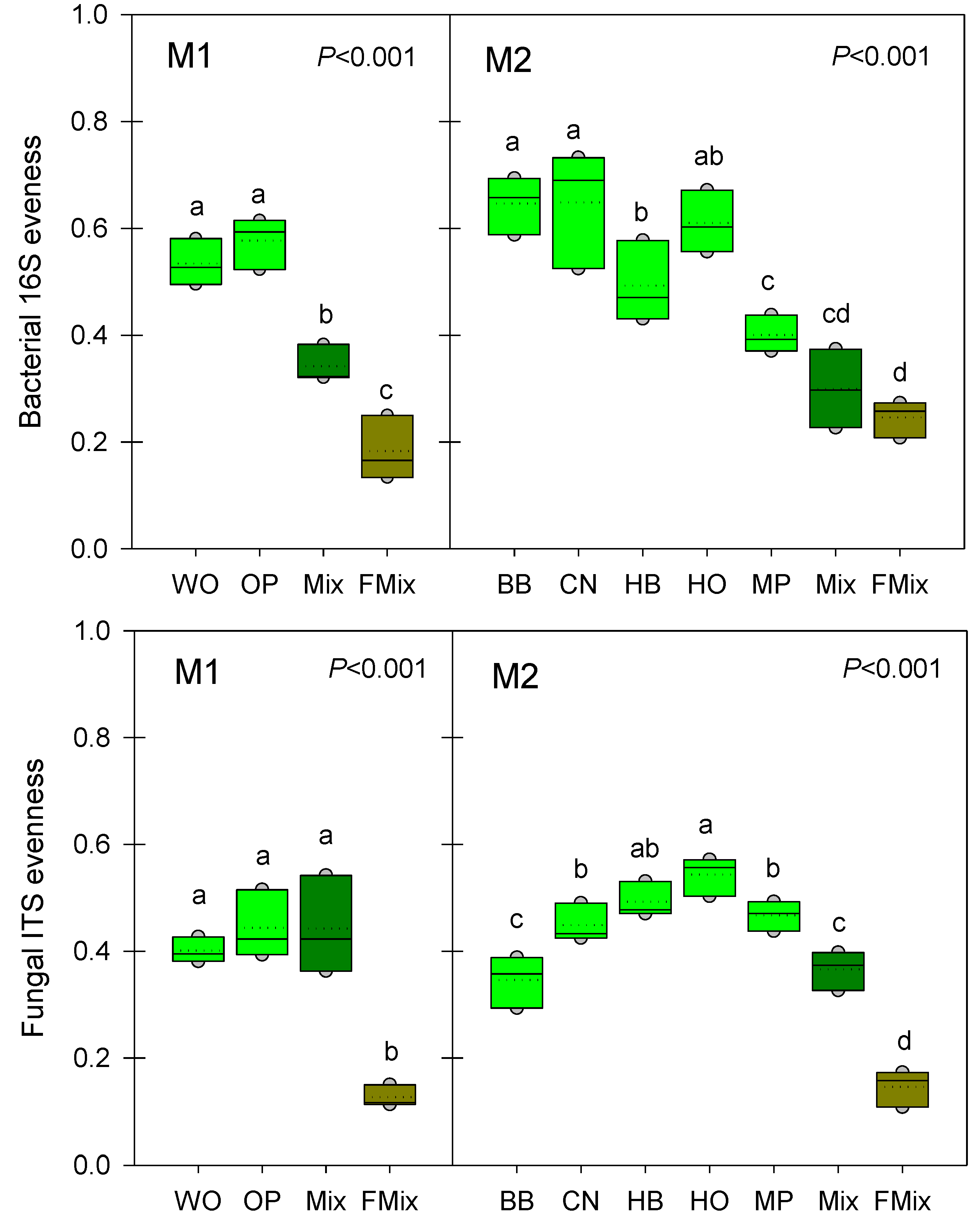
3.3. Microbial α-Diversities
3.4. Microbial β-Diversities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms-A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- De Graaff, M.A.; Hornslein, N.; Throop, H.L.; Kardol, P.; van Diepen, L.T.A. Effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: A meta-analysis. Adv. Agron. 2019, 155, 1–44. [Google Scholar]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Develop. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Siedt, M.; Schaffer, A.; Smith, K.E.C.; Nabel, M.; Ross-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Shirmohammadi, E.; Alikhani, H.A.; Pourbabaei, A.A.; Etesami, H. Improved phosphorus (P) uptake and yield of rainfed wheatfed with p fertilizer by drought-tolerant phosphate-solubilizing fluorescent pseudomonads strains: A field study in drylands. J. Soil Sci. Plant Nutr. 2020, 20, 2195–2211. [Google Scholar] [CrossRef]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant growth promoting rhizobacteria. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Elsevier: Cambridge, MA, USA, 2019; pp. 41–66. [Google Scholar]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants—Natural Biological Resources for Sustainable Plant Production. Microorganisms 2022, 10, 51. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Reith, F.; Drake, H.L.; Küsel, K. Anaerobic activities of bacteria and fungi in moderately acidic conifer and deciduous leaf litter. FEMS Microbiol. Ecol. 2002, 41, 27–35. [Google Scholar] [CrossRef]
- Kumar, B.L.; Gopal, D.V.R.S. Effective role of indigenous microorganisms for sustainable environment. Biotech 2015, 5, 867–876. [Google Scholar] [CrossRef]
- Brunel, C.; Gros, R.; Ziarelli, F.; Farnet Da Silva, A.M. Additive or non-additive effect of mixing oak in pine stands on soil properties depends on the tree species in Mediterranean forests. Sci. Tot. Env. 2017, 590–591, 676–685. [Google Scholar] [CrossRef]
- Kheir, M.; Lerch, T.Z.; Borsali, A.H.; Roche, P.; Zouidi, M.; Ziarelli, F.; Farnet Da Silva, A.M. Effect of monospecific and mixed litters on bacterial communities’ structure and functions under contrasted Mediterranean climate conditions. Appl. Soil Ecol. 2020, 155, 10368. [Google Scholar] [CrossRef]
- Kheir, M.; Lerch, T.Z.; Borsali, A.H.; Roche, P.; Ziarelli, F.; Zouidi, M.; Farnet Da Silva, A.M. Litter microbial responses to climate change: How do inland or coastal context and litter type matter across the Mediterranean? Ecol. Indic. 2021, 125, 107505. [Google Scholar] [CrossRef]
- Terre & Humanisme. Manuel de la litière forestière fermentée; Rouergue, Terre et Humanisme: Lablachère, France, 2021; p. 128. [Google Scholar]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Müller, T.; Cadisch, G. Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Nunan, N.; Lerch, T.Z.; Pouteau, V.; Mora, P.; Changey, F.; Kätterer, T.; Herrmann, A.M. Metabolising old soil carbon: Simply a matter of simple organic matter? Soil Biol. Biochem. 2015, 88, 128–136. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Dymock, D.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Moni, C.; Lerch, T.Z.; de Zarruk, K.K.; Strand, L.T.; Forte, C.; Certini, G.; Rasse, D.P. Temperature response of soil organic matter mineralisation in arctic soil profiles. Soil Biol. Biochem. 2015, 88, 236–246. [Google Scholar] [CrossRef]
- Blaud, A.; Diouf, F.; Herrmann, A.M.; Lerch, T.Z. Analysing the effect of soil organic matter on bacterial communities using T-RFLP fingerprinting: Different methods, different stories? Biol Fertil. Soils 2015, 51, 959–971. [Google Scholar] [CrossRef]
- Borsali, A.H.; Lerch, T.Z.; Besbes, R.; Gros, R.; Laffont-Schwob, I.; Boudenne, J.L.; Ziarelli, F.; Pando, A.; Farnet Da Silva, A.M. Coastal environments shape chemical and microbial properties of forest litters in circum Mediterranean region assessment. Eur. J. Soil Sci. 2021, 72, 1010–1025. [Google Scholar] [CrossRef]
- Leite, M.; Pan, Y.; Bloem, J.; Berge, H.T.; Kuramae, E.E. Organic nitrogen rearranges both structure and activity of the soil-borne microbial seedbank. Sci. Rep. 2017, 7, 42634. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simple measure of net N mineralization from stabilized soil organic matter in sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Janssen, B.H. Nitrogen mineralization in relation to C:N ratio and decomposability of organic materials. Plant Soil. 1996, 181, 39–45. [Google Scholar] [CrossRef]
- Sauer, S.; Dlugosch, L.; Milke, F.; Brinkhoff, T.; Kammerer, D.R.; Stintzing, F.C.; Simon, M. Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants. Fermentation 2022, 8, 383. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef] [PubMed]


| pHWater | Ec (μS.cm−1) | C(%) | N(%) | C/N | HI | ||
|---|---|---|---|---|---|---|---|
| Mix1: | WO | 7.12 a | 175 c | 50.37 a | 1.82 c | 27.58 a | 0.89 b |
| OP | 6.78 b | 169 c | 52.95 a | 2.61 b | 20.77 b | 0.90 b | |
| Mix1 | 6.31 c | 1238 b | 53.06 a | 2.90 ab | 18.24 bc | 0.96 a | |
| FMix1 | 4.29 d | 2134 a | 52.36 a | 3.39 a | 15.43 c | 0.97 a | |
| Mix2: | BB | 6.58 a | 71 d | 38.18 cd | 1.49 b | 25.77 bc | 0.89 d |
| CN | 6.41 a | 88 cd | 54.04 ab | 1.54 b | 35.01 b | 0.92 bc | |
| HB | 6.61 a | 34 e | 44.85 bc | 1.75 b | 25.61 bc | 0.91 cd | |
| HO | 5.38 b | 95 c | 34.40 d | 1.58 b | 24.52 cd | 0.88 d | |
| MP | 4.62 c | 92 c | 57.12 a | 0.84 c | 68.54 a | 0.95 ab | |
| Mix2 | 5.78 b | 2219 b | 51.67 ab | 3.02 a | 17.11 cd | 0.97 a | |
| FMix2 | 4.36 d | 2876 a | 52.03 ab | 3.44 a | 15.10 d | 0.96 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marois, J.; Lerch, T.Z.; Dunant, U.; Farnet Da Silva, A.-M.; Christen, P. Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers. Microorganisms 2023, 11, 306. https://doi.org/10.3390/microorganisms11020306
Marois J, Lerch TZ, Dunant U, Farnet Da Silva A-M, Christen P. Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers. Microorganisms. 2023; 11(2):306. https://doi.org/10.3390/microorganisms11020306
Chicago/Turabian StyleMarois, Johann, Thomas Z. Lerch, Ugo Dunant, Anne-Marie Farnet Da Silva, and Pierre Christen. 2023. "Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers" Microorganisms 11, no. 2: 306. https://doi.org/10.3390/microorganisms11020306
APA StyleMarois, J., Lerch, T. Z., Dunant, U., Farnet Da Silva, A.-M., & Christen, P. (2023). Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers. Microorganisms, 11(2), 306. https://doi.org/10.3390/microorganisms11020306






