Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis
Abstract
1. The Pathogenic Neisseria and Their Relationship to the Human Host
2. Meningococcal Diversity, Epidemiology, and Invasive Potential
3. Mechanisms Producing Meningococcal Variation
3.1. Natural Competence and Recombination
3.2. Phase Variation
3.3. Antigenic Variation and Hypervariable Loci
3.4. Hypermutation
4. Meningococcal Population Structure
5. Microevolution in Nme
5.1. Within-Host Evolution
5.2. Microevolution during Epidemics and Outbreaks
5.3. Microevolution in Response to Antimicrobials
5.4. Microevolution in Response to Vaccination
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Tang, C.M.; Exley, R.M. Non-pathogenic Neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiology 2015, 161, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, M.; Ong, J.J.; Fairley, C.K.; Chen, M.Y.; Williamson, D.A.; Maddaford, K.; Aung, E.T.; Carter, G.; Bradshaw, C.S.; Chow, E.P.F. Clinical presentation of asymptomatic and symptomatic heterosexual men who tested positive for urethral gonorrhoea at a sexual health clinic in Melbourne, Australia. BMC Infect. Dis. 2020, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, M.; Fairley, C.K.; Ong, J.J.; Maddaford, K.; Chen, M.Y.; Williamson, D.A.; Bradshaw, C.S.; Chow, E.P.F. Clinical presentation of asymptomatic and symptomatic women who tested positive for genital gonorrhoea at a sexual health service in Melbourne, Australia. Epidemiol. Infect. 2020, 148, e240. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Apicella, M.A. The Molecular Mechanisms Used by Neisseria gonorrhoeae To Initiate Infection Differ between Men and Women. Clin. Microbiol. Rev. 2004, 17, 965–981. [Google Scholar] [CrossRef]
- Criss, A.K.; Seifert, H.S. A bacterial siren song: Intimate interactions between Neisseria and neutrophils. Nat. Rev. Microbiol. 2012, 10, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, A.; McCluskey, N.R.; Kahler, C.M. The Host-Pathogen Interactions and Epicellular Lifestyle of Neisseria meningitidis. Front. Cell. Infect. Microbiol. 2022, 12, 862935. [Google Scholar] [CrossRef]
- Pace, D.; Pollard, A.J. Meningococcal disease: Clinical presentation and sequelae. Vaccine 2012, 30, B3–B9. [Google Scholar] [CrossRef]
- Schoen, C.; Kischkies, L.; Elias, J.; Ampattu, B.J. Metabolism and virulence in Neisseria meningitidis. Front. Cell. Infect. Microbiol. 2014, 4, 1–16. [Google Scholar] [CrossRef]
- Pelton, S.I. The Global Evolution of Meningococcal Epidemiology Following the Introduction of Meningococcal Vaccines. J. Adolesc. Health 2016, 59, S3–S11. [Google Scholar] [CrossRef]
- Caugant, D.A.; Maiden, M.C.J. Meningococcal carriage and disease—Population biology and evolution. Vaccine 2009, 27, B64–B70. [Google Scholar] [CrossRef]
- Bille, E.; Ure, R.; Gray, S.J.; Kaczmarski, E.B.; McCarthy, N.D.; Nassif, X.; Maiden, M.C.; Tinsley, C.R. Association of a bacteriophage with meningococcal disease in young adults. PLoS ONE 2008, 3, e3885. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.P.; Kriz, P.; Tzanakaki, G.; Kremastinou, J.; Kalmusova, J.; Musilek, M.; Alvestad, T.; Jolley, K.A.; Wilson, D.J.; McCarthy, N.D.; et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J. Clin. Microbiol. 2004, 42, 5146–5153. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis. 2013, 19, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Bartley, S.N.; Mowlaboccus, S.; Mullally, C.A.; Stubbs, K.A.; Vrielink, A.; Maiden, M.C.J.; Harrison, O.B.; Perkins, T.T.; Kahler, C.M. Acquisition of the capsule locus by horizontal gene transfer in Neisseria meningitidis is often accompanied by the loss of UDP-GalNAc synthesis. Sci. Rep. 2017, 7, 44442. [Google Scholar] [CrossRef]
- Lewis, L.A.; Ram, S. Complement interactions with the pathogenic Neisseriae: Clinical features, deficiency states, and evasion mechanisms. FEBS Lett. 2020, 594, 2670–2694. [Google Scholar] [CrossRef]
- Swartley, J.S.; Marfin, A.A.; Edupuganti, S.; Liu, L.J.; Cieslak, P.; Perkins, B.; Wenger, J.D.; Stephens, D.S. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 1997, 94, 271–276. [Google Scholar] [CrossRef]
- Saunders, N.J.; Snyder, L.A.S. The minimal mobile element. Microbiology 2002, 148, 3756–3760. [Google Scholar] [CrossRef]
- Schoen, C.; Blom, J.; Claus, H.; Schramm-Glück, A.; Brandt, P.; Müller, T.; Goesmann, A.; Joseph, B.; Konietzny, S.; Kurzai, O.; et al. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 2008, 105, 3473–3478. [Google Scholar] [CrossRef]
- Mustapha, M.M.; Marsh, J.W.; Krauland, M.G.; Fernandez, J.O.; de Lemos, A.P.S.; Hotopp, J.C.D.; Wang, X.; Mayer, L.W.; Lawrence, J.G.; Hiller, N.L.; et al. Genomic Investigation Reveals Highly Conserved, Mosaic, Recombination Events Associated with Capsular Switching among Invasive Neisseria meningitidis Serogroup W Sequence Type (ST)-11 Strains. Genome Biol. Evol. 2016, 8, 2065–2075. [Google Scholar] [CrossRef][Green Version]
- Bingqing, Z.; Pingping, Y.; Leyi, Z.; Yuan, G.; Li, X.; Na, X.; Zhujun, S. Genetic Analysis of Neisseria meningitidis Sequence Type 7 Serogroup X Originating from Serogroup A. Infect. Immun. 2017, 85, e01919-16. [Google Scholar] [CrossRef]
- Vogel, U.; Claus, H.; Frosch, M. Rapid Serogroup Switching in Neisseria meningitidis. N. Engl. J. Med. 2000, 342, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Shutt, K.A.; Schmink, S.E.; Marsh, J.W.; Harcourt, B.H.; Wang, X.; Whitney, A.M.; Stephens, D.S.; Cohn, A.A.; Messonnier, N.E.; et al. Population Structure and Capsular Switching of Invasive Neisseria meningitidis Isolates in the Pre-Meningococcal Conjugate Vaccine Era, United States, 2000–2005. J. Infect. Dis. 2010, 201, 1208–1224. [Google Scholar] [CrossRef] [PubMed]
- Mubaiwa, T.D.; Semchenko, E.A.; Hartley-Tassell, L.E.; Day, C.J.; Jennings, M.P.; Seib, K.L. The sweet side of the pathogenic Neisseria: The role of glycan interactions in colonisation and disease. Pathog. Dis. 2017, 75, ftx063. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Morita, M.; Kamiya, H.; Nakamura-Miwa, H.; Shimuta, K.; Ohnishi, M. Genetic characterization of clonal complex sequence type 2057 (cc2057) serogroup B Neisseria meningitidis strains unique to Japan and identification of a capsular-switched serogroup Y isolate cc2057. J. Med. Microbiol. 2022, 71, 001504. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Fazio, C.; Vacca, P.; Palmieri, A.; Ambrosio, L.; Neri, A.; Piana, A.; Castiglia, P.; Argiolas, F.; Santus, S.; et al. An outbreak of severe invasive meningococcal disease due to a capsular switched Neisseria meningitidis hypervirulent strain B:cc11. Clin. Microbiol. Infect. 2019, 25, 111.e111–111.e114. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.S.W.; Ahmad, T.; Tyler, S.; Lefebvre, B.; Deeks, S.L.; Gilca, R.; Hoang, L.; Tyrrell, G.; Van Caeseele, P.; Van Domselaar, G.; et al. Whole genome typing of the recently emerged Canadian serogroup W Neisseria meningitidis sequence type 11 clonal complex isolates associated with invasive meningococcal disease. Int. J. Infect. Dis. 2018, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yao, P.-P.; Zhang, L.-Y.; Li, Y.; Xu, F.; Mei, L.-L.; Zhu, S.-R.; Zhang, Y.-J.; Zhu, H.-P.; van der Veen, S. Capsule switching of Neisseria meningitidis sequence type 7 serogroup A to serogroup X. J. Infect. 2017, 75, 521–531. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, Z.; Du, P.; Xu, L.; Sun, X.; Gao, Y.; Shao, Z. Sequence Type 4821 Clonal Complex Serogroup B Neisseria meningitidis in China, 1978–2013. Emerg. Infect. Dis. 2015, 21, 925–932. [Google Scholar] [CrossRef]
- Pan, J.; Yao, P.; Zhang, H.; Sun, X.; He, H.; Xie, S. The case of a new sequence type 7 serogroup X Neisseria meningitidis infection in China: May capsular switching change serogroup profile? Int. J. Infect. Dis. 2014, 29, 62–64. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Z.; Wang, X.; Gao, Y.; Li, M.; Xu, L.; Xu, J.; Wang, L. Genetic Study of Capsular Switching between Neisseria meningitidis Sequence Type 7 Serogroup A and C Strains. Infect. Immun. 2010, 78, 3883–3888. [Google Scholar] [CrossRef]
- Simões, M.J.; Cunha, M.; Almeida, F.; Furtado, C.; Brum, L. Molecular surveillance of Neisseria meningitidis capsular switching in Portugal, 2002–2006. Epidemiol. Infect. 2009, 137, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Beddek Amanda, J.; Li, M.-S.; Kroll, J.S.; Jordan, T.W.; Martin Diana, R. Evidence for Capsule Switching between Carried and Disease-Causing Neisseria meningitidis Strains. Infect. Immun. 2009, 77, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, M.; Guiyoule, A.; Ruckly, C.; Hong, E.; Alonso, J.-M.; Taha, M.-K. Conserved virulence of C to B capsule switched Neisseria meningitidis clinical isolates belonging to ET-37/ST-11 clonal complex. Microbes Infect. 2006, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.S.W.; Law, D.K.S.; Tyler, S.D.; Stephens, G.S.; Bigham, M.; Zollinger, W.D. Potential Capsule Switching from Serogroup Y to B: The Characterization of Three such Neisseria meningitidis Isolates Causing Invasive Meningococcal Disease in Canada. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 216369. [Google Scholar] [CrossRef] [PubMed]
- Paola, S.; Cecilia, F.; Arianna, N.; Tonino, S.; Paola, M. First Report of Capsule Replacement among Electrophoretic Type 37 Neisseria meningitidis Strains in Italy. J. Clin. Microbiol. 2003, 41, 5783–5786. [Google Scholar] [CrossRef]
- Selander, R.K.; Caugant, D.A.; Ochman, H.; Musser, J.M.; Gilmour, M.N.; Whittam, T.S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environ. Microbiol. 1986, 51, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Caugant, D.A.; Bol, P.; Heiby, E.A.; Zanen, H.C.; Frøholm, L.O. Clones of Serogroup B Neisseria meningitidis Causing Systemic Disease in the Netherlands, 1958–1986. J. Infect. Dis. 1990, 162, 867–874. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A.; et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef]
- Urwin, R.; Maiden, M.C.J. Multi-locus sequence typing: A tool for global epidemiology. Trends Microbiol. 2003, 11, 479–487. [Google Scholar] [CrossRef]
- Mullally, C.A.; Mikucki, A.; Wise, M.J.; Kahler, C.M. Modelling evolutionary pathways for commensalism and hypervirulence in Neisseria meningitidis. Microb. Genom. 2021, 7, 000662. [Google Scholar] [CrossRef]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Malorny, B.; Müller, K.; Seiler, A.; Wang, J.-F.; Del Valle, J.; Achtman, M. Clonal descent and microevolution of Neisseria meningitidis during 30 years of epidemic spread. Mol. Microbiol. 1997, 25, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.M.; Marsh, J.W.; Krauland, M.G.; Fernandez, J.O.; de Lemos, A.P.S.; Dunning Hotopp, J.C.; Wang, X.; Mayer, L.W.; Lawrence, J.G.; Hiller, N.L.; et al. Genomic Epidemiology of Hypervirulent Serogroup W, ST-11 Neisseria meningitidis. EBioMedicine 2015, 2, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Lucidarme, J.; Hill, D.M.C.; Bratcher, H.B.; Gray, S.J.; du Plessis, M.; Tsang, R.S.W.; Vazquez, J.A.; Taha, M.-K.; Ceyhan, M.; Efron, A.M.; et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J. Infect. 2015, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Pardo de Santayana, C.; Tin Tin Htar, M.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed]
- Balmer, P.; Burman, C.; Serra, L.; York, L.J. Impact of meningococcal vaccination on carriage and disease transmission: A review of the literature. Hum. Vaccines Immunother. 2018, 14, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.; Chandrakumar, A.; Wang, H.L.R.; Clarke, M.; Sullivan, T.R.; Andrews, R.M.; Ramsay, M.; Marshall, H.S. Effectiveness of Meningococcal Vaccines at Reducing Invasive Meningococcal Disease and Pharyngeal Neisseria meningitidis Carriage: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2020, 73, e609–e619. [Google Scholar] [CrossRef] [PubMed]
- Miellet, W.R.; Pluister, G.; Sikking, M.; Tappel, M.; Karczewski, J.; Visser, L.J.; Bosch, T.; Trzciński, K.; Mariman, R. Surveillance of Neisseria meningitidis Carriage Four Years After menACWY Vaccine Implementation in the Netherlands Reveals Decline in Vaccine-type and Rise in Genogroup E Circulation. medRxiv 2023. [Google Scholar] [CrossRef]
- Lemos, A.P.S.d.; Gorla, M.C.O.; de Moraes, C.; Willemann, M.C.; Sacchi, C.T.; Fukasawa, L.O.; Camargo, C.H.; Barreto, G.; Rodrigues, D.S.; Gonçalves, M.G.; et al. Emergence of Neisseria meningitidis W South American sublineage strain variant in Brazil: Disease and carriage. J. Med. Microbiol. 2022, 71, 001484. [Google Scholar] [CrossRef]
- He, B.; Jia, Z.; Zhou, H.; Wang, Y.; Jiang, X.; Ma, H.; Qian, Z.; Liu, X.; Shao, Z.; Chen, S.; et al. CC4821 serogroup W meningococcal disease in China. Int. J. Infect. Dis. 2014, 29, 113–114. [Google Scholar] [CrossRef]
- Li, J.H.; Wu, D.; Yin, Z.D.; Li, Y.X. Analysis of epidemic characteristics for meningococcal meningitis in China during 2015–2017. Chin. J. Prev. Med. 2019, 53, 159–163. [Google Scholar] [CrossRef]
- Fukusumi, M.; Kamiya, H.; Takahashi, H.; Kanai, M.; Hachisu, Y.; Saitoh, T.; Ohnishi, M.; Oishi, K.; Sunagawa, T. National surveillance for meningococcal disease in Japan, 1999–2014. Vaccine 2016, 34, 4068–4071. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, G. Regulatory Networks of Neisseria meningitidis and Their Implications for Pathogenesis. Ph.D. Thesis, Univerity of Bologna, Bologna, Italy, 2015. [Google Scholar] [CrossRef]
- Blokesch, M. Natural competence for transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.-L.; Cehovin, A.; McDowell, M.A.; Lea, S.M.; Pelicic, V. Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Neisseria Species. PLoS Genet. 2013, 9, e1004014. [Google Scholar] [CrossRef]
- Frye, S.A.; Nilsen, M.; Tønjum, T.; Ambur, O.H. Dialects of the DNA Uptake Sequence in Neisseriaceae. PLoS Genet. 2013, 9, e1003458. [Google Scholar] [CrossRef]
- Budroni, S.; Siena, E.; Hotopp, J.C.D.; Seib, K.L.; Serruto, D.; Nofroni, C.; Comanducci, M.; Riley, D.R.; Daugherty, S.C.; Angiuoli, S.V.; et al. Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. Proc. Natl. Acad. Sci. USA 2011, 108, 4494. [Google Scholar] [CrossRef] [PubMed]
- Schoen, C.; Tettelin, H.; Parkhill, J.; Frosch, M. Genome flexibility in Neisseria meningitidis. Vaccine 2009, 27, B103–B111. [Google Scholar] [CrossRef]
- Michod, R.E.; Bernstein, H.; Nedelcu, A.M. Adaptive value of sex in microbial pathogens. Infect. Genet. Evol. 2008, 8, 267–285. [Google Scholar] [CrossRef]
- Thompson, E.A.L.; Feavers, I.M.; Maiden, M.C.J. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 2003, 149, 1849–1858. [Google Scholar] [CrossRef]
- Boan, P.; Metasan, N.; Tempone, S.; Harnett, G.; Speers, D.J.; Keil, A.D. Neisseria meningitidis porA, fetA and fHbp gene distribution in Western Australia 2000 to 2011. BMC Infect. Dis. 2014, 14, 686. [Google Scholar] [CrossRef]
- Bennett, J.S.; Thompson, E.A.L.; Kriz, P.; Jolley, K.A.; Maiden, M.C.J. A common gene pool for the Neisseria FetA antigen. Int. J. Med. Microbiol. 2009, 299, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Brehony, C.; Wilson, D.J.; Maiden, M.C.J. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology 2009, 155, 4155–4169. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.K.K.; Carlone, G.M.; Borrow, R. Advances in the development of vaccines against Neisseria meningitidis. N. Engl. J. Med. 2010, 362, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Bambini, S.; Muzzi, A.; Olcen, P.; Rappuoli, R.; Pizza, M.; Comanducci, M. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 2009, 27, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Bambini, S.; Chiara, M.D.; Muzzi, A.; Mora, M.; Lucidarme, J.; Brehony, C.; Borrow, R.; Masignani, V.; Comanducci, M.; Maiden, M.C.J.; et al. Neisseria adhesin A variation and revised nomenclature scheme. Clin. Vaccine Immunol. 2014, 21, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cohn, A.; Comanducci, M.; Andrew, L.; Zhao, X.; MacNeil, J.R.; Schmink, S.; Muzzi, A.; Bambini, S.; Rappuoli, R.; et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 2011, 29, 4739–4744. [Google Scholar] [CrossRef]
- Muzzi, A.; Mora, M.; Pizza, M.; Rappuoli, R.; Donati, C. Conservation of meningococcal antigens in the genus Neisseria. mBio 2013, 4, e00163-13. [Google Scholar] [CrossRef]
- Bambini, S.; Piet, J.; Muzzi, A.; Keijzers, W.; Comandi, S.; De Tora, L.; Pizza, M.; Rappuoli, R.; van de Beek, D.; van der Ende, A.; et al. An analysis of the sequence variability of meningococcal fHbp, NadA and NHBA over a 50-Year period in the Netherlands. PLoS ONE 2013, 8, e65043. [Google Scholar] [CrossRef]
- Mowlaboccus, S.; Perkins, T.T.; Smith, H.; Sloots, T.; Tozer, S.; Prempeh, L.-J.; Tay, C.Y.; Peters, F.; Speers, D.; Keil, A.D.; et al. Temporal Changes in BEXSERO® Antigen Sequence Type Associated with Genetic Lineages of Neisseria meningitidis over a 15-Year Period in Western Australia. PLoS ONE 2016, 11, e0158315. [Google Scholar] [CrossRef]
- Gasparini, R.; Comanducci, M.; Amicizia, D.; Ansaldi, F.; Canepa, P.; Orsi, A.; Icardi, G.; Rizzitelli, E.; Angelis, G.D.; Bambini, S.; et al. Molecular and serological diversity of Neisseria meningitidis carrier strains isolated from Italian students aged 14 to 22 years. J. Clin. Microbiol. 2014, 52, 1901–1910. [Google Scholar] [CrossRef]
- Parkhill, J.; Achtman, M.; James, K.D.; Bentley, S.D.; Churcher, C.; Klee, S.R.; Morelli, G.; Basham, D.; Brown, D.; Chillingworth, T.; et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 2000, 404, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Bille, E.; Zahar, J.-R.; Perrin, A.; Morelle, S.; Kriz, P.; Jolley, K.A.; Maiden, M.C.J.; Dervin, C.; Nassif, X.; Tinsley, C.R. A chromosomally integrated bacteriophage in invasive meningococci. J. Exp. Med. 2005, 201, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Uchiyama, I.; Kobayashi, I. Genome Comparison In Silico in Neisseria Suggests Integration of Filamentous Bacteriophages by their Own Transposase. DNA Res. 2005, 12, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bille, E.; Meyer, J.; Jamet, A.; Euphrasie, D.; Barnier, J.-P.; Brissac, T.; Larsen, A.; Pelissier, P.; Nassif, X. A virulence-associated filamentous bacteriophage of Neisseria meningitidis increases host-cell colonisation. PLoS Pathog. 2017, 13, e1006495. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Vernikos, G.S.; Snyder, L.A.S.; Churcher, C.; Arrowsmith, C.; Chillingworth, T.; Cronin, A.; Davis, P.H.; Holroyd, N.E.; Jagels, K.; et al. Meningococcal Genetic Variation Mechanisms Viewed through Comparative Analysis of Serogroup C Strain FAM18. PLoS Genet. 2007, 3, e23. [Google Scholar] [CrossRef] [PubMed]
- Liu Shi, V.; Saunders Nigel, J.; Jeffries, A.; Rest Richard, F. Genome Analysis and Strain Comparison of Correia Repeats and Correia Repeat-Enclosed Elements in Pathogenic Neisseria. J. Bacteriol. 2002, 184, 6163–6173. [Google Scholar] [CrossRef] [PubMed]
- Cehovin, A.; Lewis, S.B. Mobile genetic elements in Neisseria gonorrhoeae: Movement for change. Pathog. Dis. 2017, 75, ftx071. [Google Scholar] [CrossRef]
- Snyder, L.A.S.; Cole, J.A.; Pallen, M.J. Comparative analysis of two Neisseria gonorrhoeae genome sequences reveals evidence of mobilization of Correia Repeat Enclosed Elements and their role in regulation. BMC Genom. 2009, 10, 70. [Google Scholar] [CrossRef]
- Packiam, M.; Shell, D.M.; Liu, S.V.; Liu, Y.-B.; McGee, D.J.; Srivastava, R.; Seal, S.; Rest, R.F. Differential expression and transcriptional analysis of the alpha-2,3-sialyltransferase gene in pathogenic Neisseria spp. Infect. Immun. 2006, 74, 2637–2650. [Google Scholar] [CrossRef]
- Rouquette-Loughlin, C.E.; Balthazar, J.T.; Hill, S.A.; Shafer, W.M. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 2004, 54, 731–741. [Google Scholar] [CrossRef]
- Roberts, S.B.; Spencer-Smith, R.; Shah, M.; Nebel, J.-C.; Cook, R.T.; Snyder, L.A.S. Correia Repeat Enclosed Elements and Non-Coding RNAs in the Neisseria Species. Microorganisms 2016, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Zelewska, M.A.; Pulijala, M.; Spencer-Smith, R.; Mahmood, H.A.; Norman, B.; Churchward, C.P.; Calder, A.; Snyder, L.A.S. Phase variable DNA repeats in Neisseria gonorrhoeae influence transcription, translation, and protein sequence variation. Microb. Genom. 2016, 2, e000078. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.R.; Owen, P.; Nataro, J.P. Molecular switches—The ON and OFF of bacterial phase variation. Mol. Microbiol. 1999, 33, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Levinson, G.; Gutman, G.A. Slipped-strand mispairing: A major mechanism for DNA sequence evolution. Mol. Biol. Evol. 1987, 4, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.R.; Pytlos-Sinden, M.J.; Potaman, V.N. Slipped strand DNA structures. Front. Biosci. 2007, 12, 4788–4799. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.J.; Jeffries, A.C.; Peden, J.F.; Hood, D.W.; Tettelin, H.; Rappuoli, R.; Moxon, E.R. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol. 2000, 37, 207–215. [Google Scholar] [CrossRef]
- Martin, P.; Van De Ven, T.; Mouchel, N.; Jeffries, A.C.; Hood, D.W.; Moxon, E.R. Experimentally revised repertoire of putative contingency loci in Neisseria meningitidis strain MC58: Evidence for a novel mechanism of phase variation. Mol. Microbiol. 2003, 50, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Siena, E.; D’Aurizio, R.; Riley, D.; Tettelin, H.; Guidotti, S.; Torricelli, G.; Moxon, E.R.; Medini, D. In-silico prediction and deep-DNA sequencing validation indicate phase variation in 115 Neisseria meningitidis genes. BMC Genom. 2016, 17, 843. [Google Scholar] [CrossRef]
- Snyder, L.A.S.; Butcher, S.A.; Saunders, N.J. Comparative whole-genome analyses reveal over 100 putative phase-variable genes in the pathogenic Neisseria spp. Microbiology 2001, 147, 2321–2332. [Google Scholar] [CrossRef]
- Klughammer, J.; Dittrich, M.; Blom, J.; Mitesser, V.; Vogel, U.; Frosch, M.; Goesmann, A.; Müller, T.; Schoen, C. Comparative Genome Sequencing Reveals Within-Host Genetic Changes in Neisseria meningitidis during Invasive Disease. PLoS ONE 2017, 12, e0169892. [Google Scholar] [CrossRef]
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Hill, D.M.C.; Harrison, O.B.; Srikhanta, Y.N.; Jennings, M.P.; Maiden, M.C.J.; Seib, K.L. Distribution of the type III DNA methyltransferases modA, modB and modD among Neisseria meningitidis genotypes: Implications for gene regulation and virulence. Sci. Rep. 2016, 6, 21015. [Google Scholar] [CrossRef] [PubMed]
- Blyn, L.B.; Braaten, B.A.; Low, D.A. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990, 9, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- Haagmans, W.; van Der Woude, M. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol. 2000, 35, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Low, D. DNA methylation-dependent regulation of pef expression in Salmonella typhimurium. Mol. Microbiol. 2000, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Srikhanta, Y.N.; Dowideit, S.J.; Edwards, J.L.; Falsetta, M.L.; Wu, H.-J.; Harrison, O.B.; Fox, K.L.; Seib, K.L.; Maguire, T.L.; Wang, A.H.-J. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog. 2009, 5, e1000400. [Google Scholar] [CrossRef]
- Srikhanta, Y.N.; Fox, K.L.; Jennings, M.P. The phasevarion: Phase variation of type III DNA methyltransferases controls coordinated switching in multiple genes. Nat. Rev. Microbiol. 2010, 8, 196–206. [Google Scholar] [CrossRef]
- Kennouche, P.; Charles-Orszag, A.; Nishiguchi, D.; Goussard, S.; Imhaus, A.-F.; Dupré, M.; Chamot-Rooke, J.; Duménil, G. Deep mutational scanning of the Neisseria meningitidis major pilin reveals the importance of pilus tip-mediated adhesion. EMBO J. 2019, 38, e102145. [Google Scholar] [CrossRef]
- Georgiadou, M.; Castagnini, M.; Karimova, G.; Ladant, D.; Pelicic, V. Large-scale study of the interactions between proteins involved in type IV pilus biology in Neisseria meningitidis: Characterization of a subcomplex involved in pilus assembly. Mol. Microbiol. 2012, 84, 857–873. [Google Scholar] [CrossRef]
- Brown, D.R.; Helaine, S.; Carbonnelle, E.; Pelicic, V. Systematic functional analysis reveals that a set of seven genes is involved in fine-tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis. Infect. Immun. 2010, 78, 3053–3063. [Google Scholar] [CrossRef]
- Wörmann, M.E.; Horien, C.L.; Bennett, J.S.; Jolley, K.A.; Maiden, M.C.J.; Tang, C.M.; Aho, E.L.; Exley, R.M. Sequence, distribution and chromosomal context of class I and class II pilin genes of Neisseria meningitidis identified in whole genome sequences. BMC Genom. 2014, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, T.S.; Dempsey, J.A.F.; Cohen, M.S.; Cannon, J.G. Antigenic variation of gonococcal pilin expression in vivo: Analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology 2001, 147, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Prister, L.L.; Ozer, E.A.; Cahoon, L.A.; Seifert, H.S. Transcriptional initiation of a small RNA, not R-loop stability, dictates the frequency of pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 2019, 112, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.S. Above and Beyond Watson and Crick: Guanine Quadruplex Structures and Microbes. Annu. Rev. Microbiol. 2018, 72, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Rusniok, C.; Vallenet, D.; Floquet, S.; Ewles, H.; Mouzé-Soulama, C.; Brown, D.; Lajus, A.; Buchrieser, C.; Médigue, C.; Glaser, P.; et al. NeMeSys: A biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis. Genome Biol. 2009, 10, R110. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Schipper, K.; van Ulsen, P.; van der Ende, A.; Tommassen, J. Domain exchange at the 3′ end of the gene encoding the fratricide meningococcal two-partner secretion protein A. BMC Genom. 2013, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Jamet, A.; Jousset, A.B.; Euphrasie, D.; Mukorako, P.; Boucharlat, A.; Ducousso, A.; Charbit, A.; Nassif, X. A New Family of Secreted Toxins in Pathogenic Neisseria Species. PLoS Pathog. 2015, 11, e1004592. [Google Scholar] [CrossRef] [PubMed]
- Virji, M.; Makepeace, K.; Ferguson, D.J.P.; Achtman, M.; Moxon, E.R. Meningococcal Opa and Opc proteins: Their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 1993, 10, 499–510. [Google Scholar] [CrossRef]
- Bhat, K.S.; Gibbs, C.P.; Barrera, O.; Morrison, S.G.; Jähnig, F.; Stern, A.; Kupsch, E.M.; Meyer, T.F.; Swanson, J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 1991, 5, 1889–1901. [Google Scholar] [CrossRef]
- Martin, J.C.; Keith, A.J.; Martin, C.J.M. Opacity-Associated Adhesin Repertoire in Hyperinvasive Neisseria meningitidis. Infect. Immun. 2006, 74, 5085–5094. [Google Scholar] [CrossRef]
- De Jonge, M.I.; Hamstra, H.J.; Van Alphen, L.; Dankert, J.; Van Der Ley, P. Mapping the binding domains on meningococcal Opa proteins for CEACAM1 and CEA receptors. Mol. Microbiol. 2003, 50, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Pollard, A.J.; Gray-Owen, S.D. Opa proteins and CEACAMs: Pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol. Rev. 2011, 35, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, M.; Giovanna, M.; Barica, K.; Jan, K.; Mark, A. Sequence Diversity, Predicted Two-Dimensional Protein Structure, and Epitope Mapping of Neisserial Opa Proteins. J. Bacteriol. 1998, 180, 1323–1330. [Google Scholar] [CrossRef]
- Islam, E.A.; Anipindi, V.C.; Francis, I.; Shaik-Dasthagirisaheb, Y.; Xu, S.; Leung, N.; Sintsova, A.; Amin, M.; Kaushic, C.; Wetzler, L. Specific binding to differentially-expressed human CEACAMs determines the outcome of Neisseria gonorrhoeae infections along the female reproductive tract. Infect. Immun. 2018, 86, e00092-18. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J.; Buckee, C.O.; Jolley, K.A.; Kriz, P.; Maiden, M.C.J.; Gupta, S. The Effect of Immune Selection on the Structure of the Meningococcal Opa Protein Repertoire. PLoS Pathog. 2008, 4, e1000020. [Google Scholar] [CrossRef] [PubMed]
- Marinus, M.G. DNA Mismatch Repair. EcoSal Plus 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Baquero, F.; Blázquez, J. The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: Molecular characterization of naturally occurring mutants. Mol. Microbiol. 2002, 43, 1641–1650. [Google Scholar] [CrossRef]
- Rosche, W.A.; Foster, P.L. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 1999, 96, 6862–6867. [Google Scholar] [CrossRef]
- Viberg, L.T.; Sarovich, D.S.; Kidd, T.J.; Geake, J.B.; Bell, S.C.; Currie, B.J.; Price, E.P. Within-host evolution of Burkholderia pseudomallei during chronic infection of seven Australasian cystic fibrosis patients. mBio 2017, 8, e00356-17. [Google Scholar] [CrossRef]
- Hammerstrom, T.G.; Beabout, K.; Clements, T.P.; Saxer, G.; Shamoo, Y. Acinetobacter baumannii Repeatedly Evolves a Hypermutator Phenotype in Response to Tigecycline That Effectively Surveys Evolutionary Trajectories to Resistance. PLoS ONE 2015, 10, e0140489. [Google Scholar] [CrossRef]
- Jayaraman, R. Hypermutation and stress adaptation in bacteria. J. Genet. 2011, 90, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Płaczkiewicz, J.; Adamczyk-Popławska, M.; Lasek, R.; Bącal, P.; Kwiatek, A. Inactivation of Genes Encoding MutL and MutS Proteins Influences Adhesion and Biofilm Formation by Neisseria gonorrhoeae. Microorganisms 2019, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.R.; Yu, Z.; Popovic, T.; Stojiljkovic, I. Mutator clones of Neisseria meningitidis in epidemic serogroup A disease. Proc. Natl. Acad. Sci. USA 2002, 99, 6103–6107. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.R.; Stojiljkovic, I. Mismatch repair and the regulation of phase variation in Neisseria meningitidis. Mol. Microbiol. 2001, 40, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Rotman, E.; Seifert, H.S. Neisseria gonorrhoeae MutS affects pilin antigenic variation through mismatch correction and not by pilE guanine quartet binding. J. Bacteriol. 2015, 197, 1828–1838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bayliss Christopher, D.; Hoe, J.C.; Makepeace, K.; Martin, P.; Hood Derek, W.; Moxon, E.R. Neisseria meningitidis Escape from the Bactericidal Activity of a Monoclonal Antibody Is Mediated by Phase Variation of lgtG and Enhanced by a Mutator Phenotype. Infect. Immun. 2008, 76, 5038–5048. [Google Scholar] [CrossRef]
- Fraser, C.; Hanage, W.P.; Spratt, B.G. Neutral microepidemic evolution of bacterial pathogens. Proc. Natl. Acad. Sci. USA 2005, 102, 1968–1973. [Google Scholar] [CrossRef]
- Corander, J.; Fraser, C.; Gutmann, M.U.; Arnold, B.; Hanage, W.P.; Bentley, S.D.; Lipsitch, M.; Croucher, N.J. Frequency-dependent selection in vaccine-associated pneumococcal population dynamics. Nat. Ecol. Evol. 2017, 1, 1950–1960. [Google Scholar] [CrossRef]
- McNally, A.; Kallonen, T.; Connor, C.; Abudahab, K.; Aanensen, D.M.; Horner, C.; Peacock, S.J.; Parkhill, J.; Croucher, N.J.; Corander, J. Diversification of Colonization Factors in a Multidrug-Resistant Escherichia coli Lineage Evolving under Negative Frequency-Dependent Selection. mBio 2019, 10, e00644-19. [Google Scholar] [CrossRef]
- Diallo, K.; Maiden, M.C.J. An introductory narrative to the population genomics of pathogenic bacteria, exemplified by Neisseria meningitidis. In Population Genomics: Microorganisms; Polz, M.F., Rajora, O.P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–143. [Google Scholar]
- Feil, E.J.; Holmes, E.C.; Bessen, D.E.; Chan, M.-S.; Day, N.P.J.; Enright, M.C.; Goldstein, R.; Hood, D.W.; Kalia, A.; Moore, C.E.; et al. Recombination within natural populations of pathogenic bacteria: Short-term empirical estimates and long-term phylogenetic consequences. Proc. Natl. Acad. Sci. USA 2001, 98, 182–187. [Google Scholar] [CrossRef]
- Feil, E.J.; Maiden, M.C.; Achtman, M.; Spratt, B.G. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol. Biol. Evol. 1999, 16, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Smith, N.H.; O’Rourke, M.; Spratt, B.G. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 1993, 90, 4384–4388. [Google Scholar] [CrossRef] [PubMed]
- Tibayrenc, M.; Ayala, F.J. How clonal are Neisseria species? The epidemic clonality model revisited. Proc. Natl. Acad. Sci. USA 2015, 112, 8909–8913. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Wilson, D.J.; Kriz, P.; McVean, G.; Maiden, M.C.J. The Influence of Mutation, Recombination, Population History, and Selection on Patterns of Genetic Diversity in Neisseria meningitidis. Mol. Biol. Evol. 2004, 22, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Buckee, C.O.; Jolley, K.A.; Recker, M.; Penman, B.; Kriz, P.; Gupta, S.; Maiden, M.C.J. Role of selection in the emergence of lineages and the evolution of virulence in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 2008, 105, 15082. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, G.; Medini, D. Horizontal Gene Transfer and the Role of Restriction-Modification Systems in Bacterial Population Dynamics. In Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life; Pontarotti, P., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 169–190. [Google Scholar]
- Oliveira, P.H.; Touchon, M.; Rocha, E.P.C. Regulation of genetic flux between bacteria by restriction–modification systems. Proc. Natl. Acad. Sci. USA 2016, 113, 5658–5663. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.R.; Bull, J.J. Short-sighted evolution and the virulence of pathogenic microorganisms. Trends Microbiol. 1994, 2, 76–81. [Google Scholar] [CrossRef]
- Meyers, L.A.; Levin, B.R.; Richardson, A.R.; Stojiljkovic, I. Epidemiology, hypermutation, within–host evolution and the virulence of Neisseria meningitidis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1667–1677. [Google Scholar] [CrossRef]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef]
- Omer, H.; Rose, G.; Jolley, K.A.; Frapy, E.; Zahar, J.-R.; Maiden, M.C.J.; Bentley, S.D.; Tinsley, C.R.; Nassif, X.; Bille, E. Genotypic and Phenotypic Modifications of Neisseria meningitidis after an Accidental Human Passage. PLoS ONE 2011, 6, e17145. [Google Scholar] [CrossRef]
- Lecuyer, H.; Nassif, X.; Coureuil, M. Two strikingly different signaling pathways are induced by meningococcal type IV pili on endothelial and epithelial cells. Infect. Immun. 2012, 80, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.A.; Kremer, P.H.C.; Manso, A.S.; Croucher, N.J.; Ferwerda, B.; Serón, M.V.; Oggioni, M.R.; Parkhill, J.; Brouwer, M.C.; van der Ende, A.; et al. Large scale genomic analysis shows no evidence for pathogen adaptation between the blood and cerebrospinal fluid niches during bacterial meningitis. Microb. Genom. 2017, 3, e000103. [Google Scholar] [CrossRef] [PubMed]
- Alamro, M.; Bidmos Fadil, A.; Chan, H.; Oldfield Neil, J.; Newton, E.; Bai, X.; Aidley, J.; Care, R.; Mattick, C.; Turner David, P.J.; et al. Phase Variation Mediates Reductions in Expression of Surface Proteins during Persistent Meningococcal Carriage. Infect. Immun. 2014, 82, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Green, L.R.; Al-Rubaiawi, A.A.; Al-Maeni, M.A.R.M.; Harrison, O.B.; Blades, M.; Oldfield, N.J.; Turner, D.P.J.; Maiden, M.C.J.; Bayliss, C.D. Localized Hypermutation is the Major Driver of Meningococcal Genetic Variability during Persistent Asymptomatic Carriage. mBio 2020, 11, e03068-19. [Google Scholar] [CrossRef]
- Mustapha, M.M.; Marsh, J.W.; Shutt, K.A.; Schlackman, J.; Ezeonwuka, C.; Farley, M.M.; Stephens, D.S.; Wang, X.; Van Tyne, D.; Harrison, L.H. Transmission Dynamics and Microevolution of Neisseria meningitidis During Carriage and Invasive Disease in High School Students in Georgia and Maryland, 2006–2007. J. Infect. Dis. 2021, 223, 2038–2047. [Google Scholar] [CrossRef]
- Bårnes, G.K.; Brynildsrud, O.B.; Børud, B.; Workalemahu, B.; Kristiansen, P.A.; Beyene, D.; Aseffa, A.; Caugant, D.A. Whole genome sequencing reveals within-host genetic changes in paired meningococcal carriage isolates from Ethiopia. BMC Genom. 2017, 18, 407. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Cleary, D.W.; Laver, J.R.; Gorringe, A.; Deasy, A.M.; Dale, A.P.; Morris, P.D.; Didelot, X.; Maiden, M.C.J.; Read, R.C. Microevolution of Neisseria lactamica during nasopharyngeal colonisation induced by controlled human infection. Nat. Commun. 2018, 9, 4753. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Seiler, A.; Achtman, M.; Cannon, J.G. Microevolution within a clonal population of pathogenic bacteria: Recombination, gene duplication and horizontal genetic exchange in the opa gene family of Neisseria meningitidis. Mol. Microbiol. 1994, 12, 171–180. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Malorny, B.; Prasad, P.; Morelli, G.; Kusecek, B.; Heckels, J.E.; Cannon, J.G.; Achtman, M. Recombinational reassortment among opa genes from ET-37 complex Neisseria meningitidis isolates of diverse geographical origins. Microbiology 1998, 144, 157–166. [Google Scholar] [CrossRef]
- Zhu, P.; van der Ende, A.; Falush, D.; Brieske, N.; Morelli, G.; Linz, B.; Popovic, T.; Schuurman, I.G.A.; Adegbola, R.A.; Zurth, K.; et al. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. Proc. Natl. Acad. Sci. USA 2001, 98, 5234–5239. [Google Scholar] [CrossRef]
- Achtman, M. Microevolution during epidemic spread of Neisseria meningitidis (minireview). Electrophoresis 1998, 19, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Jelfs, J.; Munro, R.; Wedege, E.; Caugant, D.A. Sequence Variation in the porA Gene of a Clone of Neisseria meningitidis during Epidemic Spread. Clin. Diagn. Lab. Immunol. 2000, 7, 390–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kriz, P.; Giorgini, D.; Musilek, M.; Larribe, M.; Taha, M.-K. Microevolution through DNA exchange among strains of Neisseria meningitidis isolated during an outbreak in the Czech Republic. Res. Microbiol. 1999, 150, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Diallo, K.; Gamougam, K.; Daugla, D.M.; Harrison, O.B.; Bray, J.E.; Caugant, D.A.; Lucidarme, J.; Trotter, C.L.; Hassan-King, M.; Stuart, J.M.; et al. Hierarchical genomic analysis of carried and invasive serogroup A Neisseria meningitidis during the 2011 epidemic in Chad. BMC Genom. 2017, 18, 398. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, A.; Harris Simon, R.; Röltgen, K.; Dangy, J.-P.; Hauser, J.; Kingsley Robert, A.; Connor Thomas, R.; Sie, A.; Hodgson, A.; Dougan, G.; et al. Emergence of a New Epidemic Neisseria meningitidis Serogroup A Clone in the African Meningitis Belt: High-Resolution Picture of Genomic Changes That Mediate Immune Evasion. mBio 2014, 5, e01974-14. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, A.; Hauser, J.; Dangy, J.P.; Hamid, A.M.; Röltgen, K.; Abdul Sater, M.R.; Hodgson, A.; Sie, A.; Junghanss, T.; Harris, S.R.; et al. Emergence and genomic diversification of a virulent serogroup W:ST-2881(CC175) Neisseria meningitidis clone in the African meningitis belt. Microb. Genom. 2017, 3, e000120. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, N.J.; Harrison, O.B.; Bayliss, C.D.; Maiden, M.C.J.; Ala’Aldeen, D.A.A.; Turner, D.P.J. Genomic Analysis of Serogroup Y Neisseria meningitidis Isolates Reveals Extensive Similarities Between Carriage-Associated and Disease-Associated Organisms. J. Infect. Dis. 2016, 213, 1777–1785. [Google Scholar] [CrossRef]
- Lamelas, A.; Hamid, A.-W.M.; Dangy, J.-P.; Hauser, J.; Jud, M.; Röltgen, K.; Hodgson, A.; Junghanss, T.; Harris, S.R.; Parkhill, J.; et al. Loss of Genomic Diversity in a Neisseria meningitidis Clone Through a Colonization Bottleneck. Genome Biol. Evol. 2018, 10, 2102–2109. [Google Scholar] [CrossRef]
- Mulhall, R.M.; Brehony, C.; O’Connor, L.; Meyler, K.; Jolley, K.A.; Bray, J.; Bennett, D.; Maiden, M.C.J.; Cunney, R. Resolution of a Protracted Serogroup B Meningococcal Outbreak with Whole-Genome Sequencing Shows Interspecies Genetic Transfer. J. Clin. Microbiol. 2016, 54, 2891–2899. [Google Scholar] [CrossRef]
- Mustapha, M.M.; Marsh, J.W.; Harrison, L.H. Global epidemiology of capsular group W meningococcal disease (1970–2015): Multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine 2016, 34, 1515–1523. [Google Scholar] [CrossRef]
- Lucidarme, J.; Lekshmi, A.; Parikh, S.R.; Bray, J.E.; Hill, D.M.; Bratcher, H.B.; Gray, S.J.; Carr, A.D.; Jolley, K.A.; Findlow, J.; et al. Frequent capsule switching in ‘ultra-virulent’ meningococci—Are we ready for a serogroup B ST-11 complex outbreak? J. Infect. 2017, 75, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Deghmane, A.-E.; Taha, S.; Taha, M.-K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: A narrative review. Infect. Dis. 2022, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guiddir, T.; Gros, M.; Hong, E.; Terrade, A.; Denizon, M.; Deghmane, A.-E.; Taha, M.-K. Unusual Initial Abdominal Presentations of Invasive Meningococcal Disease. Clin. Infect. Dis. 2018, 67, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.; Raith, E.; Williams, E.; Burrell, A.J.C. Severe meningococcal serogroup W sepsis presenting as myocarditis: A case report and review of literature. J. Intensive Care Soc. 2019, 20, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Ezeoke, I.; Galac, M.R.; Lin, Y.; Liem, A.T.; Roth, P.A.; Kilianski, A.; Gibbons, H.S.; Bloch, D.; Kornblum, J.; Del Rosso, P.; et al. Tracking a serial killer: Integrating phylogenetic relationships, epidemiology, and geography for two invasive meningococcal disease outbreaks. PLoS ONE 2018, 13, e0202615. [Google Scholar] [CrossRef]
- Taha, M.-K.; Claus, H.; Lappann, M.; Veyrier, F.J.; Otto, A.; Becher, D.; Deghmane, A.-E.; Frosch, M.; Hellenbrand, W.; Hong, E.; et al. Evolutionary Events Associated with an Outbreak of Meningococcal Disease in Men Who Have Sex with Men. PLoS ONE 2016, 11, e0154047. [Google Scholar] [CrossRef]
- Odile, B.H.; Kevin, C.; Joanna, P.; Fiona, C.; Gillian, D.; David, W.E.; John, P.; Martin, C.J.M. Genomic analysis of urogenital and rectal Neisseria meningitidis isolates reveals encapsulated hyperinvasive meningococci and coincident multidrug-resistant gonococci. Sex. Transm. Infect. 2017, 93, 445. [Google Scholar] [CrossRef]
- Retchless, A.C.; Kretz, C.B.; Chang, H.-Y.; Bazan, J.A.; Abrams, A.J.; Norris Turner, A.; Jenkins, L.T.; Trees, D.L.; Tzeng, Y.-L.; Stephens, D.S.; et al. Expansion of a urethritis-associated Neisseria meningitidis clade in the United States with concurrent acquisition of N. gonorrhoeae alleles. BMC Genom. 2018, 19, 176. [Google Scholar] [CrossRef]
- Nadel, S.; Carcillo, J. Treatment of meningococcal disease. In Handbook of Meningococcal Disease Management; Springer: Berlin/Heidelberg, Germany, 2016; pp. 75–90. [Google Scholar]
- Rostamian, M.; Chegene Lorestani, R.; Jafari, S.; Mansouri, R.; Rezaeian, S.; Ghadiri, K.; Akya, A. A systematic review and meta-analysis on the antibiotic resistance of Neisseria meningitidis in the last 20 years in the world. Indian J. Med. Microbiol. 2022, 40, 323–329. [Google Scholar] [CrossRef]
- Golparian, D.; Unemo, M. Antimicrobial resistance prediction in Neisseria gonorrhoeae: Current status and future prospects. Expert Rev. Mol. Diagn. 2022, 22, 29–48. [Google Scholar] [CrossRef]
- McNamara, L.A.; Potts, C.; Blain, A.E.; Retchless, A.C.; Reese, N.; Swint, S.; Lonsway, D.; Karlsson, M.; Lunquest, K.; Sweitzer, J.J.; et al. Detection of Ciprofloxacin-Resistant, β-Lactamase-Producing Neisseria meningitidis Serogroup Y Isolates—United States, 2019–2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Morlot, C.; Taha, M.-K. Resistance to β-Lactams in Neisseria ssp. Due to Chromosomally Encoded Penicillin-Binding Proteins. Antibiotics 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Thulin, S.; Olcén, P.; Fredlund, H.; Unemo, M. Total Variation in the penA Gene of Neisseria meningitidis: Correlation between Susceptibility to β-Lactam Antibiotics and penA Gene Heterogeneity. Antimicrob. Agents Chemother. 2006, 50, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Tomberg, J.; Unemo, M.; Davies, C.; Nicholas, R.A. Molecular and Structural Analysis of Mosaic Variants of Penicillin-Binding Protein 2 Conferring Decreased Susceptibility to Expanded-Spectrum Cephalosporins in Neisseria gonorrhoeae: Role of Epistatic Mutations. Biochemistry 2010, 49, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.S.; Jolley, K.A.; Earle, S.G.; Corton, C.; Bentley, S.D.; Parkhill, J.; Maiden, M.C.J. A genomic approach to bacterial taxonomy: An examination and proposed reclassification of species within the genus Neisseria. Microbiology 2012, 158, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Vigue, L.; Eyre-Walker, A. The comparative population genetics of Neisseria meningitidis and Neisseria gonorrhoeae. PeerJ 2019, 7, e7216. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.-K.; Martin, I.; Liu, G.; Bryden, L. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2002, 46, 3020–3025. [Google Scholar] [CrossRef]
- Chisholm, S.A.; Dave, J.; Ison, C.A. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob. Agents Chemother. 2010, 54, 3812–3816. [Google Scholar] [CrossRef]
- Hagman, K.E.; Pan, W.; Spratt, B.G.; Balthazar, J.T.; Judd, R.C.; Shafer, W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995, 141, 611–622. [Google Scholar] [CrossRef]
- McCarthy, P.C.; Sharyan, A.; Laleh Sheikhi, M. Meningococcal Vaccines: Current Status and Emerging Strategies. Vaccines 2018, 6, 12. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Read, A.F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. R. Soc. B Biol. Sci. 2017, 284, 20162562. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.A.; Read, A.F. Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 12878–12886. [Google Scholar] [CrossRef] [PubMed]
- Singleton, R.J.; Hennessy, T.W.; Bulkow, L.R.; Hammitt, L.L.; Zulz, T.; Hurlburt, D.A.; Butler, J.C.; Rudolph, K.; Parkinson, A. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007, 297, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Chewapreecha, C.; Hanage, W.P.; Harris, S.R.; McGee, L.; van der Linden, M.; Song, J.-H.; Ko, K.S.; de Lencastre, H.; Turner, C.; et al. Evidence for soft selective sweeps in the evolution of pneumococcal multidrug resistance and vaccine escape. Genome Biol. Evol. 2014, 6, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Spry, L.; Wee-Hee, A.; Ortika Belinda, D.; Boelsen Laura, K.; Batinovic, S.; Mazarakis, N.; Ford Rebecca, L.; Lo Stephanie, W.; Bentley Stephen, D.; et al. Variants of Streptococcus pneumoniae serotype 14 from Papua New Guinea with the potential to be mistyped and escape vaccine-induced protection. Microbiol. Spectr. 2022, 10, e01524-22. [Google Scholar] [CrossRef] [PubMed]
- Coffey, T.J.; Enright, M.C.; Daniels, M.; Morona, J.K.; Morona, R.; Hryniewicz, W.; Paton, J.C.; Spratt, B.G. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 1998, 27, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.S.W. A Narrative Review of the Molecular Epidemiology and Laboratory Surveillance of Vaccine Preventable Bacterial Meningitis Agents: Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Streptococcus agalactiae. Microorganisms 2021, 9, 449. [Google Scholar] [CrossRef]
- Meyler, K.; Meehan, M.; Bennett, D.; Mulhall, R.; Harrison, O.; Gavin, P.; Drew, R.J.; Cunney, R. Spontaneous capsule loss in Haemophilus influenzae serotype b associated with Hib conjugate vaccine failure and invasive disease. Clin. Microbiol. Infect. 2019, 25, 390–391. [Google Scholar] [CrossRef]
- McElligott, M.; Meyler, K.; Bennett, D.; Mulhall, R.; Drew, R.J.; Cunney, R. Epidemiology of Haemophilus influenzae in the Republic of Ireland, 2010–2018. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2335–2344. [Google Scholar] [CrossRef]
- Steens, A.; Stanoeva, K.R.; Knol, M.J.; Mariman, R.; de Melker, H.E.; van Sorge, N.M. Increase in invasive disease caused by Haemophilus influenzae b, the Netherlands, 2020 to 2021. Eurosurveillance 2021, 26, 2100956. [Google Scholar] [CrossRef]
- Scheifer, C.; Rolland-Debord, C.; Badell, E.; Reibel, F.; Aubry, A.; Perignon, A.; Patey, O.; Brisse, S.; Caumes, E. Re-emergence of Corynebacterium diphtheriae. Méd. Mal. Infect. 2019, 49, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Rohani, P. Perplexities of pertussis: Recent global epidemiological trends and their potential causes. Epidemiol. Infect. 2014, 142, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Jayasundara, D.; Lee, E.; Octavia, S.; Lan, R.; Tanaka, M.M.; Wood, J.G. Emergence of pertactin-deficient pertussis strains in Australia can be explained by models of vaccine escape. Epidemics 2020, 31, 100388. [Google Scholar] [CrossRef] [PubMed]
- Solans, L.; Debrie, A.-S.; Coutte, L.; Locht, C. Construction and evaluation of a pertactin-deficient live attenuated pertussis vaccine candidate BPZE1 derivative. Vaccine 2021, 39, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Will, R.C.; Ramamurthy, T.; Sharma, N.C.; Veeraraghavan, B.; Sangal, L.; Haldar, P.; Pragasam, A.K.; Vasudevan, K.; Kumar, D.; Das, B.; et al. Spatiotemporal persistence of multiple, diverse clades and toxins of Corynebacterium diphtheriae. Nat. Commun. 2021, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhujin, D.; Marana, M.H.; Dalsgaard, I.; Rzgar, J.; Heidi, M.; Asma, K.M.; Per, K.W.; Kurt, B. Immersion vaccines against Yersinia ruckeri infection in rainbow trout: Comparative effects of strain differences. J. Fish Dis. 2021, 44, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Riborg, A.; Colquhoun, D.J.; Gulla, S. Biotyping reveals loss of motility in two distinct Yersinia ruckeri lineages exclusive to Norwegian aquaculture. J. Fish Dis. 2022, 45, 641–653. [Google Scholar] [CrossRef]
- Millard Candice, M.; Baiano Justice, C.F.; Chan, C.; Yuen, B.; Aviles, F.; Landos, M.; Chong Roger, S.M.; Benedict, S.; Barnes Andrew, C. Evolution of the capsular operon of Streptococcus iniae in response to vaccination. Appl. Environ. Microbiol. 2012, 78, 8219–8226. [Google Scholar] [CrossRef]
- Oleksandra, S.; Jan, E.; Andrew, C.B. Evolutionary epidemiology of Streptococcus iniae: Linking mutation rate dynamics with adaptation to novel immunological landscapes. Infect. Genet. Evol. 2020, 85, 104435. [Google Scholar] [CrossRef]
- Omaleki, L.; Blackall, P.J.; Cuddihy, T.; White, R.T.; Courtice, J.M.; Turni, C.; Forde, B.M.; Beatson, S.A. Phase variation in the glycosyltransferase genes of Pasteurella multocida associated with outbreaks of fowl cholera on free-range layer farms. Microb. Genom. 2022, 8, 000772. [Google Scholar] [CrossRef]
- Ribeiro, G.S.; Reis, J.N.; Cordeiro, S.M.; Lima, J.B.T.; Gouveia, E.L.; Petersen, M.; Salgado, K.; Silva, H.R.; Zanella, R.C.; Almeida, S.C.G. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J. Infect. Dis. 2003, 187, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Martcheva, M.; Bolker, B.M.; Holt, R.D. Vaccine-induced pathogen strain replacement: What are the mechanisms? J. R. Soc. Interface 2008, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Swarthout, T.D.; Fronterre, C.; Lourenço, J.; Obolski, U.; Gori, A.; Bar-Zeev, N.; Everett, D.; Kamng’ona, A.W.; Mwalukomo, T.S.; Mataya, A.A.; et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat. Commun. 2020, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.R.; Kalizang’Oma, A.; Gori, A.; Gupta, S.; Heyderman, R.S. Factors affecting antimicrobial resistance in Streptococcus pneumoniae following vaccination introduction. Trends Microbiol. 2022, 30, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- CDC. Diphtheria, tetanus, and pertussis: Recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory committee (ACIP). MMWR Recomm. Rep. 1991, 40, 1–28. [Google Scholar]
- Quenee, L.E.; Schneewind, O. Plague vaccines and the molecular basis of immunity against Yersinia pestis. Hum. Vaccines 2009, 5, 817–823. [Google Scholar] [CrossRef]
- Demeure, C.E.; Dussurget, O.; Mas Fiol, G.; Le Guern, A.-S.; Savin, C.; Pizarro-Cerdá, J. Yersinia pestis and plague: An updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019, 20, 357–370. [Google Scholar] [CrossRef]
- Clark, S.A.; Borrow, R. Herd Protection against Meningococcal Disease through Vaccination. Microorganisms 2020, 8, 1675. [Google Scholar] [CrossRef]
- Gandhi, A.; Balmer, P.; York, L.J. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba®). Postgrad. Med. 2016, 128, 548–556. [Google Scholar] [CrossRef]
- Gorringe, A.R.; Pajón, R. Bexsero. Hum. Vaccines Immunother. 2012, 8, 174–183. [Google Scholar] [CrossRef]
- Harrison, L.H.; Jolley, K.A.; Shutt, K.A.; Marsh, J.W.; O’Leary, M.; Sanza, L.T.; Maiden, M.C.J.; the Maryland Emerging Infections, P. Antigenic Shift and Increased Incidence of Meningococcal Disease. J. Infect. Dis. 2006, 193, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.C.J. The Impact of Nucleotide Sequence Analysis on Meningococcal Vaccine Development and Assessment. Front. Immunol. 2019, 9, 3151. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.E.; Jolley, K.A.; Feavers, I.M.; Maiden, M.C.J.; Suker, J. PorA Variable Regions of Neisseria meningitidis. Emerg. Infect. Dis. J. 2004, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Green, L.R.; Lucidarme, J.; Dave, N.; Chan, H.; Clark, S.; Borrow, R.; Bayliss, C.D.; Onderdonk, A.B. Phase Variation of NadA in Invasive Neisseria meningitidis Isolates Impacts on Coverage Estimates for 4C-MenB, a MenB Vaccine. J. Clin. Microbiol. 2018, 56, e00204-18. [Google Scholar] [CrossRef] [PubMed]
- Fagnocchi, L.; Biolchi, A.; Ferlicca, F.; Boccadifuoco, G.; Brunelli, B.; Brier, S.; Norais, N.; Chiarot, E.; Bensi, G.; Kroll, J.S.; et al. Transcriptional Regulation of the nadA Gene in Neisseria meningitidis Impacts the Prediction of Coverage of a Multicomponent Meningococcal Serogroup B Vaccine. Infect. Immun. 2013, 81, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Muhamed-Kheir, T.; Edouard, B.; Anne, P.; Jean-Michel, A. Circumvention of Herd Immunity during an Outbreak of Meningococcal Disease Could Be Correlated to Escape Mutation in the porA Gene of Neisseria meningitidis. Infect. Immun. 2001, 69, 1971–1973. [Google Scholar] [CrossRef][Green Version]
- Lisbeth, M.N.; Ingunn, S.M.; Dominique, A.C.; Afework, K.; Abraham, A.; Bente, B. Genetic, Functional, and Immunogenic Analyses of the O-Linked Protein Glycosylation System in Neisseria meningitidis Serogroup A ST-7 Isolates. J. Bacteriol. 2023, 205, e00458-22. [Google Scholar] [CrossRef]
- Rune Andersen, S.; Kolberg, J.; Høiby, E.A.; Namork, E.; Caugant, D.A.; Oddvar Frøholm, L.; Jantzen, E.; Bjune, G. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: Possible consequences for vaccine development. Microb. Pathog. 1997, 23, 139–155. [Google Scholar] [CrossRef]
- Acevedo, R.; Bai, X.; Borrow, R.; Caugant, D.A.; Carlos, J.; Ceyhan, M.; Christensen, H.; Climent, Y.; De Wals, P.; Dinleyici, E.C.; et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev. Vaccines 2019, 18, 15–30. [Google Scholar] [CrossRef]
- Haidara, F.C.; Umesi, A.; Sow, S.O.; Ochoge, M.; Diallo, F.; Imam, A.; Traore, Y.; Affleck, L.; Doumbia, M.F.; Daffeh, B.; et al. Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia. N. Engl. J. Med. 2023, 388, 1942–1955. [Google Scholar] [CrossRef]
- Guo, P.B.; Zhu, B.Q.; Xu, L.; Gao, W.Y.; Gao, Y.; Liang, H.; Zhang, M.J.; Shao, Z.J. Comparison of the pathogenicity of Neisseria meningitidis isolates of hyperinvasive sequence type 7 belonging to serogroups A, B, C, and X. Biomed. Environ. Sci. 2020, 33, 114. [Google Scholar] [CrossRef]
- Schipper, K.; Preusting, L.C.; van Sorge, N.M.; Pannekoek, Y.; van der Ende, A. Meningococcal virulence in zebrafish embryos depends on capsule polysaccharide structure. Front. Cell. Infect. Microbiol. 2022, 12, 1020201. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Bailey, S.E.; Benmechernene, Z.; Tzitzilonis, C.; Griffiths, N.J.; Virji, M.; Derrick, J.P. Recognition of saccharides by the OpcA, OpaD, and OpaB outer membrane proteins from Neisseria meningitidis. J. Biol. Chem. 2005, 280, 31489–31497. [Google Scholar] [CrossRef] [PubMed]
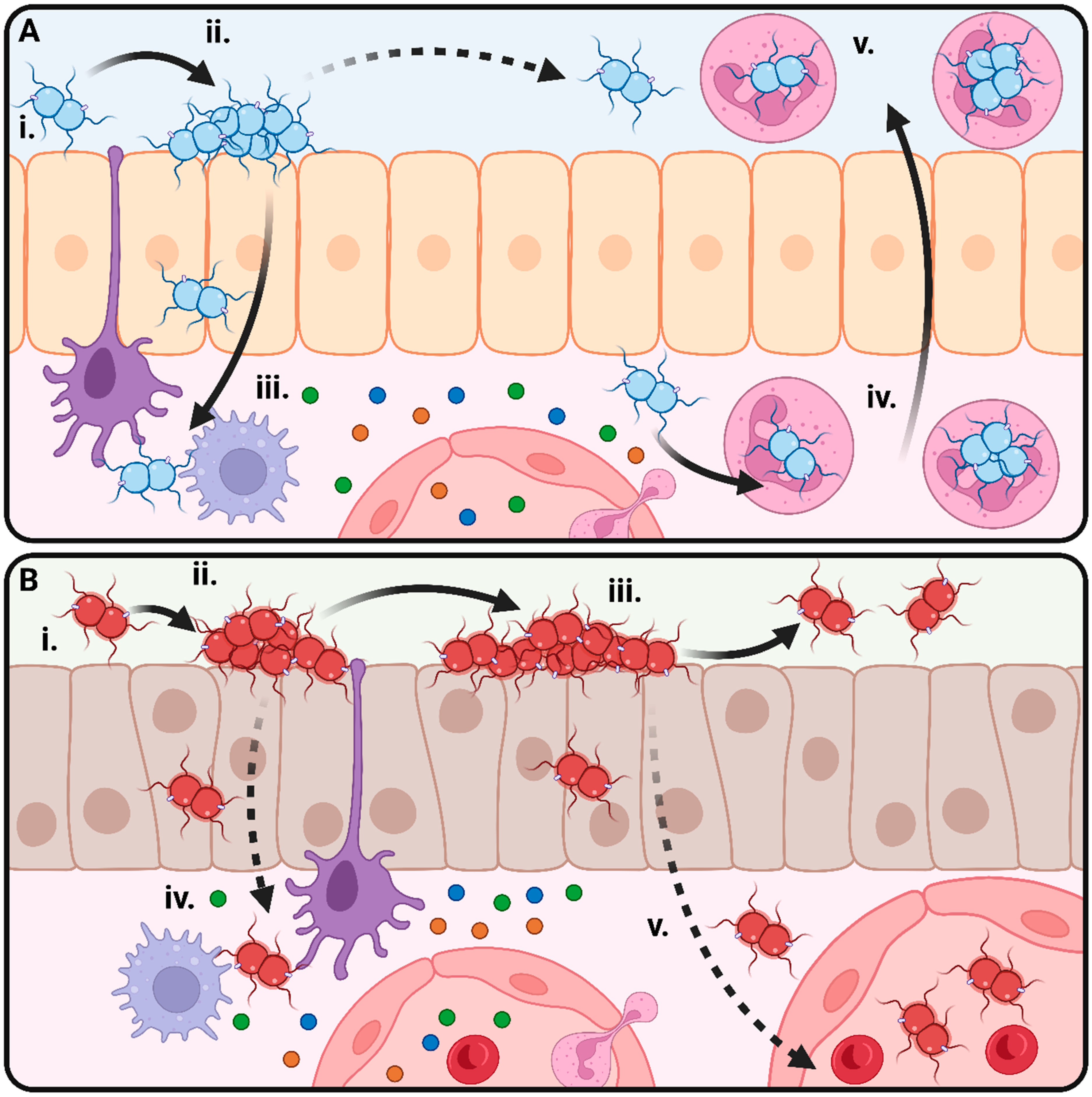
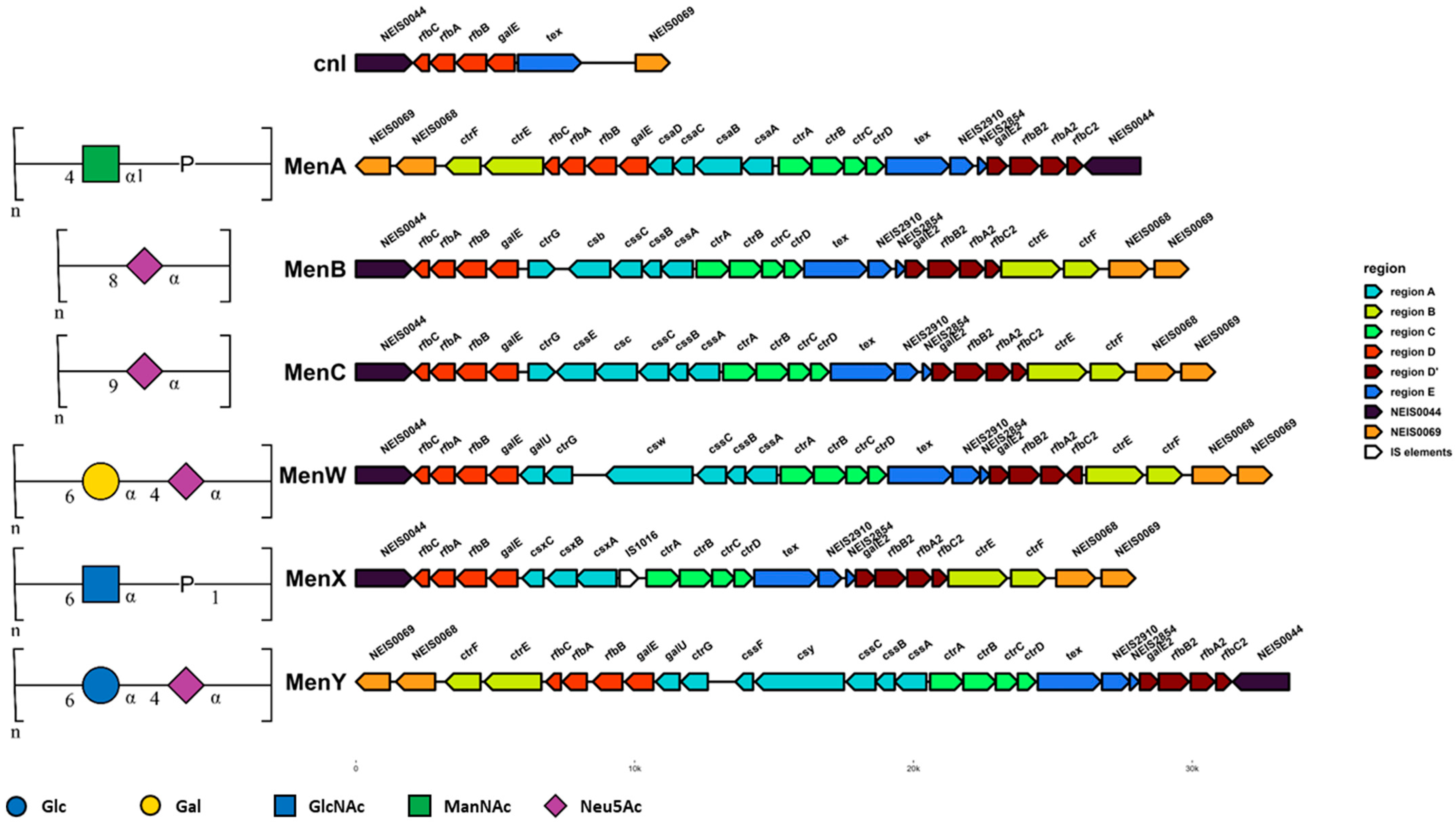
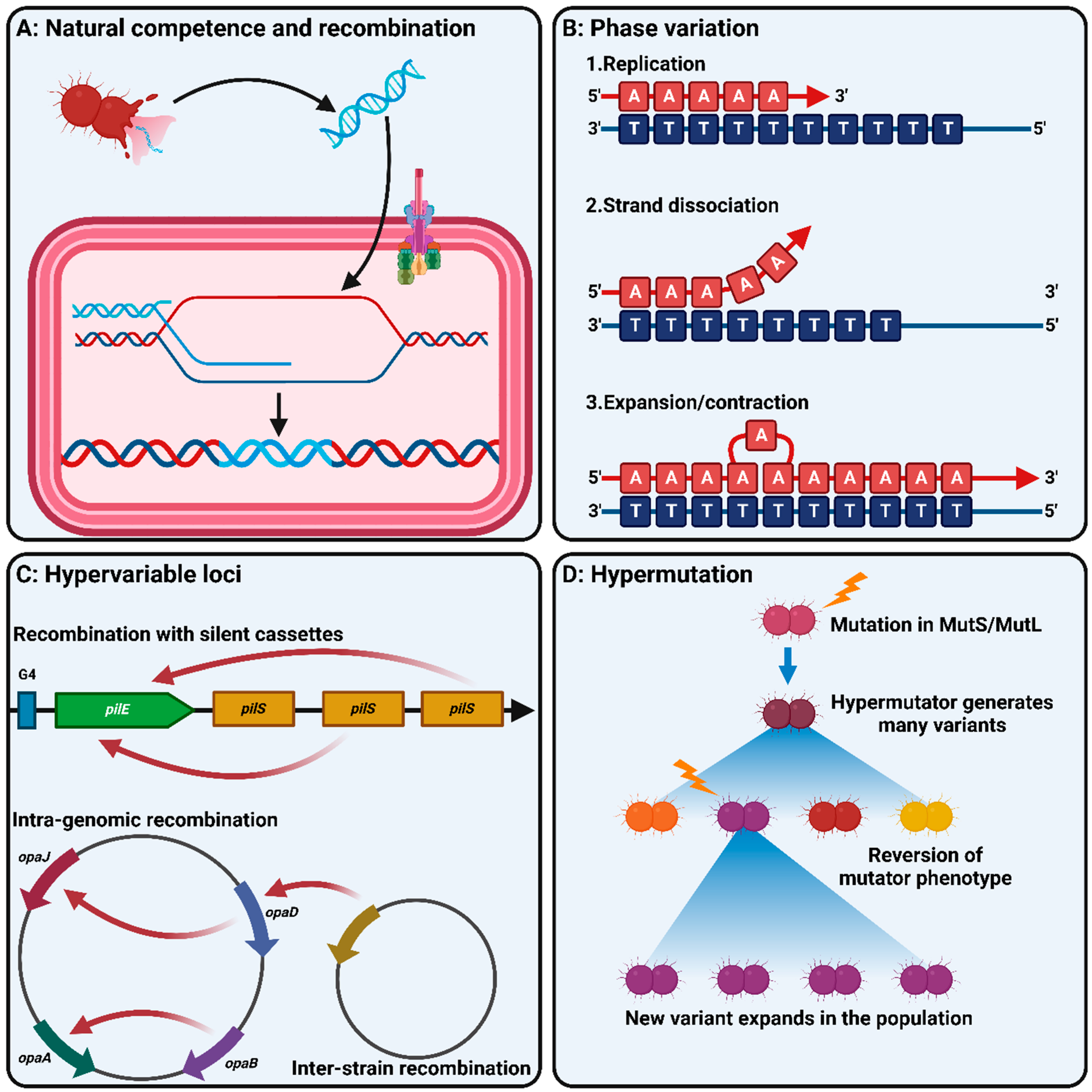


| Species | Vaccine(s) | Does Vaccination Protect against Colonisation and Transmission? | Proposed Mechanism of Vaccine Escape | Impact of Vaccination on Disease Rates | Notes | Reference |
|---|---|---|---|---|---|---|
| Neisseria meningitidis | Conjugate polysaccharide, OMVs | Conjugate vaccines most likely prevent or reduce carriage, but OMV vaccines do not. | Capsule switching, antigenic variation. | Global disease incidence has decreased, changes in dominant serogroups and lineages. | See text | |
| Haemophilus influenzae serotype b | Conjugate polysaccharide | No. | Loss of capsule expression, serotype replacement, possible increased transmission/colonisation of Hib. | Recent increases in the Netherlands. | [191,192,193,194,205,206] | |
| Streptococcus pneumoniae | Conjugate polysaccharide vaccines | Incomplete control of carriage. | Serotype replacement, loss of capsule expression, capsule switching. | Increase in prevalence of nonvaccine serotypes. | [187,188,189,190,206,207,208] | |
| Bordetella pertussis | Killed whole-cell vaccine | No. | Loss of pertactin antigen. | Recent increases in global disease rates due to pertactin-deficient strains. A decrease in fitness of pertactin-deficient strains is observed. | A live-attenuated pertactin-deficient vaccine is already in development. | [196,197,198] |
| Corynebacterium diphtheriae | Diphtheria toxoid vaccine | No. | Antigenic variation of tox. | Recent global increases, unclear if vaccine escape is playing a role. | [195,199,209] | |
| Yersinia pestis | Killed whole-cell vaccine | Loss of F1 pili expression due to IS element insertion. | Vaccine largely discontinued due to short-lived protection. Concerns about F1 expression largely impact host natural immunity. | [210,211] | ||
| Pasturella multocida | Killed whole-cell vaccine | - | Phase variation of LPS structures. | Causative agent of fowl cholera, vaccine used in poultry farming. | [204] | |
| Streptococcus iniae | Killed whole-cell vaccine | - | Capsule antigenic variation. | Pathogen of farmed fish. | [202,203] | |
| Yersinia ruckeri | Killed whole-cell vaccine | - | Loss of flagella expression. | Pathogen of farmed fish. | [200,201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikucki, A.; Kahler, C.M. Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis. Microorganisms 2023, 11, 3005. https://doi.org/10.3390/microorganisms11123005
Mikucki A, Kahler CM. Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis. Microorganisms. 2023; 11(12):3005. https://doi.org/10.3390/microorganisms11123005
Chicago/Turabian StyleMikucki, August, and Charlene M. Kahler. 2023. "Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis" Microorganisms 11, no. 12: 3005. https://doi.org/10.3390/microorganisms11123005
APA StyleMikucki, A., & Kahler, C. M. (2023). Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis. Microorganisms, 11(12), 3005. https://doi.org/10.3390/microorganisms11123005






