Medically Significant Vector-Borne Viral Diseases in Iran
Abstract
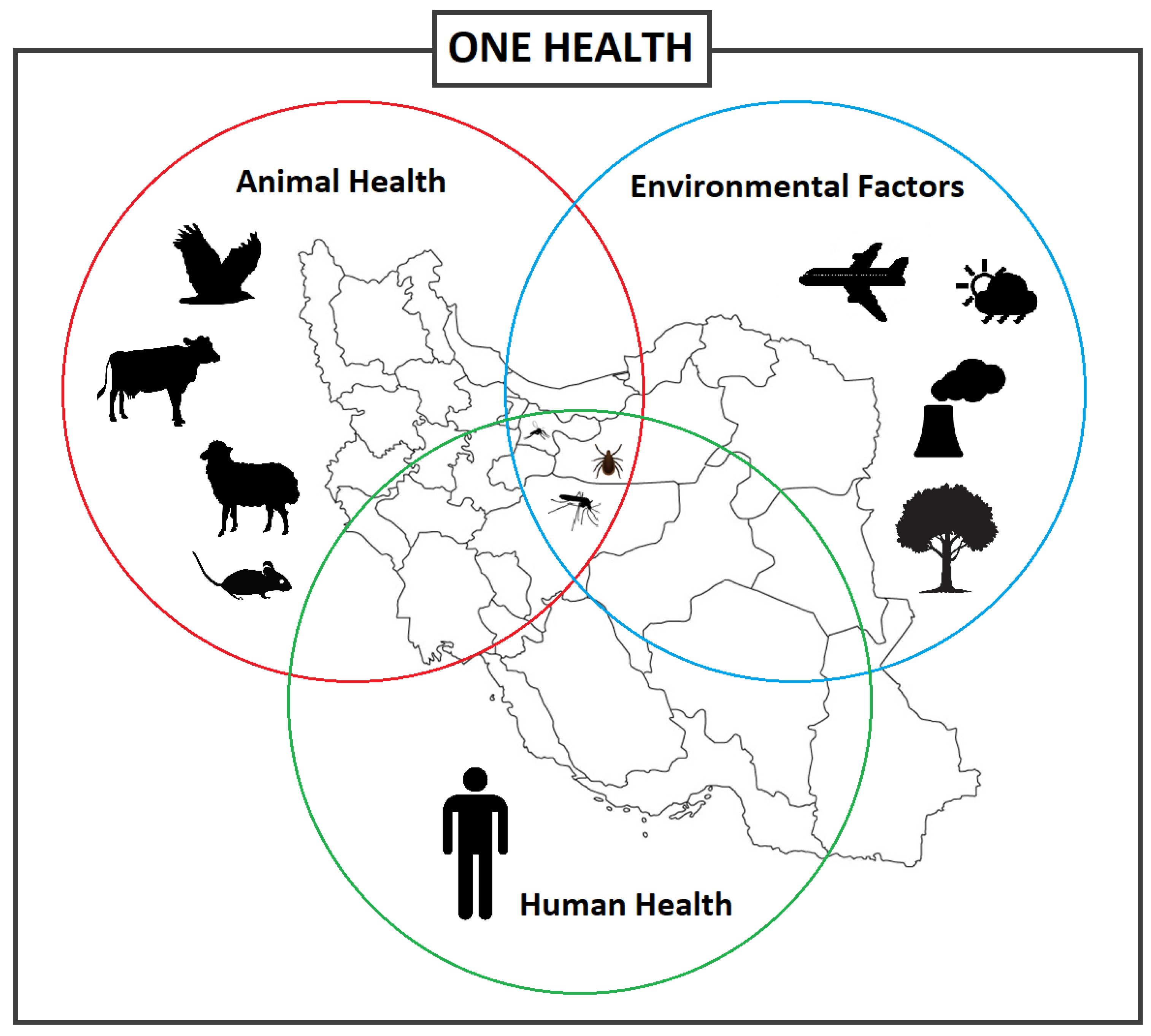
1. Introduction
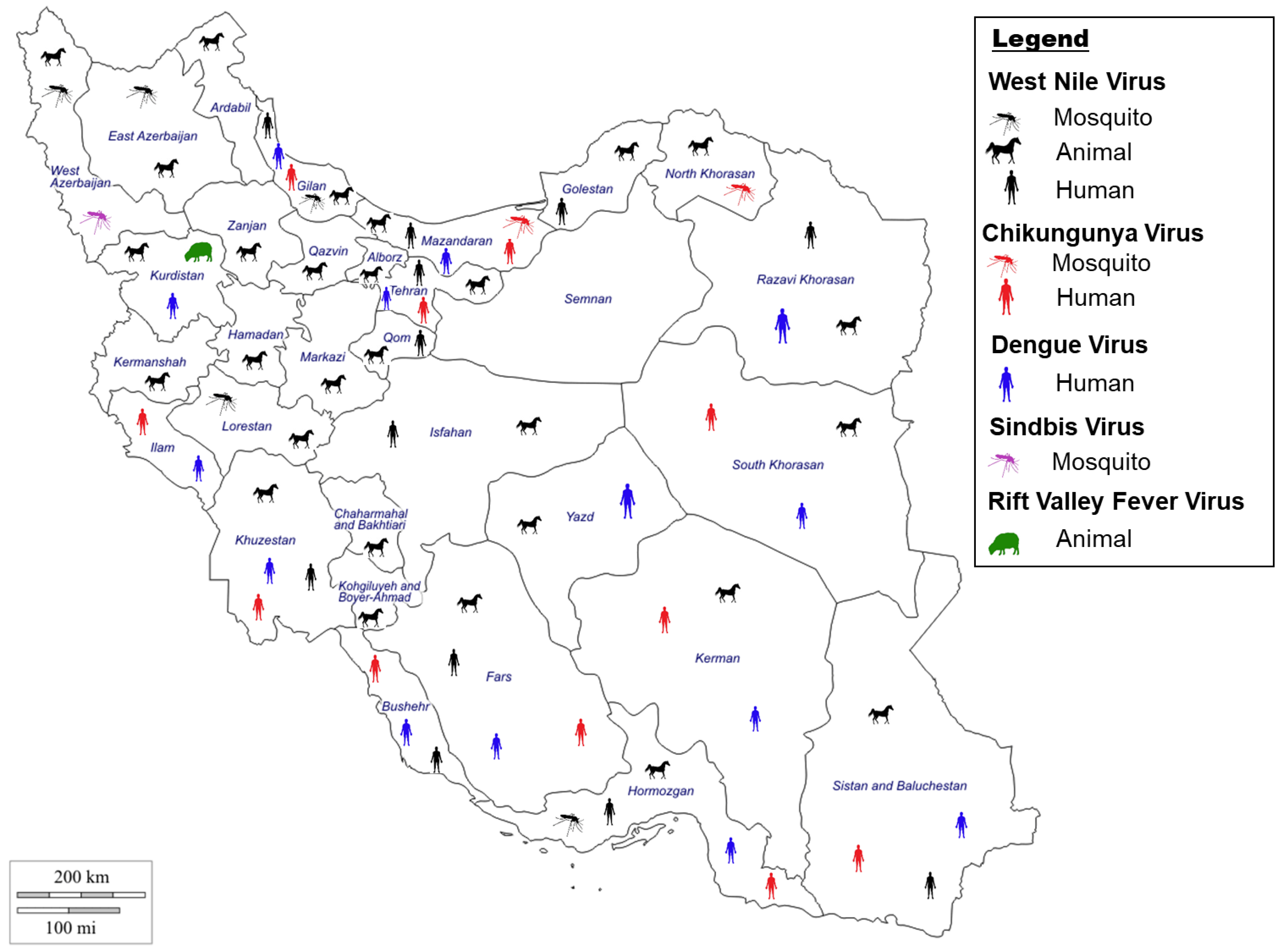
2. Mosquito-Borne Viruses in Iran
2.1. Chikungunya Virus
2.1.1. Chikungunya Virus Situation in Iran
2.1.2. Chikungunya Virus Vectors in Iran
2.2. Dengue Virus
2.2.1. Dengue Virus Situation in Iran
2.2.2. Dengue Virus Vectors in Iran
2.3. Sindbis Virus
2.3.1. Sindbis Virus Situation in Iran
2.3.2. Sindbis Virus Vectors in Iran
2.4. West Nile Virus
2.4.1. West Nile Virus Situation in Iran
2.4.2. West Nile Virus Vector in Iran
2.5. Other Related Mosquito-Borne Viruses in Iran
2.5.1. Rift Valley Fever Virus Situation in Iran
2.5.2. Zika Virus Situation in Iran
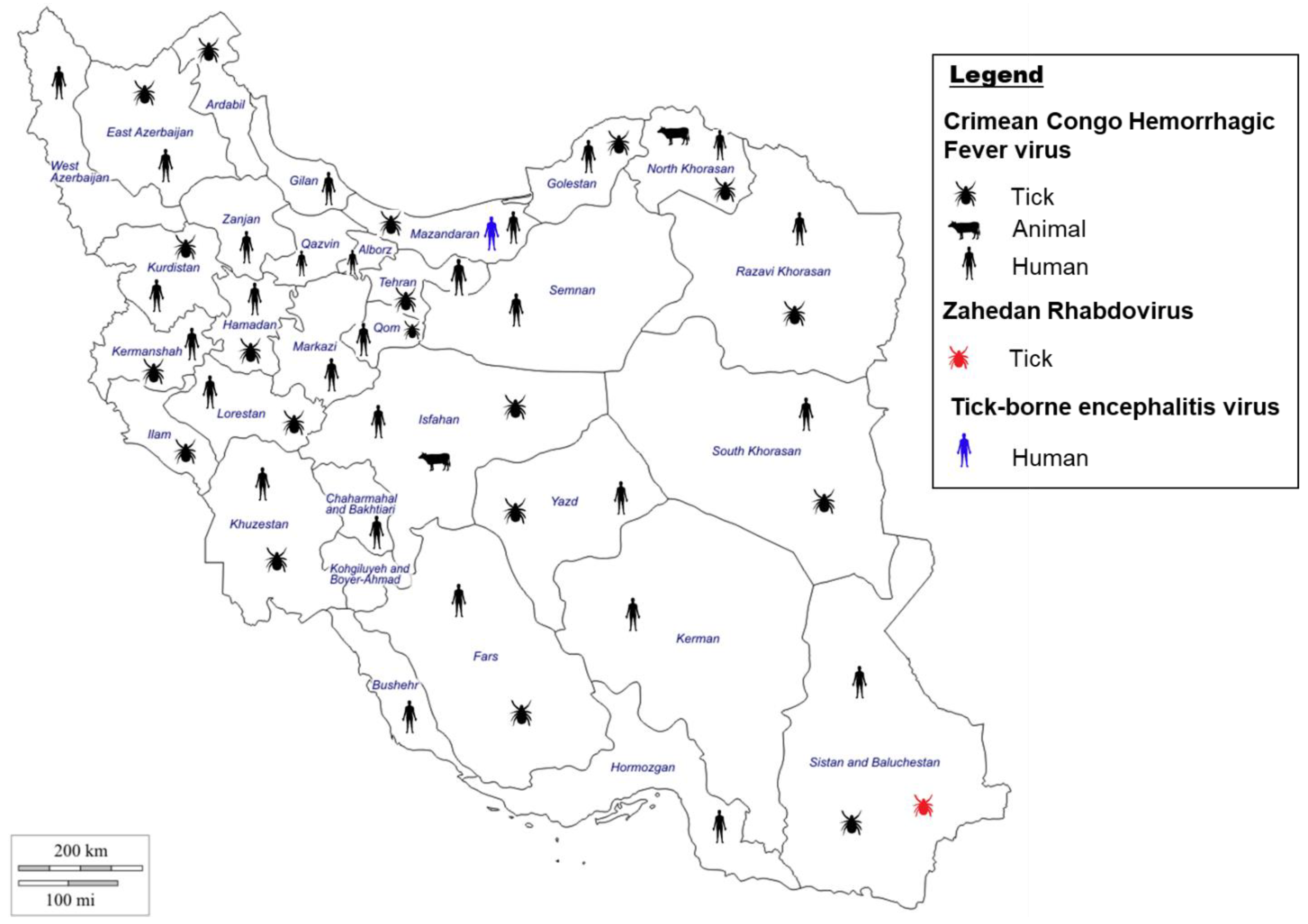
3. Tick-Borne Viruses in Iran
3.1. Crimean–Congo Hemorrhagic Fever Virus
3.1.1. Crimean–Congo Hemorrhagic Fever Virus Situation in Iran
3.1.2. Crimean–Congo Hemorrhagic Fever Virus Vectors in Iran
3.2. Other Related Tick Borne Viruses in Iran
3.2.1. Zahedan Rhabdovirus Situation in Iran
3.2.2. Tick-Borne Encephalitis Virus Situation in Iran
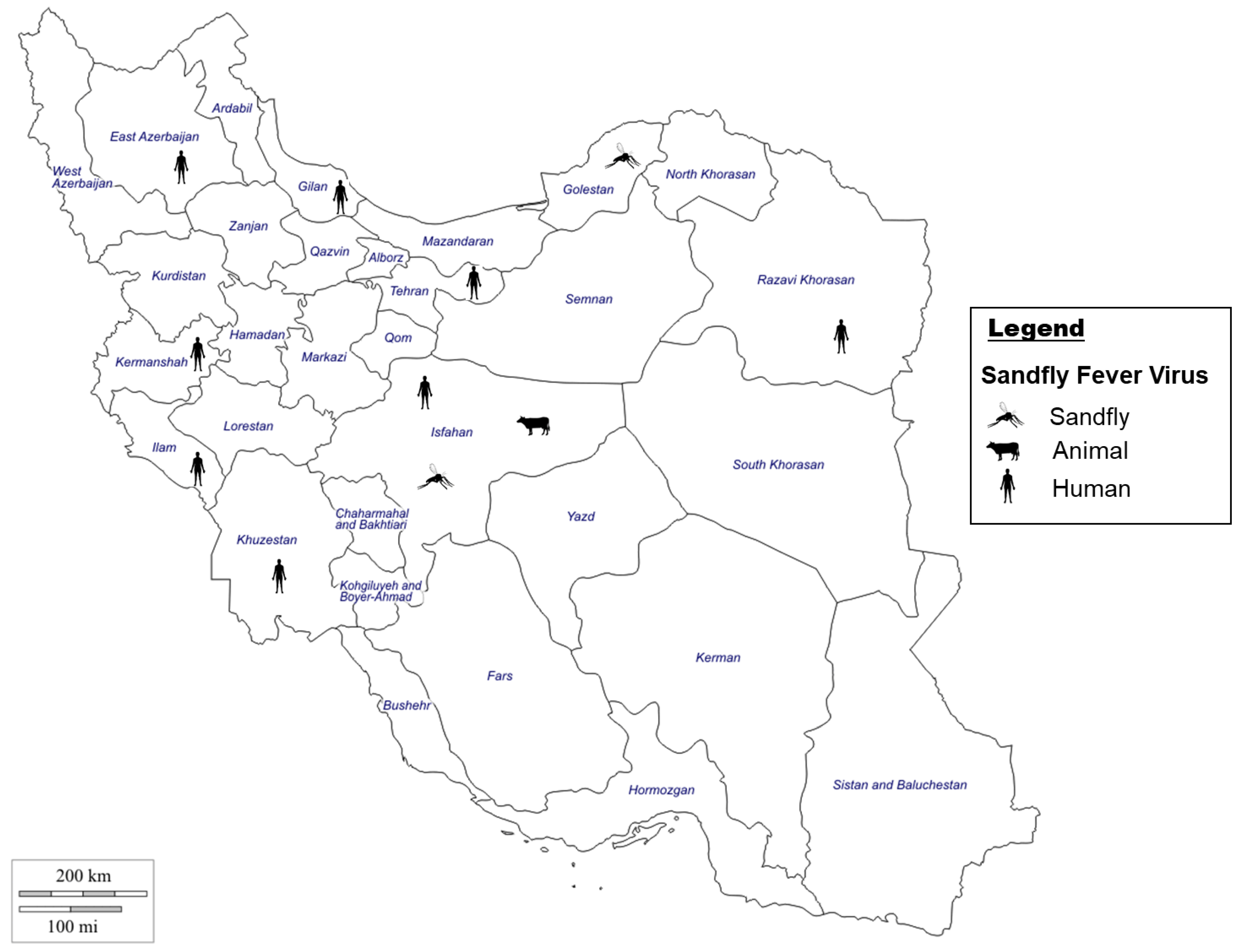
4. Sandfly-Borne Viruses in Iran
4.1. Sandfly-Borne Phleboviruses
4.1.1. Sandfly-Borne Viruses Situation in Iran
4.1.2. Sandfly-Borne Viruses Vectors in Iran
5. Rodent-Borne Viruses in Iran
5.1. Hantavirus
5.1.1. Hantavirus Situation in Iran
5.1.2. Hantavirus Vectors in Iran
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef] [PubMed]
- Parhizgari, N.; Piazak, N.; Mostafavi, E. Vector-borne diseases in Iran: Epidemiology and key challenges. Future Microbiol. 2021, 16, 51–69. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vector-Borne Diseases 2020 [updated 9 September 2022]. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 2 March 2023).
- Mangat, R.; Louie, T. Viral Hemorrhagic Fevers. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, I.; Adriana, C.; Jeegan, P.; Tatiana, G. Which Plagues are Coming Next? In Contemporary Developments and Perspectives in International Health Security; Stanislaw, P.S., Thomas, J.P., Sagar, C.G., Andrew, C.M., Michael, S.F., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Rabiee, M.H.; Mahmoudi, A.; Siahsarvie, R.; Kryštufek, B.; Mostafavi, E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl. Trop. Dis. 2018, 12, e0006256. [Google Scholar] [CrossRef] [PubMed]
- Beacham, A.M.; Hand, P.; Barker, G.C.; Denby, K.J.; Teakle, G.R.; Walley, P.G.; Monaghan, J.M. Addressing the threat of climate change to agriculture requires improving crop resilience to short-term abiotic stress. Outlook Agric. 2018, 47, 270–276. [Google Scholar] [CrossRef]
- Jánová, E. Emerging and threatening vector-borne zoonoses in the world and in Europe: A brief update. Pathog. Glob. Health 2019, 113, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mansouri Daneshvar, M.R.; Ebrahimi, M.; Nejadsoleymani, H. An overview of climate change in Iran: Facts and statistics. Environ. Syst. Res. 2019, 8, 7. [Google Scholar] [CrossRef]
- Park, K.B.; Jo, Y.H.; Kim, N.-Y.; Lee, W.-G.; Lee, H.-I.; Cho, S.-H.; Patnaik, B.B.; Han, Y.S. Tick-borne viruses: Current trends in large-scale viral surveillance. Entomol. Res. 2020, 50, 379–392. [Google Scholar] [CrossRef]
- Soltan-Alinejad, P.; Soltani, A. Vector-borne diseases and tourism in Iran: Current issues and recommendations. Travel. Med. Infect. Dis. 2021, 43, 102108. [Google Scholar] [CrossRef]
- Jaberhashemi, S.-A.; Azari-Hamidian, S.; Soltani, A.; Azizi, K.; Dorzaban, H.; Norouzi, M.; Daghighi, E. The Fauna, Diversity, and Bionomics of Culicinae (Diptera: Culicidae) in Hormozgan Province, Southern Iran. J. Med. Entomol. 2022, 59, 987–996. [Google Scholar] [CrossRef]
- Azari-Hamidian, S.; Norouzi, B.; Harbach, R.E. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019, 194, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, F.; Rezaei, F.; Shafiei-Jandaghi, N.Z.; Shadab, A.; Mokhtari-Azad, T. Seroepidemiology of dengue and chikungunya fever in patients with rash and fever in Iran, 2017. Epidemiol. Infect. 2020, 148, e42. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, V.; Salehi-Vaziri, M.; Jalali, T.; Azad-Manjiri, S.; Mohammadi, T.; Khakifirouz, S.; Fazlalipour, M. An imported case of dengue fever in Iran, 2015. Iran. J. Virol. 2016, 10, 31–34. [Google Scholar] [CrossRef]
- Bakhshi, H.; Beck, C.; Lecollinet, S.; Monier, M.; Mousson, L.; Zakeri, S.; Raz, A.; Arzamani, K.; Nourani, L.; Dinparast-Djadid, N.; et al. Serological evidence of West Nile virus infection among birds and horses in some geographical locations of Iran. Vet. Med. Sci. 2021, 7, 204–209. [Google Scholar] [CrossRef]
- Azari-Hamidian, S.; Abai, M.R.; Norouzi, B. Mansonia uniformis (Diptera: Culicidae), a genus and species new to southwestern Asia, with a review of its medical and veterinary importance. Zootaxa 2020, 4772, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Yavarian, J.; Shafiei-Jandaghi, N.Z.; Mokhtari-Azad, T. Possible viral infections in flood disasters: A review considering 2019 spring floods in Iran. Iran J. Microbiol. 2019, 11, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Hosseini-Chegeni, A.; Tavakoli, M.; Telmadarraiy, Z.J.S.; Acarology, A. The updated list of ticks (Acari: Ixodidae & Argasidae) occurring in Iran with a key to the identification of species. Syst. Appl. Acarol. 2019, 24, 2133–2166. [Google Scholar]
- Khoobdel, M.; Jafari, A.; Telmadarraiy, Z.; Sedaghat, M.; Bakhshi, H. Tick-borne pathogens in Iran: A meta-analysis. Asian Pac. J. Trop. Med. 2021, 14, 486–504. [Google Scholar] [CrossRef]
- Hanafi-Bojd, A.A.; Jafari, S.; Telmadarraiy, Z.; Abbasi-Ghahramanloo, A.; Moradi-Asl, E. Spatial Distribution of Ticks (Arachniada: Argasidae and Ixodidae) and Their Infection Rate to Crimean-Congo Hemorrhagic Fever Virus in Iran. J. Arthropod Borne Dis. 2021, 15, 41–59. [Google Scholar] [CrossRef]
- Bhowmick, S.; Kasi, K.K.; Gethmann, J.; Fischer, S.; Conraths, F.J.; Sokolov, I.M.; Lentz, H.H.K. Ticks on the Run: A Mathematical Model of Crimean-Congo Haemorrhagic Fever (CCHF)—Key Factors for Transmission. Epidemiologia 2022, 3, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Moin Vaziri, V.; Ayhan, N.; Badakhshan, M.; Bichaud, L.; Rahbarian, N.; Javadian, E.-A.; Alten, B.; de Lamballerie, X.; Charrel, R.N. Isolation and sequencing of Dashli virus, a novel Sicilian-like virus in sandflies from Iran; genetic and phylogenetic evidence for the creation of one novel species within the Phlebovirus genus in the Phenuiviridae family. PLoS Negl. Trop. Dis. 2017, 11, e0005978. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, R.; Kassiri, H.; Khodkar, I.; Karami, S. A comprehensive overview on sandfly fever. J. Acute Dis. 2021, 10, 98–106. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef]
- Velkers, F.C.; Blokhuis, S.J.; Veldhuis Kroeze, E.J.B.; Burt, S.A. The role of rodents in avian influenza outbreaks in poultry farms: A review. Vet. Q. 2017, 37, 182–194. [Google Scholar] [CrossRef]
- Kazemi-Moghaddam, V.; Dehghani, R.; Hadei, M.; Dehqan, S.; Sedaghat, M.M.; Latifi, M.; Alavi-Moghaddam, S. Rodent-borne and rodent-related diseases in Iran. Comp. Clin. Pathol. 2019, 28, 893–905. [Google Scholar] [CrossRef]
- Salehi-Vaziri, M.; Kaleji, A.S.; Fazlalipour, M.; Jalali, T.; Mohammadi, T.; Khakifirouz, S.; Baniasadi, V.; Pouriayevali, M.H.; Mahmoudi, A.; Tordo, N.; et al. Hantavirus infection in Iranian patients suspected to viral hemorrhagic fever. J. Med. Virol. 2019, 91, 1737–1742. [Google Scholar] [CrossRef]
- Khongwichit, S.; Chansaenroj, J.; Chirathaworn, C.; Poovorawan, Y. Chikungunya virus infection: Molecular biology, clinical characteristics, and epidemiology in Asian countries. J. Biomed. Sci. 2021, 28, 84. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.N.; Javed, F.; Ejaz, M.; Ali, M.; Mujaddadi, N.; Khan, A.A.; Khattak, A.A.; Zaib, A.; Ahmad, I.; Saeed, W.K. The global emergence of Chikungunya infection: An integrated view. Rev. Med. Virol. 2021, 32, e2287. [Google Scholar] [CrossRef] [PubMed]
- Pouriayevali, M.H.; Rezaei, F.; Jalali, T.; Baniasadi, V.; Fazlalipour, M.; Mostafavi, E.; Khakifirouz, S.; Mohammadi, T.; Fereydooni, Z.; Tavakoli, M.; et al. Imported cases of Chikungunya virus in Iran. BMC Infect. Dis. 2019, 19, 1004. [Google Scholar] [CrossRef] [PubMed]
- Bala Murugan, S.; Sathishkumar, R. Chikungunya infection: A potential re-emerging global threat. Asian Pac. J. Trop. Med. 2016, 9, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I. Clinical features. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. A laboratory technique for studying the insect transmission of animal viruses, employing a bat-wing membrane, demonstrated with two African viruses. J. Hyg. 1956, 54, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.J.; Rolph, M.S.; Rulli, N.E.; Mahalingam, S.; Heise, M.T. Chikungunya: A re-emerging virus. Lancet 2012, 379, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Mala, W.; Wilairatana, P.; Kotepui, K.U.; Kotepui, M. Prevalence of Malaria and Chikungunya Co-Infection in Febrile Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2021, 6, 119. [Google Scholar] [CrossRef]
- Dehghani, R.; Kassiri, H.; Kasiri, R.; Dehghani, M.; Kasiri, M. Global distribution of human chikungunya arbovirus infection: A review. J. Acute Dis. 2020, 9, 145–151. [Google Scholar] [CrossRef]
- Darwish, M.A.; Hoogstraal, H.; Roberts, T.J.; Ahmed, I.P.; Omar, F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 442–445. [Google Scholar] [CrossRef]
- Malik, M.R.; Mnzava, A.; Mohareb, E.; Zayed, A.; Al Kohlani, A.; Thabet, A.A.; El Bushra, H. Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: Epidemiological characterization and key lessons learned for early detection and control. J. Epidemiol. Glob. Health 2014, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Alomar, I.; Memish, Z.A. Chikungunya virus: Emergence of an arthritic arbovirus in Jeddah, Saudi Arabia. East. Mediterr. Health J. 2013, 19, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Mousson, L.; Moutailler, S.; Vazeille, M.; Piorkowski, G.; Zakeri, S.; Raz, A.; de Lamballerie, X.; Dinparast-Djadid, N.; Failloux, A.-B. Detection of arboviruses in mosquitoes: Evidence of circulation of chikungunya virus in Iran. PLoS Negl. Trop. Dis. 2020, 14, e0008135. [Google Scholar] [CrossRef] [PubMed]
- Solgi, A.; Karimi, A.; Armin, S. Seropositivity of Chikungunya and West Nile Viruses in Iranian Children in 2018. Arch. Pediatr. Infect. Dis. 2020, 8, e94416. [Google Scholar] [CrossRef]
- Ahmadi Vasmehjani, A.; Rezaei, F.; Farahmand, M.; Mokhtari-Azad, T.; Yaghoobi-Ershadi, M.R.; Keshavarz, M.; Baseri, H.R.; Zaim, M.; Iranpour, M.; Turki, H.; et al. Epidemiological evidence of mosquito-borne viruses among persons and vectors in Iran: A study from North to South. Virol. Sin. 2022, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Doosti, S.; Yaghoobi-Ershadi, M.R.; Schaffner, F.; Moosa-Kazemi, S.H.; Akbarzadeh, K.; Gooya, M.M.; Vatandoost, H.; Shirzadi, M.R.; Mosta-Favi, E. Mosquito Surveillance and the First Record of the Invasive Mosquito Species Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) in Southern Iran. Iran. J. Public Health 2016, 45, 1064–1073. [Google Scholar] [PubMed]
- Dehghani, R.; Kassiri, H. A review on epidemiology of dengue viral infection as an emerging disease. Res. J. Pharm. Technol. 2021, 14, 2296–2301. [Google Scholar] [CrossRef]
- Chinikar, S.; Shah-Hosseini, N. Dengue fever in Asia and Africa. In Neglected Tropical Diseases—Middle East and North Africa. Neglected Tropical Diseases; Springer: Vienna, Austria, 2014; pp. 193–215. [Google Scholar] [CrossRef]
- WHO. Dengue and Severe Dengue. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 8 September 2022).
- Zeng, Z.; Zhan, J.; Chen, L.; Chen, H.; Cheng, S. Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine 2021, 32, 100712. [Google Scholar] [CrossRef]
- Simon, A.Y.; Sutherland, M.R.; Pryzdial, E.L. Dengue virus binding and replication by platelets. Blood 2015, 126, 378–385. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, J.; Guo, X.; Mo, L.; Hu, N.; Sun, J.; Wu, M.; Zhou, H.; Hu, Y. Full-length genome and molecular characterization of dengue virus serotype 2 isolated from an imported patient from Myanmar. Virol. J. 2018, 15, 131. [Google Scholar] [CrossRef]
- Begam, N.N.; Kumar, A.; Sahu, M.; Soneja, M.; Bhatt, M.; Vishwakarma, V.K.; Sethi, P.; Baitha, U.; Barua, K.; Biswas, A. Management of dengue with co-infections: An updated narrative review. Drug Discov. Ther. 2021, 15, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Kaur, S.; Kaur, T.; Ghai, R.; Kaur, K.; Kaur, R.; Kaur, J.; Sharma, P.; Amritpal Kaur, H. Serological Evidence of Co-infection of Dengue, Leptospirosis and Scrub Typhus in Patients Presenting with Acute Febrile Illness in a Tertiary care Hospital. Eur. J. Mol. Clin. Med. 2022, 9, 2009–2020. [Google Scholar]
- Heydari, M.; Metanat, M.; Rouzbeh-Far, M.-A.; Tabatabaei, S.M.; Rakhshani, M.; Sepehri-Rad, N.; Keshtkar-Jahromi, M. Dengue fever as an emerging infection in southeast Iran. Am. J. Trop. Med. Hyg. 2018, 98, 1469. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Aflatoonian, M.R.; Hemati, M.; Mostafavi, E.; Aflatoonian, B. Dengue Fever Serology in Febrile Patients in Southeast Iran. J. Kerman Univ. Med. Sci. 2019, 26, 90–94. [Google Scholar]
- Mardani, M.; Abbasi, F.; Aghahasani, M.; Ghavam, B. First Iranian imported case of dengue. Int. J. Prev. Med. 2013, 4, 1075–1077. [Google Scholar] [PubMed]
- Shahhosseini, N.; Chinikar, S.; Nowotny, N.; Fooks, A.R.; Schmidt-Chanasit, J. Genetic analysis of imported dengue virus strains by Iranian travelers. Asian Pac. J. Trop. Dis. 2016, 6, 850–853. [Google Scholar] [CrossRef]
- Chinikar, S.; Ghiasi, S.M.; Shah-Hosseini, N.; Mostafavi, E.; Moradi, M.; Khakifirouz, S.; Rasi Varai, F.S.; Rafigh, M.; Jalali, T.; Goya, M.M.; et al. Preliminary study of dengue virus infection in Iran. Travel. Med. Infect. Dis. 2013, 11, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, A.; Aaskov, J.; Chinikar, S.; Niedrig, M.; Banazadeh, S.; Mohammadpour, H.K. Frequency of dengue virus infection in blood donors in Sistan and Baluchestan province in Iran. Transfus. Apher. Sci. 2014, 50, 59–62. [Google Scholar] [CrossRef]
- Higa, Y. Dengue Vectors and their Spatial Distribution. Trop. Med. Health 2011, 39, 17–27. [Google Scholar] [CrossRef]
- Dorzaban, H.; Soltani, A.; Alipour, H.; Hatami, J.; Jaberhashemi, S.A.; Shahriari-Namadi, M.; Paksa, A.; Safari, R.; Talbalaghi, A.; Azizi, K. Mosquito surveillance and the first record of morphological and molecular-based identification of invasive species Aedes (Stegomyia) aegypti (Diptera: Culicidae), southern Iran. Exp. Parasitol. 2022, 236, 108235. [Google Scholar] [CrossRef]
- Yaghoobi-Ershadi, M.R.; Doosti, S.; Schaffner, F.; Moosa-Kazemi, S.H.; Akbarzadeh, K.; Yaghoobi-Ershadi, N. Morphological studies on adult mosquitoes (Diptera: Culicidae) and first report of the potential Zika virus vector Aedes (Stegomyia) unilineatus (Theobald, 1906) in Iran. Bull. Soc. Pathol. Exot. 2017, 110, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Gideon Informatics, I.; Berger, S. Infectious Diseases of Iran, 2022 ed.; Gideon Informatics: Los Angeles, CA, USA, 2022. [Google Scholar]
- Ling, J.; Smura, T.; Lundström, J.O.; Pettersson, J.H.-O.; Sironen, T.; Vapalahti, O.; Lundkvist, Å.; Hesson, J.C. Introduction and Dispersal of Sindbis Virus from Central Africa to Europe. J. Virol. 2019, 93, e00620-19. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B. Arthritides caused by mosquito-borne viruses. Annu. Rev. Med. 1982, 33, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Norder, H.; Lundström, J.O.; Kozuch, O.; Magnius, L.O.J.V. Genetic relatedness of Sindbis virus strains from Europe, Middle East, and Africa. Virology 1996, 222, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen—Epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef]
- Gylfe, Å.; Ribers, Å.; Forsman, O.; Bucht, G.; Alenius, G.M.; Wållberg-Jonsson, S.; Ahlm, C.; Evander, M. Mosquitoborne Sindbis Virus Infection and Long-Term Illness. Emerg. Infect. Dis. 2018, 24, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, M.T.; Uusitalo, R.; Otte Im Kampe, E.; Vuorinen, T.; Kurkela, S.; Vapalahti, O.; Dub, T.; Huhtamo, E.; Korhonen, E.M. Sindbis virus outbreak and evidence for geographical expansion in Finland, 2021. Eurosurveillance 2022, 27, 2200580. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.O.; Pfeffer, M. Phylogeographic Structure and Evolutionary History of Sindbis Virus. Vector-Borne Zoonotic Dis. 2010, 10, 889–907. [Google Scholar] [CrossRef]
- Hanafi-Bojd, A.A.; Motazakker, M.; Vatandoost, H.; Dabiri, F.; Chavshin, A.R. Sindbis virus infection of mosquito species in the wetlands of northwestern Iran and modeling the probable ecological niches of SINV vectors in the country. Acta Trop. 2021, 220, 105952. [Google Scholar] [CrossRef]
- Laine, M.; Luukkainen, R.; Toivanen, A. Sindbis viruses and other alphaviruses as cause of human arthritic disease. J. Intern. Med. 2004, 256, 457–471. [Google Scholar] [CrossRef]
- Moin-Vaziri, V.; Charrel, R.N.; Badakhshan, M.; de Lamballerie, X.; Rahbarian, N.; Bavani, M.M.; Azari-Hamidian, S. A Molecular Screening of Mosquitoes (Diptera: Culicidae) for Flaviviruses in a Focus of West Nile Virus Infection in Northern Iran. J. Arthropod-Borne Dis. 2019, 13, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Friedrich, J.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Kayedi, M.H.; Tannich, E.; Schmidt-Chanasit, J.; Lühken, R. Host-feeding patterns of Culex mosquitoes in Iran. Parasites Vectors 2018, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Öborn, L.; Sjöberg, V.; Lundkvist, Å.; Hesson, J.C. Vertical Transmission of Sindbis Virus in Culex Mosquitoes. Viruses 2022, 14, 1915. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Eybpoosh, S.; Fazlalipour, M.; Baniasadi, V.; Pouriayevali, M.H.; Sadeghi, F.; Ahmadi Vasmehjani, A.; Karbalaie Niya, M.H.; Hewson, R.; Salehi-Vaziri, M. Epidemiology of West Nile Virus in the Eastern Mediterranean region: A systematic review. PLOS Negl. Trop. Dis. 2019, 13, e0007081. [Google Scholar] [CrossRef] [PubMed]
- Bondre, V.P.; Jadi, R.; Mishra, A.; Yergolkar, P.; Arankalle, V. West Nile virus isolates from India: Evidence for a distinct genetic lineage. J. Gen. Virol. 2007, 88, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Athanasiou, L.V.; Touloudi, A.; Papatsiros, V.; Spyrou, V.; Petrovska, L.; Billinis, C. West Nile Virus: Basic Principles, Replication Mechanism, Immune Response and Important Genetic Determinants of Virulence. In Viral Replication; inTech: London, UK, 2013; pp. 43–68. [Google Scholar]
- Vázquez, A.; Sánchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernández, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative new lineage of West Nile virus, Spain. Emerg. Infect. Dis. 2010, 16, 549. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Chowdhury, P.; Khan, S.A. Global emergence of West Nile virus: Threat & preparedness in special perspective to India. Indian. J. Med. Res. 2021, 154, 36–50. [Google Scholar] [CrossRef]
- Smithburn, K.; Hughes, T.; Burke, A.; Paul, J. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. 1940, 20, 471–472. [Google Scholar] [CrossRef]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Eurosurveillance 2020, 25, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert Rev. Anti-Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.; Monath, T. West Nile Fever. In The Arboviruses: Epidemiology and Ecology; CRC Press: Boca Raton, FL, USA, 1989; Volume V, pp. 59–88. [Google Scholar]
- Olejnik, E. Infectious adenitis transmitted by Culex molestus. Bull. Res. Counc. Isr. 1952, 2, 210–211. [Google Scholar]
- Meshkat, Z.; Chinikar, S.; Shakeri, M.; Manavifar, L.; Moradi, M.; Mirshahabi, H.; Jalali, T.; Khakifirouz, S.; Shahhosseini, N. Prevalence of West Nile virus in Mashhad, Iran: A population–based study. Asian Pac. J. Trop. Med. 2015, 8, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.; Tesh, R.; Javadian, E.; Nadim, A. The prevalence of human infection with West Nile virus in Iran. Iran. J. Public Health 1976, 5, 8–13. [Google Scholar]
- Sharifi, Z.; Shooshtari, M.M.; Talebian, A. A study of West Nile virus infection in Iranian blood donors. Arch. Iran. Med. 2010, 13, 1–4. [Google Scholar] [PubMed]
- Bagheri, M.; Terenius, O.; Oshaghi, M.A.; Motazakker, M.; Asgari, S.; Dabiri, F.; Vatandoost, H.; Mohammadi Bavani, M.; Chavshin, A.R. West Nile Virus in Mosquitoes of Iranian Wetlands. Vector-Borne Zoonotic Dis. 2015, 15, 750–754. [Google Scholar] [CrossRef]
- Chinikar, S.; Javadi, A.; Ataei, B.; Shakeri, H.; Moradi, M.; Mostafavi, E.; Ghiasi, S. Detection of West Nile virus genome and specific antibodies in Iranian encephalitis patients. Epidemiol. Infect. 2012, 140, 1525–1529. [Google Scholar] [CrossRef]
- Shah-Hosseini, N.; Chinikar, S.; Ataei, B.; Fooks, A.R.; Groschup, M.H. Phylogenetic analysis of West Nile virus genome, Iran. Emerg. Infect. Dis. 2014, 20, 1419. [Google Scholar] [CrossRef]
- Chinikar, S.; Shah-Hosseini, N.; Mostafavi, E.; Moradi, M.; Khakifirouz, S.; Jalali, T.; Goya, M.M.; Shirzadi, M.R.; Zainali, M.; Fooks, A.R. Seroprevalence of west nile virus in iran. Vector-Borne Zoonotic Dis. 2013, 13, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, A.; Aaskov, J.; Chinikar, S.; Niedrig, M.; Banazadeh, S.; Mohammadpour, H.K. Frequency of West Nile Virus Infection in Iranian Blood Donors. Indian J. Hematol. Blood Transfus. 2016, 32, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Ziyaeyan, M.; Behzadi, M.A.; Leyva-Grado, V.H.; Azizi, K.; Pouladfar, G.; Dorzaban, H.; Ziyaeyan, A.; Salek, S.; Jaber Hashemi, A.; Jamalidoust, M. Widespread circulation of West Nile virus, but not Zika virus in southern Iran. PLoS Negl. Trop. Dis. 2018, 12, e0007022. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Chinikar, S.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Kayedi, M.H.; Lühken, R.; Schmidt-Chanasit, J. West Nile Virus lineage-2 in culex specimens from Iran. Trop. Med. Int. Health 2017, 22, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Kayedi, M.H.; Sedaghat, M.M.; Racine, T.; Kobinger, G.P.; Moosa-Kazemi, S.H. DNA barcodes corroborating identification of mosquito species and multiplex real-time PCR differentiating Culex pipiens complex and Culex torrentium in Iran. PLoS ONE 2018, 13, e0207308. [Google Scholar] [CrossRef]
- Dehghan, H.; Sadraei, J.; Moosa-Kazemi, S.; Baniani, N.A.; Nowruzi, F. The molecular and morphological variations of Culex pipiens complex (Diptera: Culicidae) in Iran. J. Vector Borne Dis. 2013, 50, 111. [Google Scholar] [PubMed]
- Kayedi, M.H.; Sepahvand, F.; Mostafavi, E.; Chinikar, S.; Mokhayeri, H.; Sharafi, A.C.; Wong, G.; Shahhosseini, N.; Kazemi, S.H.M. Morphological and molecular identification of Culicidae mosquitoes (Diptera: Culicidae) in Lorestan province, Western Iran. Heliyon 2020, 6, e04480. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Chinikar, S. Genetic evidence for circulation of Kunjin-related West Nile virus strain in Iran. J. Vector Borne Dis. 2016, 53, 384. [Google Scholar]
- Shahhosseini, N.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Wong, G.; Chinikar, S.; Hajivand, Z.; Mokhayeri, H.; Nowotny, N.; Kayedi, M.H. Autochthonous Transmission of West Nile Virus by a New Vector in Iran, Vector-Host Interaction Modeling and Virulence Gene Determinants. Viruses 2020, 12, 1449. [Google Scholar] [CrossRef]
- Isfahani, E.B.; Dayer, M.S.; Kazemi, S.H.M. First Field Evidence On Circulation and Vertical Transmission of West Nile Virus Lineage-1a in Mosquitoes of Southern Iran. 2021. Available online: https://www.researchsquare.com/article/rs-842108/v1 (accessed on 2 March 2023).
- Fakour, S.; Naserabadi, S.; Ahmadi, E. A serological and hematological study on Rift valley fever and associated risk factors in aborted sheep at Kurdistan province in west of Iran. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101620. [Google Scholar] [CrossRef]
- Ikegami, T.; Makino, S. The pathogenesis of Rift Valley fever. Viruses 2011, 3, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Kassiri, H.; Dehghani, R.; Kasiri, M.; Dehghani, M. Neglected tropical disease of rift valley fever and its impact on human, and animal health with emphasis on Iran: A review article. Entomol. Appl. Sci. Lett. 2020, 7, 68–75. [Google Scholar]
- Ahmad, K. More deaths from Rift Valley fever in Saudi Arabia and Yemen. Lancet 2000, 356, 1422. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Shahhosseini, N.; Mostafavi, E.; Moradi, M.; Khakifirouz, S.; Jalali, T.; Fooks, A.R. Surveillance of rift valley fever in Iran between 2001 and 2011. All Results J. Biol. 2013, 4, 16–18. [Google Scholar]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Kwaśnik, M.; Rożek, W.; Rola, J. Rift Valley Fever—A Growing Threat To Humans and Animals. J. Vet. Res. 2021, 65, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, F.; Abdolmohammadi, K.; Fatahi, Y.; Dalili, H.; Rasoolinejad, M.; Rezaei, F.; Salehi-Vaziri, M.; Shafiei-Jandaghi, N.Z.; Gooshki, E.S.; Zaim, M.; et al. Zika Virus Infection, Basic and Clinical Aspects: A Review Article. Iran. J. Public Health 2019, 48, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Farrokh-Eslamlou, H.; Maheri, M. Knowledge, attitude, and practice toward Zika virus among staff of comprehensive health services centers affiliated with Tehran University of Medical Sciences in 2020. J. Obstet. Gynaecol. Res. 2021, 47, 2204–2214. [Google Scholar] [CrossRef]
- Nikookar, S.H.; Fazeli-Dinan, M.; Enayati, A.; Zaim, M. Zika; a continuous global threat to public health. Environ. Res. 2020, 188, 109868. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Otarod, V.; Fallah, M.H.; Lowenski, S.; Sedighi-Moghaddam, R.; Zavareh, A.; Durand, B.; Lecollinet, S.; Sabatier, P. Spread of West Nile virus in Iran: A cross-sectional serosurvey in equines, 2008-2009. Epidemiol. Infect. 2011, 139, 1587–1593. [Google Scholar] [CrossRef]
- Amin, M.; Zaim, M.; Edalat, H.; Basseri, H.R.; Yaghoobi-Ershadi, M.R.; Rezaei, F.; Azizi, K.; Salehi-Vaziri, M.; Ghane, M.; Yousefi, S.; et al. Seroprevalence Study on West Nile Virus (WNV) Infection, a Hidden Viral Disease in Fars Province, Southern Iran. J. Arthropod Borne Dis. 2020, 14, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, R.; Kassiri, H.; Kasiri, N.; Dehghani, M. A review on epidemiology and ecology of west nile fever: An emerging arboviral disease. J. Acute Dis. 2020, 9, 93–99. [Google Scholar]
- Borujeni, M.P.; Mashadi, A.R.G.; Shapouri, M.R.S.A.; Zeinvand, M. Aserological survey on antibodies against West Nile virus in horses of Khuzestan province. Iran. J. Vet. Med. 2013, 7, 185–191. [Google Scholar]
- Sultankulova, K.T.; Shynybekova, G.O.; Kozhabergenov, N.S.; Mukhami, N.N.; Chervyakova, O.V.; Burashev, Y.D.; Zakarya, K.D.; Nakhanov, A.K.; Barakbayev, K.B.; Orynbayev, M.B. The Prevalence and Genetic Variants of the CCHF Virus Circulating among Ticks in the Southern Regions of Kazakhstan. Pathogens 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Wong, G.; Babuadze, G.; Camp, J.V.; Ergonul, O.; Kobinger, G.P.; Chinikar, S.; Nowotny, N. Crimean-Congo hemorrhagic fever virus in Asia, Africa and Europe. Microorganisms 2021, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Recent advances in understanding Crimean-Congo hemorrhagic fever virus. F1000Research 2018, 7, 1715. [Google Scholar] [CrossRef] [PubMed]
- Hewson, R.; Gmyl, A.; Gmyl, L.; Smirnova, S.E.; Karganova, G.; Jamil, B.; Hasan, R.; Chamberlain, J.; Clegg, C. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J. Gen. Virol. 2004, 85, 3059–3070. [Google Scholar] [CrossRef]
- Chinikar, S.; Shah-Hosseini, N.; Bouzari, S.; Shokrgozar, M.A.; Mostafavi, E.; Jalali, T.; Khakifirouz, S.; Groschup, M.H.; Niedrig, M. Assessment of recombination in the S-segment genome of Crimean-Congo hemorrhagic fever virus in Iran. J. Arthropod-Borne Dis. 2016, 10, 12. [Google Scholar]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef]
- Ergönül, O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Jafari, A.; Asadolahi, S.; Rasekh, M.; Saadati, D.; Faghihi, F.; Fazlalipour, M.; Telmadarraiy, Z. Distribution and biodiversity components of hard ticks as potential vectors of Crimean-Congo haemorrhagic fever virus (CCHFV) in borderline of Iran-Afghanistan. Int. J. Acarol. 2021, 47, 510–519. [Google Scholar] [CrossRef]
- Keshtkar-Jahromi, M.; Sajadi, M.M.; Ansari, H.; Mardani, M.; Holakouie-Naieni, K. Crimean-Congo hemorrhagic fever in Iran. Antivir. Res. 2013, 100, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.; Casals, J.; Faghih, M.A. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am. J. Trop. Med. Hyg. 1975, 24, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Sureau, P.; Klein, J.M. Arboviruses in Iran (author’s transl). Med. Trop. 1980, 40, 549–554. [Google Scholar]
- Chinikar, S. Crimean-Congo Hemorrhagic Fever Infection in Iran. In Crimean-Congo Hemorrhagic Fever: A Global Perspective; Ergonul, O., Whitehouse, C.A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 89–98. [Google Scholar] [CrossRef]
- Chinikar, S.; Fayaz, A.; Mirahmadi, R.; Mazaheri, V.; Mathiot, C.H.; Saron, M.F. The specific serological investigation of suspected human and domestic animals to have Crimean-Congo hemorrhagic fever in various part of Iran using ELISA techniques. Hakim J. 2002, 4, 294–300. [Google Scholar]
- Chinikar, S.; Mazaheri, V.; Mirahmadi, R.; Salehi, P.; Hosseini, N.; Nabeth, P.; Saron, M.F.; Salehi, P.; Hosseini, N.; Bouloy, M.; et al. A serological survey in suspected human patients of Crimean-Congo hemorrhagic fever in Iran by determination of IgM specific ELISA method during 2000–2004. Arch. Iran. Med. 2005, 8, 52–55. [Google Scholar]
- Chinikar, S. Seroepidemiology of Crimean-Congo haemorrhagic fever in human and domestic animals in Iran by analyzing the quantities of specific IgM and IgG against the virus of the disease by ELISA method. J. Vet. Org. 2003, 3, 69–73. [Google Scholar]
- Chinikar, S.; Ghiasi, S.M.; Moradi, M.; Goya, M.M.; Shirzadi, M.R.; Zeinali, M.; Meshkat, M.; Bouloy, M. Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 2010, 10, 705–708. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Jafarbekloo, A.; Telmadarraiy, Z.; Chinikar, S.; Haeri, A.; Nowotny, N.; Groschup, M.H.; Fooks, A.R.; Faghihi, F. Co-circulation of Crimean-Congo Hemorrhagic Fever virus strains Asia 1 and 2 between the border of Iran and Pakistan. Heliyon 2017, 3, e00439. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Mohammadshahi, J.; Bakhshzadeh, A.; Moradi-Asl, E. The First Outbreak of Crimean-Congo Hemorrhagic Fever Disease in Northwest of Iran. Acta Parasitol. 2021, 66, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Ghiasi, S.M.; Hewson, R.; Moradi, M.; Haeri, A. Crimean-Congo hemorrhagic fever in Iran and neighboring countries. J. Clin. Virol. 2010, 47, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Saghafipour, A.; Mousazadeh-Mojarrad, A.; Arzamani, N.; Telmadarraiy, Z.; Rajabzadeh, R.; Arzamani, K. Molecular and seroepidemiological survey on Crimean-Congo Hemorrhagic Fever Virus in Northeast of Iran. Med. J. Islam. Repub. Iran. 2019, 33, 41. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Keshtkar-Jahromi, M.; Ataie, B.; Adibi, P. Crimean-Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. Am. J. Trop. Med. Hyg. 2009, 81, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Alavi-Naini, R.; Moghtaderi, A.; Koohpayeh, H.R.; Sharifi-Mood, B.; Naderi, M.; Metanat, M.; Izadi, M. Crimean-Congo hemorrhagic fever in Southeast of Iran. J. Infect. 2006, 52, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Shayesteh, M.; Khakifirouz, S.; Jalali, T.; Varaie, F.S.R.; Rafigh, M.; Mostafavi, E.; Shah-Hosseini, N. Nosocomial infection of Crimean–Congo haemorrhagic fever in eastern Iran: Case report. Travel. Med. Infect. Dis. 2013, 11, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Moghadam, A.H.; Parizadeh, S.J.; Moradi, M.; Bayat, N.; Zeinali, M.; Mostafavi, E. Seroepidemiology of crimean congo hemorrhagic Fever in slaughterhouse workers in north eastern iran. Iran. J. Public Health 2012, 41, 72–77. [Google Scholar]
- Mostafavi, E.; Pourhossein, B.; Esmaeili, S.; Bagheri Amiri, F.; Khakifirouz, S.; Shah-Hosseini, N.; Tabatabaei, S.M. Seroepidemiology and risk factors of Crimean-Congo Hemorrhagic Fever among butchers and slaughterhouse workers in southeastern Iran. Int. J. Infect. Dis. 2017, 64, 85–89. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Azari-Garmjan, G.-A.; Rezaiyan, M.K.; Haeri, A.; Nowotny, N.; Fooks, A.R.; Chinikar, S.; Youssefi, M. Factors Affecting Transmission of Crimean-Congo hemorrhagic fever among slaughterhouse employees: A serosurvey in Mashhad, Iran. Jundishapur J. Microbiol. 2018, 11, e57980. [Google Scholar] [CrossRef]
- Chinikar, S.; Bouzari, S.; Shokrgozar, M.A.; Mostafavi, E.; Jalali, T.; Khakifirouz, S.; Nowotny, N.; Fooks, A.R.; Shah-Hosseini, N. Genetic diversity of Crimean Congo hemorrhagic fever virus strains from Iran. J. Arthropod-Borne Dis. 2016, 10, 127. [Google Scholar]
- Biglari, P.; Chinikar, S.; Belqeiszadeh, H.; Telmadarraiy, Z.; Mostafavi, E.; Ghaffari, M.; Javaherizadeh, S.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Phylogeny of tick-derived Crimean-Congo hemorrhagic fever virus strains in Iran. Ticks Tick-Borne Dis. 2016, 7, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Chinikar, S.; Shams, E.; Nowotny, N.; Fooks, A.R. Crimean-Congo hemorrhagic fever cases in the North of Iran have three distinct origins. Virusdisease 2017, 28, 50–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kayedi, M.H.; Chinikar, S.; Mostafavi, E.; Khakifirouz, S.; Jalali, T.; Hosseini-Chegeni, A.; Naghizadeh, A.; Niedrig, M.; Fooks, A.R.; Shahhosseini, N. Crimean–Congo hemorrhagic fever virus clade iv (Asia 1) in ticks of western Iran. J. Med. Entomol. 2015, 52, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Shah-Hosseini, N.; Bouzari, S.; Jalali, T.; Shokrgozar, M.A.; Mostafavi, E. New circulating genomic variant of Crimean-Congo hemorrhagic fever virus in Iran. Arch. Virol. 2013, 158, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Mehravaran, A.; Moradi, M.; Telmadarraiy, Z.; Mostafavi, E.; Moradi, A.R.; Khakifirouz, S.; Shah-Hosseini, N.; Varaie, F.S.R.; Jalali, T.; Hekmat, S.; et al. Molecular detection of Crimean-Congo haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks Tick-Borne Dis. 2013, 4, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.M.; Sarani, M.; Chinikar, S.; Telmadarraiy, Z.; Moghaddam, A.S.; Azam, K.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Vector prevalence and detection of Crimean-Congo haemorrhagic fever virus in Golestan Province, Iran. J. Vector Borne Dis. 2017, 54, 353. [Google Scholar] [PubMed]
- Telmadarraiy, Z.; Chinikar, S.; Vatandoost, H.; Faghihi, F.; Hosseini-Chegeni, A. Vectors of Crimean Congo hemorrhagic fever virus in Iran. J. Arthropod-Borne Dis. 2015, 9, 137. [Google Scholar] [PubMed]
- Farhadpour, F.; Telmadarraiy, Z.; Chinikar, S.; Akbarzadeh, K.; Moemenbellah-Fard, M.; Faghihi, F.; Fakoorziba, M.; Jalali, T.; Mostafavi, E.; Shahhosseini, N. Molecular detection of Crimean–Congo haemorrhagic fever virus in ticks collected from infested livestock populations in a New Endemic Area, South of Iran. Trop. Med. Int. Health 2016, 21, 340–347. [Google Scholar] [CrossRef]
- Telmadarraiy, Z.; Ghiasi, S.M.; Moradi, M.; Vatandoost, H.; Eshraghian, M.R.; Faghihi, F.; Zarei, Z.; Haeri, A.; Chinikar, S. A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand. J. Infect. Dis. 2010, 42, 137–141. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Ghiasi, S.M.; Mostafavi, E.; Moradi, M.; Piazak, N.; Mozafari, A.; Haeri, A.; Fooks, A.R.; Chinikar, S. Molecular epidemiology of Crimean- Congo hemorrhagic fever virus genome isolated from ticks of Hamadan province of Iran. J. Vector Borne Dis. 2010, 47, 211–216. [Google Scholar]
- Fakoorziba, M.R.; Golmohammadi, P.; Moradzadeh, R.; Moemenbellah-Fard, M.D.; Azizi, K.; Davari, B.; Alipour, H.; Ahmadnia, S.; Chinikar, S. Reverse transcription PCR-based detection of Crimean-Congo hemorrhagic fever virus isolated from ticks of domestic ruminants in Kurdistan province of Iran. Vector Borne Zoonotic Dis. 2012, 12, 794–799. [Google Scholar] [CrossRef]
- Mohammadian, M.; Chinikar, S.; Telmadarraiy, Z.; Vatandoost, H.; Oshaghi, M.A.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Noroozi, M.; Faghihi, F.; Jalali, T.; et al. Molecular Assay on Crimean Congo Hemorrhagic Fever Virus in Ticks (Ixodidae) Collected from Kermanshah Province, Western Iran. J. Arthropod-Borne Dis. 2016, 10, 381–391. [Google Scholar] [PubMed]
- Champour, M.; Chinikar, S.; Mohammadi, G.; Razmi, G.; Shah-Hosseini, N.; Khakifirouz, S.; Mostafavi, E.; Jalali, T. Molecular epidemiology of Crimean–Congo hemorrhagic fever virus detected from ticks of one humped camels (Camelus dromedarius) population in northeastern Iran. J. Parasit. Dis. 2016, 40, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Vaziri, M.; Vatandoost, H.; Sanei-Dehkordi, A.; Fazlalipour, M.; Pouriayevali, M.H.; Jalali, T.; Mohammadi, T.; Tavakoli, M.; Paksa, A.; Abadi, Y.S. Molecular Assay on Detection of Crimean Congo Hemorrhagic Fever (CCHF) Virus in Ixodid Ticks Collected from Livestock in Slaughterhouse from South of Iran. J. Arthropod-Borne Dis. 2020, 14, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Dilcher, M.; Faye, O.; Faye, O.; Weber, F.; Koch, A.; Sadegh, C.; Weidmann, M.; Sall, A.A. Zahedan rhabdovirus, a novel virus detected in ticks from Iran. Virol. J. 2015, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Deviatkin, A.A.; Kholodilov, I.S.; Vakulenko, Y.A.; Karganova, G.G.; Lukashev, A.N. Tick-Borne Encephalitis Virus: An Emerging Ancient Zoonosis? Viruses 2020, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef]
- Yakhchali, M.; Rostami, A.; Esmaelzadeh, M.J.R.M.V. Diversity and seasonal distribution of ixodid ticks in the natural habitat of domestic ruminants in north and south of Iran. Revue Méd. Vét. 2011, 162, 229–235. [Google Scholar]
- Yousefi, M.; Mahmoudi, A.; Kafash, A.; Khani, A.; Kryštufek, B. Biogeography of rodents in Iran: Species richness, elevational distribution and their environmental correlates. Mammalia 2022, 86, 309–320. [Google Scholar] [CrossRef]
- Salehi-Vaziri, M.; Pouriayevali, M.H.; Azad-Manjiri, S.; Ahmadi Vasmehjani, A.; Baniasadi, V.; Fazlalipour, M. The Seroprevalence of Tick-Borne Encephalitis in Rural Population of Mazandaran Province, Northern Iran (2018–2019). Arch. Clin. Infect. Dis. 2020, 15, e98867. [Google Scholar] [CrossRef]
- Nikoonejad, A.; Bijani, B. Report of the first case of Crimean Congo hemorrhagic fever in Qazvin Province (2016). J. Qazvin Univ. Med. Sci. 2016, 20, 67–73. [Google Scholar]
- Shiraly, R.; Khosravi, A.; Farahangiz, S. Seroprevalence of sandfly fever virus infection in military personnel on the western border of Iran. J. Infect. Public Health 2017, 10, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Tesh, R.B.; Savji, N.; da Rosa, A.P.T.; Guzman, H.; Bussetti, A.V.; Desai, A.; Ladner, J.; Sanchez-Seco, M.; Lipkin, W.I. Characterization of the Sandfly fever Naples species complex and description of a new Karimabad species complex (genus Phlebovirus, family Bunyaviridae). J. Gen. Virol. 2014, 95, 292. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Bichaud, L.; De Lamballerie, X.; Alten, B.; Gould, E.A.; Charrel, R.N. Sandfly-borne Phleboviruses of Eurasia and Africa: Epidemiology, genetic diversity, geographic range, control measures. Antivir. Res. 2013, 100, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Badakhshan, M.; Yaghoobi-Ershadi, M.R.; Moin-Vaziri, V.; Charrel, R.; Hanafi-Bojd, A.A.; Rezaei, F.; Akhavan, A.A.; Rassi, Y.; Oshaghi, M.-A. Spatial Distribution of Phlebotomine Sand Flies (Diptera: Psychodidae) as Phlebovirus Vectors in Different Areas of Iran. J. Med. Entomol. 2018, 55, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Erisoz Kasap, O.; Alten, B.; de Lamballerie, X.; Charrel, R.N. Sandfly-borne Phlebovirus isolations from Turkey: New insight into the sandfly fever Sicilian and sandfly fever Naples species. PLoS Negl. Trop. Dis. 2016, 10, e0004519. [Google Scholar] [CrossRef] [PubMed]
- Çarhan, A.; Uyar, Y.; Özkaya, E.; Ertek, M.; Dobler, G.; Dilcher, M.; Wang, Y.; Spiegel, M.; Hufert, F.; Weidmann, M. Characterization of a sandfly fever Sicilian virus isolated during a sandfly fever epidemic in Turkey. J. Clin. Virol. 2010, 48, 264–269. [Google Scholar] [CrossRef]
- Bryan, J.P.; Iqbal, M.; Ksiazek, T.G.; Ahmed, A.; Duncan, J.F.; Awan, B.; Krieg, R.E.; Riaz, M.; Leduc, J.W.; Nabi, S. Prevalence of sand fly fever, West Nile, Crimean-Congo hemorrhagic fever, and leptospirosis antibodies in Pakistani military personnel. Mil. Med. 1996, 161, 149–153. [Google Scholar] [CrossRef]
- Tesh, R.; Saidi, S.; Javadian, E.; Nadim, A. Studies on the epidemiology of sandfly fever in Iran. I. Virus isolates obtained from Phlebotomus. Am. J. Trop. Med. Hyg. 1977, 26, 282–287. [Google Scholar] [CrossRef]
- Tavana, A.M. The seroepidemiological studies of sand fly fever in Iran during imposed war. Iran. J. Public Health 2001, 30, 145–146. [Google Scholar]
- Shahhosseini, N.; Paquette, S.-J.; Kayedi, M.H.; Abaei, M.R.; Sedaghat, M.M. Genetic Characterization of Sandfly-Borne Viruses in Phlebotomine Sandflies in Iran. Microorganisms 2023, 11, 2754. [Google Scholar] [CrossRef] [PubMed]
- Marklewitz, M.; Tchouassi, D.P.; Hieke, C.; Heyde, V.; Torto, B.; Sang, R.; Junglen, S. Insights into the evolutionary origin of Mediterranean sandfly fever viruses. MSphere 2020, 5, e00598-20. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi-Ershadi, M. Phlebotomine Sand Flies (Diptera: Psychodidae) in Iran and their Role on Leishmania Transmission. J. Arthropod-Borne Dis. 2012, 6, 1–17. [Google Scholar] [PubMed]
- Karimi, A.; Hanafi-Bojd, A.A.; Yaghoobi-Ershadi, M.R.; Akhavan, A.A.; Ghezelbash, Z. Spatial and temporal distributions of phlebotomine sand flies (Diptera: Psychodidae), vectors of Leishmaniasis, in Iran. Acta Trop. 2014, 132, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.; Tesh, R.; Javadian, E.; Sahabi, Z.; Nadim, A. Studies on the epidemiology of sandfly fever in Iran. II. The prevalence of human and animal infection with five phlebotomus fever virus serotypes in Isfahan province. Am. J. Trop. Med. Hyg. 1977, 26, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Saidi, S.; Gajdamovič, S.J.; Rodhain, F.; Vesenjak-Hirjan, J. Serological studies of the epidemiology of sandfly fever in the Old World. Bull. World Health Organ. 1976, 54, 663. [Google Scholar] [PubMed]
- Jiang, H.; Zheng, X.; Wang, L.; Du, H.; Wang, P.; Bai, X. Hantavirus infection: A global zoonotic challenge. Virol. Sin. 2017, 32, 32–43. [Google Scholar] [CrossRef]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Zhenqiang, B.; Pierre, B.H.F.; Cathy, E.R. Hantavirus Infection: A review and global update. J. Infect. Dev. Ctries. 2008, 2, 3–23. [Google Scholar] [CrossRef]
- Muranyi, W.; Bahr, U.; Zeier, M.; van der Woude, F.J. Hantavirus Infection. J. Am. Soc. Nephrol. 2005, 16, 3669. [Google Scholar] [CrossRef] [PubMed]
- Heyman, P.; Vaheri, A.; Lundkvist, A.; Avsic-Zupanc, T. Hantavirus infections in Europe: From virus carriers to a major public-health problem. Expert Rev. Anti-Infect. Ther. 2009, 7, 205–217. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, A.; Nichol, S.T.; Spiropoulou, C.F. Hantavirus pulmonary syndrome. Virus Res. 2011, 162, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Sharma, D.; Shukla, R.; Alexander, A.; Jain, G.K.; Ahmad, F.J.; Kesharwani, P. Epidemiology, virology and clinical aspects of hantavirus infections: An overview. Int. J. Environ. Health Res. 2022, 32, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohn, C.S.; Dalrymple, J.M. Analysis of hantaan virus RNA: Evidence for a new genus of Bunyaviridae. Virology 1983, 131, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Llah, S.T.; Mir, S.; Sharif, S.; Khan, S.; Mir, M.A. Hantavirus induced cardiopulmonary syndrome: A public health concern. J. Med. Virol. 2018, 90, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Javadi, A.A.; Hajiannia, A.; Ataei, B.; Jalali, T.; Khakifirouz, S.; Nowotny, N.; Schmidt-Chanasit, J.; Shahhosseini, N. First evidence of Hantavirus in central Iran as an emerging viral disease. Adv. Infect. Dis. 2014, 4, 173. [Google Scholar] [CrossRef]
- Salehi-Vaziri, M.; Sarvari, J.; Mansurnejadan, M.; Shiri, A.; Joharinia, N.; Khoshbakht, R.; Jaberi, O.; Pouriayevali, M.H.; Azad-Manjiri, S.; Jalali, T.; et al. Evidence of Hantavirus circulation among municipal street sweepers, southwest of Iran. Virus Dis. 2021, 32, 251–254. [Google Scholar] [CrossRef]
- Kassiri, H.; Dehghani, R. Hantavirus infections as zoonotic emerging viral diseases: Current sta-tus with an emphasis on data from Iran. EASL 2022, 6, 112–128. [Google Scholar]
- Nejati, J.; Bueno-Marí, R.; Collantes, F.; Hanafi-Bojd, A.A.; Vatandoost, H.; Charrahy, Z.; Tabatabaei, S.M.; Yaghoobi-Ershadi, M.R.; Hasanzehi, A.; Shirzadi, M.R. Potential risk areas of Aedes albopictus in south-eastern Iran: A vector of dengue fever, zika, and chikungunya. Front. Microbiol. 2017, 8, 1660. [Google Scholar] [CrossRef]
- Asgarian, T.S.; Moosa-Kazemi, S.H.; Sedaghat, M.M. Impact of meteorological parameters on mosquito population abundance and distribution in a former malaria endemic area, central Iran. Heliyon 2021, 7, e08477. [Google Scholar] [CrossRef] [PubMed]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Noaman, V.; Abdigoudarzi, M.; Nabinejad, A.R. Abundance, diversity and seasonal dynamics of hard ticks infesting cattle in Isfahan province, central Iran. Arch. Razi Inst. 2017, 72, 15–21. [Google Scholar] [CrossRef]
- Fazeli-Dinan, M.; Asgarian, F.; Nikookar, S.H.; Ziapour, S.P.; Enayati, A. Defining and comparison of biodiversity components of hard ticks on domestic hosts at Highland, Woodland and Plain in Northern Iran. Trop. Biomed. 2019, 36, 114–130. [Google Scholar] [PubMed]
- Zhang, M.; Wang, K.; Wang, Y.; Guo, C.; Li, B.; Huang, H. Recovery of a rodent community in an agro-ecosystem after flooding. J. Zool. 2007, 272, 138–147. [Google Scholar] [CrossRef]
- Villar, C.H.; Naya, D.E. Climate change and temporal trends in body size: The case of rodents. Oikos 2018, 127, 1186–1194. [Google Scholar] [CrossRef]
- Lederberg, J. Infectious History. Science 2000, 288, 287–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquette, S.-J.; Simon, A.Y.; XIII, A.; Kobinger, G.P.; Shahhosseini, N. Medically Significant Vector-Borne Viral Diseases in Iran. Microorganisms 2023, 11, 3006. https://doi.org/10.3390/microorganisms11123006
Paquette S-J, Simon AY, XIII A, Kobinger GP, Shahhosseini N. Medically Significant Vector-Borne Viral Diseases in Iran. Microorganisms. 2023; 11(12):3006. https://doi.org/10.3390/microorganisms11123006
Chicago/Turabian StylePaquette, Sarah-Jo, Ayo Yila Simon, Ara XIII, Gary P. Kobinger, and Nariman Shahhosseini. 2023. "Medically Significant Vector-Borne Viral Diseases in Iran" Microorganisms 11, no. 12: 3006. https://doi.org/10.3390/microorganisms11123006
APA StylePaquette, S.-J., Simon, A. Y., XIII, A., Kobinger, G. P., & Shahhosseini, N. (2023). Medically Significant Vector-Borne Viral Diseases in Iran. Microorganisms, 11(12), 3006. https://doi.org/10.3390/microorganisms11123006