Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions
Abstract
:1. Introduction
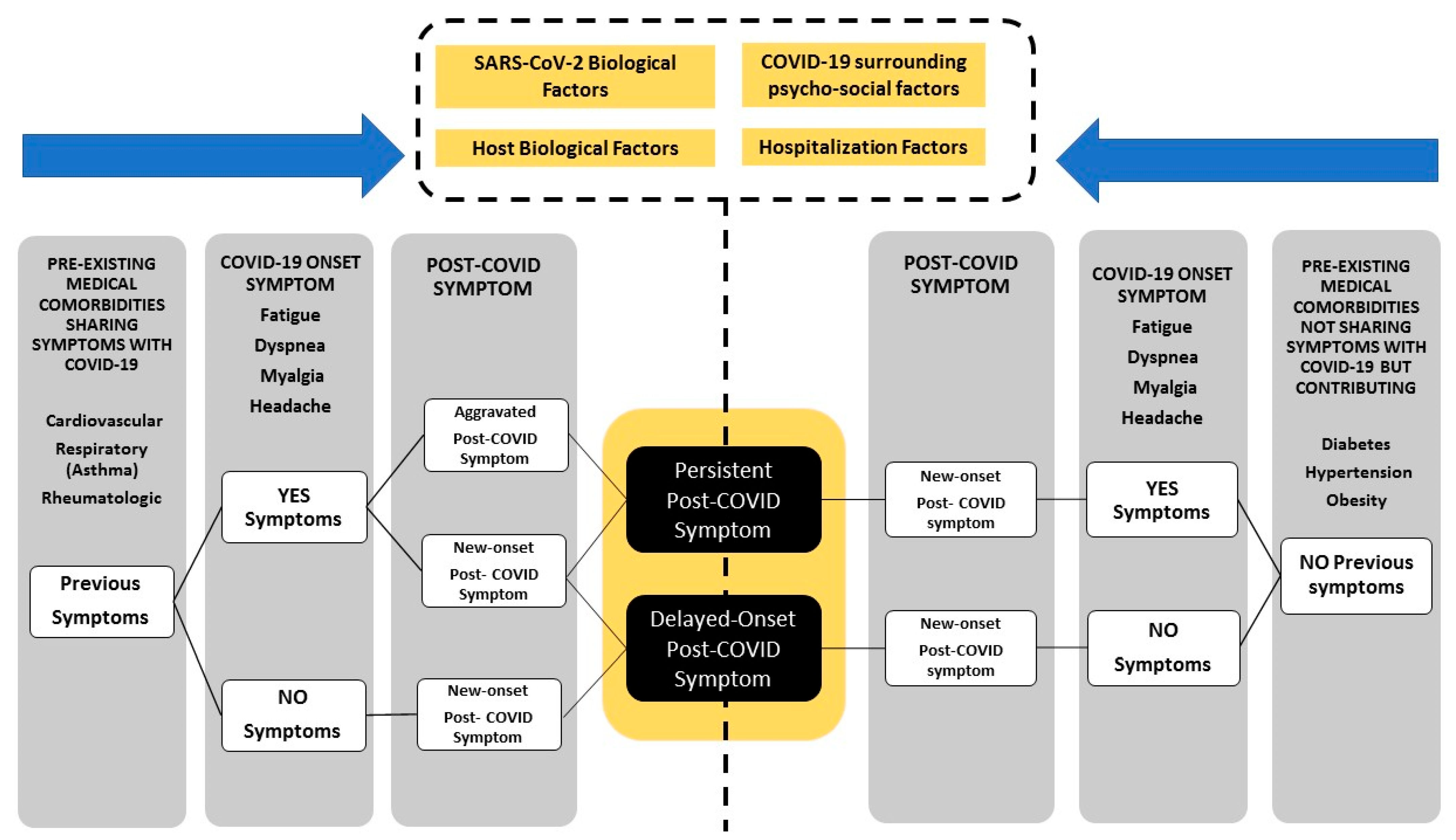
2. How Should Post-COVID Symptoms Be Defined
3. Subclassification (Clustering) and Phenotyping of Long-COVID
4. Risk Factors Associated with Long-COVID
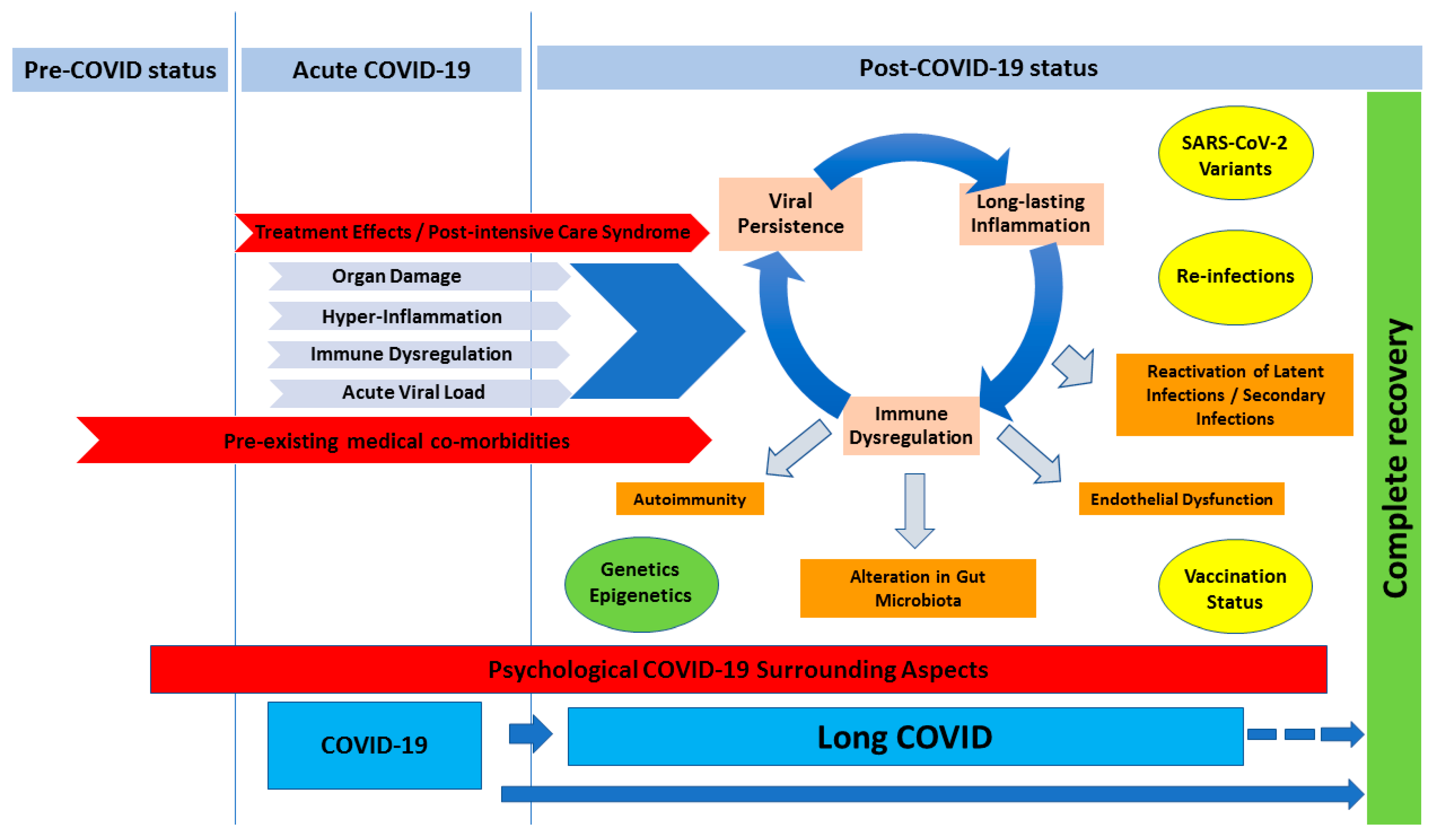
5. Current Pathophysiology Theories of Long-COVID
5.1. Viral Persistence
5.2. Long-Lasting Inflammation
5.3. Immune Dysregulation and Autoimmunity
5.4. Reactivation of Latent Infections
5.5. Endothelial Dysfunction
5.6. Alteration in Gut Microbiota
5.7. Psychological COVID-19 Surrounding Aspects
6. Genetic Influence on Long-COVID
7. Epigenetic Influence on Long-COVID
8. SARS-CoV-2 Variants, Re-Infections and Vaccination Status
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 1st October 2023).
- COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management/ (accessed on 15 September 2023).
- Therapeutic Management of Non-Hospitalized Adults with COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/ (accessed on 15 September 2023).
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, L.; Li, M.; Xie, B.; He, L.; Wang, M.; Zhang, R.; Hou, N.; Zhang, Y.; Jia, F. Real-word effectiveness of Global COVID-19 vaccines against SARS-CoV-2 Variants: A systematic review and meta-analysis. Front. Med. 2022, 9, 820544. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.D.; Ingram, J.; Sculthorpe, N.F. More Than 100 Persistent Symptoms of SARS-CoV-2 (Long COVID): A scoping review. Front. Med. 2021, 8, 750378. [Google Scholar] [CrossRef] [PubMed]
- Amdal, C.D.; Pe, M.; Falk, R.S.; Piccinin, C.; Bottomley, A.; Arraras, J.I.; Darlington, A.S.; Hofsø, K.; Holzner, B.; Jørgensen, N.M.H.; et al. Health-related quality of life issues, including symptoms, in patients with active COVID-19 or post COVID-19; a systematic literature review. Qual. Life Res. 2021, 30, 3367–3381. [Google Scholar] [CrossRef] [PubMed]
- Tene, L.; Bergroth, T.; Eisenberg, A.; Ben David, S.S.; Chodick, G. Risk factors, health outcomes, healthcare services utilization, and direct medical costs of long COVID patient. Int. J. Infect. Dis. 2023, 128, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Moens, M.; Duarte, R.V.; De Smedt, A.; Putman, K.; Callens, J.; Billot, M.; Roulaud, M.; Rigoard, P.; Goudman, L. Health-related quality of life in persons post-COVID-19 infection in comparison to normative controls and chronic pain patients. Front. Public Health 2022, 10, 991572. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kuodi, P.; Gorelik, Y.; Gausi, B.; Bernstine, T.; Edelstein, M. Characterization of post-COVID syndromes by symptom cluster and time period up to 12 months post-infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 134, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Long COVID Collaborators; Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar]
- Rahmati, M.; Udeh, R.; Yon, D.K.; Lee, S.W.; Dolja-Gore, X.; McEVoy, M.; Kenna, T.; Jacob, L.; López Sánchez, G.F.; Koyanagi, A.; et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: A call to action for neurological, physical, and psychological sciences. J. Med. Virol. 2023, 95, e28852. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Chen, C.; Zhou, M.; Wen, W.; Wang, C.; Tang, J.; Cheng, Y.; Wu, Q.; Zhang, X.; Wang, M.; et al. Post-COVID-19 fatigue among COVID-19 in patients discharged from hospital: A meta-analysis. J. Infect. 2022, 84, 722–746. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Navarro-Santana, M.; Plaza-Manzano, G.; Palacios-Ceña Arendt-Nielsen, L. Time course prevalence of Post-COVID pain symptoms of musculoskeletal origin in patients who had survived to SARS-CoV-2 infection: A systematic review and meta-analysis. Pain 2022, 163, 1220–1231. [Google Scholar] [CrossRef]
- Marjenberg, Z.; Leng, S.; Tascini, C.; Garg, M.; Misso, K.; El Guerche Seblain, C.; Shaikh, N. Risk of long COVID main symptoms after SARS-CoV-2 infection: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 15332. [Google Scholar] [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J.Ž. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef]
- Boaventura, P.; Macedo, S.; Ribeiro, F.; Jaconiano, S.; Soares, P. Post-COVID-19 condition: Where are we now? Life 2022, 12, 517. [Google Scholar] [CrossRef]
- Mumoli, N.; Conte, G.; Evangelista, I.; Cei, M.; Mazzone, A.; Colombo, A. Post-COVID or long-COVID: Two different conditions or the same? J. Infect. Public Health 2021, 14, 1349–1350. [Google Scholar] [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. Long COVID, a comprehensive systematic scoping review. Infection 2021, 49, 1163–1186. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A Multi-center study investigating long COVID-19 in healthcare workers from North-Eastern Italy: Prevalence, risk factors and the impact of pre-existing humoral immunity-ORCHESTRA Project. Vaccines 2023, 11, 1769. [Google Scholar] [CrossRef]
- Zheng, B.; Daines, L.; Han, Q.; Hurst, J.R.; Pfeffer, P.; Shankar-Hari, M.; Elneima, O.; Walker, S.; Brown, J.S.; Siddiqui, S.; et al. Prevalence, risk factors and treatments for post-COVID-19 breathlessness: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 220071. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Florencio, L.L.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Palacios-Ceña, D.; Raveendran, A.V. Proposed integrative model for post-COVID symptoms. Diabetes Metab. Syndr. 2021, 15, 102159. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C. Are patients exhibiting post-Coronavirus Disease (COVID) symptoms at 12 months the same at 5 or 9 months? The fluctuating nature of Post-COVID. Clin. Infect. Dis. 2022, 75, e1208. [Google Scholar] [CrossRef]
- Korompoki, E.; Gavriatopoulou, M.; Hicklen, R.S.; Ntanasis-Stathopoulos, I.; Kastritis, E.; Fotiou, D.; Stamatelopoulos, K.; Terpos, E.; Kotanidou, A.; Hagberg, C.A.; et al. Epidemiology and organ specific sequelae of post-acute COVID19: A narrative review. J. Infect. 2021, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE); Royal College of General Practitioners; Healthcare Improvement Scotland SIGN. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. London: National Institute for Health and Care Excellence. 2020. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 1 October 2023).
- Baig, A.M. Chronic COVID Syndrome: Need for an appropriate medical terminology for Long-COVID and COVID Long-Haulers. J. Med. Virol. 2021, 93, 2555–2556. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.; O’Connor, R.; Sivan, M. Long COVID and chronic COVID syndromes. J. Med. Virol. 2021, 93, 1242–1243. [Google Scholar] [CrossRef]
- Marshall, M. The lasting misery of coronavirus long-haulers. Nature 2020, 585, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Kräusslich, H.G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V.; et al. Post-acute sequelae of COVID-19 six to 12 months after infection: Population-based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef]
- Diem, L.; Schwarzwald, A.; Friedli, C.; Hammer, H.; Gomes-Fregolente, L.; Warncke, J.; Weber, L.; Kamber, N.; Chan, A.; Bassetti, C.; et al. Multidimensional phenotyping of the post-COVID-19 syndrome: A Swiss survey study. CNS Neurosci. Ther. 2022, 28, 1953–1963. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk factors associated with post-COVID-19 condition: A systematic review and meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Bwire, G.M. Coronavirus: Why men are more vulnerable to covid-19 than women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Spicuzza, L.; Campisi, R.; Alia, S.; Prestifilippo, S.; Giuffrida, M.L.; Angileri, L.; Ciancio, N.; Vancheri, C. Female sex affects respiratory function and exercise ability in patients recovered from COVID-19 pneumonia. J. Womens Health 2023, 32, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Qie, G.; Yao, Q.; Sun, W.; Wang, C.; Zhang, Z.; Wang, X.; Wang, P.; Jiang, J.; Bai, X.; et al. Sex differences on clinical characteristics, severity, and mortality in adult patients with COVID-19: A multicentre retrospective study. Front. Med. 2021, 8, 607059. [Google Scholar] [CrossRef] [PubMed]
- Barek, M.A.; Aziz, M.A.; Islam, M.S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon 2020, 6, e05684. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical and mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Mathew, R.; Aggarwal, P.; Nayer, J.; Bhoi, S.; Satapathy, S.; Ekka, M. Clinical determinants of severe COVID-19 Disease: A systematic review and meta-analysis. J. Glob. Infect. Dis. 2021, 13, 13–19. [Google Scholar]
- Sansone, D.; Tassinari, A.; Valentinotti, R.; Kontogiannis, D.; Ronchese, F.; Centonze, S.; Maggiore, A.; Cegolon, L.; Filon, F.L. Persistence of symptoms 15 months since COVID-19 diagnosis: Prevalence, risk factors and residual work ability. Life 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab. Syndr. 2021, 15, 145–146. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B.; RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86002. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Chappell, K.J. Chronic SARS-CoV-2, a Cause of Post-acute COVID-19 Sequelae (Long-COVID)? Front. Microbiol. 2021, 12, 724654. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B.; RECOVER Mechanistic Pathways Task Force. Viral persistence, reactivation, and mechanisms of long COVID. Elife 2023, 12, e86015. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B.; Aleman, S.; Bach, K.; Boribong, B.P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 2023, 24, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Bussani, R.; Zentilin, L.; Correa, R.; Colliva, A.; Silvestri, F.; Zacchigna, S.; Collesi, C.; Giacca, M. Persistent SARS-CoV-2 infection in patients seemingly recovered from COVID-19. J. Pathol. 2023, 259, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology 2022, 163, 495–506. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent circulating Severe Acute Respiratory Syndrome Coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Tian, F.; Chen, Z.; Feng, Q. Nirmatrelvir-ritonavir compared with other antiviral drugs for the treatment of COVID-19 patients: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e28732. [Google Scholar] [CrossRef]
- Marshall, G.D., Jr. The pathophysiology of post-acute sequelae of COVID-19 (PASC): Possible role for persistent inflammation. Asia Pac. Allergy 2023, 13, 77–84. [Google Scholar]
- PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of Long-COVID and association with one-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, 761–775. [Google Scholar] [CrossRef]
- Yin, J.X.; Agbana, Y.L.; Sun, Z.S.; Fei, S.W.; Zhao, H.Q.; Zhou, X.N.; Chen, J.H.; Kassegne, K. Increased interleukin-6 is associated with long COVID-19: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 43. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Williams, E.S.; Martins, T.B.; Shah, K.S.; Hill, H.R.; Coiras, M.; Spivak, A.M.; Planelles, V. Cytokine deficiencies in patients with long-COVID. J. Clin. Cell. Immunol. 2022, 13, 672. [Google Scholar]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef]
- Hawley, H.B. Long COVID: Clinical findings, pathology, and endothelial molecular mechanisms. Am. J. Med. 2023, in press. [Google Scholar] [CrossRef]
- Griesel, M.; Wagner, C.; Mikolajewska, A.; Stegemann, M.; Fichtner, F.; Metzendorf, M.I.; Nair, A.A.; Daniel, J.; Fischer, A.L.; Skoetz, N. Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2022, 3, CD015125. [Google Scholar]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.I.; Fischer, A.L.; Stegemann, M.; Spagl, M.; Nair, A.A.; Daniel, J.; Fichtner, F.; et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 11, CD014963. [Google Scholar]
- Badenes Bonet, D.; Caguana Vélez, O.A.; Duran Jordà, X.; Comas Serrano, M.; Posso Rivera, M.; Admetlló, M.; Herranz Blasco, A.; Cuadrado Godia, E.; Marco Navarro, E.; Martin Ezquerra, G.; et al. Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19. J. Clin. Med. 2023, 12, 4158. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Zhang, H.-Z.; Liu, C.; Dong, K. Autoantibodies in COVID-19: Frequency and function. Autoimmun. Rev. 2021, 20, 102754. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Blagova, O.; Varionchik, N.; Zaidenov, V.; Savina, P.; Sarkisova, N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur. J. Immunol. 2021, 51, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Harne, R.; Williams, B.; Abdelal, H.F.M.; Baldwin, S.L.; Coler, R.N. SARS-CoV-2 infection and immune responses. AIMS Microbiol. 2023, 9, 245–276. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation, and an uncoordinated adaptive immune response to SARS-CoV-2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Peluso, M.J.; Deveau, T.-M.; Munter, S.E.; Ryder, D.; Buck, A.; Lu, S.; Goldberg, S.A.; et al. Evidence of recent Epstein-Barr virus reactivation in individuals experiencing long COVID. medRxiv 2022. [Google Scholar] [CrossRef]
- Banko, A.; Miljanovic, D.; Cirkovic, A. Systematic review with meta-analysis of active herpesvirus infections in patients with COVID-19: Old players on the new field. Int. J. Infect. Dis. 2023, 130, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Santoro, L.; Zaccone, V.; Falsetti, L.; Ruggieri, V.; Danese, M.; Miro, C.; Di Giorgio, A.; Nesci, A.; D’Alessandro, A.; Moroncini, G.; et al. Role of endothelium in cardiovascular sequelae of long COVID. Biomedicines 2023, 11, 2239. [Google Scholar] [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 complications: Oxidative stress, inflammation, and mitochondrial and endothelial dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef]
- Shabani, Z.; Liu, J.; Su, H. Vascular dysfunctions contribute to the long-term cognitive deficits following COVID-19. Biology 2023, 12, 1106. [Google Scholar] [CrossRef]
- Zanini, G.; Selleri, V.; Roncati, L.; Coppi, F.; Nasi, M.; Farinetti, A.; Manenti, A.; Pinti, M.; Mattioli, A.V. Vascular “Long COVID”: A new vessel disease? Angiology 2023, 75. [Google Scholar] [CrossRef]
- Moreno-Corona, N.C.; López-Ortega, O.; Pérez-Martínez, C.A.; Martínez-Castillo, M.; De Jesús-González, L.A.; León-Reyes, G.; León-Juárez, M. Dynamics of the microbiota and its relationship with post-COVID-19 syndrome. Int. J. Mol. Sci. 2023, 24, 14822. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.M.; Matos, Y.L.; Lin, H.C.; Ryman, S.G.; Birg, A.; Quinn, D.K.; Parada, A.N.; Vakhtin, A.A. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front. Neurosci. 2023, 17, 1232480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Ianiro, G.; Ponziani, F.R.; Bibbò, S.; Segal, J.P.; Cammarota, G.; Gasbarrini, A. COVID-19 as a trigger of irritable bowel syndrome: A review of potential mechanisms. World J. Gastroenterol. 2021, 27, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Sheeran, P.; Fong, G.T.; Cheah, C.S.L.; Oremus, M.; Liu-Ambrose, T.; Sakib, M.N.; Butt, Z.A.; Ayaz, H.; Jandu, N.; et al. Biobehavioral Aspects of the COVID-19 Pandemic: A Review. Psychosom. Med. 2021, 83, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 depressive symptoms: Epidemiology, Pathophysiology, and pharmacological treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef]
- Al-Jassas, H.K.; Al-Hakeim, H.K.; Maes, M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome-like symptoms due to COVID-19: A nomothetic network approach. J. Affect. Disord. 2022, 15, 233–245. [Google Scholar] [CrossRef]
- Bottemanne, H.; Gouraud, C.; Hulot, J.S.; Blanchard, A.; Ranque, B.; Lahlou-Laforêt, K.; Limosin, F.; Günther, S.; Lebeaux, D.; Lemogne, C. Do anxiety and depression predict persistent physical symptoms after a severe COVID-19 episode? A prospective study. Front. Psychiatry 2021, 12, 757685. [Google Scholar] [CrossRef]
- Merikanto, I.; Dauvilliers, Y.; Chung, F.; Wing, Y.K.; De Gennaro, L.; Holzinger, B.; Bjorvatn, B.; Morin, C.M.; Penzel, T.; Benedict, C.; et al. Sleep symptoms are essential features of long-COVID—Comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II). J. Sleep Res. 2022, 32, e13754. [Google Scholar] [CrossRef]
- Pacho-Hernández, J.C.; Fernández-de-las-Peñas, C.; Fuensalida-Novo, S.; Jiménez-Antona, C.; Ortega-Santiago, R.; Cigarán-Mendez, M. Sleep quality mediates the effect of sensitization-associated symptoms, anxiety, and depression on quality of life in individuals with post-COVID-19 pain. Brain Sci. 2022, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D. Precision medicine and management of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102405. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Committee on AFfDaNToD. The National Academies Collection: Reports funded by National Institutes of Health. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; National Academies Press: Washington, DC, USA, 2011.
- Hetta, H.F.; Muhammad, K.; Algammal, A.M.; Ramadan, H.; Abdel-Rahman, M.S.; Mabrok, M.; Koneru, G.; Elkady, A.A.; El-Saber Batiha, G.; Waheed, Y.; et al. Mapping the effect of drugs on ACE2 as a novel target site for COVID-19 therapy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3923–3932. [Google Scholar] [PubMed]
- de Bruin, N.; Schneider, A.K.; Reus, P.; Talmon, S.; Ciesek, S.; Bojkova, D.; Cinatl, J.; Lodhi, I.; Charlesworth, B.; Sinclair, S.; et al. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol do not influence ACE2 expression and activity in vitro or in mice and do not exacerbate in-vitro SARS-CoV-2 infection. Int. J. Mol. Sci. 2022, 23, 1049. [Google Scholar] [CrossRef]
- Darbeheshti, F.; Abolhassani, H.; Bashashati, M.; Ghavami, S.; Shahkarami, S.; Zoghi, S.; Gupta, S.; Orange, J.S.; Ochs, H.D.; Rezaei, N. Coronavirus: Pure infectious disease or genetic predisposition. Adv. Exp. Med. Biol. 2021, 1318, 91–107. [Google Scholar]
- Darbeheshti, F.; Rezaei, N. Genetic Predisposition Models to COVID-19 Infection. Med. Hypotheses 2020, 142, 109818. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Sharifi, N.; Erzurum, S.; Eng, C.; Cheng, F. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef]
- Saengsiwaritt, W.; Jittikoon, J.; Chaikledkaew, U.; Udomsinprasert, W. Genetic polymorphisms of ACE1, ACE2, and TMPRSS2 associated with COVID-19 severity: A systematic review with meta-analysis. Rev. Med. Virol. 2022, 8, e2323. [Google Scholar] [CrossRef]
- Gupta, K.; Kaur, G.; Pathak, T.; Banerjee, I. Systematic review and meta-analysis of human genetic variants contributing to COVID-19 susceptibility and severity. Gene 2022, 844, 146790. [Google Scholar] [CrossRef]
- Acosta, P.L.; Byrne, A.B.; Hijano, D.R.; Talarico, L.B. Human type I interferon antiviral effects in respiratory and reemerging viral infections. J. Immunol. Res. 2020, 2020, 1372494. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Zhao, Y.; Zhang, P.; Xu, B.; Li, K.; Liang, L.; Zhang, C.; Dai, Y.; Feng, Y.; et al. Interferon-induced transmembrane protein 3 genetic variant Rs12252-C associated with disease severity in Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 34–37. [Google Scholar] [CrossRef]
- Karcioglu Batur, L.; Hekim, N. Correlation between interleukin gene polymorphisms and current prevalence and mortality rates due to novel coronavirus disease 2019 (COVID-2019) in 23 countries. J. Med. Virol. 2021, 93, 5853–5863. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Arendt-Nielsen, L.; Díaz-Gil, G.; Gómez-Esquer, F.; Gil-Crujera, A.; Gómez-Sánchez, S.M.; Ambite-Quesada, S.; Palomar-Gallego, M.A.; Pellicer-Valero, O.J.; Giordano, R. Genetic association between ACE2 (rs2285666 and rs2074192) and TMPRSS2 (rs12329760 and rs2070788) polymorphisms with post-COVID symptoms in previously hospitalized COVID-19 survivors. Genes 2022, 13, 1935. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Giordano, R.; Díaz-Gil, G.; Gómez-Esquer, F.; Ambite-Quesada, S.; Palomar-Gallego, M.A.; Arendt-Nielsen, L. Post-COVID pain is not associated with inflammatory polymorphisms in people who had been hospitalized by COVID-19. J. Clin. Med. 2022, 11, 5645. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Giordano, R.; Díaz-Gil, G.; Gil-Crujera, A.; Gómez-Sánchez, S.M.; Ambite-Quesada, S.; Arendt-Nielsen, L. Are pain polymorphisms associated with the risk and phenotype of post-COVID pain in previously hospitalized COVID-19 survivors? Genes 2022, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Atlante, S.; Mongelli, A.; Barbi, V.; Martelli, F.; Farsetti, A.; Gaetano, C. The epigenetic implication in coronavirus infection and therapy. Clin. Epigenet. 2020, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Capp, J.P. Interplay between genetic, epigenetic, and gene expression variability: Considering complexity in evolvability. Evol. Appl. 2021, 14, 893–901. [Google Scholar] [CrossRef]
- Mantovani, A.; Netea, M.G. Trained innate immunity, epigenetics, and COVID-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef]
- Corley, M.J.; Pang, A.P.S.; Dody, K.; Mudd, P.A.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-Wide DNA Methylation profiling of peripheral blood reveals an epigenetic signature associated with severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef]
- Dey, A.; Vaishak, K.; Deka, D.; Radhakrishnan, A.K.; Paul, S.; Shanmugam, P.; Daniel, A.P.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. Epigenetic perspectives associated with COVID-19 infection and related cytokine storm: An updated review. Infection 2023, 51, 1603–1618. [Google Scholar] [CrossRef]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Chieng, H.C.; Tiwari, A.; Vincent, C.E.; Chopra, A.; Vincent, P.A.; Robek, M.D.; et al. Blood DNA Methylation and COVID-19 outcomes. Clin. Epigenet. 2021, 13, 118. [Google Scholar] [CrossRef]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Adhikari, A.; Vancavage, R.; Singer, H.A.; Alisch, R.S.; Jaitovich, A. Whole-Genome methylation sequencing reveals that COVID-19-induced epigenetic dysregulation remains 1 year after hospital discharge. Am. J. Respir. Cell Mol. Biol. 2023, 68, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Riskedal, E.; Kalleberg, K.T.; Istre, M.; Lind, A.; Lund-Johansen, F.; Reiakvam, O.; Søraas, A.V.L.; Harris, J.R.; Dahl, J.A.; et al. EWAS of post-COVID-19 patients shows methylation differences in the immune-response associated gene, IFI44L, three months after COVID-19 infection. Sci. Rep. 2022, 12, 11478. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated biological aging in COVID-19 patients. Nat. Commun. 2022, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Dantzer, R.; Khandaker, G.M. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology 2021, 131, 105295. [Google Scholar] [CrossRef]
- Schultz, I.C.; Bertoni, A.P.S.; Wink, M.R. MSC-exosomes carrying MiRNA—Could they enhance tocilizumab activity in neuropathology of COVID-19? Stem Cell Rev. Rep. 2023, 19, 279–283. [Google Scholar] [CrossRef]
- Yong, S.J.; Halim, A.; Halim, M.; Liu, S.; Aljeldah, M.; Al Shammari, B.R.; Alwarthan, S.; Alhajri, M.; Alawfi, A.; Alshengeti, A.; et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers. Rev. Med. Virol. 2023, 33, e2424. [Google Scholar] [CrossRef]
- Jankovic, M.; Nikolic, D.; Novakovic, I.; Petrovic, B.; Lackovic, M.; Santric-Milicevic, M. MiRNAs as a potential biomarker in the COVID-19 infection and complications course, severity, and outcome. Diagnostics 2023, 13, 1091. [Google Scholar] [CrossRef]
- Parra-Lucares, A.; Segura, P.; Rojas, V.; Pumarino, C.; Saint-Pierre, G.; Toro, L. Emergence of SARS-CoV-2 variants in the world: How could this happen? Life 2022, 12, 194. [Google Scholar] [CrossRef]
- Thye, A.Y.; Law, J.W.; Pusparajah, P.; Letchumanan, V.; Chan, K.G.; Lee, L.H. Emerging SARS-CoV-2 Variants of Concern (VOCs): An Impending Global Crisis. Biomedicines 2021, 9, 1303. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: A systematic review of the literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ma, Y.; Deng, J.; Liu, M.; Liu, J. Comparison of long COVID-19 caused by different SARS-CoV-2 strains: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 16010. [Google Scholar] [CrossRef]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health-Eur. 2022, 20, 100453. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef]
- Boufidou, F.; Medić, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 reinfections and long COVID in the post-Omicron phase of the pandemic. Int. J. Mol. Sci. 2023, 24, 12962. [Google Scholar] [CrossRef]
- Peghin, M.; De Martino, M.; Palese, A.; Chiappinotto, S.; Fonda, F.; Gerussi, V.; Sartor, A.; Curcio, F.; Grossi, P.A.; Isola, M.; et al. Post-COVID-19 syndrome 2 years after the first wave: The role of humoral response, vaccination and reinfection. Open Forum Infect. Dis. 2023, 10, ofad364. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. COVID-19 vaccines reduce the risk of SARS-CoV-2 reinfection and hospitalization: Meta-analysis. Front. Med. 2022, 9, 1023507. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the realworld: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Orendáčová, M.; Kvašňák, E. Effects of vaccination, new SARS-CoV-2 variants and reinfections on post-COVID-19 complications. Front. Public Health 2022, 10, 903568. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Raveendran, A.V.; Giordano, R.; Arendt-Nielsen, L. Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions. Microorganisms 2023, 11, 2959. https://doi.org/10.3390/microorganisms11122959
Fernández-de-las-Peñas C, Raveendran AV, Giordano R, Arendt-Nielsen L. Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions. Microorganisms. 2023; 11(12):2959. https://doi.org/10.3390/microorganisms11122959
Chicago/Turabian StyleFernández-de-las-Peñas, César, Arkiath Veettil Raveendran, Rocco Giordano, and Lars Arendt-Nielsen. 2023. "Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions" Microorganisms 11, no. 12: 2959. https://doi.org/10.3390/microorganisms11122959
APA StyleFernández-de-las-Peñas, C., Raveendran, A. V., Giordano, R., & Arendt-Nielsen, L. (2023). Long COVID or Post-COVID-19 Condition: Past, Present and Future Research Directions. Microorganisms, 11(12), 2959. https://doi.org/10.3390/microorganisms11122959





