Development and Validation of a Highly Sensitive Multiplex Immunoassay for SARS-CoV-2 Humoral Response Monitorization: A Study of the Antibody Response in COVID-19 Patients with Different Clinical Profiles during the First and Second Waves in Cadiz, Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Bead Functionalization
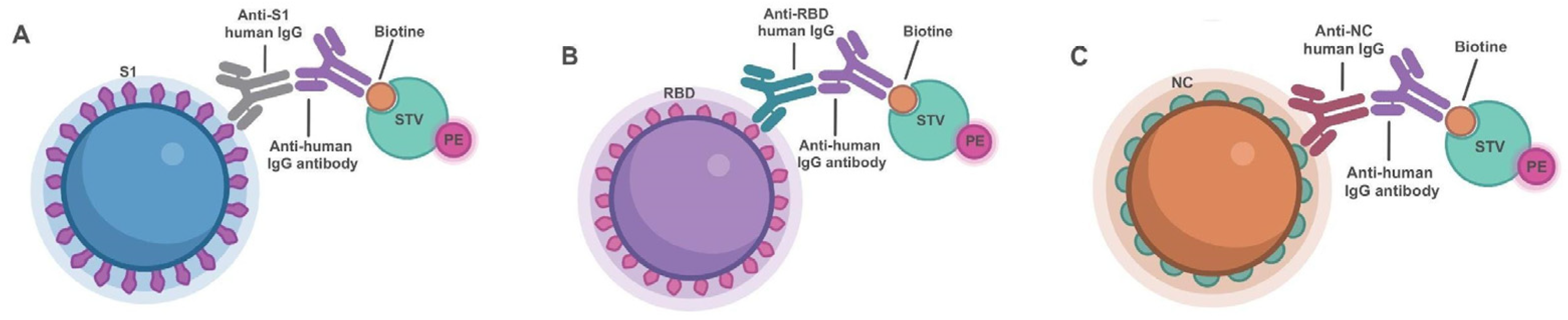
2.3. Multiplex Immunoassay
2.4. Statistical Analysis
3. Results
3.1. Multiplex Immunoassay Detects Anti-SARS-CoV-2 S1, RBD and NC IgG Antibodies with a High Specificity and Sensitivity
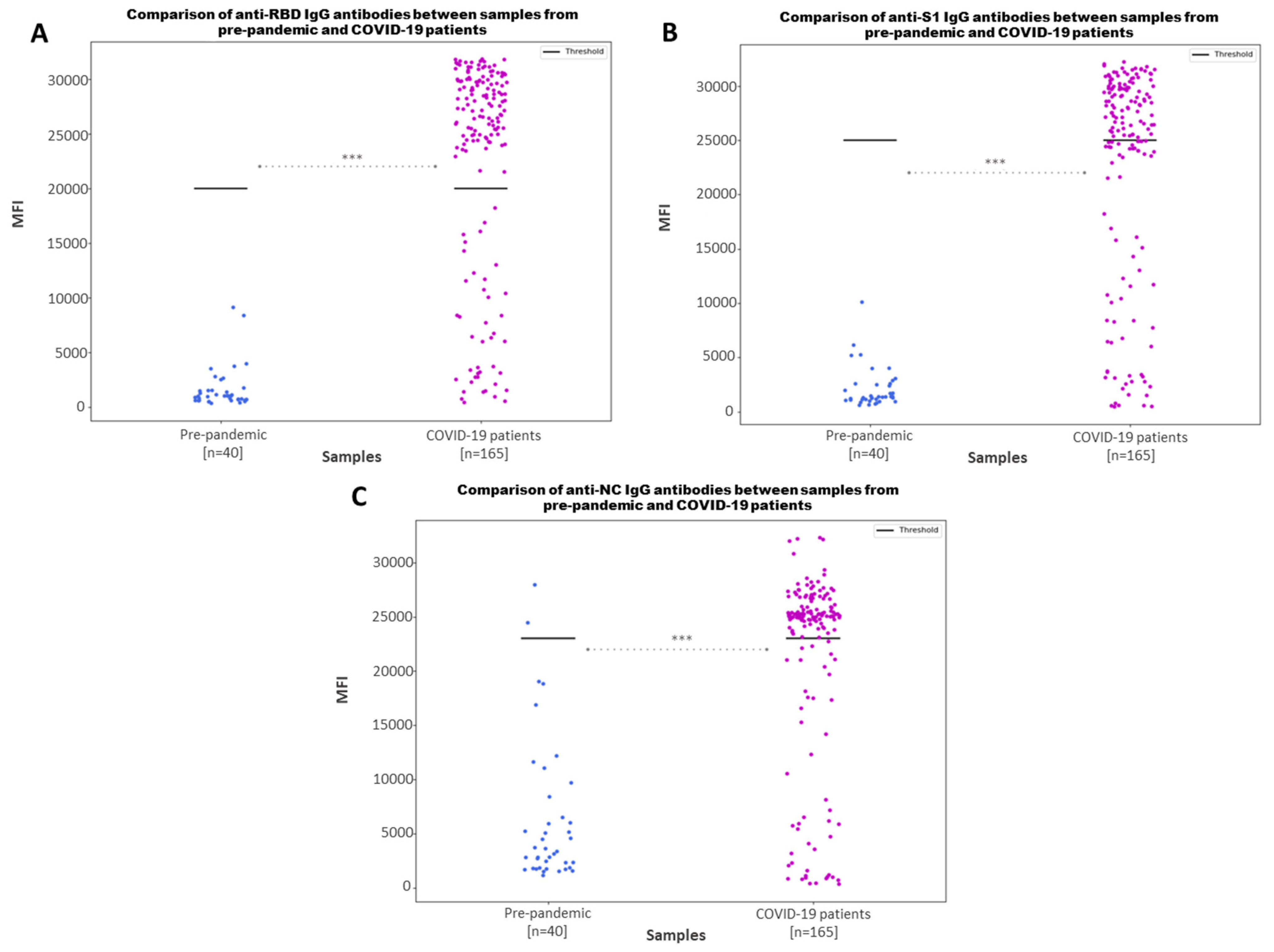
3.2. Multiplex Immunoassay Can Discriminate between Positive and Negative Samples
3.3. Comparison between Anti-S1, Anti-RBD and Anti-NC IgG Antibody Values by Different Clinical Variables
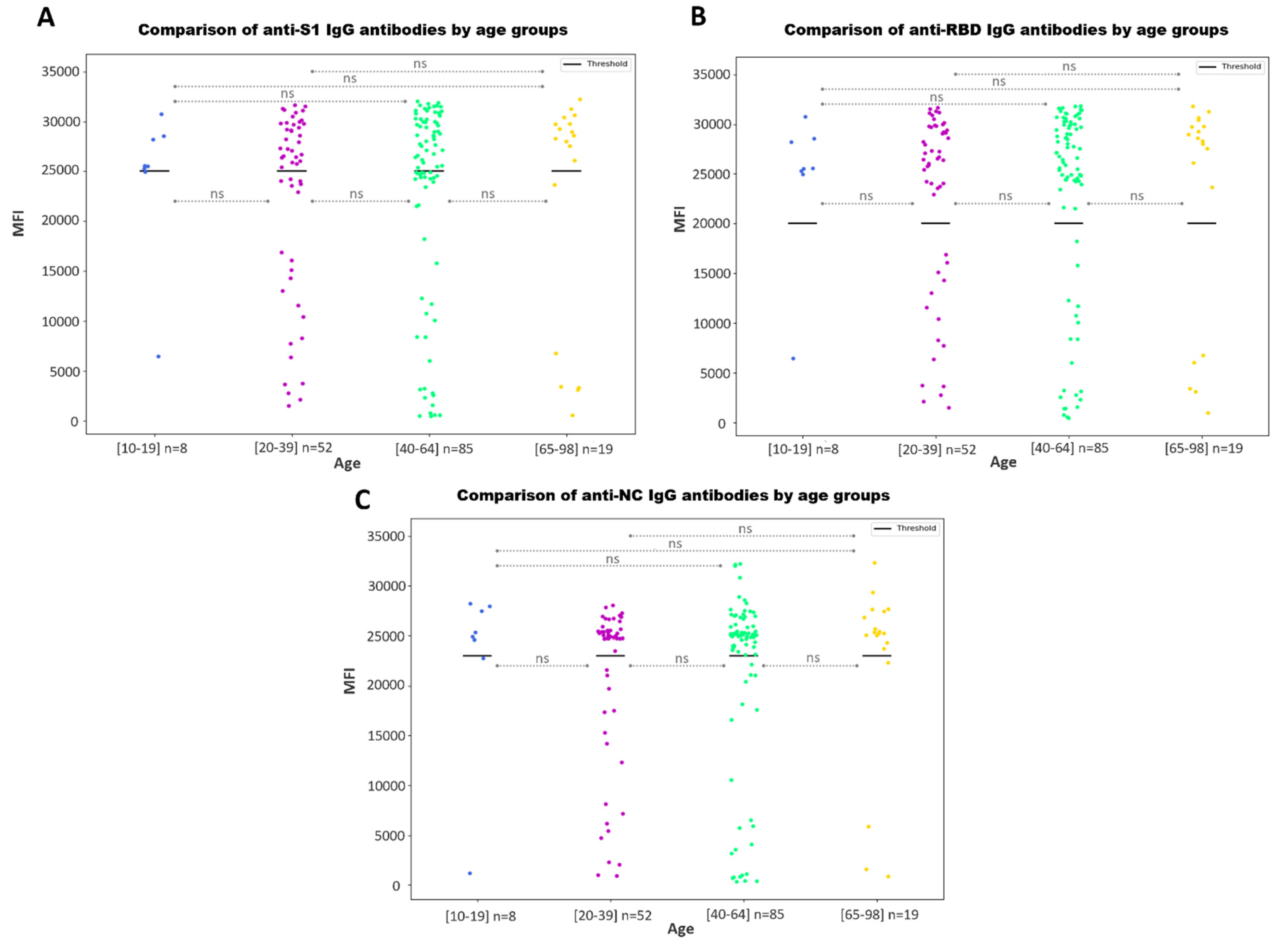
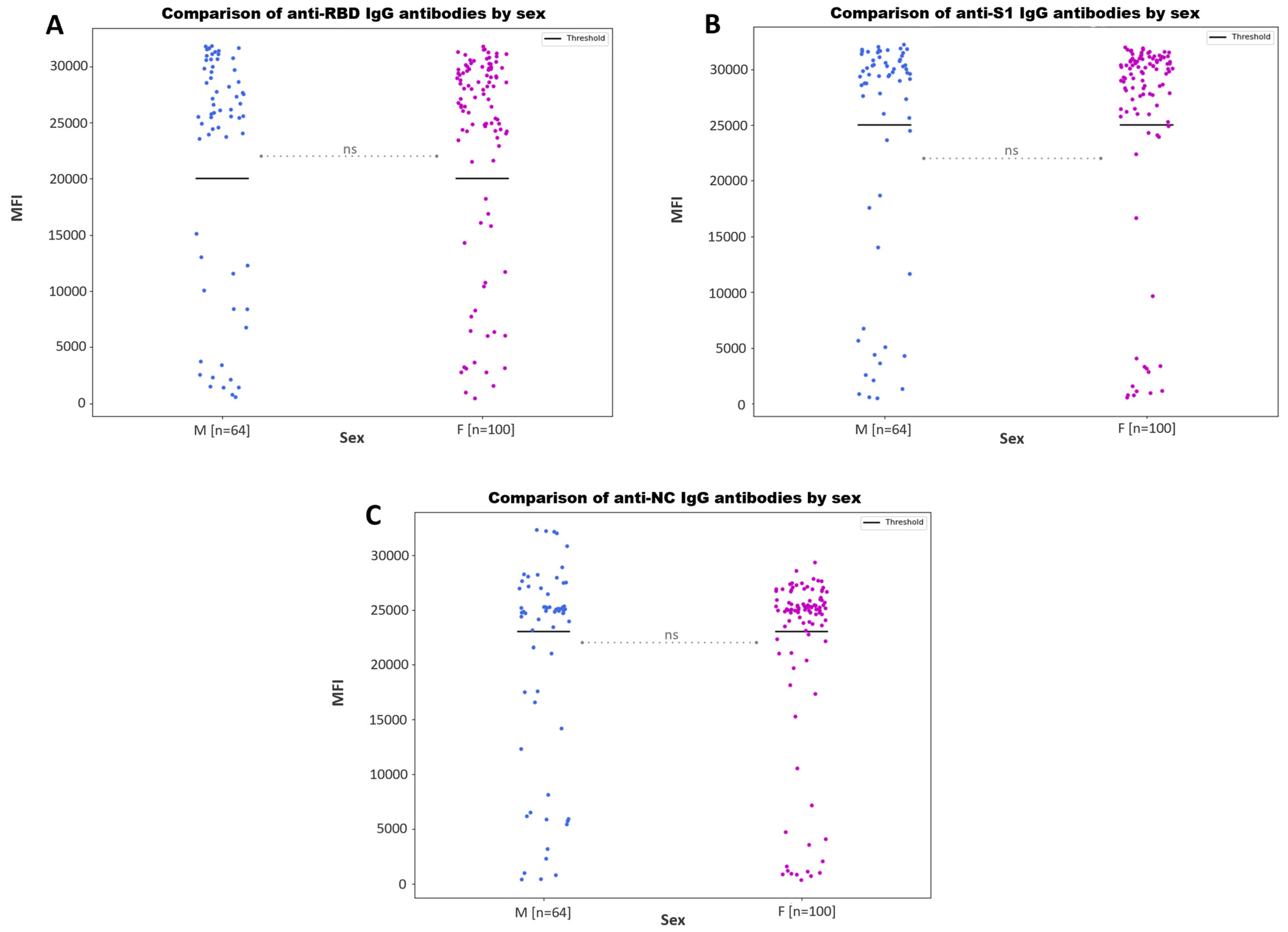
3.3.1. Antibody Responses by Age and Sex
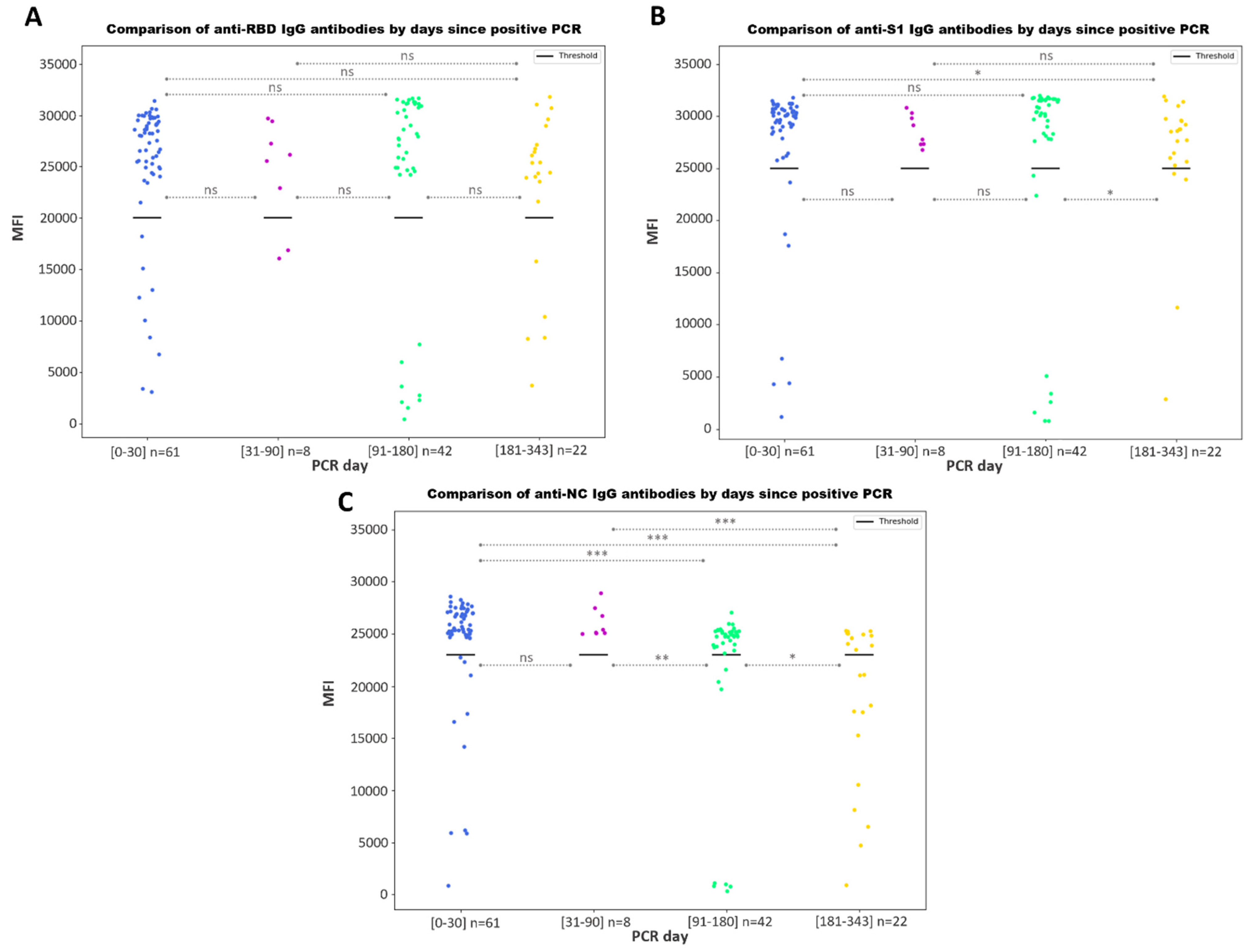
3.3.2. Antibody Responses by Days since a Positive PCR
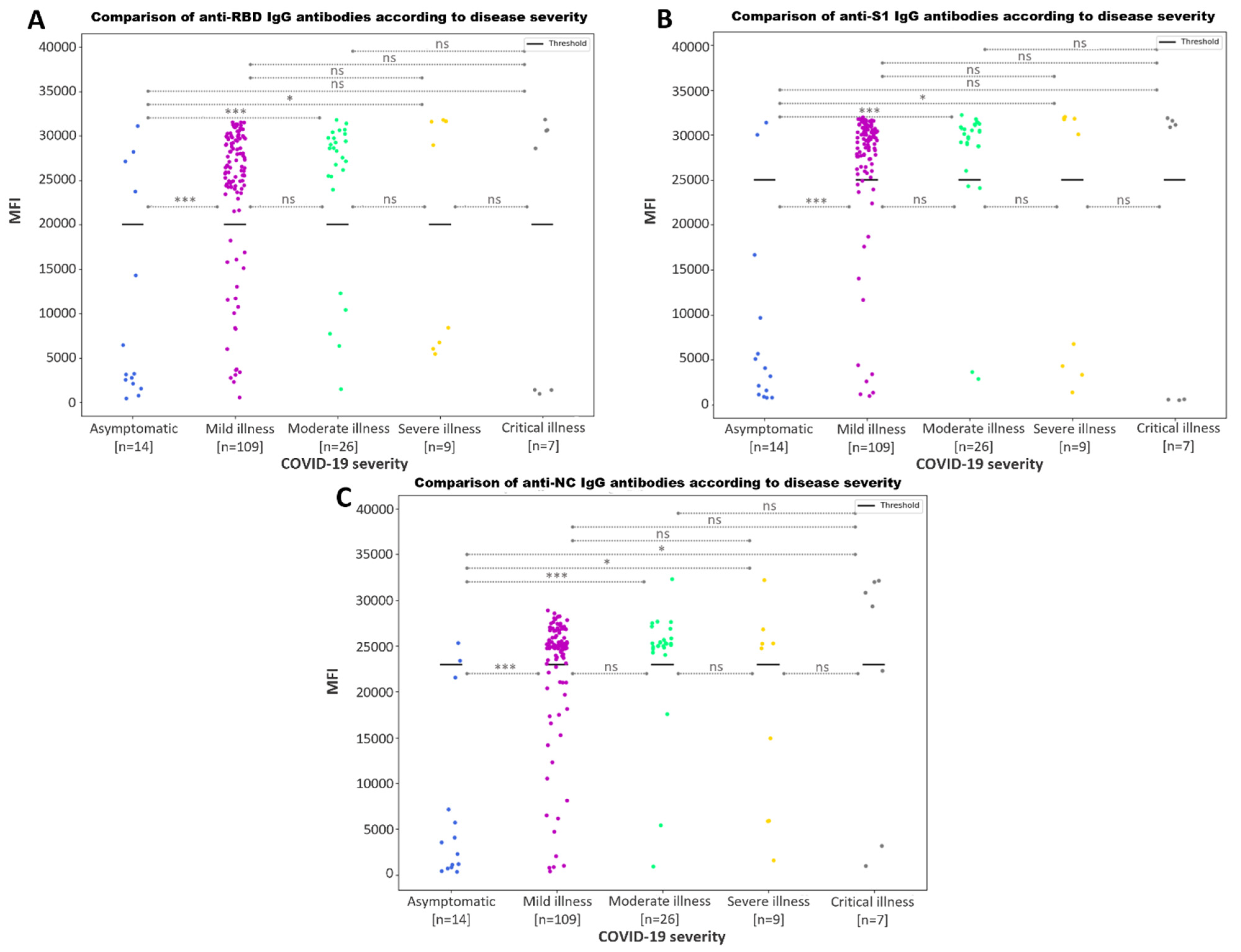
3.3.3. Association between Increased SARS-CoV-2 IgG Antibody Response and Disease Severity
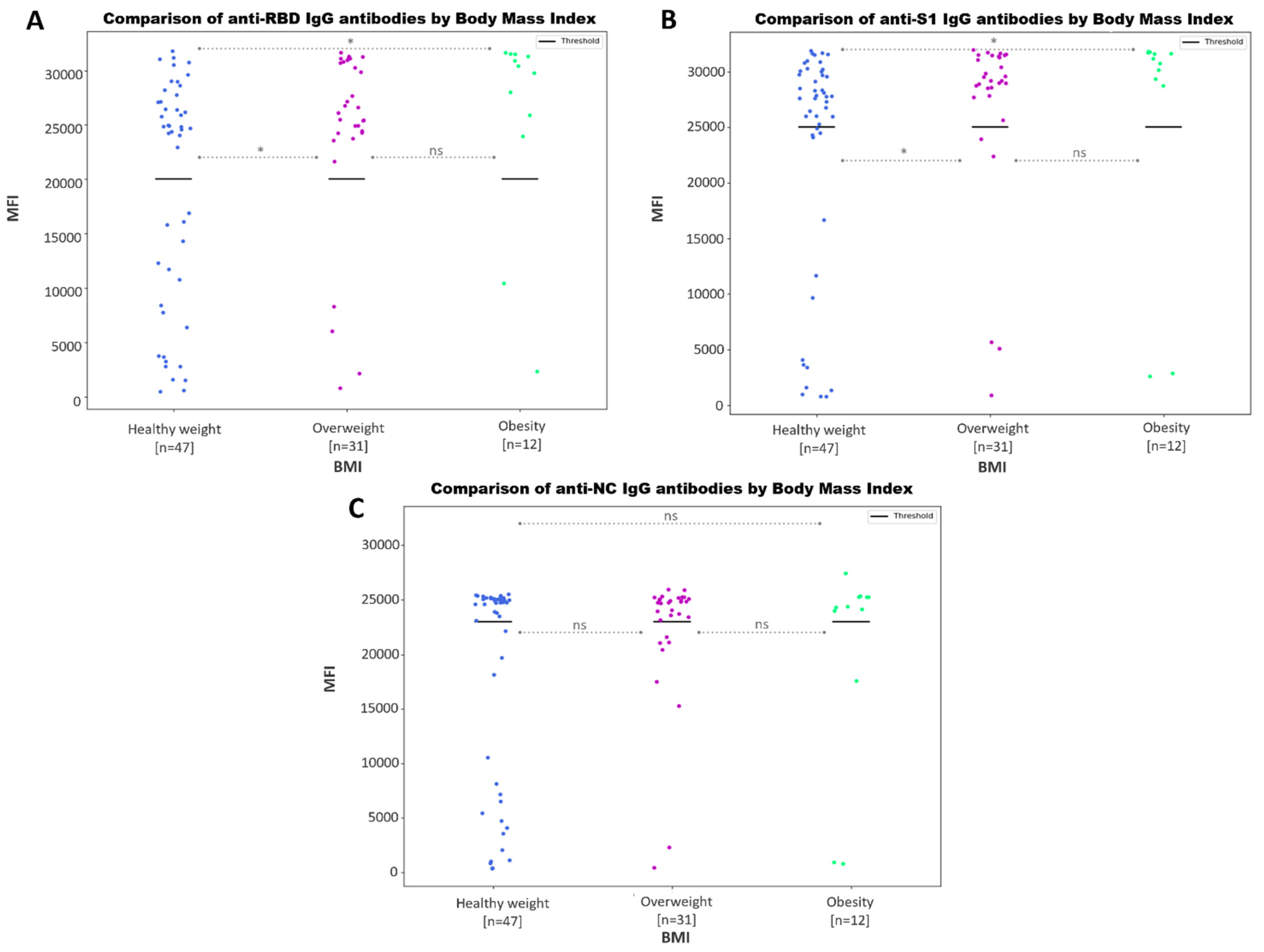
3.3.4. Negative Association between SARS-CoV-2 Antibody Values and Body Mass Index
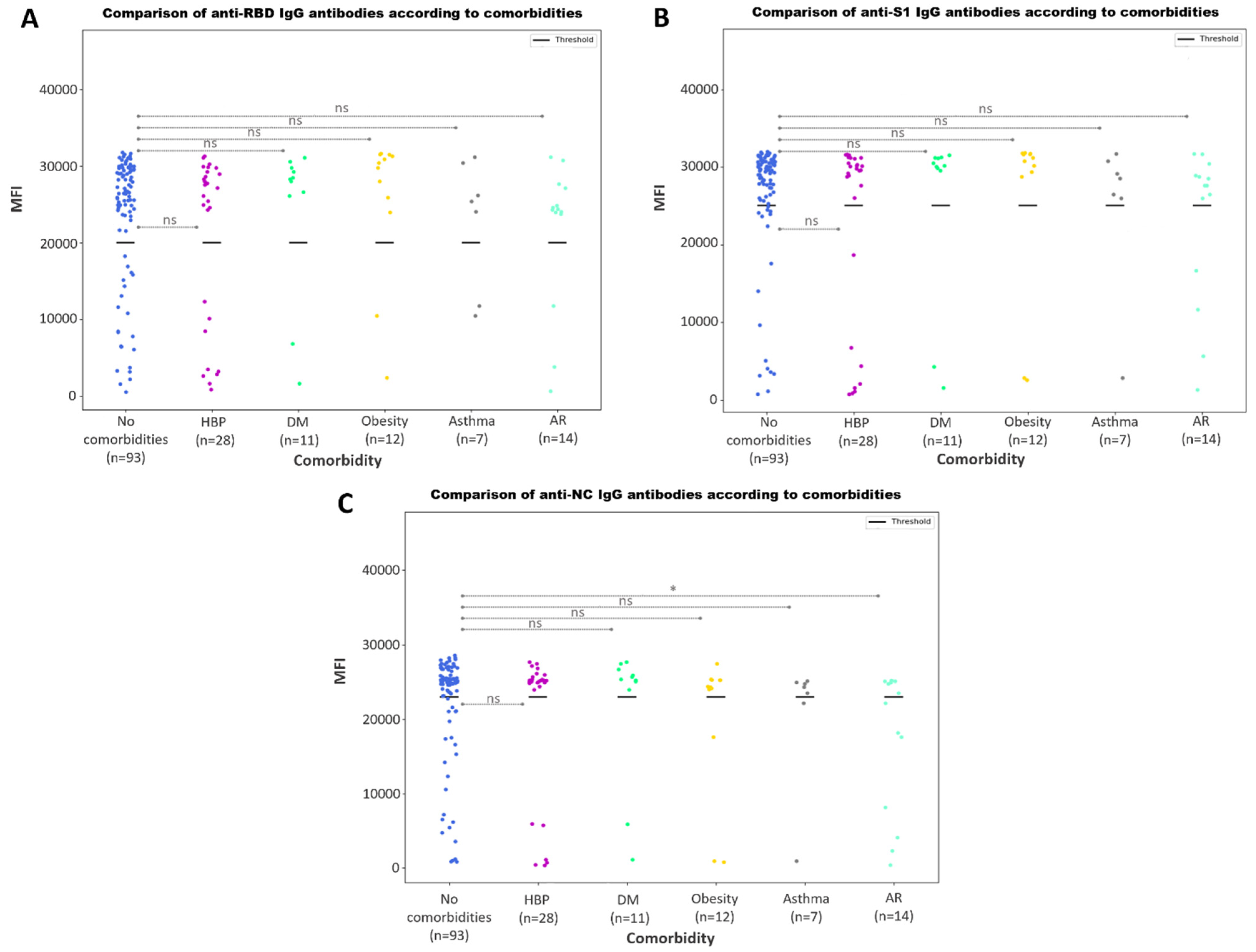
3.3.5. Antibody Responses According to Comorbidities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from Pandemic Response to Control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 Detection: A Comprehensive Review of the FDA-EUA COVID-19 Testing Landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. In Vitro Diagnostics EUAs. 2022. Available online: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas (accessed on 30 October 2023).
- Mathur, G.; Mathur, S. Antibody Testing for COVID-19 Can It Be Used as a Screening Tool in Areas With Low Prevalence? Am. J. Clin. Pathol. 2020, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.K.; Lippi, G.; Horvath, A.; Sethi, S.; Koch, D.; Ferrari, M.; Wang, C.-B.; Mancini, N.; Steele, S.; Adeli, K. Molecular, Serological, and Biochemical Diagnosis and Monitoring of COVID-19: IFCC Taskforce Evaluation of the Latest Evidence. Clin. Chem. Lab. Med. 2020, 58, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef]
- Chambers, J.P.; Yu, J.; Valdes, J.J.; Arulanandam, B.P. SARS-CoV-2, Early Entry Events. J. Pathog. 2020, 2020, 9238696. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sens. 2021, 6, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The Humoral Response and Antibodies against SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; Lindsley, A.; Courter, J.; Assa’ad, A. Clinical Testing for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J.; et al. Seasonal Human Coronavirus Antibodies Are Boosted upon SARS-CoV-2 Infection but Not Associated with Protection. Cell 2021, 184, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Surenaud, M.; Manier, C.; Richert, L.; Thiébaut, R.; Levy, Y.; Hue, S.; Lacabaratz, C. Optimization and Evaluation of Luminex Performance with Supernatants of Antigen-Stimulated Peripheral Blood Mononuclear Cells. BMC Immunol. 2016, 17, 44. [Google Scholar] [CrossRef]
- Breen, E.J.; Tan, W.; Khan, A. The Statistical Value of Raw Fluorescence Signal in Luminex xMAP Based Multiplex Immunoassays. Sci. Rep. 2016, 6, 26996. [Google Scholar] [CrossRef]
- Narasimhan, M.; Mahimainathan, L.; Araj, E.; Clark, A.E.; Markantonis, J.; Green, A.; Xu, J.; SoRelle, J.A.; Alexis, C.; Fankhauser, K.; et al. Clinical Evaluation of the Abbott Alinity SARS-CoV-2 Spike-Specific Quantitative IgG and IgM Assays among Infected, Recovered, and Vaccinated Groups. J. Clin. Microbiol. 2021, 59, e00388-21. [Google Scholar] [CrossRef]
- Torretta, S.; Zuccotti, G.; Cristofaro, V.; Ettori, J.; Solimeno, L.; Battilocchi, L.; D’Onghia, A.; Bonsembiante, A.; Pignataro, L.; Marchisio, P.; et al. Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature. Ear Nose Throat J. 2021, 100, 131S–138S. [Google Scholar] [CrossRef] [PubMed]
- Sule, W.F.; Oluwayelu, D.O. Real-Time RT-PCR for COVID-19 Diagnosis: Challenges and Prospects. Pan Afr. Med. J. 2020, 35, 121. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J.T. Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Testing: Important but Imperfect. Clin. Infect. Dis. 2021, 73, E3074–E3076. [Google Scholar] [CrossRef]
- Smerczak, E. SARS-CoV-2 Antibody Testing: Where Are We Now? Lab. Med. 2022, 53, E19–E29. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef]
- Knezevic, I.; Mattiuzzo, G.; Page, M.; Minor, P.; Griffiths, E.; Nuebling, M.; Moorthy, V. WHO International Standard for Evaluation of the Antibody Response to COVID-19 Vaccines: Call for Urgent Action by the Scientific Community. Lancet Microbe 2022, 3, E235–E240. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing Monoclonal Antibodies for Treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Dhawan, M.; Priyanka; Parmar, M.; Angural, S.; Choudhary, O.P. Convalescent Plasma Therapy against the Emerging SARS-CoV-2 Variants: Delineation of the Potentialities and Risks. Int. J. Surg. 2022, 97, 106204. [Google Scholar] [CrossRef]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What Defines an Efficacious COVID-19 Vaccine? A Review of the Challenges Assessing the Clinical Efficacy of Vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Bai, Z.; Cao, Y.; Liu, W.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef]
- Rak, A.; Donina, S.; Zabrodskaya, Y.; Rudenko, L.; Isakova-Sivak, I. Cross-Reactivity of SARS-CoV-2 Nucleocapsid-Binding Antibodies and Its Implication for COVID-19 Serology Tests. Viruses 2022, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Westphal, T.; Mader, M.; Karsten, H.; Cords, L.; Knapp, M.; Schulte, S.; Hermanussen, L.; Peine, S.; Ditt, V.; Grifoni, A.; et al. Evidence for Broad Cross-Reactivity of the SARS-CoV-2 NSP12-Directed CD4+ T-Cell Response with Pre-Primed Responses Directed against Common Cold Coronaviruses. Front. Immunol. 2023, 14, 1182504. [Google Scholar] [CrossRef] [PubMed]
- Ayouba, A.; Thaurignac, G.; Morquin, D.; Tuaillon, E.; Raulino, R.; Nkuba, A.; Lacroix, A.; Vidal, N.; Foulongne, V.; Le Moing, V.; et al. Multiplex Detection and Dynamics of IgG Antibodies to SARS-CoV2 and the Highly Pathogenic Human Coronaviruses SARS-CoV and MERS-CoV. J. Clin. Virol. 2020, 129, 104521. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Xu, L.; Hegazy, S.; Wheeler, B.J.; Anderson, A.R.; Critelli, N.; Yost, M.; McElroy, A.K.; Shurin, M.R.; Wheeler, S.E. Multiplex Assessment of SARS-CoV-2 Antibodies Improves Assay Sensitivity and Correlation with Neutralizing Antibodies. Clin. Biochem. 2021, 97, 54–61. [Google Scholar] [CrossRef]
- den Hartog, G.; Schepp, R.M.; Kuijer, M.; GeurtsvanKessel, C.; van Beek, J.; Rots, N.; Koopmans, M.P.G.; van der Klis, F.R.M.; van Binnendijk, R.S. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef]
- Karachaliou, M.; Moncunill, G.; Espinosa, A.; Castaño-Vinyals, G.; Jiménez, A.; Vidal, M.; Santano, R.; Barrios, D.; Puyol, L.; Carreras, A.; et al. Infection Induced SARS-CoV-2 Seroprevalence and Heterogeneity of Antibody Responses in a General Population Cohort Study in Catalonia Spain. Sci. Rep. 2021, 11, 21571. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 Affects Women Less than Men: Clinical Response to Viral Infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar] [CrossRef]
- Garcia-Basteiro, A.L.; Moncunill, G.; Tortajada, M.; Vidal, M.; Guinovart, C.; Jiménez, A.; Santano, R.; Sanz, S.; Méndez, S.; Llupià, A.; et al. Seroprevalence of Antibodies against SARS-CoV-2 among Health Care Workers in a Large Spanish Reference Hospital. Nat. Commun. 2020, 11, 3500. [Google Scholar] [CrossRef]
- Yousefi, Z.; Taheri, N.; Dargahi, M.; Chaman, R.; Binesh, E.; Emamian, M.H.; Jafari, R. Long-Term Persistence of Anti-SARS-COV-2 IgG Antibodies. Curr. Microbiol. 2022, 79, 96. [Google Scholar] [CrossRef]
- He, Z.; Ren, L.; Yang, J.; Guo, L.; Feng, L.; Ma, C.; Wang, X.; Leng, Z.; Tong, X.; Zhou, W.; et al. Seroprevalence and Humoral Immune Durability of Anti-SARS-CoV-2 Antibodies in Wuhan, China: A Longitudinal, Population-Level, Cross-Sectional Study. Lancet 2021, 397, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.R.; Da Silva Torres, M.K.; Lima, S.S.; De Sarges, K.M.L.; Santos, E.F.D.; De Brito, M.T.F.M.; Da Silva, A.L.S.; De Meira Leite, M.; Da Costa, F.P.; Cantanhede, M.H.D.; et al. Antibody Response to the SARS-CoV-2 Spike and Nucleocapsid Proteins in Patients with Different COVID-19 Clinical Profiles. Viruses 2023, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Chen, Z.; Zheng, P.; Li, L.; Zhuo, J.; Li, F.; Li, S.; Chen, D.; Wen, C.; Cai, W.; et al. Detection of Anti-SARS-CoV-2-S2 IgG Is More Sensitive Than Anti-RBD IgG in Identifying Asymptomatic COVID-19 Patients. Front. Immunol. 2021, 12, 724763. [Google Scholar] [CrossRef] [PubMed]
- Legros, V.; Denolly, S.; Vogrig, M.; Boson, B.; Siret, E.; Rigaill, J.; Pillet, S.; Grattard, F.; Gonzalo, S.; Verhoeven, P.; et al. A Longitudinal Study of SARS-CoV-2-Infected Patients Reveals a High Correlation between Neutralizing Antibodies and COVID-19 Severity. Cell Mol. Immunol. 2021, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.-H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing Antibody Titres in SARS-CoV-2 Infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef]
- Racine-Brzostek, S.E.; Yang, H.S.; Jack, G.A.; Chen, Z.; Chadburn, A.; Ketas, T.J.; Francomano, E.; Klasse, P.J.; Moore, J.P.; McDonough, K.A.; et al. Postconvalescent SARS-CoV-2 IgG and Neutralizing Antibodies Are Elevated in Individuals with Poor Metabolic Health. J. Clin. Endocrinol. Metab. 2021, 106, e2025–e2034. [Google Scholar] [CrossRef]
- Lobstein, T. COVID-19 and Obesity: The 2021 Atlas; World Obesity Federation: London, UK, 2021. [Google Scholar]
- Xu, C.; Zhao, H.; Song, Y.; Zhou, J.; Wu, T.; Qiu, J.; Wang, J.; Song, X.; Sun, Y. The Association between Allergic Rhinitis and COVID-19: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 6510332. [Google Scholar] [CrossRef]
- Troyano-Hernáez, P.; Reinosa, R.; Holguín, Á. Evolution of SARS-CoV-2 in Spain during the First Two Years of the Pandemic: Circulating Variants, Amino Acid Conservation, and Genetic Variability in Structural, Non-Structural, and Accessory Proteins. Int. J. Mol. Sci. 2022, 23, 6394. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera-Collantes, L.; Moares, N.; Fernandez-Cisnal, R.; Muñoz-Miranda, J.P.; Gonzalez-Garcia, P.; Gabucio, A.; Freyre-Carrillo, C.; Jordan-Chaves, J.d.D.; Trujillo-Soto, T.; Rodriguez-Martinez, M.P.; et al. Development and Validation of a Highly Sensitive Multiplex Immunoassay for SARS-CoV-2 Humoral Response Monitorization: A Study of the Antibody Response in COVID-19 Patients with Different Clinical Profiles during the First and Second Waves in Cadiz, Spain. Microorganisms 2023, 11, 2997. https://doi.org/10.3390/microorganisms11122997
Olvera-Collantes L, Moares N, Fernandez-Cisnal R, Muñoz-Miranda JP, Gonzalez-Garcia P, Gabucio A, Freyre-Carrillo C, Jordan-Chaves JdD, Trujillo-Soto T, Rodriguez-Martinez MP, et al. Development and Validation of a Highly Sensitive Multiplex Immunoassay for SARS-CoV-2 Humoral Response Monitorization: A Study of the Antibody Response in COVID-19 Patients with Different Clinical Profiles during the First and Second Waves in Cadiz, Spain. Microorganisms. 2023; 11(12):2997. https://doi.org/10.3390/microorganisms11122997
Chicago/Turabian StyleOlvera-Collantes, Lucia, Noelia Moares, Ricardo Fernandez-Cisnal, Juan P. Muñoz-Miranda, Pablo Gonzalez-Garcia, Antonio Gabucio, Carolina Freyre-Carrillo, Juan de Dios Jordan-Chaves, Teresa Trujillo-Soto, Maria P. Rodriguez-Martinez, and et al. 2023. "Development and Validation of a Highly Sensitive Multiplex Immunoassay for SARS-CoV-2 Humoral Response Monitorization: A Study of the Antibody Response in COVID-19 Patients with Different Clinical Profiles during the First and Second Waves in Cadiz, Spain" Microorganisms 11, no. 12: 2997. https://doi.org/10.3390/microorganisms11122997
APA StyleOlvera-Collantes, L., Moares, N., Fernandez-Cisnal, R., Muñoz-Miranda, J. P., Gonzalez-Garcia, P., Gabucio, A., Freyre-Carrillo, C., Jordan-Chaves, J. d. D., Trujillo-Soto, T., Rodriguez-Martinez, M. P., Martin-Rubio, M. I., Escuer, E., Rodriguez-Iglesias, M., Fernandez-Ponce, C., & Garcia-Cozar, F. (2023). Development and Validation of a Highly Sensitive Multiplex Immunoassay for SARS-CoV-2 Humoral Response Monitorization: A Study of the Antibody Response in COVID-19 Patients with Different Clinical Profiles during the First and Second Waves in Cadiz, Spain. Microorganisms, 11(12), 2997. https://doi.org/10.3390/microorganisms11122997





