The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Inoculum Preparation
2.2. Plant Materials, Experimental Design, and Growth Conditions
2.3. Phytohormone Extraction and Quantification in Wheat Seedlings and in the Liquid Culture Medium of Bacteria
2.4. Assessment of Lignin Deposition in Roots
2.5. Measurement of Lipid Peroxidation (LPO) and Electrolyte Leakage (EL)
2.6. Statistical Analysis
3. Results
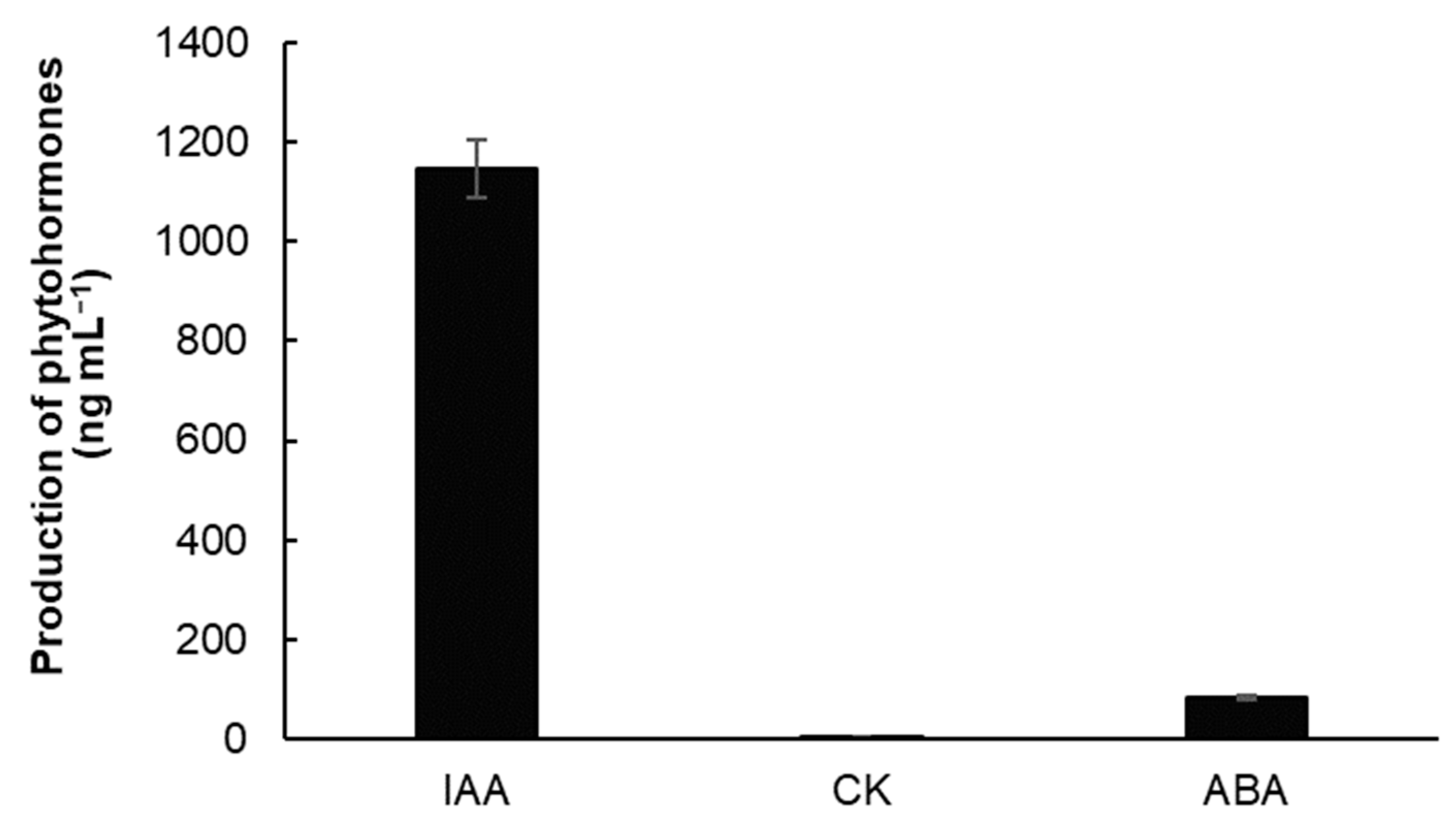
3.1. The Ability of Strain Bacillus subtilis 10-4 (BS) to Produce Phytohormones Indole-3-Acetic Acid (IAA), Cytokinins (CK), and Abscisic Acid (ABA)
3.2. The Growth Attributes (Length, Biomass, Leaf Chlorophyll) of Endophyte-Primed Hydroponically Grown Wheat Seedlings under Normal and Stress Conditions
3.3. Endogenous Phytohormones Abscisic Acid (ABA), Indole-3-Acetic Acid (IAA), and Cytokinins (CK) Concentrations in Endophyte-Primed Seedlings under Normal and Osmotic Stress Conditions
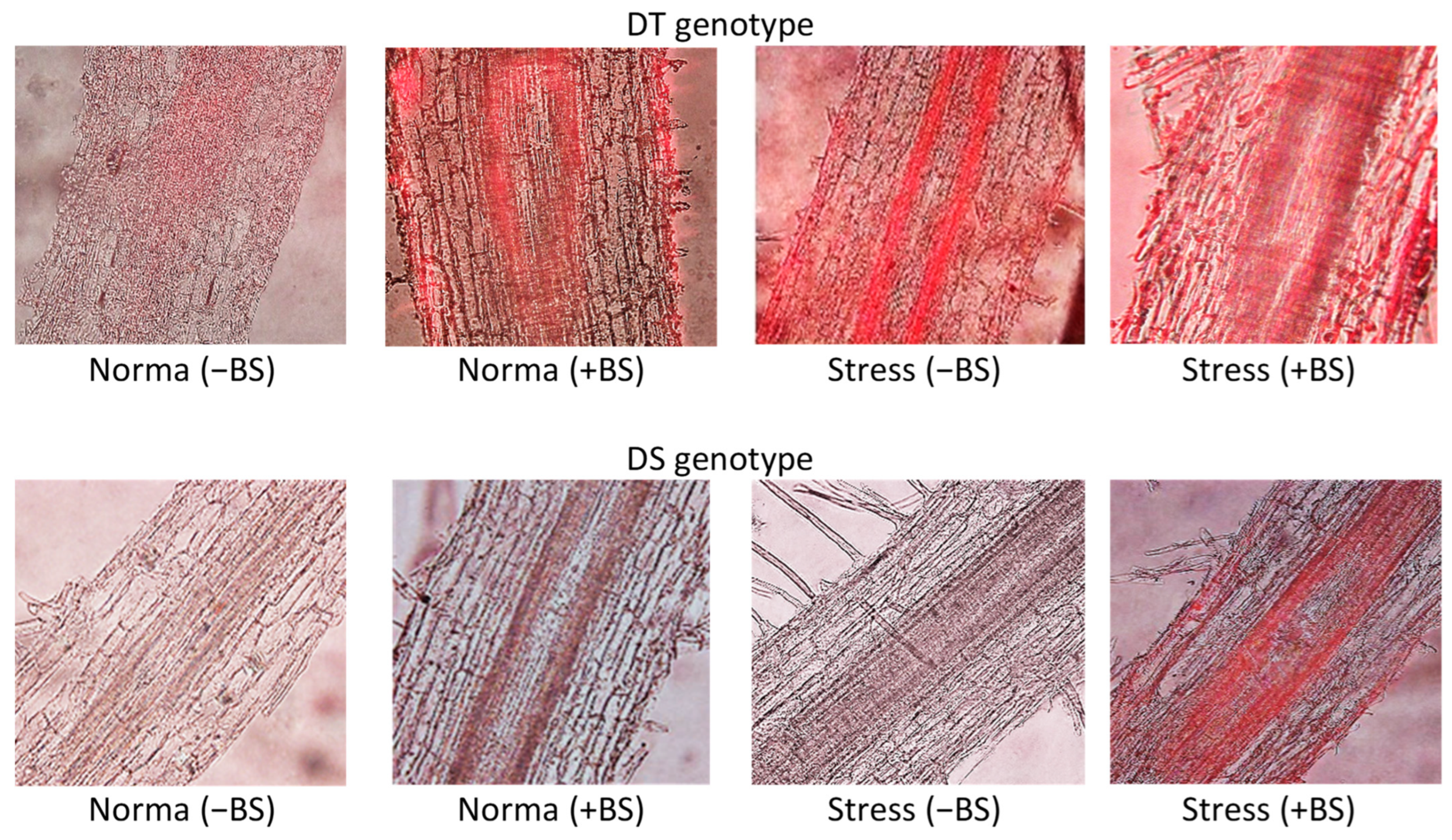
3.4. Lignin Accumalation in Roots of Endophyte-Primed Seedlings under Normal and Osmotic Stress Conditions
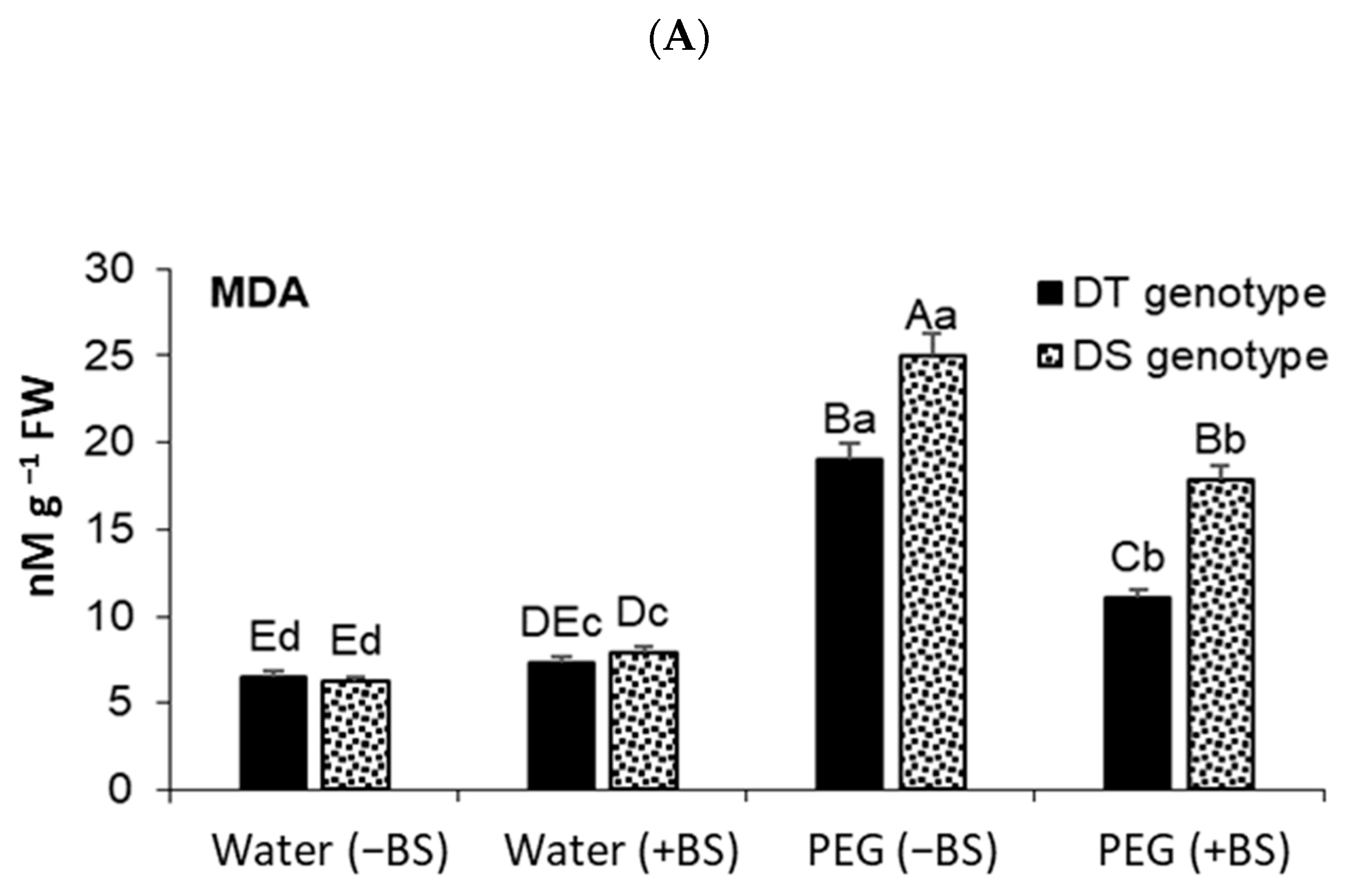
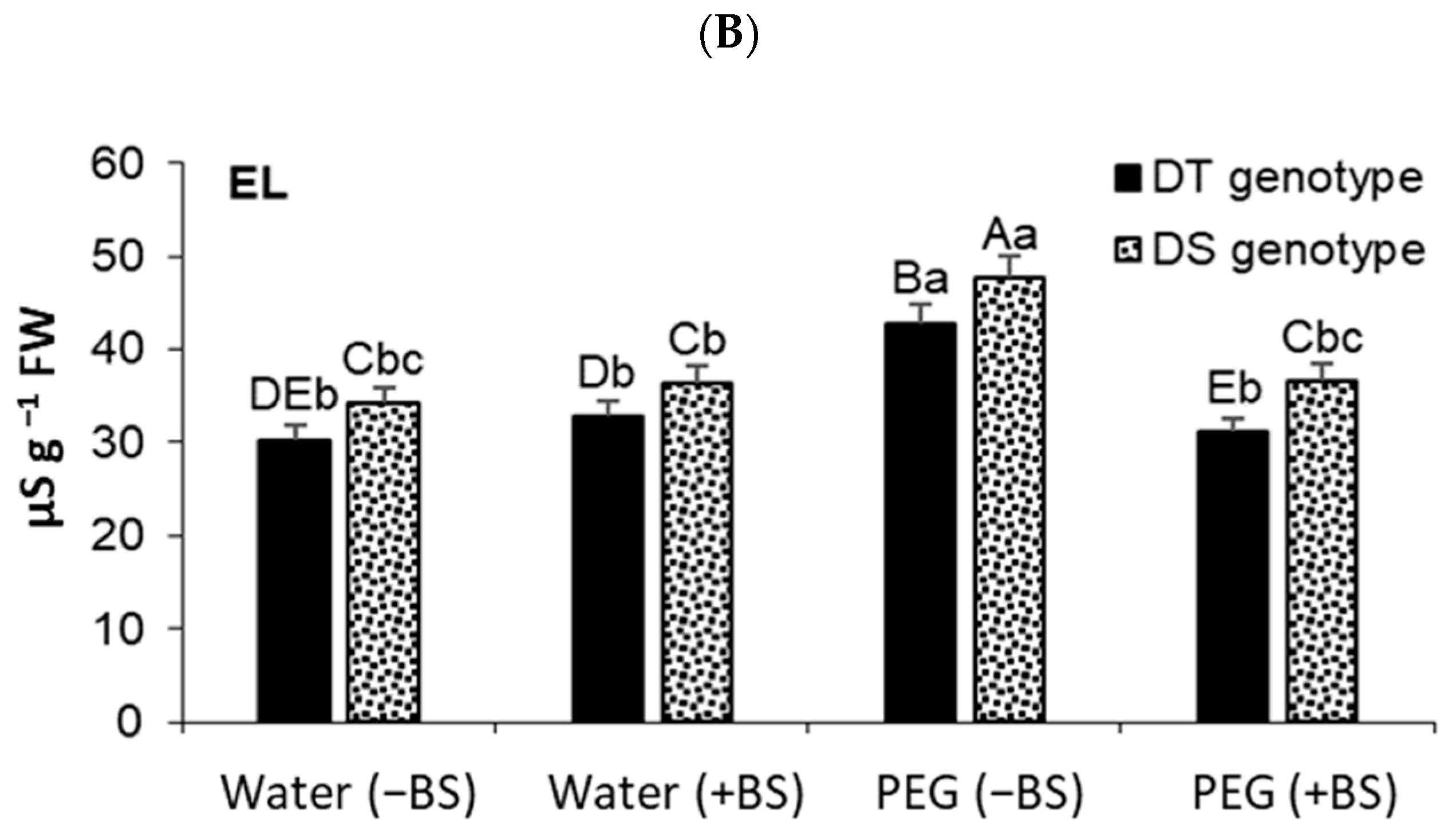
3.5. Lipid Peroxidation (LPO) and Elektrolyte Leakage (EL) Degree in Endophyte-Primed Seedlings under Osmotic Stress
3.6. Correlation Matrices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Ali Babar, M.; et al. Climate Change Impact and Adaptation for Wheat Protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought Response in Winter Wheat: Protection from Oxidative Stress and Mutagenesis Effect. Acta Physiol. Plant. 2021, 43, 1–11. [Google Scholar] [CrossRef]
- FAO. Cereal Supply and Demand Brief. 2021. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 21 March 2023).
- Altaf, A.; Shah, A.Z.; Gull, S.; Hussain, S.; Faheem, M.; Zada, A.; Saeed, A.; Miah, A.A.A.; Zhu, M.; Zhu, X. Progress in Modern Crop Science Research in Wheat Biology. J. Glob. Innov. Agric. Sci. 2022, 10, 43–49. [Google Scholar] [CrossRef]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting Desert Plant Bacillus Endophytic Strains for Their Potential to Enhance Plant Stress Tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Kudoyarova, G.R. Role of Endogenous Hormonal System in the Realization of the Antistress Action of Plant Growth Regulators on Plants. Plant Stress 2010, 4, 32–38. [Google Scholar]
- Shakirova, F.; Avalbaev, A.; Bezrukova, M.; Fatkhutdinova, R.; Maslennikova, D.; Yuldashev, R.; Allagulova, C.; Lastochkina, O. Hormonal Intermediates in the Protective Action of Exogenous Phytohormones in Wheat Plants Under Salinity. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N., Nazar, R., Iqbal, N., Anjum, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–228. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as Go-Betweens in Plant Microbiome Assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop J. 2020, CJ-00492, 13. [Google Scholar] [CrossRef]
- Casaretto, J.A.; El-kereamy, A.; Zeng, B.; Stiegelmeyer, S.M.; Chen, X.; Bi, Y.M.; Rothstein, S.J. Expression of OsMYB55 in Maize Activates Stress-Responsive Genes and Enhances Heat and Drought Tolerance. BMC Genom. 2016, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Hausman, J.F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Rao, X.; Li, L.; Dixon, R.A. Abscisic Acid Regulates Secondary Cell-Wall Formation and Lignin Deposition in Arabidopsis thaliana Through Phosphorylation of NST1. Proc. Nat. Acad. Sci. USA 2021, 118, e2010911118. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of Lignin Biosynthesis for Drought Tolerance in Plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur, N.; Marothia, D.; Singh, B.; Singh, V.; Gantet, P.; Pati, P.K. Morphological Analysis, Protein Profiling and Expression Analysis of Auxin Homeostasis Genes of Roots of Two Contrasting Cultivars of Rice Provide Inputs on Mechanisms Involved in Rice Adaptation towards Salinity Stress. Plants 2021, 10, 1544. [Google Scholar] [CrossRef]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ghorai, M.; Anand, U.; Roy, D.; Kant, N.; Mishra, T.; Mane, A.B.; Jha, N.K.; Lal, M.K.; Tiwari, R.K.; et al. Cytokinins: A Genetic Target for Increasing Yield Potential in The CRISPR Era. Front. Genet. 2022, 13, 883930. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple Levels of Crosstalk in Hormone Networks Regulating Plant Defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Sharma, M. Significance of Inoculation with Bacillus subtilis to Alleviate Drought Stress in Wheat (Triticum aestivum L.). Vegetos 2020, 33, 782–792. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Belabess, Z.; Jiang, Y.; Mokrini, F.; Peng, G. Bacillus spp.-Mediated Drought Stress Tolerance in Plants: Current and Future Prospects. In Bacilli in Agrobiotechnology. Bacilli in Climate Resilient Agriculture and Bioprospecting; Islam, M.T., Rahman, M., Pandey, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 487–518. [Google Scholar] [CrossRef]
- Azeem, M.; Javed, S.; Zahoor, A.F. Bacillus Species as Potential Plant Growth Promoting Rhizobacteria for Drought Stress Resilience. Russ. J. Plant Physiol. 2023, 70, 59. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Kalhor, M.S.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant Growth Promoting Bacteria Biotic Strategy to Cope with Abiotic Stresses in Wheat. In Wheat Production in Changing Environments: Management, Adaptation and Tolerance; Hasanuzzaman, M., Nahar, K., Hossain, A., Eds.; Springer: Singapore, 2019; pp. 579–614. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved Heat Stress Tolerance of Wheat Seedlings by Bacterial Seed treatment. Plant Soil. 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus velezensis 5113 Induced Metabolic and Molecular Reprogramming during Abiotic Stress Tolerance in Wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium Isolated from Semi-Arid Conditions Induces Systemic Tolerance of Wheat under Drought Conditions. Plant Cell Rep. 2021, 41, 549–569. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á. Molecular Aspects of Plant Growth Promotion and Protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.V. Adaptation and Tolerance of Wheat Plants to Drought Mediated by Natural Growth Regulators Bacillus spp.: Mechanisms and Practical Importance (review). Agric. Biol. 2021, 56, 843–867. [Google Scholar] [CrossRef]
- Lastochkina, O.V.; Allagulova, C.R. Mechanisms of Growth Promotional and Protective Effects of Endophytic PGP-Bacteria in Wheat Plants under the Impact of Drought (Review). Appl. Biochem. Microbiol. 2023, 59, 14–32. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Kanchana, D.; Usharani, G.; Saranraj, P. Production of Plant Growth Promoting Substance by Pseudomonas fluorescens and Bacillus subtilis isolated from paddy rhizosphere soil of Cuddalore district, Tamil Nadu, India. Int. J. Microbiol. Res. 2013, 4, 227–233. [Google Scholar] [CrossRef]
- Ishak, Z.; Mohd Iswadi, M.K.; Russman Nizam, A.H.; Ahmad Kamil, M.J.; Ernie Eileen, R.R.; Wan Syaidatul, A.; Ainon, H. Plant Growth Hormones Produced by Endophytic Bacillus subtilis Strain LKM-BK Isolated from Cocoa. Malaysian Cocoa J. 2016, 9, 127–133. [Google Scholar]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant Growth-Promoting Rhizobacteria Enhance Wheat Salt and Drought Stress Tolerance by Altering Endogenous Phytohormone Levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrichm, F.; Rahalkar, M.; et al. Functional Characteristics of An Endophyte Community Colo-Nizing Rice Roots as Revealed by Metagenomic Analysis. MPMI 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski-Sorbal, J.; Araujo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and Characterization of Soybean-Associated Bacteria and Their Potential for Plant Growth Promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Miljakovic, D.; Marinkovic, J.; Balesevic-Tubic, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Piccoli, P.N. Azospirillum brasilense Ameliorates the Response of Arabidopsis thaliana to Drought Mainly via Enhancement of ABA Levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of Abscisic Acid-Producing Endophytic Bacteria Enhances Salinity Stress Tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Ji, C.; Tian, H.; Wang, X.; Song, X.; Ju, R.; Li, H.; Liu, X. Bacillus subtilis HG-15, a Halotolerant Rhizoplane Bacterium, Promotes Growth and Salinity Tolerance in Wheat (Triticum aestivum). BioMed Res. Int. 2022, 2022, 9506227. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars Under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Maslennikova, D.; Lastochkina, O. Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity. Plants 2021, 10, 2557. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on Some Physiological and Biochemical Parameters of Triticum aestivum L. (wheat) Under Salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed Priming with Endophytic Bacillus subtilis Strain-Specifically Improves Growth of Phaseolus vulgaris Plants under Normal and Salinity Conditions and Exerts Anti-Stress Effect through Induced Lignin Deposition in Roots and Decreased Oxidative and Osmotic Damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M. Praktikum po Mikrobiologii (A Practical Course in Microbiology); Izdat. Tsentr Akademiya: Moscow, Russia, 2005; 608p. [Google Scholar]
- Mokronosova, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; 184p. [Google Scholar]
- Jefrey, S.; Humphrey, G. New Spectrophotometric Equations for Determining Chlorophylls a, b, c1 and c2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pfl. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the Hormonal Status of Wheat Seedlings Induced by Salicylic Acid and Salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Kildibekova, A.R.; Bezrukova, M.V.; Avalbaev, A.M. Wheat Germ Agglutinin Regulates Cell Division in Wheat Seedling Roots. Plant Growth Regul. 2004, 42, 175–180. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Veselov, S.Y.; Karavaiko, N.N.; Gyuli-zade, V.Z.; Cheredova, E.P.; Mustafina, A.R.; Moshkov, I.E.; Kulaeva, O.N. Immunoenzyme Test System for Assaying Cytokinins. Fiziol. Rast. (Sov. Plant Physiol.) 1990, 37, 193–199. [Google Scholar]
- Veselov, S.Y.; Simonyan, M.V. Immunoenzyme Analysis of Cytokinins as an Assay for Cytokinin Oxidase Activity. Russ. J. Plant Physiol. 2004, 51, 266–270. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Valcke, R.; Van Onckelen, H.; Kudoyarova, G.R. Cytokinin Content and Location in the Leaves of the Wild-Type and Transgenic Tobacco Plants. Russ. J. Plant Physiol. 1999, 46, 26–31. [Google Scholar]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin Producing Bacteria Enhance Plant Growth in Drying Soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Veselov, S.; Kudoyarova, G.; Egutkin, N.; Gyuli-Zade, V.; Mustafina, A.; Kof, E. Modified Solvent Partitioning Scheme Providing Increased Specificity and Rapidity of Immunoassay for Indole 3-Acetic Acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Furst, G.G. Methods of Anatomical and Histochemical Studies of Plants; Science: Moscow, Russia, 1979; p. 155. (In Russian) [Google Scholar]
- Sedlarova, M.; Lebeda, A. Histochemical Detection and Role of Phenolic Compounds in the Defense Response of Lactuca spp. to Lettuce Downy Mildew (Bremia lactacae). J. Phytopathol. 2001, 149, 693–697. [Google Scholar] [CrossRef]
- Health, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Kinetics And Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Maslennikova, D.R.; Avalbaev, A.M.; Fedorova, K.A.; Yuldashev, R.A.; Shakirova, F.M. Influence of 24-Epibrassinolide on Growth of Wheat Plants and The Content of Dehydrins Under Cadmium Stress. Russ. J. Plant Physiol. 2015, 62, 465–471. [Google Scholar] [CrossRef]
- Csiszár, J.; Galle, A.; Horvath, E.; Dancso, P.; Gombos, M.; Vary, Z.; Erdei, L.; Györgyey, J.; Tari, I. Different Peroxidase Activities and Expression of Abiotic Stress-Related Peroxidases in Apical Root Segments of Wheat Genotypes with Different Drought Stress Tolerance Under Osmotic Stress. Plant Physiol. Biochem. 2012, 52, 119–129. [Google Scholar] [CrossRef]
- Soares, G.F.; Ribeiro Júnior, W.Q.; Pereira, L.F.; Lima, C.A.; Soares, D.D.; Muller, O.; Rascher, U.; Ramos, M.L. Characterization of Wheat Genotypes for Drought Tolerance and Water Use Efficiency. Sci. Agric. 2021, 78, e20190304. [Google Scholar] [CrossRef]
- Ahmad, N.; Javed, A.; Gohar, S.; Ahmed, J.; Sher, A.; Abdullah, M.; Asghar, S.; Javed, K.; Iqbal, J.; Kumar, S.; et al. Estimation of Drought Effects on Different Bread Wheat Genotypes Using Morpho-Physiological Traits. Biochem. Syst. Ecol. 2022, 104, 104483. [Google Scholar] [CrossRef]
- Pandey, A.; Khobra, R.; Mamrutha, H.M.; Wadhwa, Z.; Krishnappa, G.; Singh, G.; Singh, G.P. Elucidating the Drought Responsiveness in Wheat Genotypes. Sustainability 2022, 14, 3957. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, Y.; Guo, R.; Liu, E.; Liu, X.; Gu, F.; Yang, Z.; Li, S.; Zhong, X.; et al. Wheat Genotypes with Higher Yield Sensitivity to Drought Overproduced Proline and Lost Minor Biomass Under Severer Water Stress. Front. Plant Sci. 2022, 13, 1035038. [Google Scholar] [CrossRef]
- Villa-Rodriguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; de los Santos-Villalobos, S. Bacillus subtilis TE3: A Promising Biological Control Agent Against Bipolaris Sorokiniana, The Causal Agent of Spot Blotch in Wheat (Triticum turgidum L. subsp. durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; del Carmen Orozco-Mosqueda, M.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de Los Santos-Villalobos, S.; Santoyo, G. Plant Growth-Promoting Bacterial Endophytes as Biocontrol Agents of Pre-and Post-Harvest Diseases: Fundamentals, Methods of Application and Future Perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Brambila, K.M.; Escalante-Beltrán, A.; Montoya-Martínez, A.C.; Díaz-Rodríguez, A.M.; López-Montoya, N.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Bacillus cabrialesii: Five Years of Research on a Novel Species of Biological Control and Plant Growth-Promoting Bacteria. Plants 2023, 12, 2419. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 Tolerates Oxidative and Nitrosative Stress and Promotes the Growth of Soybean by Modulating the Production of Phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of Inoculation with a Thermotolerant Plant Growth Promoting Pseudomonas putida Strain AKMP7 on Growth of Wheat (Triticum spp.) under Heat Stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of Drought Tolerance in Wheat by the Interaction of Plant Growth-Promoting Rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Nedukha, O. Tolerance of Plant Cell Wall to Environment. In Advances in Plant Defense Mechanisms; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Riaz, M.W.; Yousaf, M.I.; Hussain, Q.; Yasir, M.; Sajjad, M.; Shah, L. Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses. Stresses 2023, 3, 434–453. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Bacillus amyloliquefaciens Alters Gene Expression, ROS Production and Lignin Synthesis in Cotton Seedling Roots. J. Appl. Microbiol. 2017, 122, 1110–1120. [Google Scholar] [CrossRef]
- Khabbaz, S.E.; Ladhalakshmi, D.; Babu, M.; Kandan, A.; Ramamoorthy, V.; Saravanakumar, D.; Al-Mughrabi, T.; Kandasamy, S. Plant Growth Promoting Bacteria (PGPB)—A Versatile Tool for Plant Health Management. Can. J. Pestic. Pest Manag. 2019, 1, 1. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, And Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Reski, R. Small Molecules in The Move: Homeostasis, Crosstalk, And Molecular Action of Phytohormones. Plant Biol. 2006, 8, 277–280. [Google Scholar] [CrossRef][Green Version]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Alterations of Endogenous Hormonal Levels in Plants under Drought and Salinity. Am. J. Plant Sci. 2016, 7, 1357–1371. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic Acid Regulates Root Growth under Osmotic Stress Conditions Via an Interacting Hormonal Network with Cytokinin, Ethylene and Auxin. N. Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Rock, C.; Sakata, Y.; Quatrano, R. Stress Signaling I: The Role of Abscisic Acid (ABA). In Abiotic Stress Adaptation in Plants; Springer: Dordrecht, The Netherlands, 2009; pp. 33–73. [Google Scholar] [CrossRef]
- Salomon, M.V.; Bottini, R.; de Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria Isolated from Roots and Rhizosphere of Vitis vinifera Retard Water Losses, Induce Abscisic Acid Accumulation and Synthesis of Defense-Related Terpenes in In Vitro Cultured Grapevine. Physiol. Plant. 2014, 151, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Plant-Growth-Promoting Rhizobacteria: Drought Stress Alleviators to Ameliorate Crop Production in Drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.; Kumar, V.; Verma, R.; Upadhyay, R.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-Mediated Stomatal Response in Regulating Water Use during the Development of Terminal Drought in Wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Keskin, B.C.; Sarikaya, A.T.; Yüksel, B.; Memon, A.R. Abscisic Acid Regulated Gene Expression in Bread Wheat (Triticum aestivum L.). Aust. J. Crop Sci. 2010, 4, 617–625. [Google Scholar]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-Mediated Transcriptional Regulation in Response to Osmotic Stress in Plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
- Böhmer, M.; Schroeder, J.I. Quantitative Transcriptomic Analysis of Abscisic Acid-Induced and Reactive Oxygen Species-Dependent Expression Changes and Proteomic Profiling in Arabidopsis Suspension Cells. Plant J. 2011, 67, 105–118. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought Stress in Wheat during Flowering and Grain-filling Periods. CRC Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Muhammad, H.; Lee, I.-J. Plant Growth-Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Quarrie, S.A. Genetic Variability and Heritability of Drought-Induced Abscisic Acid Accumulation in Spring Wheat. Plant Cell Environ. 1981, 4, 147–151. [Google Scholar] [CrossRef]
- Ji, X.; Dong, B.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of Abscisic Acid Catabolism and Abscisic Acid Homeostasis Is Important for Reproductive Stage Stress Tolerance in Cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ Balance and Transport Regulatory Mechanisms in Weedy and Cultivated Rice (Oryza sativa L.) Under Salt Stress. BMC Plant Biol. 2018, 8, 375. [Google Scholar] [CrossRef]
- Barnawal, D.; Singh, R.; Singh, R.P. Role of Plant Growth Promoting Rhizobacteria in Drought Tolerance: Regulating Growth Hormones and Osmolytes. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 107–128. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128158791000069 (accessed on 23 January 2023).
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza sativa, Via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-N.; Khan, M.A.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Enhancement of Drought-Stress Tolerance of Brassica oleracea Var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic Acid Metabolizing Rhizobacteria Decrease ABA Concentrations in Planta and Alter Plant Growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought Stress Amelioration in Wheat Through Inoculation with Burkholderia phytofirmans Strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Feoktistova, A.; Timergalin, M.; Chetverikov, S.; Nazarov, A.; Kudoyarova, G. Effects on Pseudomonas plecoglossicida 2,4-D and Humic Substances on the Growth, Pigment Indices and Concentration of Hormones in Wheat Seedlings Grown under Water Deficit. Microorganisms 2023, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.Y.; Al-Qutami, M.A.; Ali, H.M.; Khan, M.N. Sodium Nitroprusside and Indole Acetic Acid Improve the Tolerance of Tomato Plants to Heat Stress by Protecting Against DNA Damage. J. Plant Interact. 2017, 12, 177–186. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a Source of Phytohormones for Use in Agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of Shape During Stress: A Key Role for Auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sikdar, S.; Tiwari, S. Application of Plant Growth Promoting Rhizobacteria (PGPR) in Crop Productivity Improvement and Sustainable Agriculture. In Agricultural Biotechnology: Latest Research and Trends; Springer: Singapore, 2021; pp. 635–660. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere Bacteria Help Plants Tolerate Abiotic Stress. Trends Plant Sci. 2009, 14, 1–14. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin Production by Rhizobacteria was Associated with Improved Yield of Wheat (Triticum aestivum L.) under Drought Stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Cox, C.E.; Brandl, M.T.; de Moraes, M.H.; Gunasekera, S.; Teplitski, M. Production of The Plant Hormone Auxin by Salmonella and its Role in The Interactions with Plants and Animals. Front. Microbiol. 2018, 8, 2668. [Google Scholar] [CrossRef]
- Meena, K.K.; Bitla, U.M.; Sorty, A.M.; Singh, D.P.; Gupta, V.K.; Wakchaure, G.C.; Kumar, S. Mitigation of Salinity Stress in Wheat Seedlings Due to The Application of Phytohormone-Rich Culture Filtrate Extract of Methylotrophic actinobacterium Nocardioides sp. NIMMe6. Front. Microbiol. 2020, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frebort, I.; Pospısilova, H.; Martınez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Lutts, S.; Dodd, I.C.; et al. Root-synthesized Cytokinins Improve Shoot Growth and Fruit Yield in Salinized Tomato (Solanum lycopersicum L.) Plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Veselov, D.S.; Kudoyarova, G.R.; Kudryakova, N.V.; Kusnetsov, V.V. Role of Cytokinins in Stress Resistance of Plants. Russ. J. Plant Physiol. 2017, 64, 15–27. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-Producing, Plant Growth-Promoting Rhizobacteria That Confer Resistance to Drought Stress in Platycladus orientalis Container Seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants with Microbial Pathogens and Pest Insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic Microbes: Biodiversity, Plant Growth-Promoting Mechanisms and Potential Applications for Agricultural Sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]


| Treatments | Shoot Length (cm) | Root Length (cm) | Shoot (g) | Root (g) | Chl Content (mg g−1 FW) | |||
|---|---|---|---|---|---|---|---|---|
| FW | DW | FW | DW | |||||
| DT genotype | ||||||||
| Water | (−BS) | 6.9 ± 0.2 b | 5.8 ± 0.1 b | 0.51 ± 0.02 b | 0.05 ± 0.008 b | 0.55 ± 0.01 c | 0.055 ± 0.009 b | 1.23 ± 0.08 c |
| (+BS) | 7.9 ± 0.3 a | 7.5 ± 0.2 a | 0.59 ± 0.03 a | 0.06 ± 0.006 a | 0.66 ± 0.04 a | 0.060 ± 0.003 ab | 1.38 ± 0.11 a | |
| PEG | (−BS) | 4.1 ± 0.1 d | 4.5 ± 0.2 c | 0.39 ± 0.04 c | 0.04 ± 0.002 c | 0.53 ± 0.01 d | 0.052 ± 0.006 c | 1.10 ± 0.06 d |
| (+BS) | 6.6 ± 0.2 c | 6.2 ± 0.3 b | 0.51 ± 0.01 b | 0.05 ± 0.007 b | 0.60 ± 0.03 b | 0.062 ± 0.005 a | 1.31 ± 0.07 b | |
| DS genotype | ||||||||
| Water | (−BS) | 5.1 ± 0.3 ab | 5.0 ± 0.2 ab | 0.49 ± 0.01 b | 0.055 ± 0.005 b | 0.50 ± 0.03 b | 0.06 ± 0.007 b | 1.14 ± 0.03 ab |
| (+BS) | 5.4 ± 0.4 a | 5.2 ± 0.2 a | 0.57 ± 0.06 a | 0.062 ± 0.006 a | 0.58 ± 0.04 a | 0.07 ± 0.009 a | 1.19 ± 0.04 a | |
| PEG | (−BS) | 3.0 ± 0.2 bc | 3.8 ± 0.3 b | 0.34 ± 0.03 c | 0.031 ± 0.002 d | 0.35 ± 0.02 d | 0.037 ± 0.005 c | 0.81 ± 0.02 c |
| (+BS) | 3.3 ± 0.3 b | 3.9 ± 0.2 b | 0.34 ± 0.02 c | 0.036 ± 0.005 c | 0.37 ± 0.03 c | 0.036 ± 0.008 c | 1.09 ± 0.05 b | |
| Water | PEG | |||
|---|---|---|---|---|
| (−BS) | (+BS) | (−BS) | (+BS) | |
| DT genotype | + | +++ | +++ | ++++ |
| DS genotype | +/− | ++ | ++/− | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lastochkina, O.; Yuldashev, R.; Avalbaev, A.; Allagulova, C.; Veselova, S. The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress. Microorganisms 2023, 11, 2955. https://doi.org/10.3390/microorganisms11122955
Lastochkina O, Yuldashev R, Avalbaev A, Allagulova C, Veselova S. The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress. Microorganisms. 2023; 11(12):2955. https://doi.org/10.3390/microorganisms11122955
Chicago/Turabian StyleLastochkina, Oksana, Ruslan Yuldashev, Azamat Avalbaev, Chulpan Allagulova, and Svetlana Veselova. 2023. "The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress" Microorganisms 11, no. 12: 2955. https://doi.org/10.3390/microorganisms11122955
APA StyleLastochkina, O., Yuldashev, R., Avalbaev, A., Allagulova, C., & Veselova, S. (2023). The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress. Microorganisms, 11(12), 2955. https://doi.org/10.3390/microorganisms11122955









