A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California
Abstract
:1. Introduction
2. Materials and Methods
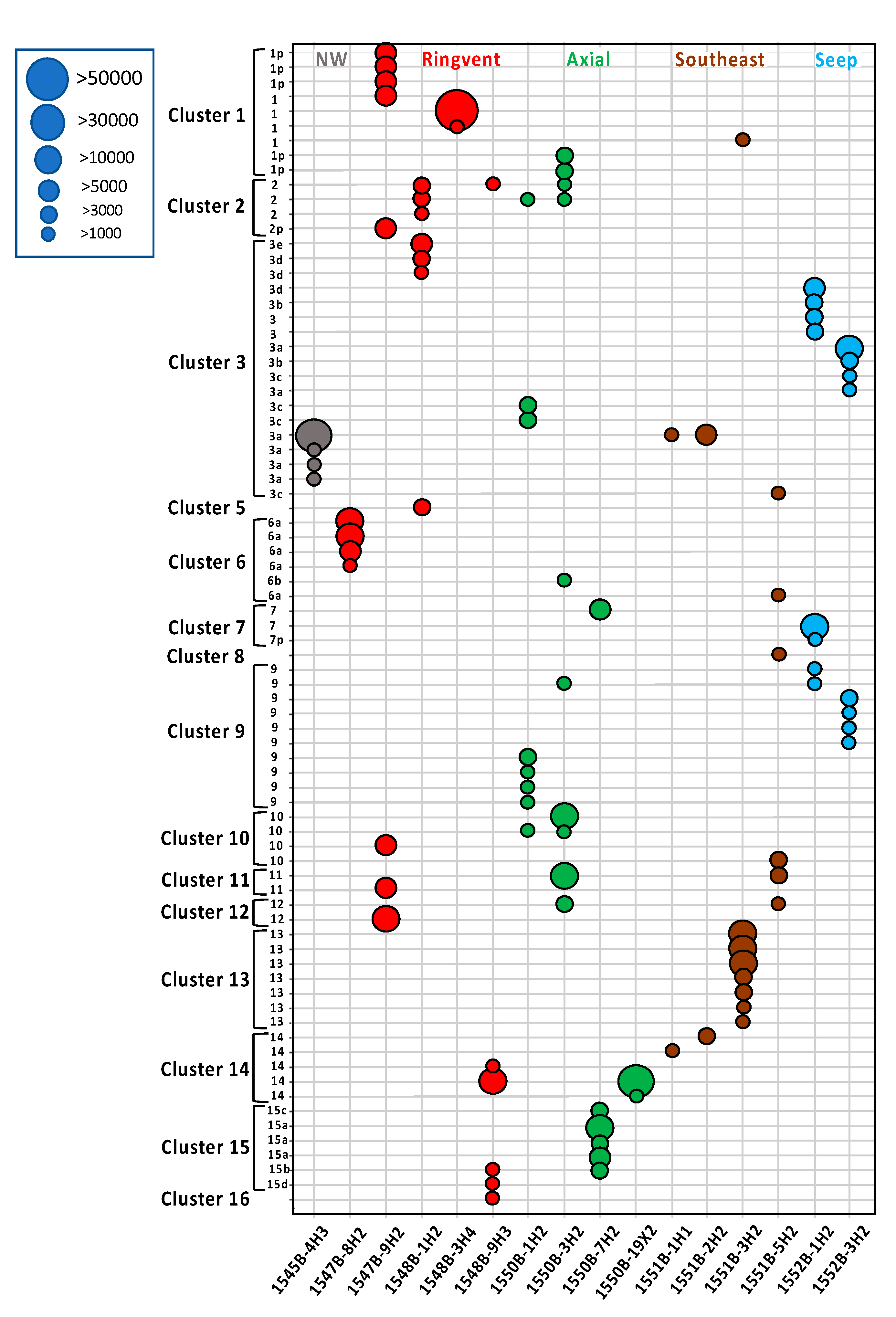
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simoneit, B.R.T.; Summerhayes, C.P.; Meyers, P.A. Sources and hydrothermal alteration of organic matter in Quaternary sediments: A synthesis of studies from the Central Gulf of California. Mar. Pet. Geol. 1986, 3, 282–297. [Google Scholar] [CrossRef]
- Teske, A.P.; McKay, L.J.; Ravelo, A.C.; Aiello, I.; Mortera, C.; Núñez-Useche, F.; Canet, C.; Chanton, J.P.; Brunner, B.; Hensen, C.; et al. Characteristics and Evolution of sill-driven off-axis hydrothermalism in Guaymas Basin—The Ringvent site. Sci. Rep. 2019, 9, 13847. [Google Scholar] [CrossRef] [PubMed]
- Bojanova, D.P.; De Anda, V.Y.; Haghnegahdar, M.A.; Teske, A.P.; Ash, J.L.; Young, E.D.; Baker, B.J.; LaRowe, D.E.; Amend, J.P.; IODP Expedition Scientists. Well-hidden methanogenesis in deep, organic-rich sediments of Guaymas Basin, Gulf of California. ISME J. 2023, 17, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.J.; Leigh, J.A.; Mayer, F.; Woese, C.R.; Wolfe, R.S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 1983, 136, 254–261. [Google Scholar] [CrossRef]
- Kurr, M.; Huber, R.; König, H.; Jannasch, H.W.; Fricke, H.; Trincone, A.; Kristjansson, J.K.; Stetter, K.O. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110 °C. Arch. Microbiol. 1991, 156, 239–247. [Google Scholar] [CrossRef]
- Dhillon, A.; Lever, M.; Lloyd, K.G.; Albert, D.B.; Sogin, M.L.; Teske, A. Methanogen Diversity Evidenced by Molecular Characterization of Methyl Coenzyme M Reductase A (mcrA) Genes in Hydrothermal Sediments of the Guaymas Basin. Appl. Environ. Microbiol. 2005, 71, 4592–4601. [Google Scholar] [CrossRef]
- Lever, M.A.; Teske, A.P. Diversity of Methane-Cycling Archaea in Hydrothermal Sediment Investigated by General and Group-Specific PCR Primers. Appl. Environ. Microbiol. 2015, 81, 1426–1441. [Google Scholar] [CrossRef]
- Nobu, M.K.; Narihiro, T.; Kuroda, K.; Mei, R.; Liu, W.T. Chasing the elusive Euryarchaeota class WSA2: Genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J. 2016, 10, 2478–2487. [Google Scholar] [CrossRef]
- Teske, A.; Hinrichs, K.-U.; Edgcomb, V.; de Vera Gomez, A.; Kysela, D.; Sylva, S.P.; Sogin, M.L.; Jannasch, H.W. Microbial Diversity of Hydrothermal Sediments in the Guaymas Basin: Evidence for Anaerobic Methanotrophic Communities. Appl. Environ. Microbiol. 2002, 68, 1994–2007. [Google Scholar] [CrossRef]
- Biddle, J.F.; Cardman, Z.; Mendlovitz, H.; Albert, D.B.; Lloyd, K.G.; Boetius, A.; Teske, A. Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J. 2012, 6, 1018–1031. [Google Scholar] [CrossRef]
- Dowell, F.; Cardman, Z.; Dasarathy, S.; Kellermann, M.Y.; McKay, L.J.; MacGregor, B.J.; Ruff, S.E.; Biddle, J.F.; Lloyd, K.G.; Lipp, J.S.; et al. Microbial Communities in Methane and Short Alkane-Rich Hydrothermal Sediments of Guaymas Basin. Front. Microbiol. 2016, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.J.; Klokman, V.W.; Mendlovitz, H.P.; LaRowe, D.E.; Hoer, D.R.; Albert, D.; Amend, J.P.; Teske, A. Thermal and geochemical influences on microbial biogeography in the hydrothermal sediments of Guaymas Basin, Gulf of California. Environ. Microbiol. Rep. 2016, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Holler, T.; Widdel, F.; Knittel, K.; Amann, R.; Kellermann, M.Y.; Hinrichs, K.U.; Teske, A.P.; Boetius, A.; Wegener, G. Thermophilic anaerobic oxidation of methane by marine microbial consortia. ISME J. 2011, 5, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intracellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Benito-Merino, D.; Zehnle, H.; Teske, A.P.; Wegener, G. Deep-branching ANME-1c archaea grow at the upper temperature limit of anaerobic oxidation of methane. Front. Microbiol. 2022, 13, 988871. [Google Scholar] [CrossRef] [PubMed]
- Speth, D.R.; Yu, F.B.; Connon, S.A.; Lim, S.; Magyar, J.S.; Pena-Salinas, M.E.; Quake, S.R.; Orphan, V.J. Microbial communities of Auka hydrothermal sediments shed light on vent biogeography and the evolutionary history of thermophily. ISME J. 2022, 16, 1750–1764. [Google Scholar] [CrossRef] [PubMed]
- Laso-Pérez, R.; Wu, F.; Crémière, A.; Speth, D.R.; Magyar, J.S.; Zhao, K.; Krupovic, M.; Orphan, V.J. Evolutionary diversification of methanotrophic Ca. Methanophagales (ANME-1) and their expansive virome. Nat. Microbiol. 2023, 8, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Culbertson, C.; Simoneit, B.R.T. 1982. Methanogenic activity in sediment from Leg 64, Gulf of California. In Initial Reports of the Deep Sea Drilling Project; Curray, J.R., Moore, D.G., Aguayo, J.E., Aubry, M.-P., Einsele, G., Fornari, D., Gieskes, J., Guerrero-Garcia, J., Kastner, M., Kelts, K., et al., Eds.; U.S. Government Printing Office: Washington, DC, USA, 1982; Volume 64, pp. 759–762. [Google Scholar] [CrossRef]
- Thauer, R.K. Methyl (Alkyl)-Coenzyme M Reductases: Nickel F-430-Containing Enzymes Involved in Anaerobic Methane Formation and in Anaerobic Oxidation of Methane or of Short Chain Alkanes. Biochemistry 2019, 58, 5198–5220. [Google Scholar] [CrossRef]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef]
- Hallam, S.J.; Girguis, P.R.; Preston, C.M.; Richardson, P.M.; DeLong, E.F. Identification of Methyl Coenzyme M Reductase A (mcrA) Genes Associated with Methane-Oxidizing Archaea. Appl. Environ. Microbiol. 2003, 69, 5483–5491. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Evans, P.N.; Parks, D.H.; Jensen, P.D.; Woodcroft, B.J.; Hugenholtz, P.; Tyson, G.W. Methylotrophic meth-anogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat. Microbiol. 2016, 1, 16170. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.J.; Hatzenpichler, R.; Inskeep, W.P.; Fields, M.W. Occurrence and expression of novel methyl-coenzyme M reductase gene (mcrA) variants in hot spring sediments. Sci. Rep. 2017, 7, 7252. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, B.A.; Yu, F.B.; Schulz, F.; Blainey, P.C.; Woyke, T.; Quake, S.R. Hydrogenotrophic methanogenesis in archaeal phylum Verstraetearchaeota reveals the shared ancestry of all methanogens. Proc. Natl. Acad. Sci. USA 2019, 116, 5037–5044. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.M.; Krukenberg, V.; Jay, Z.J.; Kohtz, A.J.; Gobrogge, C.A.; Spietz, R.L.; Hatzenpichler, R. Diversity and function of methyl-coenzyme M reductase-encoding archaea in Yellowstone hot springs revealed by metagenomics and mesocosm experiments. ISME Commun. 2023, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Expedition summary. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Heesemann, M.; Villinger, H.; Fisher, A.T.; Tréhu, A.M.; White, S.A. Data report: Testing and deployment of the new APC3 tool to determine in situ temperatures while piston coring. In Proceedings of the IODP; Riedel, M., Collett, T.S., Malone, M.J., the Expedition 311 Scientists, Eds.; Integrated Ocean Drilling Program Management International, Inc.: Washington, DC, USA, 2021; Volume 311. [Google Scholar] [CrossRef]
- Ramírez, G.A.; Mara, P.; Sehein, T.; Wegener, G.; Chambers, C.R.; Joye, S.B.; Peterson, R.N.; Philippe, A.; Burgard, G.; Edgcomb, V.P.; et al. Environmental factors shaping bacterial, archaeal and fungal community structure in hydrothermal sediments of Guaymas Basin, Gulf of California. PLoS ONE 2021, 16, e0256321. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable, and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.; Negrete-Aranda, R.; Harris, R.N.; Contreras, J.; Galerne, C.Y.; Peña-Salinas, M.S.; Splez, R.M.; Teske, A.P.; Lizarralde, D.; Höfig, T.W.; et al. Heat flow and thermal regime in the Guaymas Basin, Gulf of California: Estimates of conductive and advective heat transport. Basin Res. 2023, 35, 1308–1328. [Google Scholar] [CrossRef]
- Persad, L.D.; Marsaglia, K.M. Data Report: Detailed lithologic columns for IODP Expeditions 385 and DSDP Leg 64 Sites in the Guaymas Basin, Gulf of California, Mexico. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2023; Volume 385. [Google Scholar] [CrossRef]
- Torres, M.E.; Kim, J.-H. Data report: Concentration and carbon isotopic composition in pore fluids from IODP Expedition 385. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2022; Volume 385. [Google Scholar] [CrossRef]
- Parkes, R.J.; Webster, G.; Cragg, B.A.; Weightman, A.J.; Newberry, C.J.; Ferdelman, T.G.; Kallmeyer, J.; Jørgensen, B.B.; Aiello, I.W.; Fry, J.C. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature 2005, 436, 390–394. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1545. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1546. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Lizarralde, D.; Teske, A.; Höfig, T.W.; González-Fernández, A.; the IODP Expedition 385 Scientists. Carbon released by sill intrusion into young sediments measured through scientific drilling. Geology 2023, 51, 329–333. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Sites U1547 and U1548. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1549. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1550. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1551. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Teske, A.; Lizarralde, D.; Höfig, T.W.; Aiello, I.W.; Ash, J.L.; Bojanova, D.P.; Buatier, M.D.; Edgcomb, V.P.; Galerne, C.Y.; Gontharet, S.; et al. Site U1552. In Guaymas Basin Tectonics and Biosphere. Proceedings of the International Ocean Discovery Program; Teske, A., Lizarralde, D., Höfig, T.W., the Expedition 385 Scientists, Eds.; International Ocean Discovery Program: College Station, TX, USA, 2021; Volume 385. [Google Scholar] [CrossRef]
- Stewart, L.C.; Jung, J.-H.; Kim, Y.-T.; Kwon, S.-W.; Park, C.-S.; Holden, J.F. M ethanocaldococcus bathoardescens sp. nov., a hyperthermophilic methanogen isolated from a volcanically active deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2015, 65, 1280–1283. [Google Scholar] [CrossRef]
- Lazar, C.S.; L’Haridon, S.; Pignet, P.; Toffib, L. Archaeal Populations in Hypersaline Sediments Underlying Orange Microbial Mats in the Napoli Mud Volcano. Appl. Environ. Microbiol. 2011, 77, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Underwood, S.; Lapham, L.; Teske, A.; Lloyd, K.G. Microbial community structure and methane-cycling activity of subsurface sediments at Mississippi Canyon 118 before the Deepwater Horizon disaster. Deep. Sea Res. II 2016, 129, 148–156. [Google Scholar] [CrossRef]
- Teske, A.; Wegener, G.; Chanton, J.P.; White, D.; MacGregor, B.; Hoer, D.; de Beer, D.; Zhuang, G.; Saxton, M.A.; Joye, S.B.; et al. Microbial Communities Under Distinct Thermal and Geochemical Regimes in Axial and Off-Axis Sediments of Guaymas Basin. Front. Microbiol. 2021, 12, 633649. [Google Scholar] [CrossRef] [PubMed]
- Rattray, J.E.; Chakraborty, A.; Elizondo, G.; Ellefson, E.; Bernard, B.; Brooks, J.; Hubert, C.R.J. Endospores associated with deep seabed geofluid features in the eastern Gulf of Mexico. Geobiology 2022, 20, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Announcement of the Non-Renewal of the JOIDES Resolution Operations and Maintenance Cooperative Agreement. 2023; National Science Foundation. Available online: https://www.nsf.gov/news/news_summ.jsp?cntn_id=306986&org=OCE (accessed on 12 August 2023).
- Morono, Y.; Teske, A.; Galerne, C.; Bojanova, D.; Edgcomb, V.; Meyer, N.; Schubert, F.; Toffin, L.; the Expedition 385 Scientists. Microbial cell distribution in the Guaymas subseafloor biosphere, a young marginal rift basin with rich organics and steep temperature gradients. In Proceedings of the EGU General Assembly Conference Abstracts, EGU22-3312, Vienna, Austria, 23–27 May 2022. [Google Scholar] [CrossRef]
- Pérez Castro, S.; Borton, M.A.; Regan, K.; Hrabe de Angelis, I.; Wrighton, K.C.; Teske, A.P.; Strous, M.; Ruff, S.E. Degradation of biological macromolecules supports uncultured microbial populations in Guaymas Basin hydrothermal sediments. ISME J. 2021, 15, 3480–3497. [Google Scholar] [CrossRef] [PubMed]
- Mara, P.; Zhou, Y.-L.; Teske, A.; Morono, Y.; Beaudoin, D.; Edgcomb, V. Microbial gene expression in Guaymas Basin subsurface sediments responds to hydrothermal stress and energy limitation. ISME J. 2023, 17, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, N.; Teske, A.P.; Baker, B.J. Expansive microbial metabolic versatility and biodiversity in dynamic Guaymas Basin hydrothermal sediments. Nat. Commun. 2018, 9, 4999. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Lüke, C.; Jetten, M.S.M. Enrichment of anaerobic nitrate-dependent methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef]
- Wellsbury, P.; Goodman, K.; Barth, T.; Cragg, B.A.; Barnes, S.P.; Parkes, R.J. Deep marine biosphere fuelled by increasing organic matter availability during burial and heating. Nature 1997, 388, 573–576. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Koch, B.P.; Feseker, T.; Ziervogel, K.; Goldhammer, T.; Schmidt, F.; Witt, M.; Kellermann, M.Y.; Zabel, M.; Teske, A.; et al. Near-surface Heating of Young Rift Sediment Causes Mass Production and Discharge of Reactive Dissolved Organic Matter. Sci. Rep. 2017, 7, srep44864. [Google Scholar] [CrossRef]
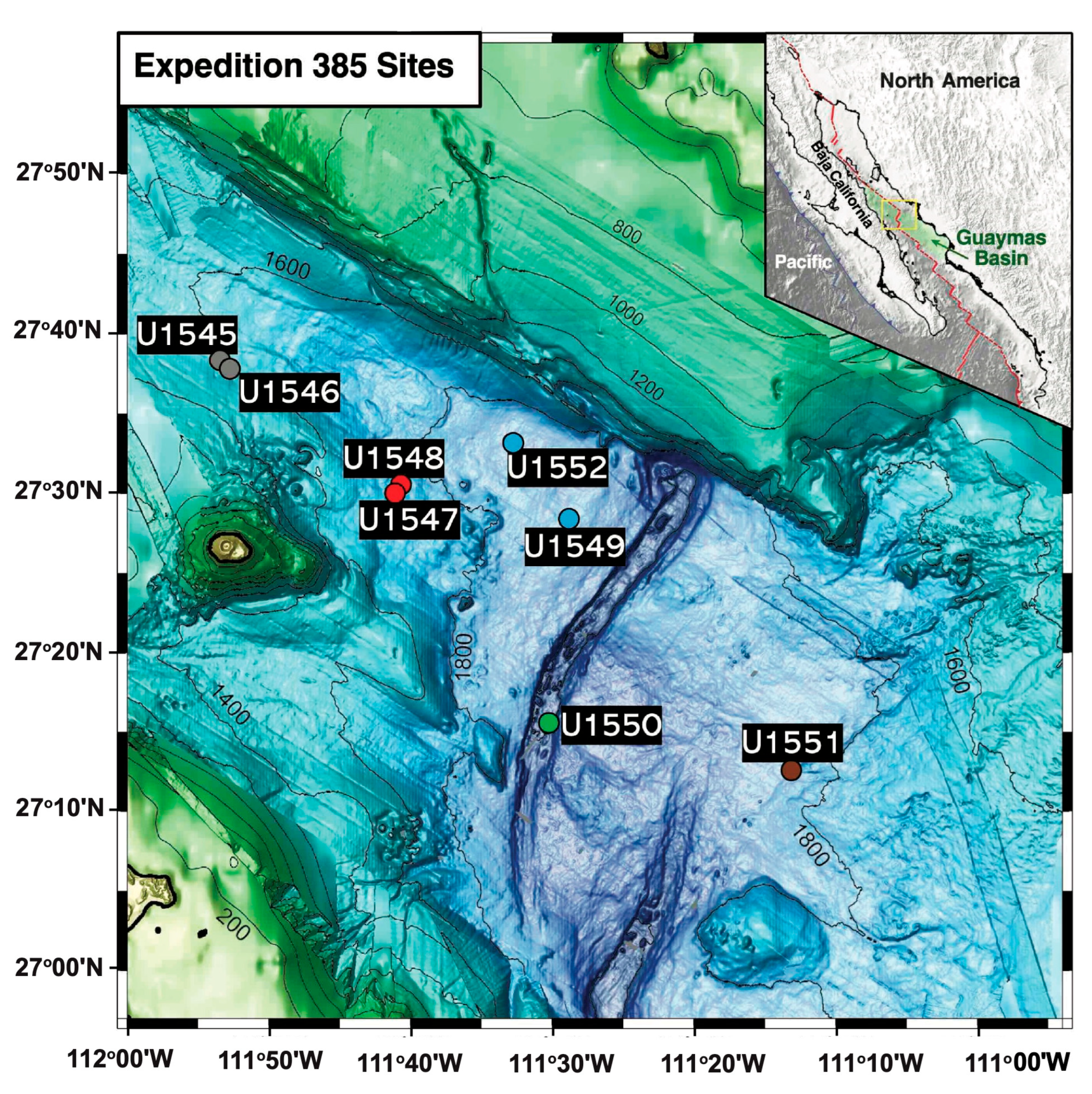

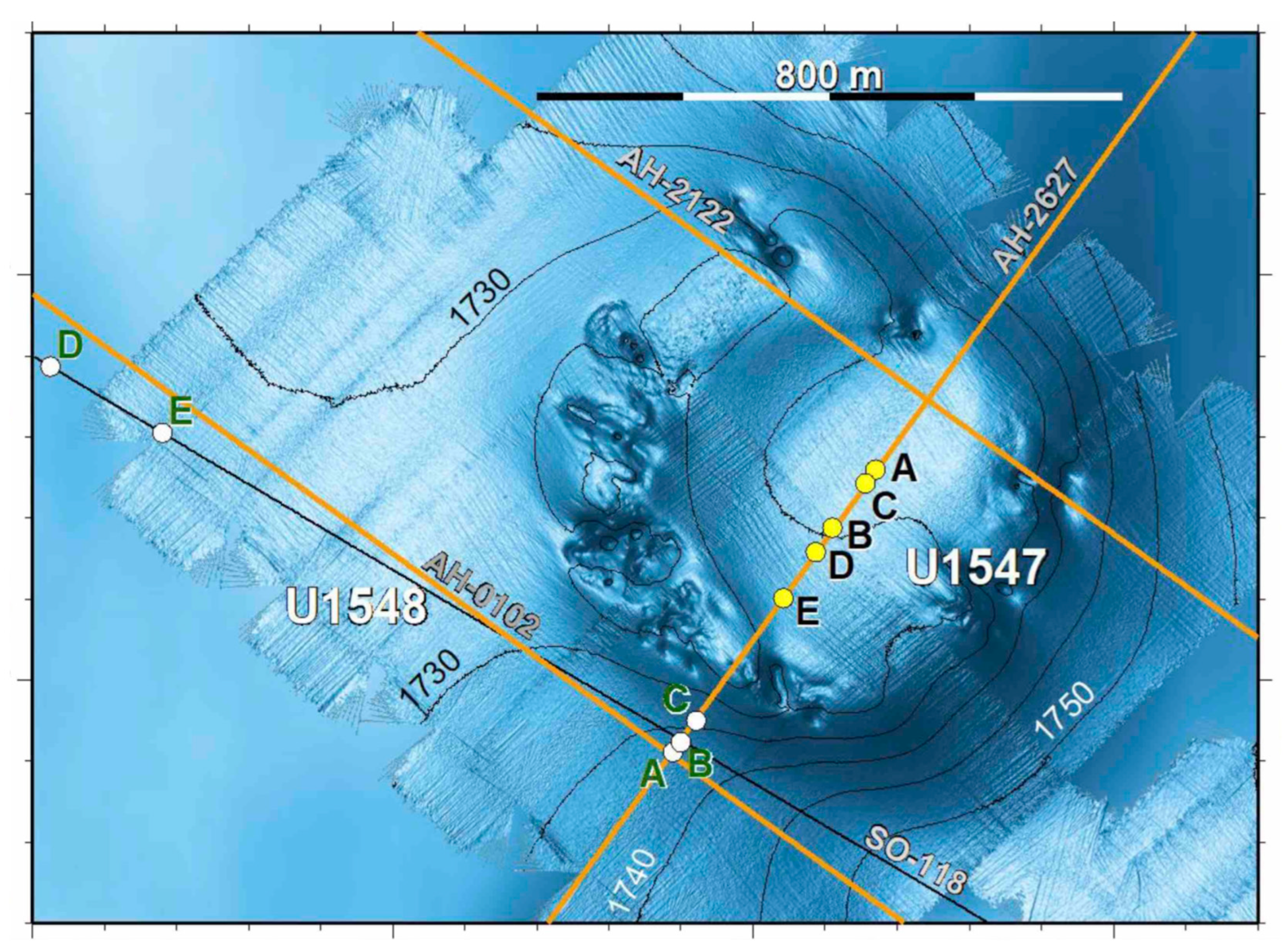
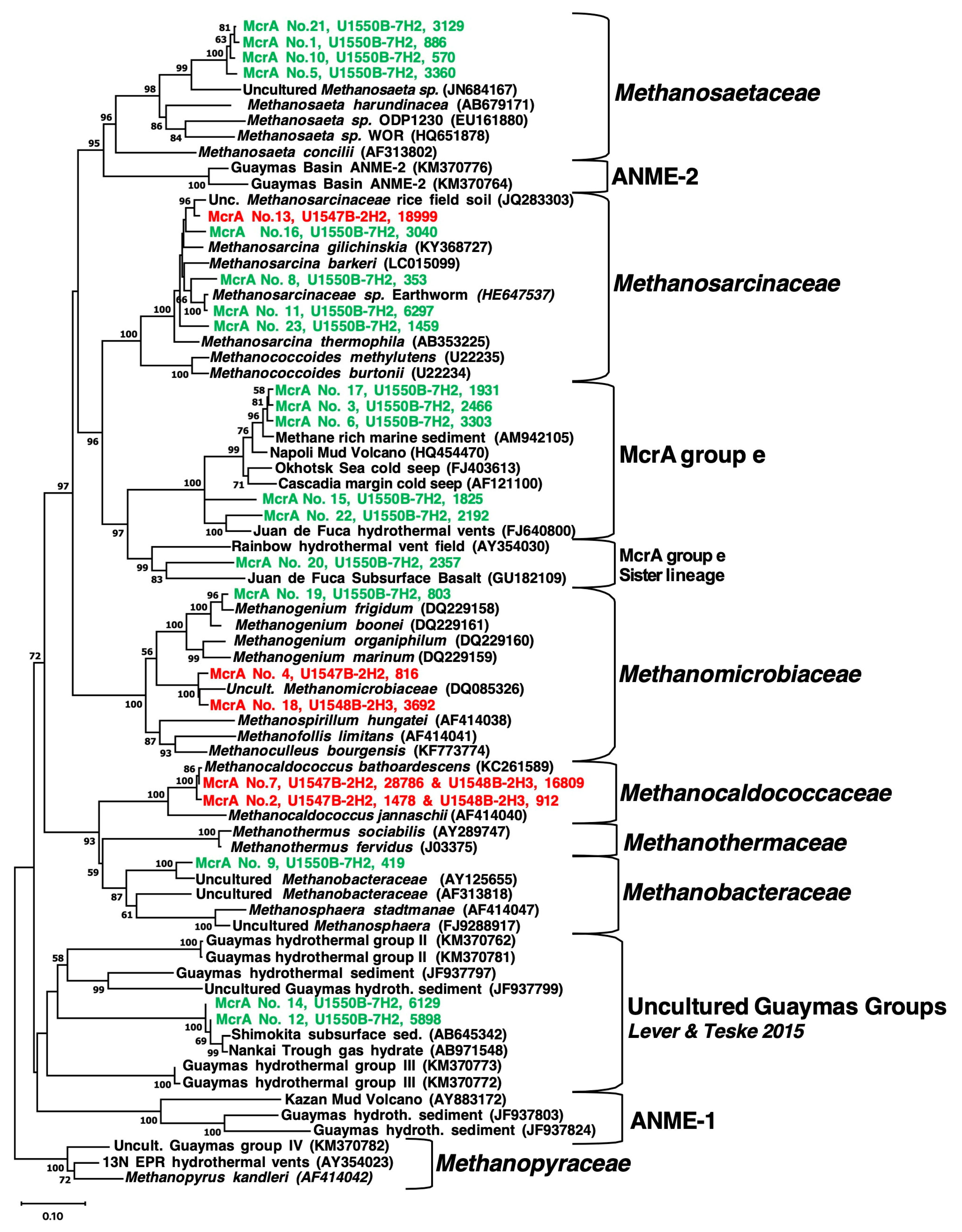
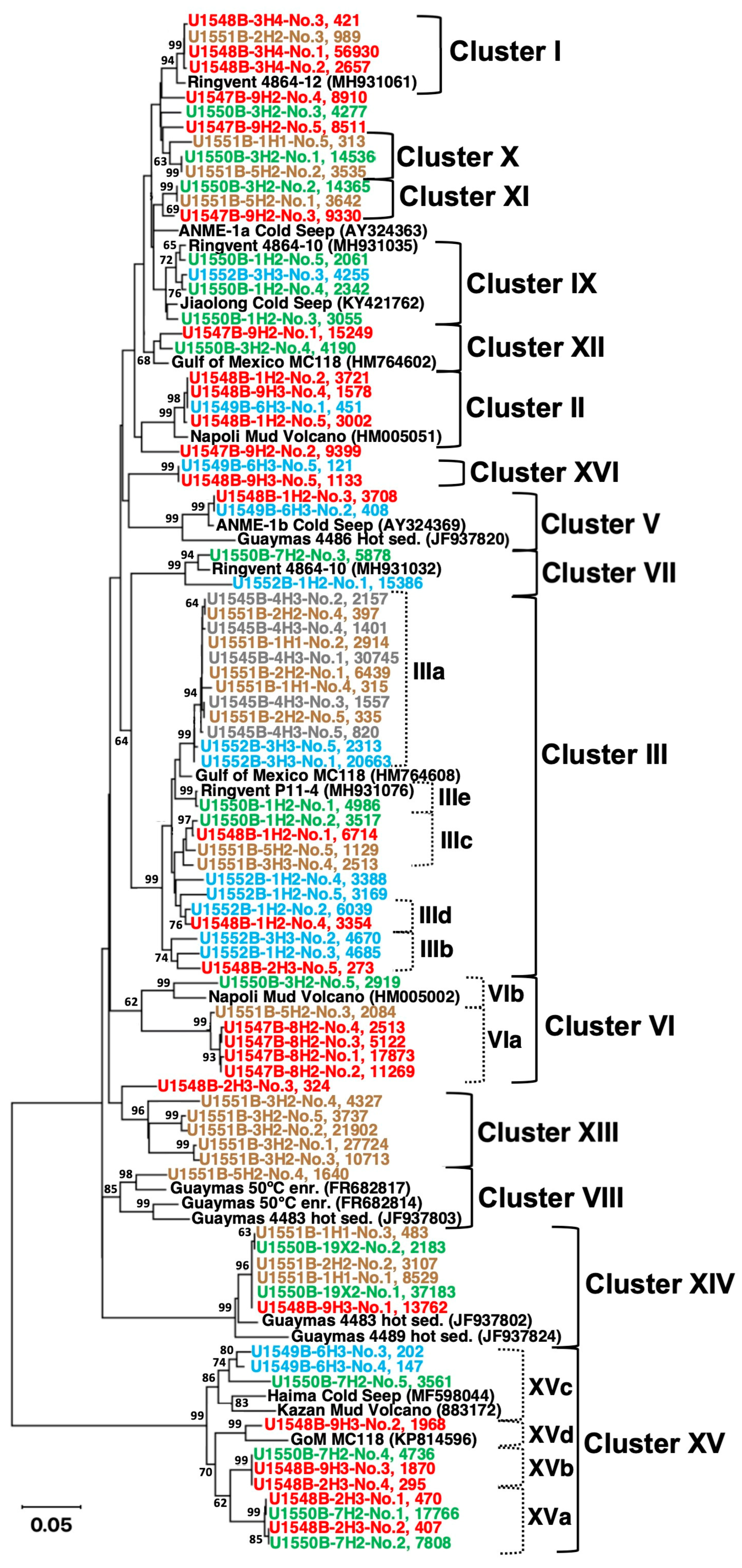
| Site (Position) | Sample ID | Depth (mbsf) | T (°C) | Geochemical Zone | mcrA ANME-1 Specific Primers | mcrA General Primers (mcrIRD) |
|---|---|---|---|---|---|---|
| U1545B (27°38.2301′ N, 111°53.3295′ W) | 1545B_1H2 | 1.7 | 5.3 | SR | − | − |
| 1545B_4H3 | 25.8 | 10.7 | SMTZ | + | − | |
| U1547B (27°30.4128′ N, 111°40.7341′ W) | 1547B_2H2 | 8.6 | 17.4 | SR | − | + |
| 1547B_8H2 | 65.7 | 46.6 | SR | + | − | |
| 1547B_9H2 | 74.2 | 51.0 | SR | + | − | |
| U1548B (27°30.2540′ N, 111°40.8601′ W) | 1548B_1H2 | 2.1 | 8.2 | SR | + | − |
| 1548B_2H3 | 9.1 | 13.7 | SR | + | − | |
| 1548B_3H4 | 20.4 | 22.9 | SR | + | + | |
| 1548B_9H3 | 76.5 | 68.0 | SR | + | − | |
| U1549B (27°28.3383′ N, 111°28.7927′ W) | 1549B_6H3 | 45.6 | 12.1 | SMTZ | + | − |
| U1550B (27°15.1704′ N, 111°30.4451′ W) | 1550B_1H2 | 2.0 | 3.8 | SR | + | − |
| 1550B_3H2 | 16.9 | 5.8 | MR | + | − | |
| 1550B_7H2 | 54.8 | 10.9 | MR | + | + | |
| 1550B_19X2 | 142.0 | 22.7 | MR | + | − | |
| U1551B (27°12.3832′ N, 111°13.1841′ W) | 1551B_1H1 | 0.8 | 4.8 | SR | + | − |
| 1551B_2H2 | 5.8 | 5.3 | SR | + | − | |
| 1551B_3H2 | 15.4 | 6.3 | SR | + | − | |
| 1551B_5H2 | 34.2 | 8.2 | MR | + | − | |
| U1552B (27°33.2885′ N, 111°32.9640′ W) | 1552B_1H2 | 0.8 | 3.8 | SR | + | − |
| 1552B_3H3 | 19.2 | 8.6 | MR | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinkle, J.E.; Mara, P.; Beaudoin, D.J.; Edgcomb, V.P.; Teske, A.P. A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California. Microorganisms 2023, 11, 2956. https://doi.org/10.3390/microorganisms11122956
Hinkle JE, Mara P, Beaudoin DJ, Edgcomb VP, Teske AP. A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California. Microorganisms. 2023; 11(12):2956. https://doi.org/10.3390/microorganisms11122956
Chicago/Turabian StyleHinkle, John E., Paraskevi Mara, David J. Beaudoin, Virginia P. Edgcomb, and Andreas P. Teske. 2023. "A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California" Microorganisms 11, no. 12: 2956. https://doi.org/10.3390/microorganisms11122956
APA StyleHinkle, J. E., Mara, P., Beaudoin, D. J., Edgcomb, V. P., & Teske, A. P. (2023). A PCR-Based Survey of Methane-Cycling Archaea in Methane-Soaked Subsurface Sediments of Guaymas Basin, Gulf of California. Microorganisms, 11(12), 2956. https://doi.org/10.3390/microorganisms11122956





