Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies
Abstract
:1. Introduction
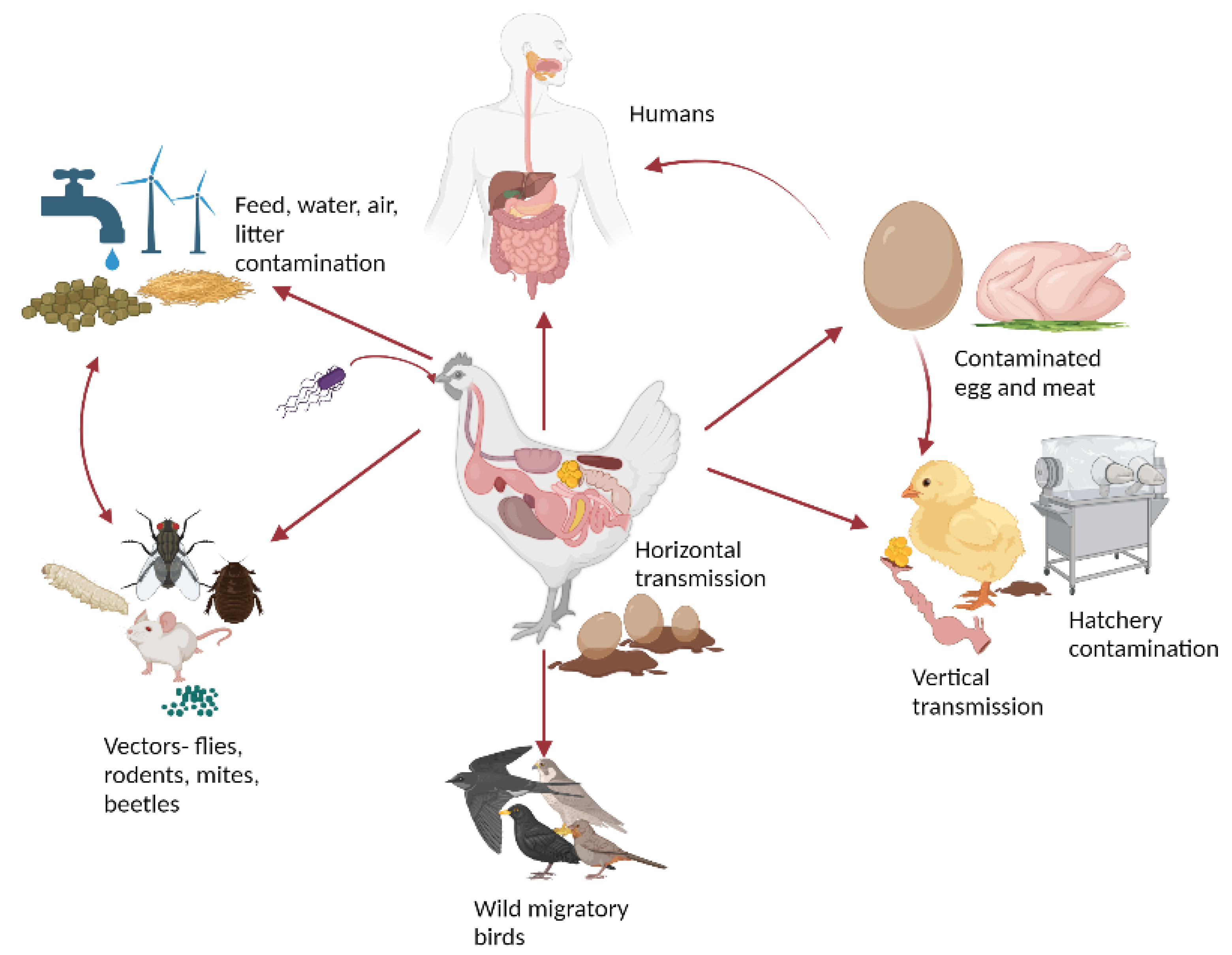
2. Etiology and Transmission
3. Cell Wall and Nomenclature
4. Pathogenesis
5. Immune Response to Salmonella
5.1. Innate Immune System
5.2. Adaptive Immunity
5.2.1. CD4+ T-Cells, CD8+ T-Cells
5.2.2. Regulatory T-Cells
5.2.3. Cytokines
5.2.4. B-Cells and Immunoglobulins
6. Salmonella Control Strategies in Poultry
6.1. Biosecurity
6.2. Antibiotics
6.3. Prebiotics
6.4. Probiotics
6.5. Synbiotics
6.6. Postbiotics
6.7. Phytobiotics
6.8. Bacteriophages
6.9. Vaccination against Salmonella in Poultry
6.9.1. Live-Attenuated Vaccine
6.9.2. Killed or Inactivated Vaccine
6.9.3. Subunit Vaccine
6.9.4. Ghost Vaccine
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, B.; Park, S.; Kim, E.; Yoon, H.; Hahn, T. Salmonella Typhimurium lacking phoBR as a live vaccine candidate against poultry infection. Vet. Microbiol. 2022, 266, 109342. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. International Collaboration on Enteric Disease “Burden of Illness” Studies. Glob. Burd. Nontyphoidal Salmonella Gastroenteritis Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar]
- Cosby, D.E.; Cox, N.A.; Harrison, M.A.; Wilson, J.L.; Buhr, R.J.; Fedorka-Cray, P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015, 24, 408–426. [Google Scholar] [CrossRef]
- Tajkarimi, M. Salmonella spp. Calif. Dep. Food Agric. 2007, 1–8. Available online: https://www.cdfa.ca.gov/ahfss/Animal_Health/PHR250/2007/25007Sal.pdf (accessed on 19 June 2023).
- Howard, Z.R.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Salmonella Enteritidis in shell eggs: Current issues and prospects for control. Food Res. Int. 2012, 45, 755–764. [Google Scholar] [CrossRef]
- Ricke, S.C.; Kim, S.A.; Shi, Z.; Park, S.H. Molecular-based identification and detection of Salmonella in food production systems: Current perspectives. J. Appl. Microbiol. 2018, 125, 313–327. [Google Scholar] [CrossRef]
- Chousalkar, K.; Gast, R.; Martelli, F.; Pande, V. Review of egg-related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 2018, 44, 290–303. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407. [Google Scholar] [CrossRef]
- Gieraltowski, L.; Higa, J.; Peralta, V.I.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H. National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Alshahrani, O.A.; Saghir, S.A.; Al-Wajeeh, A.S.; Al-Shargi, O.Y.; Taha, A.E.; Mesalam, N.M.; Abdel-Moneim, A.E. Prebiotics can restrict Salmonella populations in poultry: A review. Anim. Biotechnol. 2022, 33, 1668–1677. [Google Scholar] [CrossRef]
- Bhunia, A.K. Salmonella enterica. In Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: New York, NY, USA, 2008; pp. 271–287. [Google Scholar]
- Wibisono, F.M.; Wibisono, F.J.; Effendi, M.H.; Plumeriastuti, H.; Hidayatullah, A.R.; Hartadi, E.B.; Sofiana, E.D. A review of salmonellosis on poultry farms: Public health importance. Syst. Rev. Pharm. 2020, 11, 481–486. [Google Scholar]
- Center for Disease Control and Prevention. Salmonella. Available online: https://www.cdc.gov/Salmonella/index.html (accessed on 30 June 2023).
- O’Bryan, C.A.; Ricke, S.C.; Marcy, J.A. Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control 2022, 132, 108539. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Padron, M. Salmonella typhimurium penetration through the eggshell of hatching eggs. Avian Dis. 1990, 34, 463–465. [Google Scholar] [PubMed]
- Kopanic, R.J., Jr.; Sheldon, B.W.; Wright, C.G. Cockroaches as vectors of Salmonella: Laboratory and field trials. J. Food Prot. 1994, 57, 125–131. [Google Scholar] [CrossRef]
- Sparagano, O. Control of poultry mites: Where do we stand? Exp. Appl. Acarol. 2009, 48, 1–2. [Google Scholar] [CrossRef]
- Leffer, A.M.; Kuttel, J.; Martins, L.M.; Pedroso, A.C.; Astolfi-Ferreira, C.S.; Ferreira, F.; Ferreira, A.J.P. Vectorial competence of larvae and adults of Alphitobius diaperinus in the transmission of Salmonella Enteritidis in poultry. Vector-Borne Zoonotic Dis. 2010, 10, 481–487. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Kijlstra, A. Role of rodents in transmission of Salmonella and Campylobacter. J. Sci. Food Agric. 2007, 87, 2774–2781. [Google Scholar] [CrossRef]
- Raufu, I.A.; Ahmed, O.A.; Aremu, A.; Odetokun, I.A.; Raji, M.A. Salmonella transmission in poultry farms: The roles of rodents, lizards and formites. Savannah Vet. J. 2019, 2, 1–4. [Google Scholar]
- Hughes, L.A.; Shopland, S.; Wigley, P.; Bradon, H.; Leatherbarrow, A.H.; Williams, N.J.; Bennett, M.; de Pinna, E.; Lawson, B.; Cunningham, A.A. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet. Res. 2008, 4, 4. [Google Scholar] [CrossRef]
- Alley, M.R.; Connolly, J.H.; Fenwick, S.G.; Mackereth, G.F.; Leyland, M.J.; Rogers, L.E.; Haycock, M.; Nicol, C.; Reed, C. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N. Z. Vet. J. 2002, 50, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.; Werther, K.; Berchieri Júnior, A. Assessment of Newcastle and infectious bronchitis pathogens, and Salmonella spp. in wild birds captured near poultry facilities. Arq. Bras. Med. Veterinária Zootec. 2010, 62, 219–223. [Google Scholar] [CrossRef]
- Fu, Y.; M’ikanatha, N.M.; Lorch, J.M.; Blehert, D.S.; Berlowski-Zier, B.; Whitehouse, C.A.; Li, S.; Deng, X.; Smith, J.C.; Shariat, N.W. Salmonella enterica Serovar Typhimurium Isolates from Wild Birds in the United States Represent Distinct Lineages Defined by Bird Type. Appl. Environ. Microbiol. 2022, 88, 1979. [Google Scholar] [CrossRef] [PubMed]
- Obukhovska, O. The natural reservoirs of Salmonella Enteritidis in populations of wild birds. Online J. Public Health Inform. 2013, 5. [Google Scholar] [CrossRef]
- Authority, E.F.S. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSa J. 2018, 16, e05500. [Google Scholar]
- Yavari, L. Antibiotic Resistance in Salmonella enterica and the Role of Animal and Animal Food Control A literature review of Europe and USA. 2012. Available online: https://www.researchgate.net/profile/Leila-Yavari/publication/280531461_Antibiotic_Resistance_in_Salmonella_enterica_and_the_Role_of_Animal_and_Animal_Food_Control/links/55b7b5b608aed621de048679/Antibiotic-Resistance-in-Salmonella-enterica-and-the-Role-of-Animal-and-Animal-Food-Control (accessed on 3 July 2023).
- Lüderitz, O.; Westphal, O.; Staub, A.M.; Nikaido, H. Isolation and chemical and immunological characterization of bacterial lipopolysaccharides. Microb. Toxins 2016, 4, 145–233. [Google Scholar]
- Lüderitz, O.; Staub, A.M.; Westphal, O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol. Rev. 1966, 30, 192–255. [Google Scholar] [CrossRef]
- Reeves, M.W.; Evins, G.M.; Heiba, A.A.; Plikaytis, B.D.; Farmer, J.J., 3rd. Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J. Clin. Microbiol. 1989, 27, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Stackebrandt, E.; Schleifer, K. The Prokaryotes: A Handbook on the Biology of Bacteria; Springer: New York, NY, USA, 2006; Volume 7. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Arya, G.; Holtslander, R.; Robertson, J.; Yoshida, C.; Harris, J.; Parmley, J.; Nichani, A.; Johnson, R.; Poppe, C. Epidemiology, pathogenesis, genoserotyping, antimicrobial resistance, and prevention and control of non-typhoidal Salmonella serovars. Curr. Clin. Microbiol. Rep. 2017, 4, 43–53. [Google Scholar] [CrossRef]
- Old, D.C. Nomenclature of Salmonella. J. Med. Microbiol. 1992, 37, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, M.A.; Langridge, G.C.; Wain, J. Salmonella nomenclature in the genomic era: A time for change. Sci. Rep. 2021, 11, 7494. [Google Scholar] [CrossRef]
- Grimont, P.A.; Weill, F. Antigenic formulae of the Salmonella serovars. WHO Collab. Cent. Ref. Res. Salmonella 2007, 9, 1–166. [Google Scholar]
- Carter, A.J.; Adams, M.R.; Woodward, M.J.; La Ragione, R.M. Control strategies for Salmonella colonization of poultry: The probiotic perspective. Food Sci. Technol. 2009, 5, 103–115. [Google Scholar]
- Hickman-Brenner, F.W.; Stubbs, A.D.; Farmer, J.J., 3rd. Phage typing of Salmonella enteritidis in the United States. J. Clin. Microbiol. 1991, 29, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.R.; Bangtrakulnonth, A.; Tishyadhigama, P.; Saroj, S.D.; Bandekar, J.R.; Hendriksen, R.S.; Kapadnis, B.P. Serotyping, PCR, phage-typing and antibiotic sensitivity testing of Salmonella serovars isolated from urban drinking water supply systems of Nepal. Lett. Appl. Microbiol. 2007, 44, 588–594. [Google Scholar] [CrossRef]
- Crabb, H.K.; Allen, J.L.; Devlin, J.M.; Firestone, S.M.; Stevenson, M.; Wilks, C.R.; Gilkerson, J.R. Traditional Salmonella Typhimurium typing tools (phage typing and MLVA) are sufficient to resolve well-defined outbreak events only. Food Microbiol. 2019, 84, 103237. [Google Scholar] [CrossRef]
- Ha, A.J.; Perez, L.G.S.; Kim, T.; Mizan, M.F.R.; Nahar, S.; Park, S.; Chun, H.; Ha, S. Research Note: Identification and characterization of Salmonella spp. in mechanically deboned chickens using pulsed-field gel electrophoresis. Poult. Sci. 2021, 100, 100961. [Google Scholar] [CrossRef]
- Khan, S.A.; Foley, S.; Stefanova, R. Subtyping of Salmonella enterica Serovar Typhimurium from Clinical Samples by Multiple-Locus Variable-Number Tandem Repeat Analysis and Pulsed-Field Gel Electrophoresis. Microbiol. Infect. Dis. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Mechesso, A.F.; Moon, D.C.; Kim, S.; Song, H.; Kang, H.Y.; Na, S.H.; Choi, J.; Kim, H.; Yoon, S.; Lim, S. Nationwide surveillance on serotype distribution and antimicrobial resistance profiles of non-typhoidal Salmonella serovars isolated from food-producing animals in South Korea. Int. J. Food Microbiol. 2020, 335, 108893. [Google Scholar] [CrossRef]
- Ferrato, C.; Chui, L.; King, R.; Louie, M. Utilization of a molecular serotyping method for Salmonella enterica in a routine laboratory in Alberta Canada. J. Microbiol. Methods 2017, 135, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, C.K.; Peters, T.M.; Gharbia, S.E.; Logan, J.M.; Arnold, C. Towards the development of a DNA-sequence based approach to serotyping of Salmonella enterica. BMC Microbiol. 2004, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.; Barretto, C.; Portmann, A.; Fournier, C.; Karczmarek, A.; Voets, G.; Li, S.; Deng, X.; Klijn, A. Salmonella serotyping; comparison of the traditional method to a microarray-based method and an in silico platform using whole genome sequencing data. Front. Microbiol. 2019, 10, 2554. [Google Scholar] [CrossRef] [PubMed]
- Higginson, E.E.; Simon, R.; Tennant, S.M. Animal models for salmonellosis: Applications in vaccine research. Clin. Vaccine Immunol. 2016, 23, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Ohlson, M.B.; Monack, D.M. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes 2012, 3, 62–70. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The roles of Peyer’s patches and microfold cells in the gut immune system: Relevance to autoimmune diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Buchmeier, N.A.; Heffron, F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 1991, 59, 2232–2238. [Google Scholar] [CrossRef]
- Velge, P.; Cloeckaert, A.; Barrow, P. Emergence of Salmonella epidemics: The problems related to Salmonella enterica serotyp Enteritidis and multiple antibiotic resistance in other major serotypes. Vet. Res. 2005, 36, 267–288. [Google Scholar] [CrossRef]
- World Health Organization. Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Coburn, B.; Grassl, G.A.; Finlay, B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007, 85, 112–118. [Google Scholar] [CrossRef]
- Bhunia, A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: New York, NY, USA, 2018. [Google Scholar]
- Knuff, K.; Finlay, B.B. What the SIF is happening—The role of intracellular Salmonella-induced filaments. Front. Cell. Infect. Microbiol. 2017, 7, 335. [Google Scholar] [CrossRef]
- McGourty, K.; Thurston, T.L.; Matthews, S.A.; Pinaud, L.; Mota, L.J.; Holden, D.W. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012, 338, 963–967. [Google Scholar] [CrossRef]
- Tahoun, A.; Mahajan, S.; Paxton, E.; Malterer, G.; Donaldson, D.S.; Wang, D.; Tan, A.; Gillespie, T.L.; O’Shea, M.; Roe, A.J. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 2012, 12, 645–656. [Google Scholar] [CrossRef]
- Dieye, Y.; Ameiss, K.; Mellata, M.; Curtiss, R. The Salmonella Pathogenicity Island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium. BMC Microbiol. 2009, 9, 3. [Google Scholar] [CrossRef]
- Jones, M.A.; Hulme, S.D.; Barrow, P.A.; Wigley, P. The Salmonella pathogenicity island 1 and Salmonella pathogenicity island 2 type III secretion systems play a major role in pathogenesis of systemic disease and gastrointestinal tract colonization of Salmonella enterica serovar Typhimurium in the chicken. Avian Pathol. 2007, 36, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Pico-Rodríguez, J.T.; Martínez-Jarquín, H.; Gómez-Chávez, J.D.J.; Juárez-Ramírez, M.; Martínez-Chavarría, L.C. Effect of Salmonella pathogenicity island 1 and 2 (SPI-1 and SPI-2) deletion on intestinal colonization and systemic dissemination in chickens. Vet. Res. Commun. 2023, 1–12. [Google Scholar] [CrossRef]
- Merino, L.; Trejo, F.M.; De Antoni, G.; Golowczyc, M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res. Int. 2019, 123, 258–265. [Google Scholar] [CrossRef]
- Barrow, P.A. Salmonella infections: Immune and non-immune protection with vaccines. Avian Pathol. 2007, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mon, K.K.; Zhu, Y.; Chanthavixay, G.; Kern, C.; Zhou, H. Integrative analysis of gut microbiome and metabolites revealed novel mechanisms of intestinal Salmonella carriage in chicken. Sci. Rep. 2020, 10, 4809. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Lichtman, A.; Pillai, S. Cellular and Molecular Immunology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Geier, M.S.; Hughes, R.J.; Denman, S.E.; Moore, R.J. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 2013, 8, e84290. [Google Scholar] [CrossRef]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000, 24, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Temperley, N.D.; Berlin, S.; Paton, I.R.; Griffin, D.K.; Burt, D.W. Evolution of the chicken Toll-like receptor gene family: A story of gene gain and gene loss. BMC Genom. 2008, 9, 62. [Google Scholar] [CrossRef]
- Werling, D.; Hope, J.C.; Howard, C.J.; Jungi, T.W. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology 2004, 111, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Matsumoto, W.; Seike, F.; Tanaka, Y.; Teratani, C.; Tozuka, M.; Kashimoto, T.; Takehara, K.; Nakamura, M.; Yoshikawa, Y. Efficacy of soluble recombinant FliC protein from Salmonella enterica serovar Enteritidis as a potential vaccine candidate against homologous challenge in chickens. Avian Dis. 2012, 56, 354–358. [Google Scholar] [CrossRef]
- Khan, S.; Chousalkar, K.K. Transcriptome profiling analysis of caeca in chicks challenged with Salmonella Typhimurium reveals differential expression of genes involved in host mucosal immune response. Appl. Microbiol. Biotechnol. 2020, 104, 9327–9342. [Google Scholar] [CrossRef]
- MacKinnon, K.M.; He, H.; Nerren, J.R.; Swaggerty, C.L.; Genovese, K.J.; Kogut, M.H. Expression profile of toll-like receptors within the gastrointestinal tract of 2-day-old Salmonella enteriditis-infected broiler chickens. Vet. Microbiol. 2009, 137, 313–319. [Google Scholar] [CrossRef]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef]
- Ferro, P.J.; Swaggerty, C.L.; Kaiser, P.; Pevzner, I.Y.; Kogut, M.H. Heterophils isolated from chickens resistant to extra-intestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 2004, 132, 1029–1037. [Google Scholar] [CrossRef]
- Kogut, M.H.; Iqbal, M.; He, H.; Philbin, V.; Kaiser, P.; Smith, A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005, 29, 791–807. [Google Scholar] [CrossRef]
- Kogut, M.H.; Chiang, H.; Swaggerty, C.L.; Pevzner, I.Y.; Zhou, H. Gene expression analysis of Toll-like receptor pathways in heterophils from genetic chicken lines that differ in their susceptibility to Salmonella enteritidis. Front. Genet. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Zhang, Q.; Sánchez, A.L.B.; Bo, Z.; Qiao, W.; Zheng, M.; Li, Q.; Cui, H.; Jie, W.; Zhao, G. Transcriptome analysis of the spleen of heterophils to lymphocytes ratio-selected chickens revealed their mechanism of differential resistance to Salmonella. J. Integr. Agric. 2022, 21, 2372–2383. [Google Scholar]
- Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Zheng, M.; Wen, J.; Zhao, G.; Wang, Q. Association of heterophil/lymphocyte ratio with intestinal barrier function and immune response to salmonella enteritidis infection in chicken. Animals 2021, 11, 3498. [Google Scholar] [CrossRef] [PubMed]
- Withanage, G.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef]
- Withanage, G.; Sasai, K.; Fukata, T.; Miyamoto, T.; Lillehoj, H.S.; Baba, E. Increased lymphocyte subpopulations and macrophages in the ovaries and oviducts of laying hens infected with Salmonella enterica serovar Enteritidis. Avian Pathol. 2003, 32, 583–590. [Google Scholar] [CrossRef]
- Sáenz, L.; Guzmán, M.; Vidal, S.; Caruffo, M.; Siel, D.; Zayas, C.; Paredes, R.; Valenzuela, C.; Hidalgo, H.; Pérez, O. Efficacy of multivalent, cochleate-based vaccine against Salmonella Infantis, S. Enteritidis and S. Typhimurium in laying hens. Vaccines 2022, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Peters, T.; Hoekman, A.; Rebel, J.; Smits, M.A. Differences in the early response of hatchlings of different chicken breeding lines to Salmonella enterica serovar Enteritidis infection. Poult. Sci. 2012, 91, 346–353. [Google Scholar] [CrossRef]
- Meijerink, N.; Van den Biggelaar, R.H.; Van Haarlem, D.A.; Stegeman, J.A.; Rutten, V.P.; Jansen, C.A. A detailed analysis of innate and adaptive immune responsiveness upon infection with Salmonella enterica serotype Enteritidis in young broiler chickens. Vet. Res. 2021, 52, 1–21. [Google Scholar] [CrossRef]
- Sutton, K.M.; Morris, K.M.; Borowska, D.; Sang, H.; Kaiser, P.; Balic, A.; Vervelde, L. Characterization of conventional dendritic cells and macrophages in the spleen using the CSF1R-reporter transgenic chickens. Front. Immunol. 2021, 12, 636436. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Kogut, M.H.; Arsenault, R.J.; Swaggerty, C.L.; Cole, K.; Reddish, J.M.; Selvaraj, R.K. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult. Sci. 2015, 94, 1828–1835. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Acevedo, K.; Mortada, M.; Akerele, G.; Applegate, T.J.; Kogut, M.H.; Selvaraj, R.K. Effects of Salmonella enterica ser. Enteritidis and Heidelberg on host CD4 CD25 regulatory T cell suppressive immune responses in chickens. PLoS ONE 2021, 16, e0260280. [Google Scholar] [CrossRef]
- Penha Filho, R.A.C.; Moura, B.S.; de Almeida, A.M.; Montassier, H.J.; Barrow, P.A.; Junior, A.B. Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens. Vaccine 2012, 30, 7637–7643. [Google Scholar] [CrossRef]
- Chaussé, A.; Grépinet, O.; Bottreau, E.; Robert, V.; Hennequet-Antier, C.; Lalmanach, A.; Lecardonnel, J.; Beaumont, C.; Velge, P. Susceptibility to Salmonella carrier-state: A possible Th2 response in susceptible chicks. Vet. Immunol. Immunopathol. 2014, 159, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.H. Avian Immunology, Immunogenetics, and Host Immune Response to Salmonella Enterica Serovar Enteritidis Infection in Chickens; Iowa State University: Ames, IA, USA, 2007. [Google Scholar]
- Beal, R.K.; Wigley, P.; Powers, C.; Hulme, S.D.; Barrow, P.A.; Smith, A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004, 100, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Withanage, G.; Sasai, K.; Fukata, T.; Miyamoto, T.; Baba, E.; Lillehoj, H.S. T lymphocytes, B lymphocytes, and macrophages in the ovaries and oviducts of laying hens experimentally infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 1998, 66, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Lillehoj, H.S.; Raybourne, R.B.; Babu, U.S.; Heckert, R.A. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: Lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-γ production. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 255–272. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Restif, O.; Goh, Y.S.; Palayret, M.; Grant, A.J.; McKinley, T.J.; Clark, M.R.; Mastroeni, P. Quantification of the effects of antibodies on the extra-and intracellular dynamics of Salmonella enterica. J. R. Soc. Interface 2013, 10, 20120866. [Google Scholar] [CrossRef] [PubMed]
- Männe, C.; Takaya, A.; Yamasaki, Y.; Mursell, M.; Hojyo, S.; Wu, T.; Sarkander, J.; McGrath, M.A.; Cornelis, R.; Hahne, S. Salmonella SiiE prevents an efficient humoral immune memory by interfering with IgG plasma cell persistence in the bone marrow. Proc. Natl. Acad. Sci. USA 2019, 116, 7425–7430. [Google Scholar] [CrossRef]
- Dar, M.A.; Urwat, U.; Ahmad, S.M.; Ahmad, R.; Kashoo, Z.A.; Dar, T.A.; Bhat, S.A.; Mumtaz, P.T.; Shabir, N.; Shah, R.A. Gene expression and antibody response in chicken against Salmonella Typhimurium challenge. Poult. Sci. 2019, 98, 2008–2013. [Google Scholar] [CrossRef]
- Arnold, J.W.; Holt, P.S. Response to Salmonella enteritidis infection by the immunocompromised avian host. Poult. Sci. 1995, 74, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.K.; Powers, C.; Davison, T.F.; Barrow, P.A.; Smith, A.L. Clearance of enteric Salmonella enterica serovar Typhimurium in chickens is independent of B-cell function. Infect. Immun. 2006, 74, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.W.; Williams, N.T.; Powell, L.F.; Cook, A. Reducing Campylobacter and salmonella infection: Two studies of the economic cost and attitude to adoption of on-farm biosecurity measures. Zoonoses Public Health 2010, 57, e109–e115. [Google Scholar] [CrossRef] [PubMed]
- Gosling, R.J.; Martelli, F.; Wintrip, A.; Sayers, A.R.; Wheeler, K.; Davies, R.H. Assessment of producers’ response to Salmonella biosecurity issues and uptake of advice on laying hen farms in England and Wales. Br. Poult. Sci. 2014, 55, 559–568. [Google Scholar] [CrossRef]
- Sylejmani, D.; Musliu, A.; Ramadani, N.; Sparagano, O.; Hamidi, A. Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. 2016, 60, 454–459. [Google Scholar] [CrossRef]
- Volkova, V.V.; Wills, R.W.; Hubbard, S.A.; Magee, D.L.; Byrd, J.A.; Bailey, R.H. Risk factors associated with detection of Salmonella in broiler litter at the time of new flock placement. Zoonoses Public Health 2011, 58, 158–168. [Google Scholar] [CrossRef]
- Eid, S.; Hassan, H.M.; Al-Atfeehy, N.M.; Selim, K.M.; El Oksh, A.S. Composting: A biosecurity measure to maximize the benefit of broilers’ litter. Anim. Res. 2023, 10, 458–468. [Google Scholar] [CrossRef]
- Abdulghaffar, T.A.; El Bahgy, H.E. Effect of some disinfectants on some pathogenic microorganisms isolated from poultry farm. Benha Vet. Med. J. 2016, 31, 154–158. [Google Scholar]
- Meher, M.M.; Sharif, M.A.; Al Bayazid, A. Seroprevalence of Salmonella spp. infection in different types of poultry and biosecurity measures associated with Salmonellosis. Int. J. Agric. Environ. Food Sci. 2022, 6, 557–567. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Eckert, N.H.; Lee, J.T.; Hyatt, D.; Stevens, S.M.; Anderson, S.; Anderson, P.N.; Beltran, R.; Schatzmayr, G.; Mohnl, M.; Caldwell, D.J. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. J. Appl. Poult. Res. 2010, 19, 59–67. [Google Scholar] [CrossRef]
- Karon, A.E.; Archer, J.R.; Sotir, M.J.; Monson, T.A.; Kazmierczak, J.J. Human multidrug-resistant Salmonella newport infections, Wisconsin, 2003–2005. Emerg. Infect. Dis. 2007, 13, 1777. [Google Scholar] [CrossRef] [PubMed]
- Palamidi, I.; Fegeros, K.; Mohnl, M.; Abdelrahman, W.; Schatzmayr, G.; Theodoropoulos, G.; Mountzouris, K.C. Probiotic form effects on growth performance, digestive function, and immune related biomarkers in broilers. Poult. Sci. 2016, 95, 1598–1608. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef]
- Hajati, H.; Rezaei, M. The application of prebiotics in poultry production. Int. J. Poult. Sci. 2010, 9, 298–304. [Google Scholar] [CrossRef]
- Bogusławska-Tryk, M.; Piotrowska, A.; Burlikowska, K. Dietary fructans and their potential beneficial influence on health and performance parametrs in broiler chickens. J. Cent. Eur. Agric. 2012, 13. Available online: https://hrcak.srce.hr/83284 (accessed on 10 November 2023). [CrossRef]
- Fomentini, M.; Haese, D.; Kill, J.L.; Sobreiro, R.P.; Puppo, D.D.; Haddade, I.R.; Lima, A.L.; Saraiva, A. Prebiotic and antimicrobials on performance, carcass characteristics, and antibody production in broilers. Ciência Rural 2016, 46, 1070–1075. [Google Scholar] [CrossRef]
- Adhikari, P.A.; Kim, W.K. Overview of prebiotics and probiotics: Focus on performance, gut health and immunity—A review. Ann. Anim. Sci. 2017, 17, 949–966. [Google Scholar] [CrossRef]
- Pourabedin, M.; Guan, L.; Zhao, X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 2015, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Shanmugasundaram, R.; Sifri, M.; Selvaraj, R. Yeasts and Yeast-based Products in Poultry Nutrition. J. Appl. Poult. Res. 2023, 32, 100345. [Google Scholar] [CrossRef]
- Markazi, A.D.; Perez, V.; Sifri, M.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of whole yeast cell product supplementation (CitriStim®) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017, 96, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Selvaraj, R.K. Effect of killed whole yeast cell prebiotic supplementation on broiler performance and intestinal immune cell parameters. Poult. Sci. 2012, 91, 107–111. [Google Scholar] [CrossRef]
- Kim, G.; Seo, Y.M.; Kim, C.H.; Paik, I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011, 90, 75–82. [Google Scholar] [CrossRef]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Franca, M.S.; Williams, S.M.; Gogal, R.M., Jr.; Ritz, C.W.; Kim, W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 2018, 97, 2525–2533. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wu, Y.S.; Chen, J.; Chen, Y. Modulations of growth performance, gut microbiota, and inflammatory cytokines by trehalose on Salmonella Typhimurium-challenged broilers. Poult. Sci. 2020, 99, 4034–4043. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Simon, O.; Jadamus, A.; Vahjen, W. Probiotic feed additives-effectiveness and expected modes of action. J. Anim. Feed. Sci. 2001, 10, 51–68. [Google Scholar] [CrossRef]
- Kabir, S.L.; Rahman, M.M.; Rahman, M.B.; Rahman, M.M.; Ahmed, S.U. The dynamics of probiotics on growth performance and immune response in broilers. Int. J. Poult. Sci. 2004, 3, 361–364. [Google Scholar]
- Krysiak, K.; Konkol, D.; Korczyński, M. Overview of the use of probiotics in poultry production. Animals 2021, 11, 1620. [Google Scholar] [CrossRef]
- Menconi, A.; Kallapura, G.; Latorre, J.D.; Morgan, M.J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci. Microbiota Food Health 2014, 33, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Kang, D. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian-Australas. J. Anim. Sci. 2017, 30, 1332. [Google Scholar] [CrossRef]
- Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.Y.; Chio, J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013, 92, 663–670. [Google Scholar] [CrossRef]
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020, 11, 29. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Applegate, T.J.; Selvaraj, R.K. Effect of Bacillus subtilis and Bacillus licheniformis probiotic supplementation on cecal Salmonella load in broilers challenged with salmonella. J. Appl. Poult. Res. 2020, 29, 808–816. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; Rizk, A.M.; Suzuki, T.; Elmonir, W.; Nassef, E.; Shukry, M.; Germoush, M.O.; Farrag, F.; Bin-Jumah, M. Evaluation of Bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals 2020, 10, 1023. [Google Scholar] [CrossRef]
- Menconi, A.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Immuno-modulation and anti-inflammatory effects of antibiotic growth promoters versus probiotics in the intestinal tract. J. Microbiol. Res. Rev. 2014, 2, 62–67. [Google Scholar]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals 2019, 10, 70. [Google Scholar] [CrossRef]
- Dianawati, D.; Mishra, V.; Shah, N.P. Effect of drying methods of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris on secondary protein structure and glass transition temperature as studied by Fourier transform infrared and differential scanning calorimetry. J. Dairy Sci. 2013, 96, 1419–1430. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Khomayezi, R.; Adewole, D. Probiotics, prebiotics, and synbiotics: An overview of their delivery routes and effects on growth and health of broiler chickens. Worlds Poult. Sci. J. 2022, 78, 57–81. [Google Scholar] [CrossRef]
- Yadav, A.S.; Kolluri, G.; Gopi, M.; Karthik, K.; Singh, Y. Exploring alternatives to antibiotics as health promoting agents in poultry—A review. J. Exp. Biol. 2016, 4, 368–383. [Google Scholar]
- Mohammed, A.A.; Jacobs, J.A.; Murugesan, G.R.; Cheng, H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018, 97, 1101–1108. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Żbikowski, A.; Szeleszczuk, P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 2020, 10, 4281. [Google Scholar] [CrossRef]
- Luoma, A.; Markazi, A.; Shanmugasundaram, R.; Murugesan, G.R.; Mohnl, M.; Selvaraj, R. Effect of synbiotic supplementation on layer production and cecal Salmonella load during a Salmonella challenge. Poult. Sci. 2017, 96, 4208–4216. [Google Scholar] [CrossRef]
- Villagrán-de la Mora, Z.; Vázquez-Paulino, O.; Avalos, H.; Ascencio, F.; Nuño, K.; Villarruel-López, A. Effect of a synbiotic mix on lymphoid organs of broilers infected with salmonella typhimurium and clostridium perfringens. Animals 2020, 10, 886. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 2020, 10, 323–330. [Google Scholar] [CrossRef]
- Van Thu, T.; Foo, H.L.; Loh, T.C.; Bejo, M.H. Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. Afr. J. Biotechnol. 2011, 10, 1359–1363. [Google Scholar]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Zulkifli, I.; Samsudin, A.A.; Mustapha, N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021, 100, 100908. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017, 96, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.W.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Awis, Q.S. Egg production, faecal pH and microbial population, small intestine morphology, and plasma and yolk cholesterol in laying hens given liquid metabolites produced by Lactobacillus plantarum strains. Br. Poult. Sci. 2012, 53, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chaney, W.E.; Naqvi, S.A.; Gutierrez, M.; Gernat, A.; Johnson, T.J.; Petry, D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms 2022, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Chaney, W.E.; McBride, H.; Girgis, G. Effect of a Saccharomyces cerevisiae Postbiotic Feed Additive on Salmonella Enteritidis Colonization of Cecal and Ovarian Tissues in Directly Challenged and Horizontally Exposed Layer Pullets. Animals 2023, 13, 1186. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Nabi, F.; Shah, Q.A.; Alagawany, M.; Fazlani, S.A.; Khalid, M.; Soomro, F.; Khand, F.M.; Farag, M.R. The role of early feeding in improving performance and health of poultry: Herbs and their derivatives. Worlds Poult. Sci. J. 2022, 78, 499–513. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in poultry and swine nutrition—A review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Pourali, M.; Mirghelenj, S.A.; Kermanshahi, H. Effects of garlic powder on productive performance and immune response of broiler chickens challenged with Newcastle Disease Virus. Glob. Vet. 2010, 4, 616–621. [Google Scholar]
- Chalghoumi, R.; Belgacem, A.; Trabelsi, I.; Bouatour, Y.; Bergaoui, R. Effect of dietary supplementation with probiotic or essential oils on growth performance of broiler chickens. Int. J. Poult. Sci. 2013, 12, 538–544. [Google Scholar] [CrossRef]
- Asadi, N.; Husseini, S.D.; Tohidian, M.; Abdali, N.; Mimandipoure, A.; Rafieian-Kopaei, M.; Bahmani, M. Performance of broilers supplemented with peppermint (Mentha piperita L.) powder. J. Evid. Based Complement. Altern. Med. 2017, 22, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.B.; Al-Rubaee, M.A.; Jalil, A.G. Effect of Ginger (Zingiber officinale) on Performance and. Int. J. Poult. Sci. 2012, 11, 143–146. [Google Scholar] [CrossRef]
- Ghosh, T.; Kumar, A.; Sati, A.; Mondal, B.C.; Singh, S.K.; Kumar, R. Effect of dietary supplementation of herbal feed additives (black cumin, garlic and turmeric) in combination with linseed oil on production performance of white leghorn laying chickens. J. Entomol. Zool. Stud. 2020, 8, 478–482. [Google Scholar]
- El-Ghany, A. Phytobiotics in poultry industry as growth promoters, antimicrobials and immunomodulators—A review. J. World’s Poult. Res. 2020, 10, 571–579. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Filippova, V.A.; Kochish, I.I.; Yildirim, E.A.; Ilina, L.A.; Dubrovin, A.V.; Brazhnik, E.A.; Novikova, N.I.; Novikova, O.B.; Dmitrieva, M.E. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella Enteritidis and fed a phytobiotic. Animals 2019, 9, 615. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Li, C.; Wang, M.; Chen, W.; Zhou, C.; Wei, P. The effect of oregano essential oil on the prevention and treatment of Salmonella pullorum and Salmonella gallinarum infections in commercial Yellow-chicken breeders. Front. Vet. Sci. 2022, 9, 1058844. [Google Scholar] [CrossRef]
- Salem, W.M.; El-Hamed, D.M.S.; Sayed, W.F.; Elamary, R.B. Alterations in virulence and antibiotic resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: The bactericidal efficacy of Allium sativum. Microb. Pathog. 2017, 108, 91–100. [Google Scholar] [CrossRef]
- Kollanoor-Johny, A.; Mattson, T.; Baskaran, S.A.; Amalaradjou, M.A.; Babapoor, S.; March, B.; Valipe, S.; Darre, M.; Hoagland, T.; Schreiber, D. Reduction of Salmonella enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012, 78, 2981–2987. [Google Scholar] [CrossRef]
- Vicente, J.L.; Lopez, C.; Avila, E.; Morales, E.; Hargis, B.M.; Tellez, G. Effect of dietary natural capsaicin on experimental Salmonella enteritidis infection and yolk pigmentation in laying hens. Int. J. Poult. Sci. 2007, 6, 393–396. [Google Scholar] [CrossRef]
- Voyles, B.A. The Biology of Viruses; Mosby-Year Book. Inc.: St. Louis, MO, USA, 1993. [Google Scholar]
- Iqbal, A.; Hasni, S.; Sajjad-ur-Rahman; Aslam, R.; Khan, K. Preparation and evaluation of bacteriophage lysate specific for Salmonella typhimurium. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 828–835. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Guenther, K.L.; Huff, W.; Donoghue, A.M.; Donoghue, D.J.; Hargis, B.M. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult. Sci. 2005, 84, 1141–1145. [Google Scholar] [CrossRef]
- Henriques, A.; Sereno, R.; Almeida, A. Reducing Salmonella horizontal transmission during egg incubation by phage therapy. Foodborne Pathog. Dis. 2013, 10, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef] [PubMed]
- Kimminau, E.A.; Russo, K.N.; Karnezos, T.P.; Oh, H.G.; Lee, J.J.; Tate, C.C.; Baxter, J.A.; Berghaus, R.D.; Hofacre, C.L. Bacteriophage in-feed application: A novel approach to preventing Salmonella Enteritidis colonization in chicks fed experimentally contaminated feed. J. Appl. Poult. Res. 2020, 29, 930–936. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Li, P.; Huang, J.; Cui, K.; Liang, L.; Lin, F.; Lu, Z.; Sun, S. Research Note: Therapeutic effect of a Salmonella phage combination on chicks infected with Salmonella Typhimurium. Poult. Sci. 2023, 102, 102715. [Google Scholar] [CrossRef]
- Malik, D.J. Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.; Roach, D.R. Phage therapy in the resistance era: Where do we stand and where are we going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousalkar, K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines 2020, 8, 696. [Google Scholar] [CrossRef]
- Tennant, S.M.; Levine, M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine 2015, 33, C36–C41. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Methner, U.; Rychlik, I.; Nagy, B.; Velge, P.; Martin, G.; Foster, N.; Ducatelle, R.; Barrow, P.A. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol. Infect. 2005, 133, 959–978. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lu, T.; Chen, Y.; Yu, H.; Wu, C.; Yang, W. Safety of bivalent live attenuated Salmonella vaccine and its protection against bacterial shedding and tissue invasion in layers challenged with Salmonella. Poult. Sci. 2022, 101, 101943. [Google Scholar] [CrossRef]
- Groves, P.J.; Sharpe, S.M.; Muir, W.I.; Pavic, A.; Cox, J.M. Live and inactivated vaccine regimens against caecal Salmonella Typhimurium colonisation in laying hens. Aust. Vet. J. 2016, 94, 387–393. [Google Scholar] [CrossRef]
- Tan, S.; Gyles, C.L.; Wilkie, B.N. Evaluation of an aroA mutant Salmonella typhimurium vaccine in chickens using modified semisolid Rappaport Vassiliadis medium to monitor faecal shedding. Vet. Microbiol. 1997, 54, 247–254. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Park, S.; Park, J.; Kirthika, P.; Lee, J.H. O-antigen-deficient, live, attenuated Salmonella typhimurium confers efficient uptake, reduced cytotoxicity, and rapid clearance in chicken macrophages and lymphoid organs and induces significantly high protective immune responses that protect chickens against Salmonella infection. Dev. Comp. Immunol. 2020, 111, 103745. [Google Scholar] [PubMed]
- Eeckhaut, V.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Oral vaccination with a live Salmonella Enteritidis/Typhimurium bivalent vaccine in layers induces cross-protection against caecal and internal organ colonization by a Salmonella Infantis strain. Vet. Microbiol. 2018, 218, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Huang, T.; Shen, H.; Meng, C.; Jiao, X.; Pan, Z. Salmonella Enteritidis Subunit Vaccine Candidate Based on SseB Protein Co-Delivered with Simvastatin as Adjuvant. Pathogens 2022, 11, 443. [Google Scholar] [CrossRef]
- Crouch, C.F.; Nell, T.; Reijnders, M.; Donkers, T.; Pugh, C.; Patel, A.; Davis, P.; van Hulten, M.C.; de Vries, S.P. Safety and efficacy of a novel inactivated trivalent Salmonella enterica vaccine in chickens. Vaccine 2020, 38, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Rabie, N.S.; Amin Girh, Z. Bacterial vaccines in poultry. Bull. Natl. Res. Cent. 2020, 44, 15. [Google Scholar] [CrossRef]
- Singh, B.R. Salmonella vaccines for animals and birds and their future perspective. Open Vaccine J. 2009, 2, 100–112. [Google Scholar] [CrossRef]
- Deguchi, K.; Yokoyama, E.; Honda, T.; Mizuno, K. Efficacy of a novel trivalent inactivated vaccine against the shedding of Salmonella in a chicken challenge model. Avian Dis. 2009, 53, 281–286. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.Y.; Akerele, G.O.; Al Hakeem, W.G.; Renu, S.; Shanmugasundaram, R.; Selvaraj, R.K. A Novel approach against Salmonella: A review of polymeric nanoparticle vaccines for broilers and layers. Vaccines 2021, 9, 1041. [Google Scholar] [CrossRef]
- Huberman, Y.D.; Caballero-García, M.; Rojas, R.; Ascanio, S.; Olmos, L.H.; Malena, R.; Lomónaco, J.; Nievas, P.; Chero, P.; Lévano-Gracía, J. The efficacy of a trivalent inactivated salmonella vaccine combined with the live s. gallinarum 9R vaccine in young layers after experimental infections with s. enteritidis, s. typhimurium, and s. infantis. Vaccines 2022, 10, 1113. [Google Scholar] [CrossRef]
- Marouf, S.; Ibrahim, H.M.; El-Naggar, M.S.; Swelum, A.A.; Alqhtani, A.H.; El-Saadony, M.T.; El-Tarabily, K.A.; Salem, H.M. Inactivated pentavalent vaccine against mycoplasmosis and salmonellosis for chickens. Poult. Sci. 2022, 101, 102139. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.Y.; Renu, S.; Shanmugasundaram, R.; Akerele, G.O.; Gourapura, R.J.; Selvaraj, R.K. Salmonella chitosan nanoparticle vaccine administration is protective against Salmonella Enteritidis in broiler birds. PLoS ONE 2021, 16, e0259334. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.; Akerele, G.; Al-Hakeem, W.; Adams, D.; Gourapura, R.; Selvaraj, R. Immunization of broiler chickens with a killed chitosan nanoparticle Salmonella vaccine decreases Salmonella enterica serovar enteritidis load. Front. Physiol. 2022, 13, 920777. [Google Scholar] [CrossRef]
- Desin, T.S.; Wisner, A.L.S.; Lam, P.S.; Berberov, E.; Mickael, C.S.; Potter, A.A.; Köster, W. Evaluation of Salmonella enterica serovar Enteritidis pathogenicity island-1 proteins as vaccine candidates against S. Enteritidis challenge in chickens. Vet. Microbiol. 2011, 148, 298–307. [Google Scholar] [CrossRef]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens. Int. J. Nanomed. 2020, 15, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Vinod, N.; Oh, S.; Kim, S.; Choi, C.W.; Kim, S.C.; Jung, C. Chemically induced Salmonella enteritidis ghosts as a novel vaccine candidate against virulent challenge in a rat model. Vaccine 2014, 32, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.; Hensel, A.; Szostak, M.; Resch, S.; Lubitz, W. Bacterial ghosts as vaccine candidates for veterinary applications. J. Control. Release 2002, 85, 17–25. [Google Scholar] [CrossRef]
- Haidinger, W.; Szostak, M.P.; Jechlinger, W.; Lubitz, W. Online monitoring of Escherichia coli ghost production. Appl. Environ. Microbiol. 2003, 69, 468–474. [Google Scholar] [CrossRef]
- Jawale, C.V.; Lee, J.H. Comparative evaluation of Salmonella Enteritidis ghost vaccines with a commercial vaccine for protection against internal egg contamination with Salmonella. Vaccine 2014, 32, 5925–5930. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of chicken with flagellin adjuvanted Salmonella enteritidis bacterial ghosts confers complete protection against chicken salmonellosis. Poult. Sci. 2021, 100, 101205. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Poh, C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef]
- Gao, X.; Xu, K.; Yang, G.; Shi, C.; Huang, H.; Wang, J.; Yang, W.; Liu, J.; Liu, Q.; Kang, Y. Construction of a novel DNA vaccine candidate targeting F gene of genotype VII Newcastle disease virus and chicken IL-18 delivered by Salmonella. J. Appl. Microbiol. 2019, 126, 1362–1372. [Google Scholar] [CrossRef]
- Yu, X.; Jia, R.; Huang, J.; Shu, B.; Zhu, D.; Liu, Q.; Gao, X.; Lin, M.; Yin, Z.; Wang, M. Attenuated Salmonella typhimurium delivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge. Vet. Res. 2012, 43, 56. [Google Scholar] [CrossRef] [PubMed]
- Oshop, G.L.; Elankumaran, S.; Heckert, R.A. DNA vaccination in the avian. Vet. Immunol. Immunopathol. 2002, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Zhao, S.; Chang, X.; Liu, J.; Zeng, X.; Li, Y.; Chen, H. Protective efficacy of an H5N1 DNA vaccine against challenge with a lethal H5N1 virus in quail. Avian Dis. 2012, 56, 937–939. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. https://doi.org/10.3390/microorganisms11112814
Shaji S, Selvaraj RK, Shanmugasundaram R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms. 2023; 11(11):2814. https://doi.org/10.3390/microorganisms11112814
Chicago/Turabian StyleShaji, Syamily, Ramesh K. Selvaraj, and Revathi Shanmugasundaram. 2023. "Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies" Microorganisms 11, no. 11: 2814. https://doi.org/10.3390/microorganisms11112814
APA StyleShaji, S., Selvaraj, R. K., & Shanmugasundaram, R. (2023). Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms, 11(11), 2814. https://doi.org/10.3390/microorganisms11112814






