Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Endophytic Bacterial Strains from C. officinalis Plants
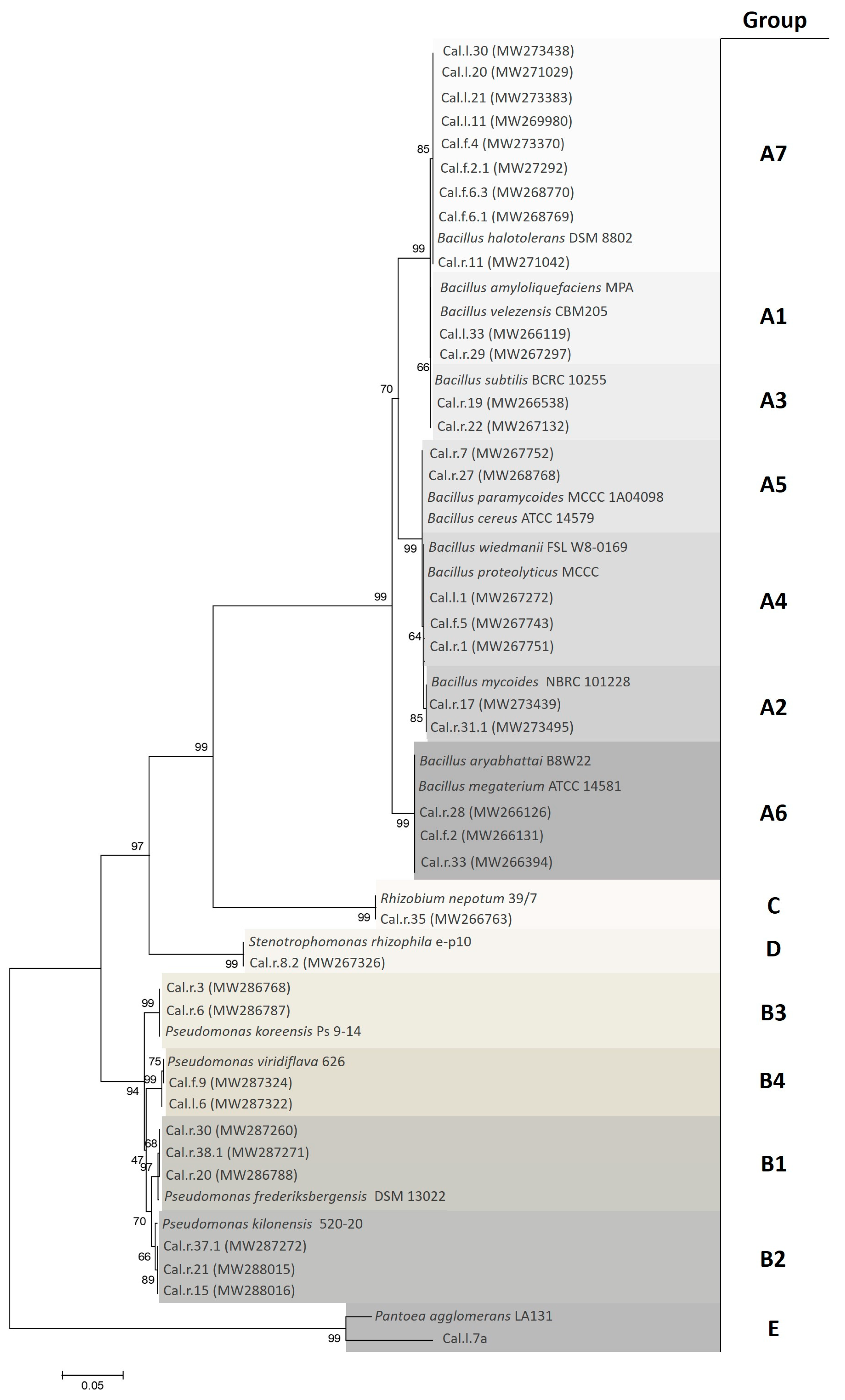
2.2. Molecular Characterization of Endophytic Bacteria Based on 16S rRNA
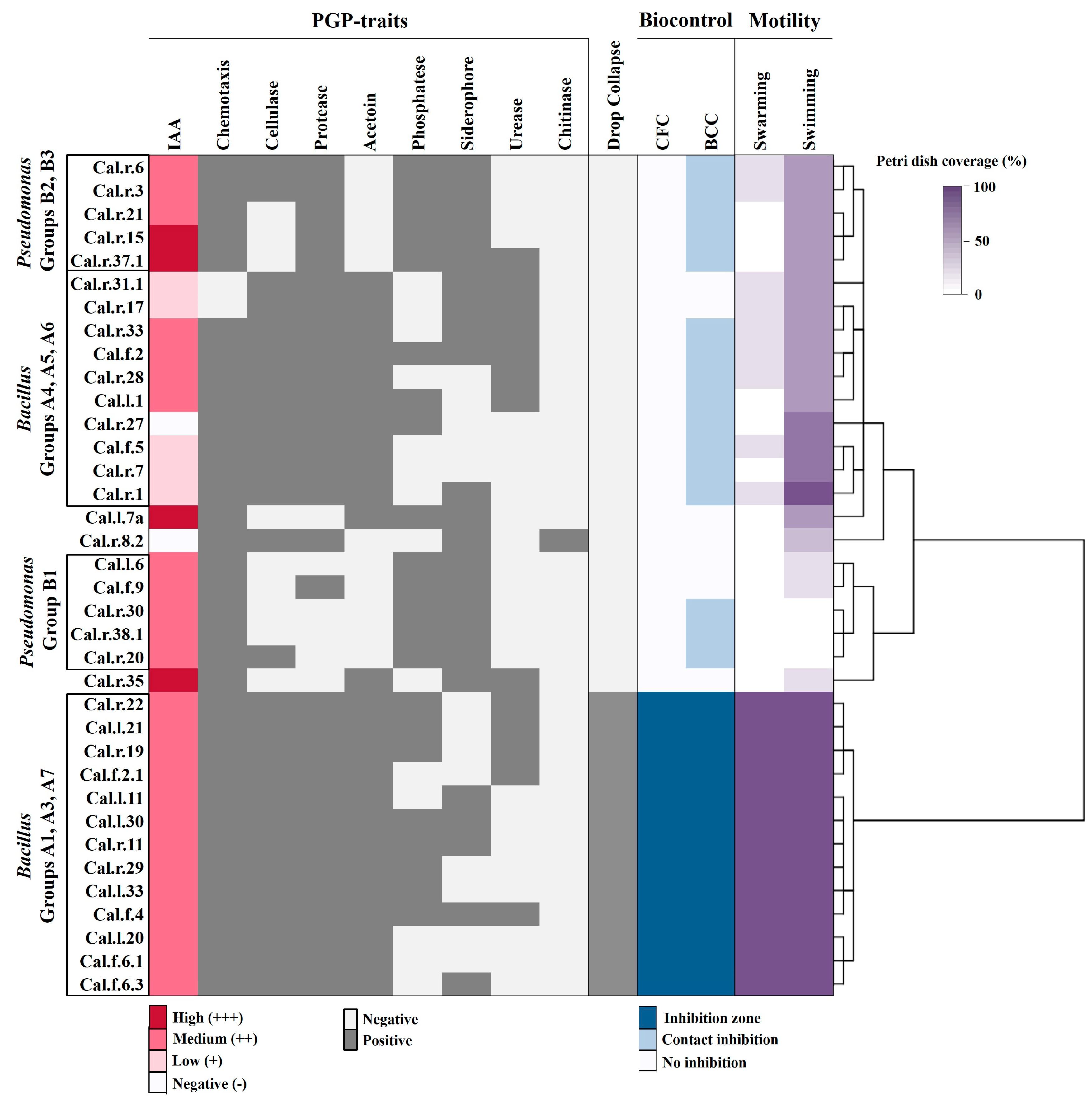
2.3. In Vitro Screening for Plant Growth Promoting (PGP) Traits
2.4. Swarming, Swimming and Chemotactic Motility
2.5. In Vitro Biocontrol Activity
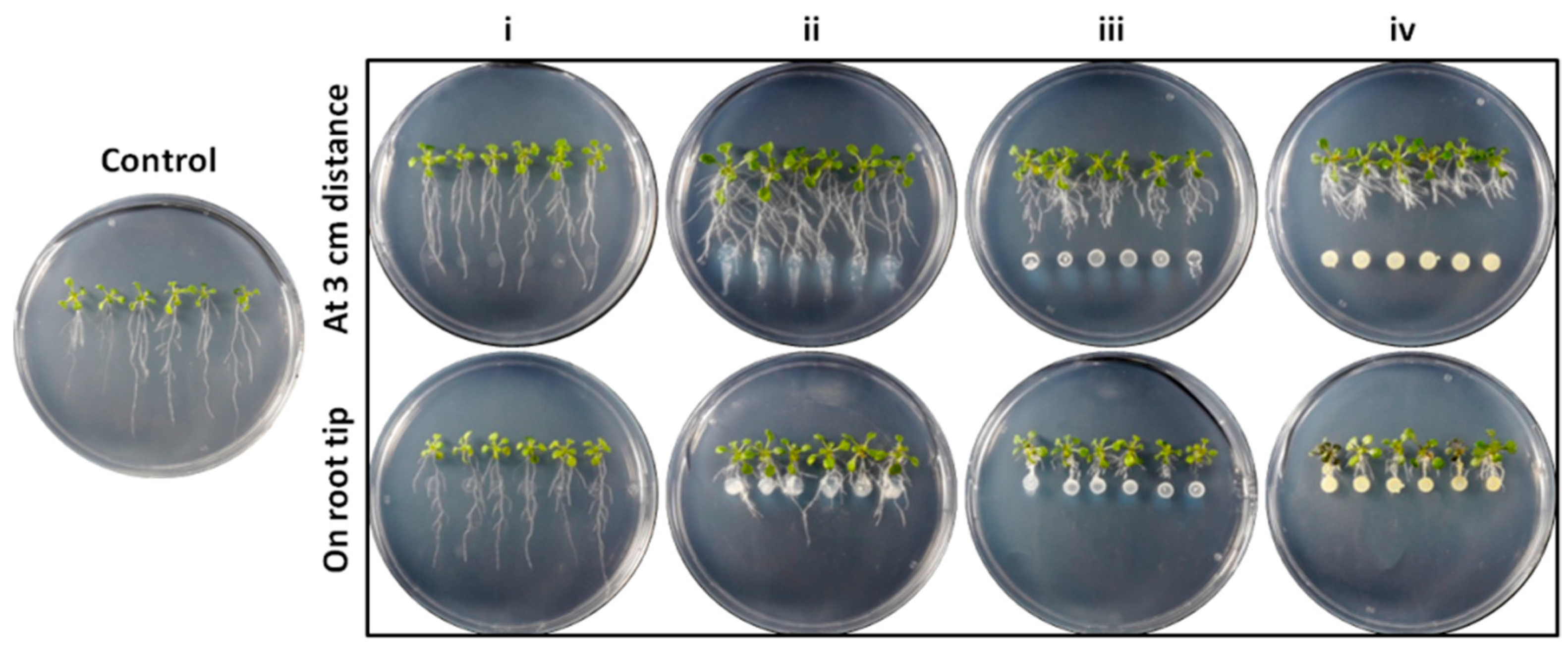
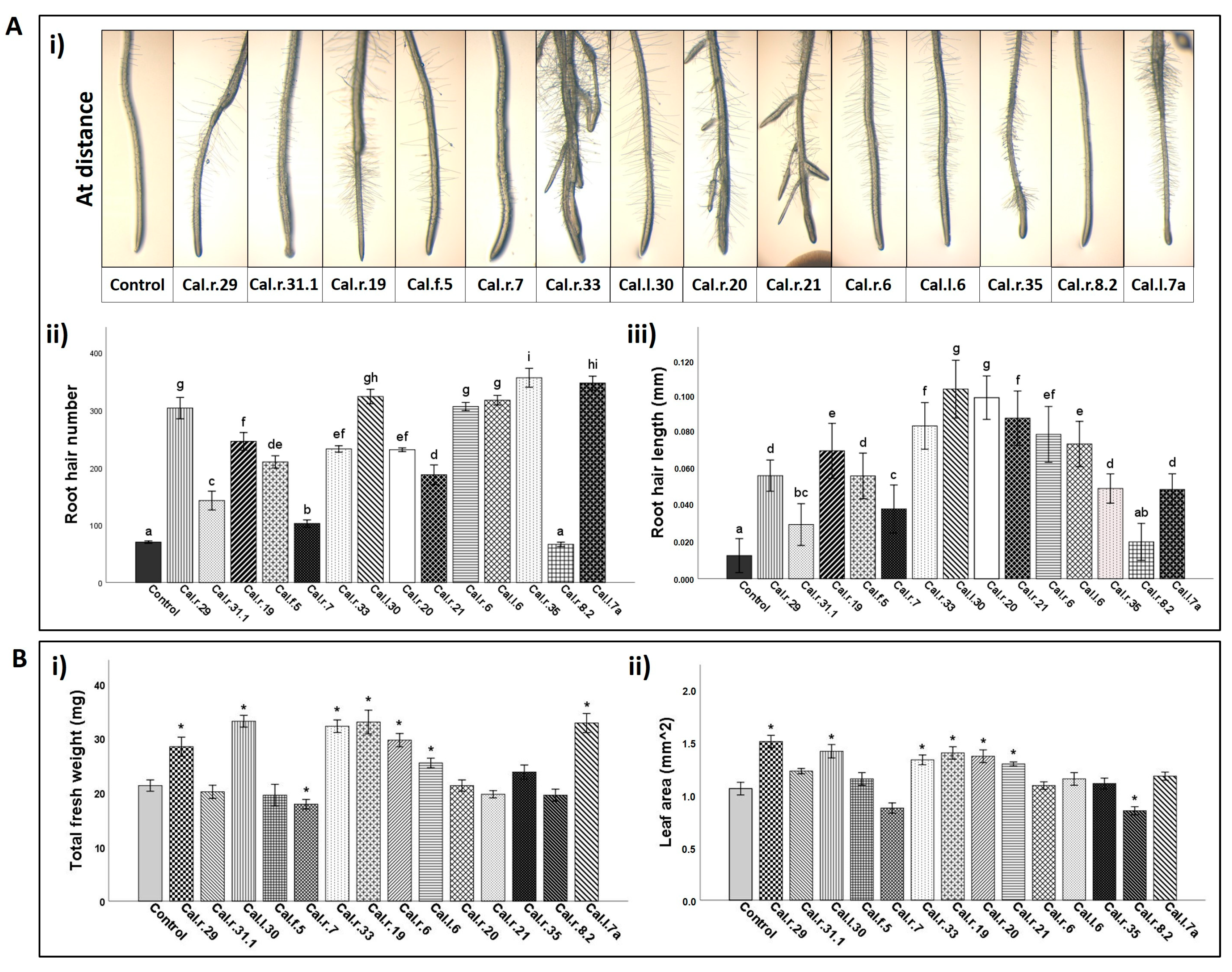
2.6. Bacterial Inoculation on A. thaliana Seedlings
2.7. Phenotypic and Data Analysis of A. thaliana Plantlets
2.8. Bio-Priming of S. lycopersicum var. Chondrokatsari Messinias Seeds with Endophytic Bacteria
2.9. Detached Fruit Assay
2.10. Extraction and Evaluation of Bacterial Agar Diffusible Secreted Metabolites
2.11. Statistical Analysis
3. Results
3.1. Identification and Characterization of the Isolated Endophytic Bacterial Strains
3.2. Plant Growth Promoting Activities
3.3. Survival in Variable Growth Conditions
3.4. Effect on A. thaliana Growth Characteristics
3.5. Plant Growth Effect on S. lycopersicum var. Chondrokatsari Messinias Seedlings
3.6. Ex Vivo Biocontrol of Botrytis cinerea on Tomato Detached Fruit
3.7. Secretion of Bioactive Bacterial Agar Diffusible Secondary Metabolites When Grown Singly or against B. cinerea
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018. [Google Scholar]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.Z.; Varma, A.; Qin, S.; Xiong, Z.; Huang, H.Y.; Zhu, W.Y.; Zhao, L.X.; Xu, L.H.; Zhang, S.; et al. An endophytic Pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS ONE 2012, 7, e51410. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Gupta, S.D.; Ghosh, S. Evaluation of anti-oxidative activity and UV absorption potential of the extracts of Aloe vera L. gel from different growth periods of plants. Ind. Crops Prod. 2013, 49, 712–719. [Google Scholar] [CrossRef]
- Morsy, N. Phytochemical analysis of biologically active constituents of medicinal plants. Main Group Chem. 2014, 13, 7–21. [Google Scholar] [CrossRef]
- Song, X.; Wu, H.; Yin, Z.; Lian, M.; Yin, C. Endophytic bacteria isolated from Panax ginseng improves ginsenoside accumulation in adventitious ginseng root culture. Molecules 2017, 22, 837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, B.; Xu, M.; Cao, X.; Xue, L.; Jiang, J. Evaluation of the Effect of Plant Growth Promoting Endophytic Bacteria from Pinellia ternata Using an Efficient Organic Silica Hybrid Monolithic Column. J. Biobased Mater. Bioenergy 2017, 11, 282–290. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Mohamad, O.A.A.; Li, L.; Ma, J.B.; Hatab, S.; Xu, L.; Guo, J.W.; Rasulov, B.A.; Liu, Y.H.; Hedlund, B.P.; Li, W.J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- Shurigin, V.; Davranov, K.; Wirth, S.; Egamberdieva, D.; Bellingrath-Kimura, S.D. Medicinal plants with phytotoxic activity harbour endophytic bacteria with plant growth inhibitory properties. Environ. Sustain. 2018, 1, 209–215. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Stone, J.K.; Bacon, C.W.; White, J.F. An overview of endophytic microbes: Endophytism defined. Microb. Endophytes 2000, 3, 29–33. [Google Scholar]
- Schulz, B.; Boyle, C. What are Endophytes? In Microbial Root Endophytes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–13. [Google Scholar]
- El-Deeb, B.; Fayez, K.; Gherbawy, Y. Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant Interact. 2013, 8, 56–64. [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of grapevine flowers, berries, and seeds: Identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome analysis of Pseudomonas fluorescens PCL1751: A rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 10, e0140231. [Google Scholar] [CrossRef]
- Hassan, S.E.D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Köberl, M.; Schmidt, R.; Ramadan, E.M.; Bauer, R.; Berg, G. The microbiome of medicinal plants: Diversity and importance for plant growth, quality and health. Front. Microbial. 2013, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy Mohamad, O.A.; Ma, J.B.; Liu, Y.H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.J.; Li, L. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front. Plant Sci. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, N.; Jones, E.; Monk, J.; Ridgway, H. Community structure, diversity and potential of endophytic bacteria in the primitive New Zealand medicinal plant Pseudowintera colorata. Plants 2020, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Baldan, E.; Nigris, S.; Romualdi, C.; D’Alessandro, S.; Clocchiatti, A.; Zottini, M.; Stevanato, P.; Squartini, A.; Baldan, B. Beneficial bacteria isolated from grapevine inner tissues shape Arabidopsis thaliana roots. PLoS ONE 2015, 10, e0140252. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Kang, S.M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Monteiro, C.; Vega, A.L.; Castro, P.M. Endophytic culturable bacteria colonizing Lavandula dentata L. plants: Isolation, characterization and evaluation of their plant growth-promoting activities. Ecol. Eng. 2016, 87, 91–97. [Google Scholar] [CrossRef]
- Aeron, A.; Maheshwari, D.K.; Meena, V.S. Endophytic bacteria promote growth of the medicinal legume Clitoria ternatea L. by chemotactic activity. Arch. Microbiol. 2020, 202, 1049–1058. [Google Scholar] [CrossRef]
- Basheer, J.; Ravi, A.; Mathew, J.; Krishnankutty, R.E. Assessment of plant-probiotic performance of novel endophytic Bacillus sp. in talc-based formulation. Probiotics Antimicrob Proteins. 2019, 11, 256–263. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- de Souza, A.R.; De Souza, S.A.; De Oliveira, M.V.V.; Ferraz, T.M.; Figueiredo, F.A.M.M.A.; Da Silva, N.D.; Rangel, P.L.; Panisset, C.R.S.; Olivares, F.L.; Campostrini, E. Endophytic colonization of Arabidopsis thaliana by Gluconacetobacter diazotrophicus and its effect on plant growth promotion, plant physiology, and activation of plant defense. Plant Soil 2016, 399, 257–270. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Ebrahimi, A.; Mahzonieh, M.R.; Lotfalian, S. Antibacterial Activities of Bacterial Endophytes Isolated From Zataria multiflora, Achillea willhelmsii and Calendula officinalis L. Against Some Human Nosocomial Pathogens. Zahedan J. Res Med. Sci. 2016, 18, e2482. [Google Scholar] [CrossRef]
- Kaki, A.A.; Chaouche, N.K.; Dehimat, L.; Milet, A.; Youcef-Ali, M.; Ongena, M.; Thonart, P. Biocontrol and plant growth promotion characterization of Bacillus species isolated from Calendula officinalis rhizosphere. Indian J. Microbiol. 2013, 53, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Müller, H.; Ramadan, E.M.; Berg, G. Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS ONE 2011, 6, e24452. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Zang, W.; Ping, W.; Chi, D. Diversity and ecological distribution of endophytic fungi associated with medicinal plants. Sci. China Life Sci. 2008, 51, 751–759. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [Google Scholar] [CrossRef]
- Moore, E.; Arnscheidt, A.; Kruger, A.; Strompl, C.; Mau, M. Simplified protocols for the preparation of genomic DNA from bacterial cultures. In Molecular Microbial Ecology Manual, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 1, pp. 3–18. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Kumar, R.S.; Ayyadurai, N.; Pandiaraja, P.; Reddy, A.V.; Venkateswarlu, Y.; Prakash, O.; Sakthivel, N. Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad-spectrum antifungal activity and biofertilizing traits. J. Appl. Microbiol. 2005, 98, 145–154. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, S. Methyl Red and Voges-Proskauer Test Protocols; American Society for Microbiology: Washington, DC, USA, 2009; pp. 1–8. [Google Scholar]
- Agrawal, T.; Kotasthane, A.S. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus. 2012, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef]
- Xu, S.J.; Kim, B.S. Biocontrol of Fusarium crown and root rot and promotion of growth of tomato by Paenibacillus strains isolated from soil. Mycobiology 2014, 42, 158–166. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Thomloudi, E.E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated Genomic and Metabolomic Analysis Illuminates Key Secreted Metabolites Produced by the Novel Endophyte Bacillus halotolerans Cal. l. 30 Involved in Diverse Biological Control Activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Baira, E.; Papadimitriou, K.; Venieraki, A.; Katinakis, P. Genomic and Metabolomic Insights into Secondary Metabolites of the Novel Bacillus halotolerans Hil4, an Endophyte with Promising Antagonistic Activity against Gray Mold and Plant Growth Promoting Potential. Microorganisms 2021, 9, 2508. [Google Scholar] [CrossRef]
- Beiranvand, M.; Amin, M.; Hashemi-Shahraki, A.; Romani, B.; Yaghoubi, S.; Sadeghi, P. Antimicrobial activity of endophytic bacterial populations isolated from medical plants of Iran. Iran. J. Microbial. 2017, 9, 11. [Google Scholar]
- Khalid, K.A.; Da Silva, J.T. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- Shurigin, V.; Alaylar, B.; Davranov, K.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. Diversity and biological activity of culturable endophytic bacteria associated with marigold (Calendula officinalis L.). AIMS Microbiol. 2021, 7, 336–353. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Matzén, S.; Petersen, M.A.; Bejai, S.; Meijer, J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016, 92, fiw070. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Cueva-Yesquén, L.G.; Goulart, M.C.; Attili de Angelis, D.; Nopper Alves, M.; Fantinatti-Garboggini, F. Multiple plant growth-promotion traits in endophytic bacteria retrieved in the vegetative stage from passionflower. Front. Plant Sci. 2021, 11, 621740. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.I.; Qing, C.; Sze, D.M.Y.; Roufogalis, B.D.; Neilan, B.A. Culturable endophytes of medicinal plants and the genetic basis for their bioactivity. Microb. Ecol. 2012, 64, 431–449. [Google Scholar] [CrossRef]
- Webster, G.; Mullins, A.J.; Cunningham-Oakes, E.; Renganathan, A.; Aswathanarayan, J.B.; Mahenthiralingam, E.; Vittal, R.R. Culturable diversity of bacterial endophytes associated with medicinal plants of the Western Ghats, India. FEMS Microbiol. Ecol. 2020, 96, fiaa147. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014, 80, 758–771. [Google Scholar] [CrossRef]
- Bavaresco, L.G.; Osco, L.P.; Araujo, A.S.F.; Mendes, L.W.; Bonifacio, A.; Araújo, F.F. Bacillus subtilis can modulate the growth and root architecture in soybean through volatile organic compounds. Theor. Exp. Plant Physiol. 2020, 32, 99–108. [Google Scholar] [CrossRef]
- Chu, T.N.; Bui, L.V.; Hoang, M.T.T. Pseudomonas PS01 Isolated from Maize Rhizosphere Alters Root System Architecture and Promotes Plant Growth. Microorganisms 2020, 8, 471. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Rodríguez, R.; Coria-Arellano, J.L.; López-Bucio, J.; Sánchez-Salas, J.; Muro-Pérez, G.; Castañeda-Gaytán, G.; Sáenz-Mata, J. Halophilic rhizobacteria from Distichlis spicata promote growth and improve salt tolerance in heterologous plant hosts. Symbiosis 2017, 73, 179–189. [Google Scholar] [CrossRef]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and biopriming improve Medicago truncatula seed germination and upregulate DNA repair and antioxidant genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
- Rozier, C.; Gerin, F.; Czarnes, S.; Legendre, L. Biopriming of maize germination by the plant growth-promoting rhizobacterium Azospirillum lipoferum CRT1. J. Plant Physiol. 2019, 237, 111–119. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Shadangi, S.; Mohapatra, P.K.D.; Panneerselvam, P. Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial relationships between endophytic bacteria and medicinal plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Gao, J.L.; Khan, M.S.; Sun, Y.C.; Xue, J.; Du, Y.; Yang, C.; Chebotar, V.K.; Tikunov, V.S.; Rubanov, I.N.; Chen, X.; et al. Characterization of an Endophytic Antagonistic Bacterial Strain Bacillus halotolerans LBG-1-13 with Multiple Plant Growth-Promoting Traits, Stress Tolerance, and Its Effects on Lily Growth. BioMed Res. Int. 2022, 2022, 5960004. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.; Deravel, J.; Krier, F.; Béchet, M.; Ongena, M.; Jacques, P. Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. 2018, 25, 29910–29920. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, N.; Huang, Q.; Raza, W.; Li, R.; Vivanco, J.M.; Shen, Q. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015, 5, srep13438. [Google Scholar] [CrossRef] [PubMed]
- Aleti, G.; Lehner, S.; Bacher, M.; Compant, S.; Nikolic, B.; Plesko, M.; Schuhmacher, R.; Sessitsch, A.; Brader, G. Surfactin variants mediate species-specific biofilm formation and root colonization in Bacillus. Environ. Microbiol. 2016, 18, 2634–2645. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Adriano-Anaya, M.L.; Salvador-Figueroa, M.; Ocampo, J.A.; García-Romera, I. Hydrolytic enzyme activities in maize (Zea mays) and sorghum (Sorghum bicolor) roots inoculated with Gluconacetobacter diazotrophicus and Glomus intraradices. Soil Biol. Biochem. 2006, 38, 879–886. [Google Scholar] [CrossRef]
- Mostajeran, A.; Amooaghaie, R.; Emtiazi, G. The participation of the cell wall hydrolytic enzymes in the initial colonization of Azospirillum brasilense on wheat roots. Plant Soil 2007, 291, 239–248. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef]
- De Bruijn, I.; de Kock, M.J.; de Waard, P.; van Beek, T.A.; Raaijmakers, J.M. Massetolide A biosynthesis in Pseudomonas fluorescens. J. Bacteriol. 2008, 190, 2777–2789. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Wu, L.; Wang, L.; Gao, W.; Jiang, J.; Wu, Y. Inhibitory effect of Bacillus subtilis WL-2 and its IturinA lipopeptides against Phytophthora infestans. bioRxiv 2019, 751131. [Google Scholar]
- Ambrico, A.; Trupo, M. Efficacy of cell free supernatant from Bacillus subtilis ET-1, an Iturin A producer strain, on biocontrol of green and gray mold. Postharvest Biol. Technol. 2017, 134, 5–10. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Blanco, D.; Venturini, M.E. The role of iturin A from B. amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiol. 2019, 82, 62–69. [Google Scholar] [CrossRef]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.-E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic Analysis and Secondary Metabolites Production of the Endophytic Bacillus velezensis Bvel1: A Biocontrol Agent against Botrytis cinerea Causing Bunch Rot in Post-Harvest Table Grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef]
- Dimkić, I.; Stanković, S.; Nišavić, M.; Petković, M.; Ristivojević, P.; Fira, D.; Berić, T. The profile and antimicrobial activity of Bacillus lipopeptide extracts of five potential biocontrol strains. Front. Microbiol. 2017, 8, 925. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Meziane, H.; Van Der Sluis, I.; Van Loon, L.C.; Höfte, M.; Bakker, P.A. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef]
- Pršić, J.; Ongena, M. Elicitors of plant immunity triggered by beneficial bacteria. Front. Plant Sci. 2020, 11, 594530. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring elicitors of the beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 to induce plant systemic resistance and their interactions with plant signaling pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Liebmann, B.; Kalantidis, K.; Vassilakos, N.; Skandalis, N. Bacillus amyloliquefaciens MBI600 differentially induces tomato defense signaling pathways depending on plant part and dose of application. Sci. Rep. 2019, 9, 19120. [Google Scholar] [CrossRef]
- Tunsagool, P.; Wang, X.; Leelasuphakul, W.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Chen, G.; Li, L. Metabolomic study of stress responses leading to plant resistance in mandarin fruit mediated by preventive applications of Bacillus subtilis cyclic lipopeptides. Postharvest Biol. Technol. 2019, 156, 110946. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Leelasuphakul, W.; McCollum, G. Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PLoS ONE 2014, 9, e109386. [Google Scholar] [CrossRef]
- Thomloudi, E.E.; Tsalgatidou, P.C.; Douka, D.; Spantidos, T.N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hell. Plant Prot. J. 2019, 12, 61–77. [Google Scholar] [CrossRef]





| Group | Bacterial Species | Strain | AMG | Total Fresh Weight (mg) | Primary Root Length (cm) | Lateral Root Number (N) | |||
|---|---|---|---|---|---|---|---|---|---|
| AD A | ORT B | AD A | ORT B | AD A | ORT B | ||||
| - | - | - | Control | 10.2 ± 1.29 | 10.3 ± 2.19 | 5.8 ± 0.49 | 5.05 ± 0.16 | 8.75 ± 1.55 | 8.75 ± 1.55 |
| A1 | B. velezensis | Cal.r.29 | ii | 25.00 ± 1.02 * | 18.65 ± 1.50 * | 3.55 ± 0.25 * | 1.78 ± 0.24 * | 21.08 ± 2.35 * | 12.17 ± 2.48 * |
| A2 | B. mycoides | Cal.r.31.1 | i | 12.98 ± 0.73 | 17.53 ± 1.49.* | 5.03 ± 0.39 * | 4.24 ± 0.14 * | 8.67 ± 1.61 | 10.58 ± 1.78 |
| A3 | B. subtilis | Cal.r.19 | ii | 27.40 ± 0.76 * | 15.15 ± 1.12 * | 3.31 ± 0.22 * | 2.17 ± 0.20 * | 18.5 ± 2.47 * | 11.50 ± 2.02 * |
| A4 | B. proteolyticus | Cal.f.5 | i | 13.40 ± 0.46 * | 17.88 ± 1.20 * | 4.23 ± 0.37 * | 5.07 ± 0.25 | 12.91 ± 1.72 * | 7.50 ± 1.57 |
| A5 | B. cereus | Cal.r.7 | i | 12.63 ± 1.66 | 13.72 ± 1.62 * | 4.91 ± 0.37 * | 5.63 ± 0.24 | 12.33 ± 1.61 * | 10.00 ± 2.41 |
| A6 | B. megaterium | Cal.r.33 | iii | 13.73 ± 0.54 * | 12.72 ± 1.07 * | 2.31 ± 0.29 * | 1.13 ± 0.09 * | 14.83 ± 2.17 * | 10.25 ± 1.06 |
| A7 | B. halotolerans | Cal.l.30 | ii | 31.92 ± 1.27 * | 17.78 ± 1.38 * | 3.34 ± 0.24 * | 3.56 ± 0.29 * | 17.25 ± 1.66 * | 12.42 ± 3.58 * |
| B1 | Pseudomonas sp. | Cal.r.20 | iii | 24.03 ± 2.24 * | 13.05 ± 1.59 * | 2.51 ± 0.45 * | 1.46 ± 0.81 * | 20.92 ± 4.44 * | 14.33 ± 1.61 * |
| B2 | P. kilonensis | Cal.r.21 | iii | 23.25 ± 1.66 * | 12.60 ± 0.88 | 2.79 ± 0.37 * | 1.64 ± 0.10 * | 23.17 ± 1.95 * | 23.42 ± 2.35 * |
| B3 | P. koreensis | Cal.r.6 | iii | 22.25 ± 2.02 * | 16.23 ± 1.49 * | 2.71 ± 0.29 * | 1.39 ± 0.09 * | 22.42 ± 2.02 * | 13.42 ± 1.08 * |
| B4 | P. viridiflava | Cal.l.6 | iii | 17.32 ± 2.82 * | 18.15 ± 1.34 * | 3.08 ± 0.42 * | 2.13 ± 0.18 * | 27.17 ± 1.64 * | 21.58 ± 2.54 * |
| C | Rhizobium sp. | Cal.r.35 | iv | 23.18 ± 1.66 * | 11.32 ± 1.15 | 2.08 ± 0.15 * | 1.15 ± 0.10 * | 26.92 ± 1.93 * | 9.67 ± 0.78 |
| D | Stenotrophomonas sp. | Cal.r.8.2 | i | 13.33 ± 2.97 | 13.38 ± 0.85 * | 5.73 ± 0.44 | 4.32 ± 0.24 * | 11.67 ± 2.43 * | 11.33 ± 2.77 * |
| E | Pantoea sp. | Cal.l.7a | iv | 20.68 ± 2.49 * | 10.82 ± 1.68 | 1.87 ± 0.23 * | 1.04 ± 0.17 * | 29.5 ± 2.51 * | 9.08 ± 1.44 |
| Bacterial Strains | % Tomato Seed Germination | |
|---|---|---|
| 3 dps | 8 dps | |
| Control | 83.00 ± 1.00 | 10.3 ± 2.19 |
| Cal.r.29 | 88.00 ± 3.00 | 18.65 ± 1.50 * |
| Cal.l.30 | 91.67 ± 2.52 * | 17.53 ± 1.49 * |
| Cal.f.4 | 90.00 ± 1.00 * | 15.15 ± 1.12 * |
| Cal.f.5 | 85.00 ± 1.73 | 17.88 ± 1.20 * |
| Cal.r.11 | 82.67 ± 3.06 | 13.72 ± 1.62 * |
| Cal.f.2.1 | 78.00 ± 3.00 | 12.72 ± 1.07 * |
| Cal.l.11 | 91.67 ± 1.53 * | 17.78 ± 1.38 * |
| Cal.r.33 | 90.33 ± 1.53 * | 13.05 ± 1.59 * |
| Cal.r.19 | 89.00 ± 2.65 * | 12.60 ± 0.88 |
| Cal.l.21 | 75.67 ± 2.52 * | 16.23 ± 1.49 * |
| Cal.r.20 | 72.33 ± 2.52 * | 18.15 ± 1.34 * |
| Cal.l.7a | 85.67 ± 3.22 | 11.32 ± 1.15 |
| Cal.r.6 | 81.00 ± 2.00 | 13.38 ± 0.85 * |
| Treatment | Disease Severity Index (%) | Disease Incidence (%) |
|---|---|---|
| Control | 72.52 ± 3.61a | 91.67 ± 2.89a |
| Cal.f.4 | 12.85 ± 2.57b | 13.33 ± 2.89b |
| Cal.r.29 | 11.49 ± 1.50b | 15.00 ± 5.00b |
| Cal.r.11 | 23.66 ± 1.32cd | 31.67 ± 7.64c |
| Cal.l.11 | 22.85 ± 0.66cd | 36.67 ± 7.64c |
| Cal.f.2.1 | 29.28 ± 3.98d | 35.00 ± 5.00c |
| Cal.l.30 | 17.49 ± 1.79bc | 25.00 ± 5.00bc |
| Cal.r.19 | 30.53 ± 1.63d | 38.33 ± 2.89c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsalgatidou, P.C.; Thomloudi, E.-E.; Nifakos, K.; Delis, C.; Venieraki, A.; Katinakis, P. Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA). Microorganisms 2023, 11, 206. https://doi.org/10.3390/microorganisms11010206
Tsalgatidou PC, Thomloudi E-E, Nifakos K, Delis C, Venieraki A, Katinakis P. Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA). Microorganisms. 2023; 11(1):206. https://doi.org/10.3390/microorganisms11010206
Chicago/Turabian StyleTsalgatidou, Polina C., Eirini-Evangelia Thomloudi, Kallimachos Nifakos, Costas Delis, Anastasia Venieraki, and Panagiotis Katinakis. 2023. "Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA)" Microorganisms 11, no. 1: 206. https://doi.org/10.3390/microorganisms11010206
APA StyleTsalgatidou, P. C., Thomloudi, E.-E., Nifakos, K., Delis, C., Venieraki, A., & Katinakis, P. (2023). Calendula officinalis—A Great Source of Plant Growth Promoting Endophytic Bacteria (PGPEB) and Biological Control Agents (BCA). Microorganisms, 11(1), 206. https://doi.org/10.3390/microorganisms11010206









