Synergistic Effects of Clonostachys rosea Isolates and Succinate Dehydrogenase Inhibitors Fungicides against Gray Mold on Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Pesticide
2.2. Fungicides Sensitivity Assessments of C. rosea and B. cinerea In Vitro
2.3. Effect of SDHI Fungicides to C. rosea Conidia Germination
2.4. Greenhouse Experiments
2.5. qPCR for Specific Quantification of C. rosea and B. cinerea
2.6. Statistical Analysis
3. Results
3.1. In Vitro Mycelial Growth Inhibition of C. rosea and B. cinerea by Differernt Fungicides
3.2. Inhibition Effect of Fungicides on the Germination Rate of C. rosea Conidium
3.3. Synergistic Effects of C. rosea Isolate 67-1 and SDHI Fungicides against Tomato Gray Mold in the Greenhouse
3.4. qPCR for Specific Quantification of C. rosea and B. cinerea
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis–the Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manage. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Veloukas, T.; Kalogeropoulou, P.; Markoglou, A.N.; Karaoglanidis, G.S. Fitness and competitive ability of Botrytis cinerea field isolates with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations. Phytopathology 2013, 104, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, M.K.; van den Bosch, F.; Powers, S.J.; Paveley, N.D. Fungicide resistance risk assessment based on traits associated with the rate of pathogen evolution. Pest Manage. Sci. 2015, 71, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhao, Y.; Ma, Z. Advances in understanding fungicide resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef] [PubMed]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Lei, T.; Sohail, M.A.; Wang, J.; Wang, H. Synergistic effects of Bacillus amyloliquefaciens SDTB009 and difenoconazole on Fusarium wilt of tomato. Plant Dis. 2022, 106, 2165–2171. [Google Scholar] [CrossRef]
- Bi, Y.; Yu, Z. Diterpenoids from Streptomyces sp. SN194 and their antifungal activity against Botrytis cinerea. J. Agric. Food Chem. 2016, 64, 8525–8529. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Meng, Z.; Zhang, S.; Dong, B.; Qiao, K. Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis. 2019, 103, 1991–1997. [Google Scholar] [CrossRef]
- Cota, L.V.; Maffia, L.A.; Mizubuti, E.S.G.; Macedo, P.E.F.; Antunes, R.F. Biological control of strawberry gray mold by Clonostachys rosea under field conditions. Biol. Control 2008, 46, 515–522. [Google Scholar] [CrossRef]
- Zaldúa, S.; Sanfuentes, E. Control of Botrytis cinerea in Eucalyptus globulus mini-cuttings using Clonostachys and Trichoderma Strains. Chil. J. Agric. Res. 2010, 70, 576–582. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Colonization of geranium foliage by Clonostachys rosea f. catenulata, a biological control agent of botrytis grey mould. Botany 2012, 90, 1–10. [Google Scholar] [CrossRef]
- Borges, Á.V.; Saraiva, R.M.; Maffia, L.A. Biocontrol of gray mold in tomato plants by Clonostachys rosea. Trop. Plant Pathol. 2015, 40, 71–76. [Google Scholar] [CrossRef]
- Mouekouba, L.D.O.; Zhang, L.L.; Guan, X.; Chen, X.L.; Chen, H.Y.; Zhang, J.; Zhang, J.F.; Li, J.F.; Yang, Y.J.; Wang, A. Analysis of Clonostachys rosea-induced resistance to tomato gray mold disease in tomato leaves. PLoS ONE 2014, 9, e102690. [Google Scholar] [CrossRef]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef]
- Quazi, S.A.J.; Meon, S.; Jaafar, H.; Ahmad, Z.A.B.M. The role of phytohormones in relation to bakanae disease development and symptoms expression. Physiol. Mol. Plant Pathol. 2015, 90, 27–38. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Chai, X.; Xue, D.; Zheng, W.; Shi, Y.; Wang, A. The involvement of jasmonic acid, ethylene, and salicylic acid in the signaling pathway of Clonostachys rosea-induced resistance to gray mold disease in tomato. Phytopathology 2019, 109, 1102–1114. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef]
- Hu, M.-J.; Fernández-Ortuño, D.; Schnabel, G. Monitoring resistance to SDHI fungicides in Botrytis cinerea from strawberry fields. Plant Dis. 2016, 100, 959–965. [Google Scholar] [CrossRef]
- Hägerhäll, C. Succinate: Quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta. Bioenerg. 1997, 1320, 107–141. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Heath, S.M.; Peres, N.A. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2013, 98, 532–539. [Google Scholar] [CrossRef] [PubMed]
- FRAC. Fungicide Resistance Management. 2022. Available online: https://www.frac.info/fungicide-resistance-management/background (accessed on 20 December 2022).
- Avenot, H.F.; Sellam, A.; Karaoglanidis, G.; Michailides, T.J. Characterization of mutations in the iron-sulphur subunit of succinate dehydrogenase correlating with boscalid resistance in Alternaria alternata from California pistachio. Phytopathology 2008, 98, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, P.L.E.; Hargreaves, J.A. A single amino-acid change in the iron-sulphur protein subunit of succinate dehydrogenase confers resistance to carboxin in Ustilago maydis. Curr. Genet. 1992, 22, 117–121. [Google Scholar] [CrossRef]
- Ito, Y.; Muraguchi, H.; Seshime, Y.; Oita, S.; Yanagi, S.O. Flutolanil and carboxin resistance in Coprinus cinereus conferred by a mutation in the cytochrome b 560 subunit of succinate dehydrogenase complex (Complex II). Mol. Genet. Genomics 2004, 272, 328–335. [Google Scholar] [CrossRef]
- Matsson, M.; Ackrell, B.A.C.; Cochran, B.; Hederstedt, L. Carboxin resistance in Paracoccus denitrificans conferred by a mutation in the membrane-anchor domain of succinate:quinone reductase (complex II). Arch. Microbiol. 1998, 170, 27–37. [Google Scholar] [CrossRef]
- Skinner, W.; Bailey, A.; Renwick, A.; Keon, J.; Gurr, S.; Hargreaves, J. A single amino-acid substitution in the iron-sulphur protein subunit of succinate dehydrogenase determines resistance to carboxin in Mycosphaerella graminicola. Curr. Genet. 1998, 34, 393–398. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef]
- Hasan, R.; Lv, B.; Uddin, M.J.; Chen, Y.; Fan, L.; Sun, Z.; Sun, M.; Li, S. Monitoring mycoparasitism of Clonostachys rosea against Botrytis cinerea using GFP. J. Fungi 2022, 8, 567. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Bardas, G.A.; Karaoglanidis, G.S. Baseline sensitivity of Botrytis cinerea to pyraclostrobin and boscalid and control of anilinopyrimidine- and benzimidazole-resistant strains by these fungicides. Plant Dis. 2008, 92, 1427–1431. [Google Scholar] [CrossRef]
- Wong, F.P.; Wilcox, W.F. Sensitivity to azoxystrobin among isolates of Uncinula necator: Baseline distribution and relationship to myclobutanil sensitivity. Plant Dis. 2002, 86, 394–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karlsson, M.; Durling, M.B.; Choi, J.; Kosawang, C.; Lackner, G.; Tzelepis, G.D.; Nygren, K.; Dubey, M.K.; Kamou, N.; Levasseur, A.; et al. Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biol. Evol. 2015, 7, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-B.; Ge, C.-Y.; Zhang, X.-K.; Wang, J.-X.; Zhou, M.-G. Development and evaluation of a novel and rapid detection assay for Botrytis cinerea based on loop-mediated isothermal amplification. PLoS ONE 2014, 9, e111094. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, M.; Li, L.; Sun, L.; Xu, Y.; Gao, J.; Wang, L.; Hou, Y.; Huang, S. Synergistic effects of the combined application of Bacillus subtilis H158 and strobilurins for rice sheath blight control. Biol. Control 2018, 117, 182–187. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Xue, M.; Liu, Z.; Zhang, Q.; Hou, J.; Xing, M.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control Fusarium wilt in cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef]
- Reynoso-López, E.A.; Méndez-Hernández, J.E.; Ek-Ramos, J.; Montesinos-Matías, R.; Loera, O. Metarhizium robertsii in combination with Trichoderma asperellum reduce the malathion doses used to control ambrosia beetles: The case of Xyleborus affinis. Biocontrol Sci. Technol. 2021, 31, 1080–1097. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Fungicide: Modes of Action and Possible Impact on Nontarget Microorganisms. ISRN Ecology 2011, 2011, 130289. [Google Scholar] [CrossRef]
- Peng, D.; Li, S.; Chen, C.; Zhou, M. Combined application of Bacillus subtilis NJ-18 with fungicides for control of sharp eyespot of wheat. Biol. Control 2014, 70, 28–34. [Google Scholar] [CrossRef]
- Peng, D.; Li, S.; Wang, J.; Chen, C.; Zhou, M. Integrated biological and chemical control of rice sheath blight by Bacillus subtilis NJ-18 and jinggangmycin. Pest Manage. Sci. 2014, 70, 258–263. [Google Scholar] [CrossRef]
- Kiewnick, S.; Jacobsen, B.J.; Braun-Kiewnick, A.; Eckhoff, J.L.A.; Bergman, J.W. Integrated control of Rhizoctonia crown and root rot of sugar beet with fungicides and antagonistic bacteria. Plant Dis. 2001, 85, 718–722. [Google Scholar] [CrossRef]
- Kondoh, M.; Hirai, M.; Shoda, M. Integrated biological and chemical control of damping-off caused by Rhizoctonia solani using Bacillus subtilis RB14-C and flutolanil. J. Biosci. Bioeng. 2001, 91, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Zhen, F.; Hu, M.; Li, X.; Schnabel, G. Efficacy of SDHI fungicides, including benzovindiflupyr, against Colletotrichum species. Pest Manage. Sci. 2016, 72, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Mao, C.; Wu, J.; Zhang, C. Activity of a SDHI fungicide penflufen and the characterization of natural-resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2021, 179, 104960. [Google Scholar] [CrossRef] [PubMed]
- Morandi, M.A.B.; Maffia, L.A.; Mizubuti, E.S.G.; Alfenas, A.C.; Barbosa, J.G. Suppression of Botrytis cinerea sporulation by Clonostachys rosea on rose debris: A valuable component in Botrytis blight management in commercial greenhouses. Biol. Control 2003, 26, 311–317. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Factors influencing colonization of cucumber roots by Clonostachys rosea f. catenulata, a biological disease control agent. Biocontrol Sci. Technol. 2010, 20, 37–55. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K. Chitinase and β-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Can. J. Microbiol. 2009, 55, 356–367. [Google Scholar] [CrossRef]
- Seidl, V. Chitinases of filamentous fungi: A large group of diverse proteins with multiple physiological functions. Fungal Biol. Rev. 2008, 22, 36–42. [Google Scholar] [CrossRef]
- Fatema, U.; Broberg, A.; Jensen, D.F.; Karlsson, M.; Dubey, M. Functional analysis of polyketide synthase genes in the biocontrol fungus Clonostachys rosea. Sci. Rep. 2018, 8, 15009. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Cabrera, G.; Gozzo, F.C.; Eberlin, M.N.; Godeas, A. Clonostachys rosea BAFC3874 as a Sclerotinia sclerotiorum antagonist: Mechanisms involved and potential as a biocontrol agent. J. Appl. Microbiol. 2011, 110, 1177–1186. [Google Scholar] [CrossRef]
- Zhai, M.-M.; Qi, F.-M.; Li, J.; Jiang, C.-X.; Hou, Y.; Shi, Y.-P.; Di, D.-L.; Zhang, J.-W.; Wu, Q.-X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef]
- Gong, C.; Liu, Y.; Liu, S.-Y.; Cheng, M.-Z.; Zhang, Y.; Wang, R.-H.; Chen, H.-Y.; Li, J.-F.; Chen, X.-l.; Wang, A.-X. Analysis of Clonostachys rosea-induced resistance to grey mould disease and identification of the key proteins induced in tomato fruit. Postharvest Biol. Technol. 2017, 123, 83–93. [Google Scholar] [CrossRef]
- Crane, J.M.; Bergstrom, G.C. Spatial distribution and antifungal interactions of a Bacillus biological control agent on wheat surfaces. Biol. Control 2014, 78, 23–32. [Google Scholar] [CrossRef]
- Wei, F.; Hu, X.; Xu, X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection coditions. Sci. Rep. 2016, 6, 22611. [Google Scholar] [CrossRef] [PubMed]
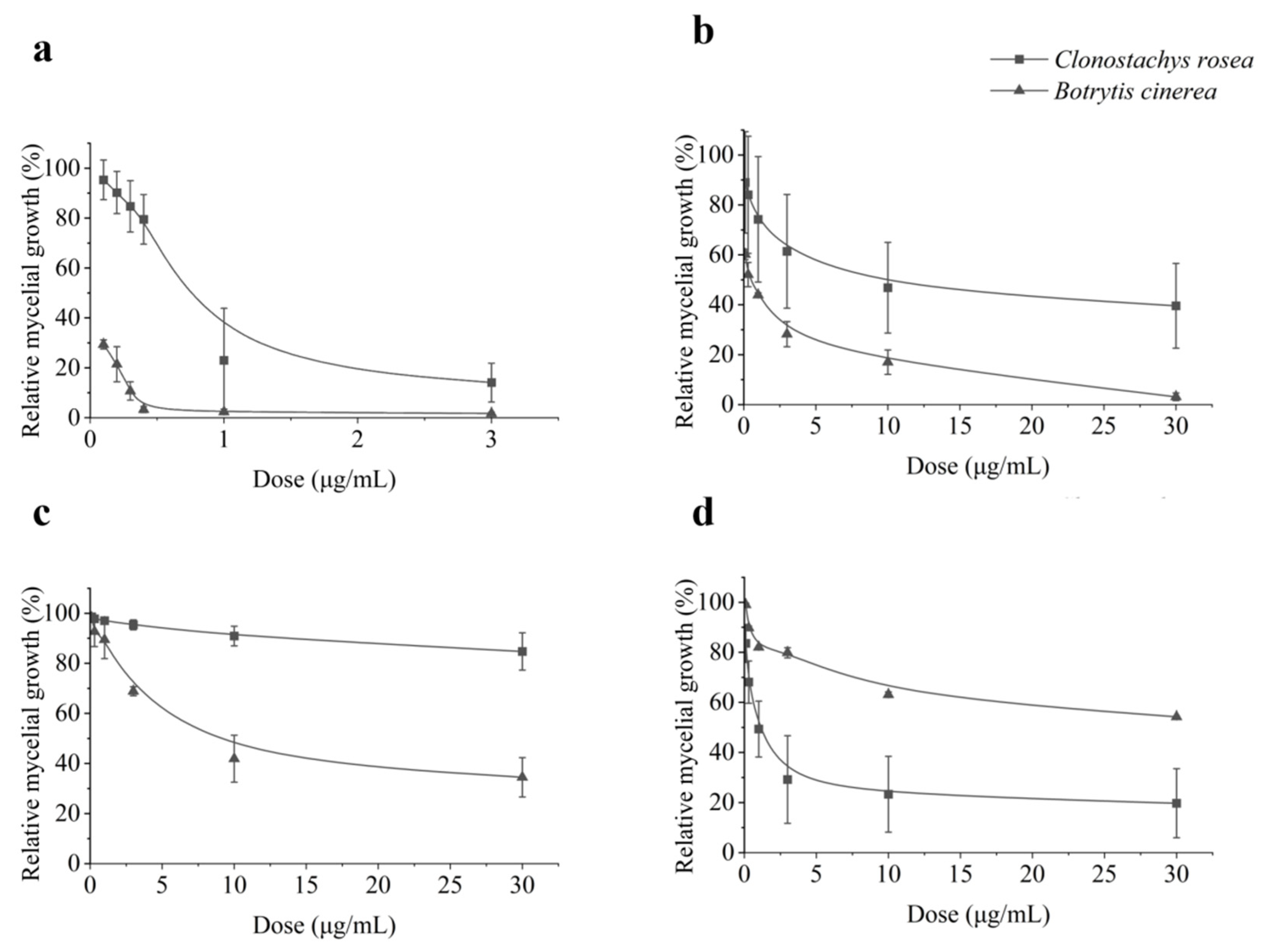
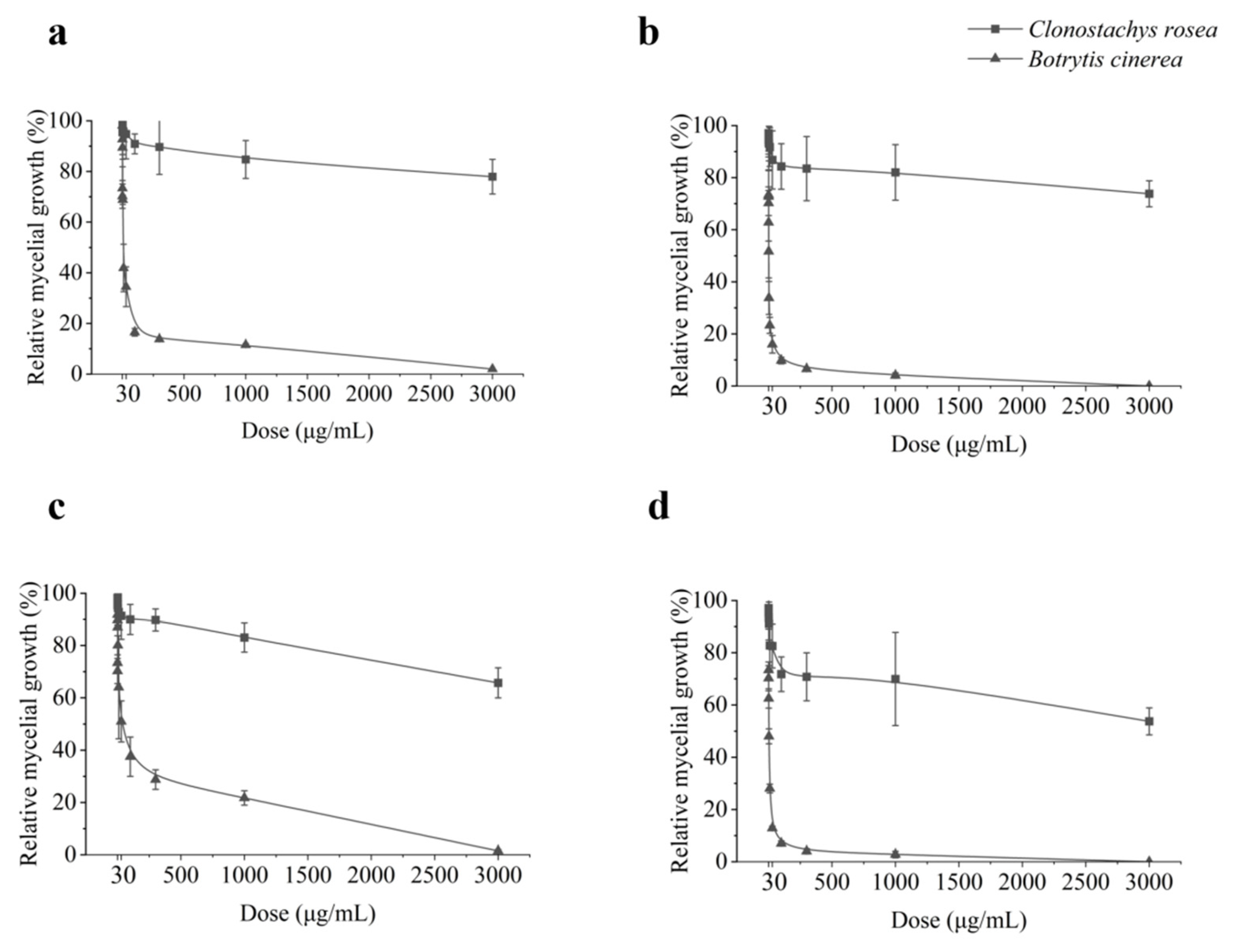

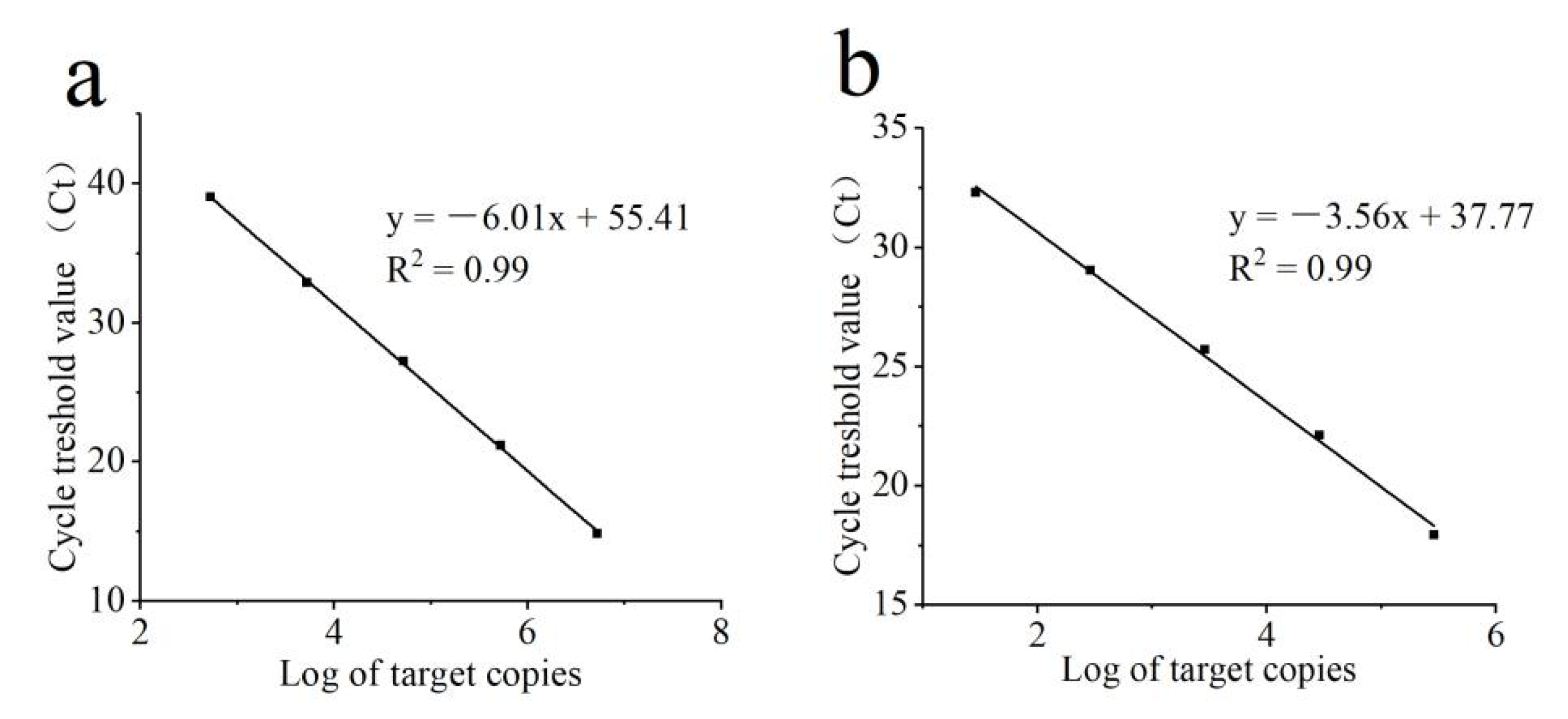
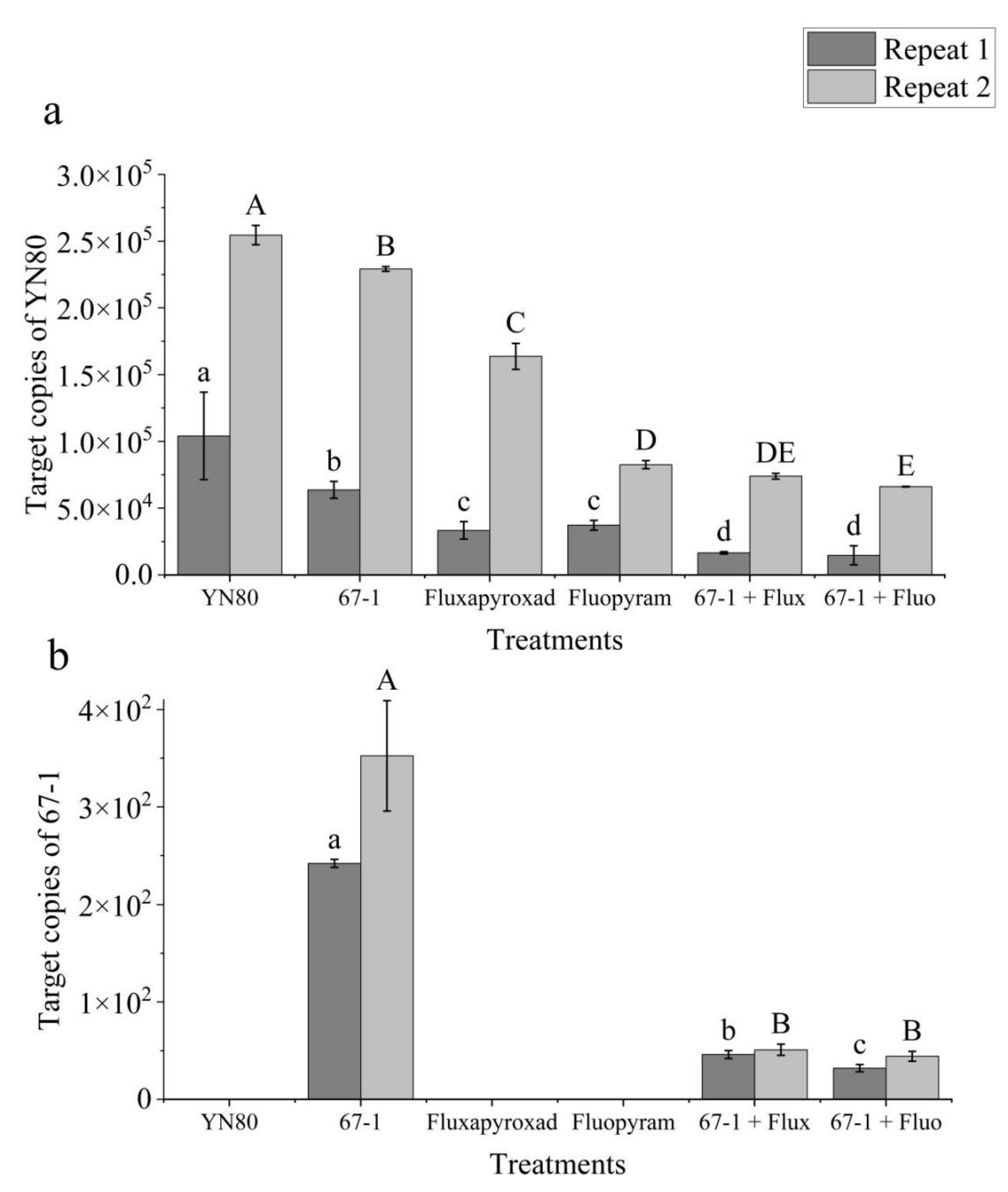
| Species | Isolate | EC50 (μg mL−1) z ± SE | ||||||
|---|---|---|---|---|---|---|---|---|
| Carbendazim | Tebuconazole | Pyraclostrobin | Boscalid | Fluxapyroxad | Fluopimomide | Fluopyram | ||
| Clonostachys rosea | JLB-7-1 | 1.66 ± 0.27 | 0.02 ± 0.01 | 11.17 ± 2.08 | >1000 | >1000 | >1000 | >1000 |
| 67-1 | 1.04 ± 0.66 | 10.24 ± 1.71 | 0.52 ± 0.29 | >1000 | >1000 | >1000 | >1000 | |
| SYP-4-2 | 0.34 ± 0.23 | 9.39 ± 1.63 | 0.59 ± 0.32 | >1000 | >1000 | >1000 | >1000 | |
| SHW-1-1 | 0.73 ± 0.48 | 21.11 ± 11.20 | 4.46 ± 3.42 | >1000 | >1000 | >1000 | >1000 | |
| YJS-3-2 | 0.50 ± 0.34 | 20.39 ± 14.54 | 0.74 ± 0.35 | >1000 | >1000 | >1000 | >1000 | |
| GS6-1 | 0.89 ± 0.57 | 7.46 ± 5.95 | 0.52 ± 0.31 | >1000 | >1000 | >1000 | >1000 | |
| NHH-48-2 | 0.71 ± 0.49 | 102.86 ± 53.70 | 3.08 ± 1.31 | >1000 | >1000 | >1000 | >1000 | |
| BD-2-1 | 0.74 ± 0.52 | 16.87 ± 11.57 | 0.66 ± 0.47 | >1000 | >1000 | >1000 | >1000 | |
| Botrytis cinerea | YN80 | 0.01 ± 0.002 | 0.27 ± 0.16 | 31.95 ± 10.97 | 15.46 ± 4.50 | 1.75 ± 1.41 | 12.96 ± 5.85 | 1.12 ± 0.67 |
| YN81 | 0.03 ± 0.006 | 0.47 ± 0.17 | 22.69 ± 6.16 | 5.95 ± 3.98 | 0.40 ± 0.33 | 33.41 ± 7.34 | 1.92 ± 1.15 | |
| Species y | Gemination Rate of Conidium at Different Fungicide Concentrations (%) z | ||||||
|---|---|---|---|---|---|---|---|
| Fungicide | Concentrations of Fungicides (µg mL−1) | ||||||
| 0 | 7.5 | 15 | 30 | 60 | 120 | ||
| Clonostachys rosea | Fluxapyroxad | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 |
| Fluopyram | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.34 ± 0.47 | 97.70 ± 0.07 | 97.34 ± 0.41 | |
| Botrytis cinerea | Fluxapyroxad | 95.71 ± 0.71 | 16.95 ± 1.86 | 8.34 ± 3.36 | 5.67 ± 3.26 | 3.61 ± 1..02 | 1.80 ± 0.08 |
| Fluopyram | 95.71 ± 0.71 | 21.05 ± 0.76 | 8.62 ± 3.71 | 10.81 ± 0.50 | 4.62 ± 0.37 | 2.00 ± 0.08 | |
| Treatments y | Control Efficacy z |
|---|---|
| 67-1 | 46.42% ± 3.14% d |
| Fluxapyroxad | 52.28% ± 4.17% c |
| Fluopyram | 58.31% ± 3.57% c |
| 67-1+Flux | 70.91% ± 3.65% b |
| 67-1+Fluo | 71.94% ± 6.34% ab |
| 67-1_Flux | 73.65% ± 1.24% ab |
| 67-1_Fluo | 77.07% ± 2.26% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Lei, T.; Hao, X.; Yuan, H.; Sun, W.; Chen, S. Synergistic Effects of Clonostachys rosea Isolates and Succinate Dehydrogenase Inhibitors Fungicides against Gray Mold on Tomato. Microorganisms 2023, 11, 20. https://doi.org/10.3390/microorganisms11010020
Song J, Lei T, Hao X, Yuan H, Sun W, Chen S. Synergistic Effects of Clonostachys rosea Isolates and Succinate Dehydrogenase Inhibitors Fungicides against Gray Mold on Tomato. Microorganisms. 2023; 11(1):20. https://doi.org/10.3390/microorganisms11010020
Chicago/Turabian StyleSong, Jiehui, Tengyu Lei, Xiaojuan Hao, Huizhu Yuan, Wei Sun, and Shuning Chen. 2023. "Synergistic Effects of Clonostachys rosea Isolates and Succinate Dehydrogenase Inhibitors Fungicides against Gray Mold on Tomato" Microorganisms 11, no. 1: 20. https://doi.org/10.3390/microorganisms11010020
APA StyleSong, J., Lei, T., Hao, X., Yuan, H., Sun, W., & Chen, S. (2023). Synergistic Effects of Clonostachys rosea Isolates and Succinate Dehydrogenase Inhibitors Fungicides against Gray Mold on Tomato. Microorganisms, 11(1), 20. https://doi.org/10.3390/microorganisms11010020








