Babesia, Theileria, Plasmodium and Hemoglobin
Abstract
:1. Introduction
2. Hemoglobin Uptake and Processing in Plasmodia
3. Hemoglobin Uptake during the Intraerythrocytic Stages of Babesia
4. Hemoglobin Processing in P. falciparum
5. Hemoglobin Processing in Babesia
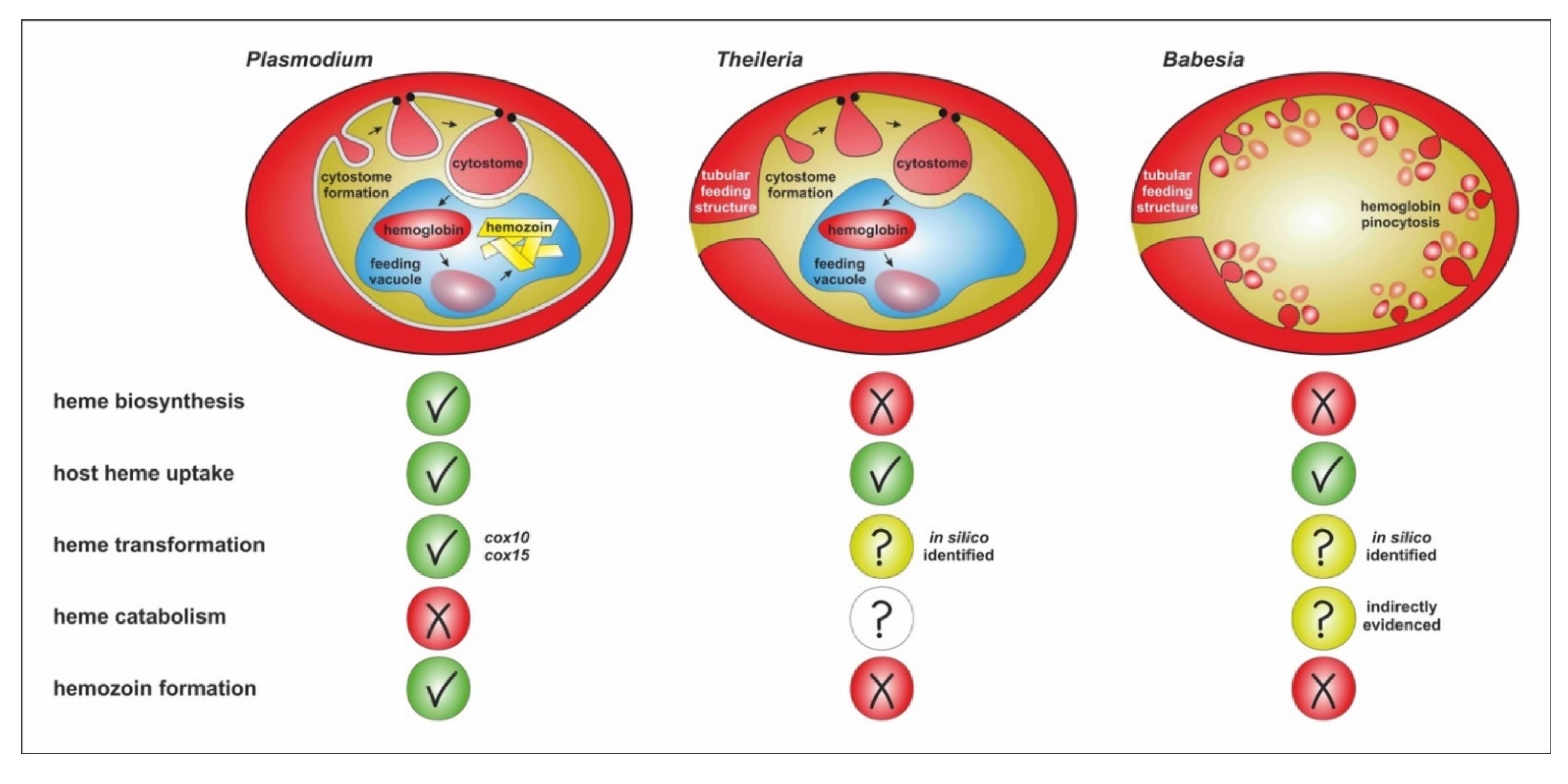
6. Summary and Outstanding Questions
- (1)
- Is the uptake and intracellular trafficking of hemoglobin by piroplasmid apicomplexans based on analogous molecular components/machineries as in P. falciparum?
- (2)
- Does the appearance of the cytostome and the FV in Theileria and not in Babesia have anything to do with the ability to create multinucleate syncytium during intra-leucocytic schizogony prior to intraerythrocytic merogony?
- (3)
- Is heme catabolized by intraerythrocytic Babesia stages, and if so, what products are formed?
- (4)
- Does the absence of hemozoin formation prevent using anti-malarials, exploiting the formation of hemozoin to exert toxicity?
- (5)
- Is there a link between the heme auxotrophy of ticks and the way of hemoglobin uptake and processing in Babesia and Theileria parasites, apparently differing from malarial plasmodia? Is this due to their co-evolution with the tick vector/definitive host?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Florin-Christensen, M.; Schnittger, L. Piroplasmids and ticks: A long-lasting intimate relationship. Front. Biosci. 2009, 14, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia life cycle–when phylogeny meets biology. Trends Parasitol. 2019, 35, 356–368. [Google Scholar] [CrossRef]
- Sevilla, E.; González, L.M.; Luque, D.; Gray, J.; Montero, E. Kinetics of the invasion and egress processes of Babesia divergens, observed by time-lapse video microscopy. Sci. Rep. 2018, 35, 14116. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J.; Shock, B.C. Natural history of zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129 (Suppl. S1), S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Djokic, V.; Primus, S.; Akoolo, L.; Chakraborti, M.; Parveen, N. Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affects disease severity. Front. Immunol. 2018, 9, 2891. [Google Scholar] [CrossRef]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbio. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Echaide, I.; Pabón, A.; Gabriel Piñeros, J.J.; Blair, S.; Tobón-Castaño, A. Babesiosis prevalence in malaria-endemic regions of Colombia. J. Vector Borne Dis. 2018, 55, 222–229. [Google Scholar] [PubMed]
- Bloch, E.M.; Kasubi, M.; Levin, A.; Mrango, Z.; Weaver, J.; Munoz, B.; West, S.K. Babesia microti and Malaria Infection in Africa: A Pilot Serosurvey in Kilosa District, Tanzania. Am. J. Trop. Med. Hyg. 2018, 99, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effect of ivermectin on the growth of Babesia and Theileria parasites in vitro and in vivo. Trop. Med. Health 2019, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Renard, I.; Ben Mamoun, C. Treatment of human babesiosis: Then and now. Pathogens 2021, 10, 1120. [Google Scholar] [CrossRef]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef]
- Rocamora, F.; Winzeler, E.A. Genomic approaches to drug resistance in malaria. Annu. Rev. Microbiol. 2020, 74, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Krugliak, M.; Zhang, J.; Ginsburg, H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 2002, 119, 249–256. [Google Scholar] [CrossRef]
- Esposito, A.; Choimet, J.B.; Skepper, J.N.; Mauritz, J.M.; Lew, V.L.; Kaminski, C.F.; Tiffert, T. Quantitative imaging of human red blood cells infected with Plasmodium falciparum. Biophys. J. 2010, 99, 953–960. [Google Scholar] [CrossRef]
- Hanssen, E.; Knoechel, C.; Dearnley, M.; Dixon, M.W.; Le Gros, M.; Larabell, C.; Tilley, L. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J. Struct. Biol. 2012, 177, 224–232. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Zimmerberg, J. Hardly Vacuous: The parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef]
- Elliott, D.A.; McIntosh, M.T.; Hosgood, H.D., III; Chen, S.; Zhang, G.; Baevova, P.; Joiner, K.A. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci USA 2008, 105, 2463–2468. [Google Scholar] [CrossRef]
- Bakar, N.A.; Klonis, N.; Hanssen, E.; Chan, C.; Tilley, L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci. 2010, 123, 441–450. [Google Scholar] [CrossRef]
- Lazarus, M.D.; Schneider, T.G.; Taraschi, T.F. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J. Cell Sci. 2008, 121, 1937–1949. [Google Scholar] [CrossRef]
- Milani, K.J.; Schneider, T.G.; Taraschi, T.F. Defining the morphology and mechanism of the hemoglobin transport pathway in Plasmodium falciparum-infected erythrocytes. Eukaryot. Cell 2015, 14, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Wendt, C.; Rachid, R.; de Souza, W.; Miranda, K. Electron tomography characterization of hemoglobin uptake in Plasmodium chabaudi reveals a stage-dependent mechanism for food vacuole morphogenesis. J. Struct. Biol. 2016, 194, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Grüring, C.; Heiber, A.; Kruse, F.; Ungefehr, J.; Gilberger, T.W.; Spielmann, T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2011, 2, 165. [Google Scholar] [CrossRef]
- Spielmann, T.; Gras, S.; Sabitzki, R.; Meissner, M. Endocytosis in Plasmodium and Toxoplasma parasites. Trends Parasitol. 2020, 36, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, C.J.; Pearce, J.A.; McFadden, G.I.; Cowman, A.F. Protein targeting to destinations of the secretory pathway in the malaria parasite Plasmodium falciparum. Curr. Opin. Microbiol. 2006, 9, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci. 2012, 4, 1424–1448. [Google Scholar]
- Dluzewski, A.R.; Ling, I.T.; Hopkins, J.M.; Grainger, M.; Margos, G.; Mitchell, G.H.; Holder, A.A.; Bannister, L.H. Formation of the food vacuole in Plasmodium falciparum: A potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)). PLoS ONE 2008, 3, e3085. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.D.; Flewett, T.H. The relation of Plasmodium berghei and Plasmodium knowlesi to their respective red-cell hosts. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 150–156. [Google Scholar] [CrossRef]
- Birnbaum, J.; Flemming, S.; Reichard, N.; Soares, A.B.; Mesén-Ramírez, P.; Jonscher, E.; Bergmann, B.; Spielmann, T. A genetic system to study Plasmodium falciparum protein function. Nat. Methods 2017, 14, 450–456. [Google Scholar] [CrossRef]
- Jonscher, E.; Flemming, S.; Schmitt, M.; Sabitzki, R.; Reichard, N.; Birnbaum, J.; Bergmann, B.; Höhn, K.; Spielmann, T. PfVPS45 is required for host cell cytosol uptake by malaria blood stage parasites. Cell Host Microbe 2019, 25, 166–173.e5. [Google Scholar] [CrossRef] [PubMed]
- McGovern, O.L.; Rivera-Cuevas, Y.; Carruthers, V.B. Emerging mechanisms of endocytosis in Toxoplasma gondii. Life 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Quevillon, E.; Spielmann, T.; Brahimi, K.; Chattopadhyay, D.; Yeramian, E.; Langsley, G. The Plasmodium falciparum family of Rab GTPases. Gene 2003, 306, 13–25. [Google Scholar] [CrossRef]
- Jackson, A.J.; Clucas, C.; Mamczur, N.J.; Ferguson, D.J.; Meissner, M. Toxoplasma gondii Syntaxin 6 is required for vesicular transport between endosomal-like compartments and the Golgi complex. Traffic 2013, 14, 1166–1181. [Google Scholar]
- Xie, S.C.; Dogovski, C.; Hanssen, E.; Chiu, F.; Yang, T.; Crespo, M.P.; Stafford, C.; Batinovic, S.; Teguh, S.; Charman, S.; et al. Haemoglobin degradation underpins the sensitivity of early ring stage Plasmodium falciparum to artemisinins. J. Cell Sci. 2016, 129, 406–416. [Google Scholar] [CrossRef]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Rudzinska, M.A. Ultrastructure of intraerythrocytic Babesia microti with emphasis on the feeding mechanism. J. Protozool. 1976, 23, 224–233. [Google Scholar] [CrossRef]
- Rudzinska, M.A.; Trager, W.; Lewengrub, S.J.; Gubert, E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976, 169, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Jalovecka, M.; Hajdusek, O.; Sojka, D.; Kopacek, P.; Malandrin, L. The complexity of piroplasms life cycles. Front. Cell. Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, W.M.; Holbrook, A.A. Feeding mechanisms of Babesia equi. J. Protozool. 1974, 21, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Kawamura, S.; Hanamatsu, K.; Yasuda, Y. Electron microscopy of Theileria sergenti in bovine erythrocytes. Jpn. J. Vet. Sci. 1984, 46, 745–748. [Google Scholar] [CrossRef]
- Fawcett, D.W.; Conrad, P.A.; Grootenhuis, J.G.; Morzaria, S.P. Ultrastructure of the intra-erythrocytic stage of Theileria species from cattle and waterbuck. Tissue Cell 1987, 19, 643–655. [Google Scholar] [CrossRef]
- Langreth, S.G. Feeding mechanisms in extracellular Babesia microti and Plasmodium lophurae. J. Protozool. 1976, 23, 215–223. [Google Scholar] [CrossRef]
- Thekkiniath, J.; Kilian, N.; Lawres, L.; Gewirtz, M.A.; Graham, M.M.; Liu, X.; Ledizet, M.; Ben Mamoun, C. Evidence for vesicle-mediated antigen export by the human pathogen Babesia microti. Life Sci. Alliance 2019, 2, e201900382. [Google Scholar] [CrossRef]
- Sun, T.; Tenenbaum, M.J.; Greenspan, J.; Teichberg, S.; Wang, R.T.; Degnan, T.; Kaplan, M.H. Morphologic and clinical observations in human infection with Babesia microti. J. Infect. Dis. 1983, 148, 239–248. [Google Scholar] [CrossRef]
- Taylor, J.H.; Guthrie, A.J.; Leisewitz, A. The effect of endogenously produced carbon monoxide on the oxygen status of dogs infected with Babesia canis. J. S. Afr. Vet. Assoc. 1991, 62, 153–155. [Google Scholar] [CrossRef]
- Goldberg, D.E. Hemoglobin degradation. Curr. Top. Microbiol. Immunol. 2005, 295, 275–291. [Google Scholar]
- Saliba, K.J.; Allen, R.J.; Zissis, S.; Bray, P.G.; Ward, S.A.; Kirk, K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J. Biol. Chem. 2003, 278, 5605–5612. [Google Scholar] [CrossRef]
- Sullivan, D.J., Jr.; Gluzman, I.Y.; Goldberg, D.E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 1996, 271, 219–222. [Google Scholar] [CrossRef]
- Teixeira, C.; Gomes, J.R.; Gomes, P. Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011, 18, 1555–1572. [Google Scholar] [CrossRef]
- Gluzman, I.Y.; Francis, S.E.; Oksman, A.; Smith, C.E.; Duffin, K.L.; Goldberg, D.E. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J. Clin. Investig. 1994, 93, 1602–1608. [Google Scholar] [CrossRef]
- Šnebergerová, P.; Bartošová-Sojková, P.; Jalovecká, M.; Sojka, D. Plasmepsin-like aspartyl proteases in Babesia. Pathogens 2021, 10, 1241. [Google Scholar] [CrossRef]
- Nasamu, A.S.; Polino, A.J.; Istvan, E.S.; Goldberg, D.E. Malaria parasite plasmepsins: More than just plain old degradative pepsins. J. Biol. Chem. 2020, 295, 8425–8441. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.L.; Rosenthal, P.J. Biosynthesis, localization, and processing of falcipain cysteine proteases of Plasmodium falciparum. Mol. Biochem. Parasitol. 2005, 139, 205–212. [Google Scholar] [CrossRef]
- Marco, M.; Coterón, J.M. Falcipain inhibition as a promising antimalarial target. Curr. Top. Med. Chem. 2012, 12, 408–444. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Chugh, M.; Sundararaman, V.; Kumar, S.; Reddy, V.S.; Siddiqui, W.A.; Stuart, K.D.; Malhotra, P. Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, K.A.; Egan, T.J. Heme detoxification in the malaria parasite: A target for antimalarial drug development. Acc. Chem. Res. 2021, 54, 2649–2659. [Google Scholar] [CrossRef]
- Fitch, C.D.; Cai, G.Z.; Chen, Y.F.; Shoemaker, J.D. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim. Biophys. Acta 1999, 1454, 31–37. [Google Scholar] [CrossRef]
- Jani, D.; Nagarkatti, R.; Beatty, W.; Angel, R.; Slebodnick, C.; Andersen, J.; Kumar, S.; Rathore, D. HDP—A novel heme detoxification protein from the malaria parasite. PLoS Pathog. 2008, 4, e1000053. [Google Scholar] [CrossRef] [PubMed]
- Burda, P.C.; Crosskey, T.; Lauk, K.; Zurborg, A.; Söhnchen, C.; Liffner, B.; Wilcke, L.; Pietsch, E.; Strauss, J.; Jeffries, C.M.; et al. Structure-based identification and functional characterization of a lipocalin in the malaria parasite Plasmodium falciparum. Cell Rep. 2020, 31, 107817. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Drepper, B.; Blum, T.B.; van Genderen, E.; Burrell, A.; Martin, P.; Stach, T.; Collinson, L.M.; Abrahams, J.P.; Matuschewski, K.; et al. A lipocalin mediates unidirectional heme biomineralization in malaria parasites. Proc. Natl. Acad. Sci. USA 2020, 117, 16546–16556. [Google Scholar] [CrossRef] [PubMed]
- Sigala, P.A.; Goldberg, D.E. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 2014, 68, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Sigala, P.A.; Miura, K.; Morrisey, J.M.; Mather, M.W.; Crowley, J.R.; Henderson, J.P.; Goldberg, D.E.; Long, C.A.; Vaidya, A.B. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J. Biol. Chem. 2014, 289, 34827–34837. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, V.A.; Sundaram, B.; Varadarajan, N.M.; Subramani, P.A.; Kalappa, D.M.; Ghosh, S.K.; Padmanaban, G. Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 2013, 9, e1003522. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Sigala, P.A. Plasmodium heme biosynthesis: To be or not to be essential? PLoS Pathog. 2017, 13, e1006511. [Google Scholar] [CrossRef] [PubMed]
- Perner, J.; Gasser, R.B.; Oliveira, P.L.; Kopáček, P. Haem Biology in Metazoan Parasites—‘The Bright Side of Haem’. Trends Parasitol. 2019, 35, 213–225. [Google Scholar] [CrossRef]
- Cornillot, E.; Hadj-Kaddour, K.; Dassouli, A.; Noel, B.; Ranwez, V.; Vacherie, B.; Augagneur, Y.; Brès, V.; Duclos, A.; Randazzo, S.; et al. Sequencing of the smallest apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012, 40, 9102–9114. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, M.; Wieser, S.N.; Suarez, C.E.; Schnittger, L. In silico Survey and characterization of Babesia microti functional and non-functional proteases. Pathogens 2021, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Dua, M.; Chishti, A.H.; Hanspal, M. Ankyrin peptide blocks falcipain-2-mediated malaria parasite release from red blood cells. J. Biol. Chem. 2003, 278, 30180–30186. [Google Scholar] [CrossRef] [PubMed]
- Mesplet, M.; Echaide, I.; Dominguez, M.; Mosqueda, J.J.; Suarez, C.E.; Schnittger, L.; Florin-Christensen, M. Bovipain-2, the falcipain-2 ortholog, is expressed in intraerythrocytic stages of the tick-transmitted hemoparasite Babesia bovis. Parasites Vectors 2010, 3, 113. [Google Scholar] [CrossRef]
- Martins, T.M.; do Rosário, V.E.; Domingos, A. Identification of papain-like cysteine proteases from the bovine piroplasm Babesia bigemina and evolutionary relationship of piroplasms C1 family of cysteine proteases. Exp. Parasitol. 2011, 127, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.M.; do Rosário, V.E.; Domingos, A. Expression and characterization of the Babesia bigemina cysteine protease BbiCPL1. Acta Trop. 2012, 121, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Yokoyama, N.; Govind, Y.; Alhassan, A.; Igarashi, I. Babesia bovis: Effects of cysteine protease inhibitors on in vitro growth. Exp. Parasitol. 2007, 117, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Carletti, T.; Barreto, C.; Mesplet, M.; Mira, A.; Weir, W.; Shiels, B.; Oliva, A.G.; Schnittger, L.; Florin-Christensen, M. Characterization of a papain-like cysteine protease essential for the survival of Babesia ovis merozoites. Ticks Tick-Borne Dis. 2016, 7, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Brayton, K.A.; Lau, A.O.; Herndon, D.R.; Hannick, L.; Kappmeyer, L.S.; Berens, S.J.; Bidwell, S.L.; Brown, W.C.; Crabtree, J.; Fadrosh, D.; et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007, 3, 1401–1413. [Google Scholar] [CrossRef]
- Kloehn, J.; Harding, C.R.; Soldati-Favre, D. Supply and demand-heme synthesis, salvage and utilization by Apicomplexa. FEBS J. 2021, 288, 382–404. [Google Scholar] [CrossRef]
- Puri, A.; Bajpai, S.; Meredith, S.; Aravind, L.; Krause, P.J.; Kumar, S. Pathogen Genomics, Genetic Variability, Immunodominant Antigens, and Pathogenesis. Front. Microbiol. 2021, 12, 697669. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M. Babesiosis in humans: A treatment review. Expert Opin. Pharmacother. 2002, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Maluf, F.V.; Bueno, V.B.; Guido, R.V.; Oliva, G.; Singh, M.; Scarpelli, P.; Costa, F.; Sartorello, R.; Catalani, L.H.; et al. Biliverdin targets enolase and eukaryotic initiation factor 2 (eIF2α) to reduce the growth of intraerythrocytic development of the malaria parasite Plasmodium falciparum. Sci. Rep. 2016, 6, 22093. [Google Scholar] [CrossRef] [PubMed]
| P. falciparum Protein | Description | Potential Analogue in Piroplasmida Species/GenBank Ref. No. |
|---|---|---|
| Kelch 13 | Located at the cytostome surface, class of Kelch/BTB/POZubiquitination adaptors, hemoglobin uptake cytostomal invagination | Babesia microti/XM_012793132.1 Babesia bovis/XM_001608959.1 Babesia bigemina/XM_012912362.1 Babesia ovata/XM_029011617.1 Theileria equi/XM_004829658.1 Theileria parva/XM_760915.1 Theileria oriantalis/XM_009690968.1 |
| VPS45 | Vacuolar protein sorting-associated protein 45 role in the trafficking of ingested hemoglobin to FV for degradation | Babesia microti/XM_012794275.1 Babesia bovis/XM_001611295.1 Babesia bigemina/XM_012913205.1 Babesia ovata/XM_029009877.1 Theileria equi/XM_004832773.1 Theileria parva/XM_758502.1 Theileria oriantalis/XM_950220.1 |
| falcipain-2(FP2) | Cysteine protease; degradation of globin chains within FV together with aspartyl proteases plasmepsins | Babesia microti/XM_012792174.1 Babesia bovis/GQ412131.1 Babesia bigemina/FJ859910.1 Babesia ovata/XM_029009877.1 Theileria equi/XM_004830311.1 Theileria parva/XM_758208.1 Theileria oriantalis/XM_009693310.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sojka, D.; Jalovecká, M.; Perner, J. Babesia, Theileria, Plasmodium and Hemoglobin. Microorganisms 2022, 10, 1651. https://doi.org/10.3390/microorganisms10081651
Sojka D, Jalovecká M, Perner J. Babesia, Theileria, Plasmodium and Hemoglobin. Microorganisms. 2022; 10(8):1651. https://doi.org/10.3390/microorganisms10081651
Chicago/Turabian StyleSojka, Daniel, Marie Jalovecká, and Jan Perner. 2022. "Babesia, Theileria, Plasmodium and Hemoglobin" Microorganisms 10, no. 8: 1651. https://doi.org/10.3390/microorganisms10081651
APA StyleSojka, D., Jalovecká, M., & Perner, J. (2022). Babesia, Theileria, Plasmodium and Hemoglobin. Microorganisms, 10(8), 1651. https://doi.org/10.3390/microorganisms10081651






