Metal Homeostasis in Pathogenic Streptococci
Abstract
1. Introduction
2. Zinc
2.1. Transport
2.2. Role in Virulence
2.2.1. Zn Limitation
2.2.2. Zn Intoxication
3. Manganese and Iron
3.1. Transport
3.2. Role in Virulence
4. Copper
4.1. Transport
4.2. Role in Virulence
5. Nickel
5.1. Transport
5.2. Role in Virulence
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Waldron, K.J.; Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Burcham, L.R.; Le Breton, Y.; Radin, J.N.; Spencer, B.L.; Deng, L.; Hiron, A.; Ransom, M.R.; Mendonça, J.D.C.; Belew, A.T.; El-Sayed, N.M.; et al. Identification of Zinc-Dependent Mechanisms Used by Group B Streptococcus to Overcome Calprotectin-Mediated Stress. mBio 2020, 11, e02302-20. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Song, W.J. Emergence of metal selectivity and promiscuity in metalloenzymes. JBIC J. Biol. Inorg. Chem. 2019, 24, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.; McDevitt, C.A.; Kitten, T. Manganese uptake and streptococcal virulence. BioMetals 2015, 28, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2013, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The Metals in the Biological Periodic System of the Elements: Concepts and Conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Chen, J. ATP-Binding Cassette Transporters in Bacteria. Annu. Rev. Biochem. 2004, 73, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Simon, A.; Titball, R.W.; Michell, S.L. Lipoproteins of Bacterial Pathogens. Infect. Immun. 2011, 79, 548–561. [Google Scholar] [CrossRef]
- Berntsson, R.P.-A.; Smits, S.H.; Schmitt, L.; Slotboom, D.-J.; Poolman, B. A structural classification of substrate-binding proteins. FEBS Lett. 2010, 584, 2606–2617. [Google Scholar] [CrossRef]
- Chandravanshi, M.; Tripathi, S.K.; Kanaujia, S.P. An updated classification and mechanistic insights into ligand binding of the substrate-binding proteins. FEBS Lett. 2021, 595, 2395–2409. [Google Scholar] [CrossRef]
- Yamashita, M.M.; Wesson, L.; Eisenman, G.; Eisenberg, D. Where metal ions bind in proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 5648–5652. [Google Scholar] [CrossRef]
- Ge, R.; Sun, X.; He, Q.-Y. Iron acquisition by Streptococcus species: An updated review. Front. Biol. China 2009, 4, 392–401. [Google Scholar] [CrossRef]
- Puccio, T.; Kunka, K.S.; An, S.; Kitten, T. Contribution of a ZIP-family protein to manganese uptake and infective endocarditis virulence in Streptococcus sanguinis. Mol. Microbiol. 2021, 117, 353–374. [Google Scholar] [CrossRef]
- Moulin, P.; Rong, V.; E Silva, A.R.; Pederick, V.G.; Camiade, E.; Mereghetti, L.; McDevitt, C.A.; Hiron, A. Defining the Role of the Streptococcus agalactiae Sht-Family Proteins in Zinc Acquisition and Complement Evasion. J. Bacteriol. 2019, 201, e00757-18. [Google Scholar] [CrossRef]
- Moulin, P.; Patron, K.; Cano, C.; Zorgani, M.A.; Camiade, E.; Borezée-Durant, E.; Rosenau, A.; Mereghetti, L.; Hiron, A. The Adc/Lmb System Mediates Zinc Acquisition in Streptococcus agalactiae and Contributes to Bacterial Growth and Survival. J. Bacteriol. 2016, 198, 3265–3277. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Goh, K.G.K.; Ulett, G.C. Cellular Management of Zinc in Group B Streptococcus Supports Bacterial Resistance against Metal Intoxication and Promotes Disseminated Infection. mSphere 2021, 6, 00105–00121. [Google Scholar] [CrossRef]
- Bray, B.A.; Sutcliffe, I.C.; Harrington, D.J. Expression of the MtsA lipoprotein of Streptococcus agalactiae A909 is regulated by manganese and iron. Antonie Van Leeuwenhoek 2009, 95, 101–109. [Google Scholar] [CrossRef]
- Shabayek, S.; Bauer, R.; Mauerer, S.; Mizaikoff, B.; Spellerberg, B. A streptococcal NRAMP homologue is crucial for the survival of Streptococcus agalactiae under low pH conditions. Mol. Microbiol. 2016, 100, 589–606. [Google Scholar] [CrossRef]
- Fernandez, A.; Lechardeur, D.; Derré-Bobillot, A.; Couvé, E.; Gaudu, P.; Gruss, A. Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in Streptococcus agalactiae. PLoS Pathog. 2010, 6, e1000860. [Google Scholar] [CrossRef]
- Clancy, A.; Loar, J.W.; Speziali, C.D.; Oberg, M.; Heinrichs, D.E.; Rubens, C.E. Evidence for siderophore-dependent iron acquisition in group B streptococcus. Mol. Microbiol. 2006, 59, 707–721. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Goh, K.G.K.; Gosling, D.; Katupitiya, L.; Ulett, G.C. Copper Intoxication in Group B Streptococcus Triggers Transcriptional Activation of the cop Operon That Contributes to Enhanced Virulence during Acute Infection. J. Bacteriol. 2021, 203, JB0031521. [Google Scholar] [CrossRef]
- Meehan, M.; Burke, F.M.; Macken, S.; Owen, P. Characterization of the haem-uptake system of the equine pathogen Streptococcus equi subsp. equi. Microbiology 2010, 156, 1824–1835. [Google Scholar] [CrossRef]
- Nygaard, T.K.; Liu, M.; McClure, M.J.; Lei, B. Identification and characterization of the heme-binding proteins SeShp and SeHtsA of Streptococcus equi subspecies equi. BMC Microbiol. 2006, 6, 82. [Google Scholar] [CrossRef]
- Heather, Z.; Holden, M.T.G.; Steward, K.F.; Parkhill, J.; Song, L.; Challis, G.L.; Robinson, C.; Davis-Poynter, N.; Waller, A.S. A novel streptococcal integrative conjugative element involved in iron acquisition. Mol. Microbiol. 2008, 70, 1274–1292. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Baker, R.A.; Jenkinson, H.F. The Adhesion-Associated sca Operon in Streptococcus gordonii Encodes an Inducible High-Affinity ABC Transporter for Mn2+ Uptake. J. Bacteriol. 1998, 180, 290–295. [Google Scholar] [CrossRef]
- Loo, C.Y.; Mitrakul, K.; Voss, I.B.; Hughes, C.V.; Ganeshkumar, N. Involvement of the adc Operon and Manganese Homeostasis in Streptococcus gordonii Biofilm Formation. J. Bacteriol. 2003, 185, 2887–2900. [Google Scholar] [CrossRef]
- Mitrakul, K.; Loo, C.Y.; Hughes, C.V.; Ganeshkumar, N. Role of a Streptococcus gordonii copper-transport operon, copYAZ, in biofilm detachment. Oral Microbiol. Immunol. 2004, 19, 395–402. [Google Scholar] [CrossRef]
- Ganguly, T.; Peterson, A.; Kajfasz, J.; Abranches, J.; Lemos, J. Zinc import mediated by AdcABC is critical for colonization of the dental biofilm by Streptococcus mutans in an animal model. Mol. Oral Microbiol. 2021, 36, 214–224. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Chen, J.; Ma, Q.; Gong, T.; Yu, S.; Zhang, Q.; Zou, J.; Li, Y. The Adc regulon mediates zinc homeostasis in Streptococcus mutans. Mol. Oral Microbiol. 2021, 36, 278–290. [Google Scholar] [CrossRef]
- Ganguly, T.; Peterson, A.; Burkholder, M.; Kajfasz, J.K.; Abranches, J.; Lemos, J.A. ZccE is a Novel P-type ATPase That Protects Streptococcus mutans against Zinc Intoxication. bioRxiv 2022. [Google Scholar] [CrossRef]
- O’Brien, J.; Pastora, A.; Stoner, A.; Spatafora, G. The S. mutans mntE gene encodes a manganese efflux transporter. Mol. Oral Microbiol. 2020, 35, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Brown, A.; Munro, C.L.; Cornelissen, C.N.; Kitten, T. The sloABCR Operon of Streptococcus mutans Encodes an Mn and Fe Transport System Required for Endocarditis Virulence and Its Mn-Dependent Repressor. J. Bacteriol. 2003, 185, 5967–5975. [Google Scholar] [CrossRef] [PubMed]
- Kajfasz, J.K.; Katrak, C.; Ganguly, T.; Vargas, J.; Wright, L.; Peters, Z.T.; Spatafora, G.A.; Abranches, J.; Lemos, J.A. Manganese Uptake, Mediated by SloABC and MntH, Is Essential for the Fitness of Streptococcus mutans. mSphere 2020, 5, e00764-19. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, G.; Moore, M.; Landgren, S.; Stonehouse, E.; Michalek, S. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology 2001, 147, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Galvao, L.C.D.C.; Miller, J.H.; Kajfasz, J.K.; Scott-Anne, K.; Freires, I.; Franco, G.C.N.; Abranches, J.; Rosalen, P.L.; Lemos, J.A. Transcriptional and Phenotypic Characterization of Novel Spx-Regulated Genes in Streptococcus mutans. PLoS ONE 2015, 10, e0124969. [Google Scholar] [CrossRef]
- Ganguly, T.; Kajfasz, J.K.; Miller, J.H.; Rabinowitz, E.; Galvao, L.C.D.C.; Rosalen, P.L.; Abranches, J.; Lemos, J.A. Disruption of a Novel Iron Transport System Reverses Oxidative Stress Phenotypes of a dpr Mutant Strain of Streptococcus mutans. J. Bacteriol. 2018, 200, e00062-18. [Google Scholar] [CrossRef]
- Vats, N.; Lee, S.F. Characterization of a copper-transport operon, copYAZ, from Streptococcus mutans. Microbiology 2001, 147, 653–662. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Tong, H.; Dong, X. PerR-Regulated Manganese Ion Uptake Contributes to Oxidative Stress Defense in an Oral Streptococcus. Appl. Environ. Microbiol. 2014, 80, 2351–2359. [Google Scholar] [CrossRef][Green Version]
- Oetjen, J.; Fives-Taylor, P.; Froeliger, E.H. The Divergently Transcribed Streptococcus parasanguis Virulence-Associated fimA Operon Encoding an Mn2+ -Responsive Metal Transporter and pepO Encoding a Zinc Metallopeptidase Are Not Coordinately Regulated. Infect. Immun. 2002, 70, 5706–5714. [Google Scholar] [CrossRef][Green Version]
- Plumptre, C.D.; Eijkelkamp, B.A.; Morey, J.R.; Behr, F.; Couñago, R.M.; Ogunniyi, A.D.; Kobe, B.; O’Mara, M.L.; Paton, J.C.; McDevitt, C.A. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 2014, 91, 834–851. [Google Scholar] [CrossRef]
- Plumptre, C.D.; Hughes, C.E.; Harvey, R.M.; Eijkelkamp, B.A.; McDevitt, C.A.; Paton, J.C. Overlapping Functionality of the Pht Proteins in Zinc Homeostasis of Streptococcus pneumoniae. Infect. Immun. 2014, 82, 4315–4324. [Google Scholar] [CrossRef]
- Kloosterman, T.G.; van der Kooi-Pol, M.M.; Bijlsma, J.J.E.; Kuipers, O.P. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 2007, 65, 1049–1063. [Google Scholar] [CrossRef]
- McAllister, L.J.; Tseng, H.-J.; Ogunniyi, A.D.; Jennings, M.P.; McEwan, A.G.; Paton, J.C. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 2004, 53, 889–901. [Google Scholar] [CrossRef]
- Manzetti, S. Quantum chemical calculations of the active site of the solute-binding protein PsaA from Streptococcus pneumoniae explain electronic selectivity of metal binding. Struct. Chem. 2018, 29, 393–401. [Google Scholar] [CrossRef]
- E Martin, J.; Le, M.T.; Bhattarai, N.; A Capdevila, D.; Shen, J.; E Winkler, M.; Giedroc, D.P. A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res. 2019, 47, 6885–6899. [Google Scholar] [CrossRef]
- Rosch, J.W.; Gao, G.; Ridout, G.; Wang, Y.-D.; Tuomanen, E.I. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 2009, 72, 12–25. [Google Scholar] [CrossRef]
- Brown, J.S.; Gilliland, S.M.; Ruiz-Albert, J.; Holden, D.W. Characterization of Pit, a Streptococcus pneumoniae Iron Uptake ABC Transporter. Infect. Immun. 2002, 70, 4389–4398. [Google Scholar] [CrossRef]
- Brown, J.S.; Gilliland, S.M.; Holden, D.W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 2001, 40, 572–585. [Google Scholar] [CrossRef]
- Miao, X.; He, J.; Zhang, L.; Zhao, X.; Ge, R.; He, Q.-Y.; Sun, X. A Novel Iron Transporter SPD_1590 in Streptococcus pneumoniae Contributing to Bacterial Virulence Properties. Front. Microbiol. 2018, 9, 1624. [Google Scholar] [CrossRef]
- Tai, S.S.; Yu, C.; Lee, J.K. A solute binding protein of Streptococcus pneumoniae iron transport. FEMS Microbiol. Lett. 2003, 220, 303–308. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Edmonds, K.A.; Raines, D.J.; Murphy, B.A.; Wu, H.; Guo, C.; Nolan, E.M.; VanNieuwenhze, M.S.; Duhme-Klair, A.-K.; Giedroc, D.P. The pneumococcal iron uptake protein A (PiuA) specifically recognizes tetradentate FeIII bis- and mono-catechole complexes. J. Mol. Biol. 2020, 432, 5390–5410. [Google Scholar] [CrossRef]
- Pramanik, A.; Braun, V. Albomycin Uptake via a Ferric Hydroxamate Transport System of Streptococcus pneumoniae R6. J. Bacteriol. 2006, 188, 3878–3886. [Google Scholar] [CrossRef]
- Shafeeq, S.; Yesilkaya, H.; Kloosterman, T.G.; Narayanan, G.; Wandel, M.; Andrew, P.W.; Kuipers, O.P.; Morrissey, J.A. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 2011, 81, 1255–1270. [Google Scholar] [CrossRef]
- Fu, Y.; Tsui, H.-C.T.; Bruce, K.E.; Sham, L.T.; Higgins, K.A.; Lisher, J.P.; Kazmierczak, K.M.; Maroney, M.J.; Dann, C.E.; Winkler, M.E.; et al. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat. Chem. Biol. 2013, 9, 177–183. [Google Scholar] [CrossRef]
- Brenot, A.; Weston, B.F.; Caparon, M.G. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 2007, 63, 1185–1196. [Google Scholar] [CrossRef]
- Tedde, V.; Rosini, R.; Galeotti, C.L. Zn2+ Uptake in Streptococcus pyogenes: Characterization of adcA and lmb Null Mutants. PLoS ONE 2016, 11, e0152835. [Google Scholar] [CrossRef]
- Sanson, M.; Makthal, N.; Flores, A.; Olsen, R.J.; Musser, J.M.; Kumaraswami, M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015, 43, 418–432. [Google Scholar] [CrossRef]
- Ong, C.-L.; Gillen, C.M.; Barnett, T.; Walker, M.; McEwan, A.G. An Antimicrobial Role for Zinc in Innate Immune Defense Against Group A Streptococcus. J. Infect. Dis. 2014, 209, 1500–1508. [Google Scholar] [CrossRef]
- Weston, B.F.; Brenot, A.; Caparon, M.G. The Metal Homeostasis Protein, Lsp, of Streptococcus pyogenes Is Necessary for Acquisition of Zinc and Virulence. Infect. Immun. 2009, 77, 2840–2848. [Google Scholar] [CrossRef]
- Janulczyk, R.; Pallon, J.; Bjorck, L. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 1999, 34, 596–606. [Google Scholar] [CrossRef]
- Janulczyk, R.; Ricci, S.; Björck, L. MtsABC Is Important for Manganese and Iron Transport, Oxidative Stress Resistance, and Virulence of Streptococcus pyogenes. Infect. Immun. 2003, 71, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ge, R.; Chiu, J.-F.; Sun, H.; He, Q.-Y. Lipoprotein MtsA of MtsABC in Streptococcus pyogenes primarily binds ferrous ion with bicarbonate as a synergistic anion. FEBS Lett. 2008, 582, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Ong, C.-L.; Gillen, C.M.; Davies, M.R.; West, N.P.; McEwan, A.G.; Walker, M.J. Manganese Homeostasis in Group A Streptococcus Is Critical for Resistance to Oxidative Stress and Virulence. mBio 2015, 6, e00278-15. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.G.; Ong, C.-L.Y.; Djoko, K.Y.; West, N.P.; Davies, M.R.; McEwan, A.G.; Walker, M.J. The PerR-Regulated P 1B-4 -Type ATPase (PmtA) Acts as a Ferrous Iron Efflux Pump in Streptococcus pyogenes. Infect. Immun. 2017, 85, e00140-17. [Google Scholar] [CrossRef]
- Montañez, G.E.; Neely, M.N.; Eichenbaum, Z. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology 2005, 151, 3749–3757. [Google Scholar] [CrossRef]
- Bates, C.S.; Montañez, G.E.; Woods, C.R.; Vincent, R.M.; Eichenbaum, Z. Identification and Characterization of a Streptococcus pyogenes Operon Involved in Binding of Hemoproteins and Acquisition of Iron. Infect. Immun. 2003, 71, 1042–1055. [Google Scholar] [CrossRef]
- Lei, B.; Liu, M.; Voyich, J.M.; Prater, C.I.; Kala, S.V.; DeLeo, F.R.; Musser, J.M. Identification and Characterization of HtsA, a Second Heme-Binding Protein Made by Streptococcus pyogenes. Infect. Immun. 2003, 71, 5962–5969. [Google Scholar] [CrossRef]
- Sook, B.R.; Block, D.R.; Sumithran, S.; Montañez, G.E.; Rodgers, K.R.; Dawson, J.H.; Eichenbaum, Z.; Dixon, D.W. Characterization of SiaA, a Streptococcal Heme-Binding Protein Associated with a Heme ABC Transport System. Biochemistry 2008, 47, 2678–2688. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Lei, B. The surface protein Shr of Streptococcus pyogenes binds heme and transfers it to the streptococcal heme-binding protein Shp. BMC Microbiol. 2008, 8, 15. [Google Scholar] [CrossRef]
- Lei, B.; Smoot, L.M.; Menning, H.M.; Voyich, J.M.; Kala, S.V.; Deleo, F.R.; Reid, S.D.; Musser, J.M. Identification and Characterization of a Novel Heme-Associated Cell Surface Protein Made by Streptococcus pyogenes. Infect. Immun. 2002, 70, 4494–4500. [Google Scholar] [CrossRef]
- Hanks, T.S.; Liu, M.; McClure, M.J.; Lei, B. ABC transporter FtsABCD of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol. 2005, 5, 62. [Google Scholar] [CrossRef]
- Young, C.A.; Gordon, L.D.; Fang, Z.; Holder, R.C.; Reid, S.D. Copper Tolerance and Characterization of a Copper-Responsive Operon, copYAZ, in an M1T1 Clinical Strain of Streptococcus pyogenes. J. Bacteriol. 2015, 197, 2580–2592. [Google Scholar] [CrossRef]
- Chen, Y.-Y.M.; Burne, R.A. Identification and Characterization of the Nickel Uptake System for Urease Biogenesis in Streptococcus salivarius 57.I. J. Bacteriol. 2003, 185, 6773–6779. [Google Scholar] [CrossRef]
- Li, K.; Gifford, A.H.; Hampton, T.H.; O’Toole, G.A. Availability of Zinc Impacts Interactions between Streptococcus sanguinis and Pseudomonas aeruginosa in Coculture. J. Bacteriol. 2020, 202, e00618-19. [Google Scholar] [CrossRef]
- Puccio, T.; An, S.; Schultz, A.C.; Lizarraga, C.A.; Bryant, A.S.; Culp, D.J.; Burne, R.A.; Kitten, T. Manganese transport by Streptococcus sanguinis in acidic conditions and its impact on growth in vitro and in vivo. Mol. Microbiol. 2022, 117, 375–393. [Google Scholar] [CrossRef]
- Crump, K.E.; Bainbridge, B.; Brusko, S.; Turner, L.S.; Ge, X.; Stone, V.; Xu, P.; Kitten, T. The relationship of the lipoprotein SsaB, manganese and superoxide dismutase in Streptococcus sanguinis virulence for endocarditis. Mol. Microbiol. 2014, 92, 1243–1259. [Google Scholar] [CrossRef]
- Aranda, J.; Teixidó, L.; Fittipaldi, N.; Cortés, P.; Llagostera, M.; Gottschalk, M.; Barbé, J. Inactivation of the gene encoding zinc-binding lipoprotein 103 impairs the infectivity of Streptococcus suis. Can. J. Vet. Res. 2012, 76, 72–76. [Google Scholar]
- Zheng, C.; Qiu, J.; Zhao, X.; Yu, S.; Wang, H.; Wan, M.; Wei, M.; Jiao, X. The AdcR-regulated AdcA and AdcAII contribute additively to zinc acquisition and virulence in Streptococcus suis. Vet. Microbiol. 2022, 269, 109418. [Google Scholar] [CrossRef]
- Shao, Z.-Q.; Pan, X.; Li, X.; Liu, W.; Han, M.; Wang, C.; Wang, J.; Zheng, F.; Cao, M.; Tang, J. HtpS, a novel immunogenic cell surface-exposed protein of Streptococcus suis, confers protection in mice. FEMS Microbiol. Lett. 2011, 314, 174–182. [Google Scholar] [CrossRef]
- Li, M.; Shao, Z.-Q.; Guo, Y.; Wang, L.; Hou, T.; Hu, D.; Zheng, F.; Tang, J.; Wang, C.; Feng, Y.; et al. The type II histidine triad protein HtpsC is a novel adhesion with the involvement of Streptococcus suis virulence. Virulence 2015, 6, 631–641. [Google Scholar] [CrossRef]
- Zheng, C.; Wei, M.; Qiu, J.; Jia, M.; Zhou, X.; Jiao, X. TroR Negatively Regulates the TroABCD System and Is Required for Resistance to Metal Toxicity and Virulence in Streptococcus suis. Appl. Environ. Microbiol. 2021, 87, e01375-21. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, C.; Cao, M.; Zeng, T.; Zhao, X.; Shi, G.; Chen, H.; Bei, W. The manganese efflux system MntE contributes to the virulence of Streptococcus suis serotype 2. Microb. Pathog. 2017, 110, 23–30. [Google Scholar] [CrossRef]
- Schreur, P.J.W.; Rebel, J.M.J.; Smits, M.A.; van Putten, J.P.M.; Smith, H.E. TroA of Streptococcus suis Is Required for Manganese Acquisition and Full Virulence. J. Bacteriol. 2011, 193, 5073–5080. [Google Scholar] [CrossRef]
- Aranda, J.; Cortés, P. Contribution of the FeoB transporter to Streptococcus suis virulence. Int. Microbiol. 2009, 12, 137–143. [Google Scholar] [CrossRef]
- Zheng, C.; Jia, M.; Gao, M.; Lu, T.; Li, L.; Zhou, P. PmtA functions as a ferrous iron and cobalt efflux pump in Streptococcus suis. Emerg. Microbes Infect. 2019, 8, 1254–1264. [Google Scholar] [CrossRef]
- Zheng, C.; Jia, M.; Lu, T.; Gao, M.; Li, L. CopA Protects Streptococcus suis against Copper Toxicity. Int. J. Mol. Sci. 2019, 20, 2969. [Google Scholar] [CrossRef]
- Smith, A.J.; Ward, P.N.; Field, T.R.; Jones, C.L.; Lincoln, R.A.; Leigh, J.A. MtuA, a Lipoprotein Receptor Antigen from Streptococcus uberis, Is Responsible for Acquisition of Manganese during Growth in Milk and Is Essential for Infection of the Lactating Bovine Mammary Gland. Infect. Immun. 2003, 71, 4842–4849. [Google Scholar] [CrossRef]
- Shafeeq, S.; Kuipers, O.P.; Kloosterman, T.G. The role of zinc in the interplay between pathogenic streptococci and their hosts. Mol. Microbiol. 2013, 88, 1047–1057. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Makthal, N.; Kumaraswami, M. Zinc’ing it out: Zinc homeostasis mechanisms and their impact on the pathogenesis of human pathogen group A streptococcus. Metallomics 2017, 9, 1693–1702. [Google Scholar] [CrossRef]
- Plumptre, C.D.; Ogunniyi, A.D.; Paton, J.C. Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol. 2012, 20, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Corbin, W.J.; Seeley, B.D.; Raab, E.H.; Feldmann, A.; Miller, J.; Torres, M.R.; Anderson, V.J.; Dattilo, K.L.; Anderson, B.M.; Dunman, K.L.; et al. Bacterial Growth in Tissue Abscesses. Science 2008, 319, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Morey, J.R.; Neville, S.L.; Tan, A.; Pederick, V.G.; Cole, N.; Singh, P.P.; Ong, C.-L.; De Vega, R.G.; Clases, D.; et al. Dietary zinc and the control of Streptococcus pneumoniae infection. PLoS Pathog. 2019, 15, e1007957. [Google Scholar] [CrossRef] [PubMed]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Ong, C.-L.Y.; Berking, O.; Walker, M.; McEwan, A.G. New Insights into the Role of Zinc Acquisition and Zinc Tolerance in Group A Streptococcal Infection. Infect. Immun. 2018, 86, e00048-18. [Google Scholar] [CrossRef]
- Makthal, N.; Nguyen, K.; Do, H.; Gavagan, M.; Chandrangsu, P.; Helmann, J.D.; Olsen, R.J.; Kumaraswami, M. A Critical Role of Zinc Importer AdcABC in Group A Streptococcus-Host Interactions during Infection and Its Implications for Vaccine Development. eBioMedicine 2017, 21, 131–141. [Google Scholar] [CrossRef]
- De Filippo, K.; Neill, D.R.; Mathies, M.; Bangert, M.; McNeill, E.; Kadioglu, A.; Hogg, N. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. FASEB J. 2014, 28, 3600–3608. [Google Scholar] [CrossRef]
- Makthal, N.; Do, H.; Wendel, B.M.; Olsen, R.J.; Helmann, J.D.; Musser, J.M.; Kumaraswami, M. Group A Streptococcus AdcR Regulon Participates in Bacterial Defense against Host-Mediated Zinc Sequestration and Contributes to Virulence. Infect. Immun. 2020, 88, e00097-20. [Google Scholar] [CrossRef]
- Brown, L.R.; Gunnell, S.M.; Cassella, A.N.; Keller, L.E.; Scherkenbach, L.A.; Mann, B.; Brown, M.; Hill, R.; Fitzkee, N.C.; Rosch, J.W.; et al. AdcAII of Streptococcus pneumoniae Affects Pneumococcal Invasiveness. PLoS ONE 2016, 11, e0146785. [Google Scholar] [CrossRef][Green Version]
- Bayle, L.; Chimalapati, S.; Schoehn, G.; Brown, J.; Vernet, T.; Durmort, C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol. Microbiol. 2011, 82, 904–916. [Google Scholar] [CrossRef]
- Shafeeq, S.; Kloosterman, T.G.; Kuipers, O.P. Transcriptional response of Streptococcus pneumoniae to Zn2+ limitation and the repressor/activator function of AdcR. Metallomics 2011, 3, 609–618. [Google Scholar] [CrossRef]
- Rosen, T.; Hadley, R.C.; Bozzi, A.T.; Ocampo, D.; Shearer, J.; Nolan, E.M. Zinc sequestration by human calprotectin facilitates manganese binding to the bacterial solute-binding proteins PsaA and MntC. Metallomics 2022, 14, mfac001. [Google Scholar] [CrossRef]
- Ogunniyi, A.D.; Grabowicz, M.; Mahdi, L.K.; Cook, J.; Gordon, D.L.; Sadlon, T.A.; Paton, J.C. Pneumococcal histidine triad proteins are regulated by the Zn2+ -dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 2009, 23, 731–738. [Google Scholar] [CrossRef]
- Song, X.-M.; Connor, W.; Hokamp, K.; A Babiuk, L.; A Potter, A. Streptococcus pneumoniae early response genes to human lung epithelial cells. BMC Res. Notes 2008, 1, 64. [Google Scholar] [CrossRef]
- Brown, L.R.; Caulkins, R.C.; Schartel, T.E.; Rosch, J.W.; Honsa, E.S.; Schultz-Cherry, S.; Meliopoulos, V.A.; Cherry, S.; Thornton, J.A. Increased Zinc Availability Enhances Initial Aggregation and Biofilm Formation of Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2017, 7, 233. [Google Scholar] [CrossRef]
- Terao, Y.; Kawabata, S.; Kunitomo, E.; Nakagawa, I.; Hamada, S. Novel Laminin-Binding Protein of Streptococcus pyogenes, Lbp, Is Involved in Adhesion to Epithelial Cells. Infect. Immun. 2002, 70, 993–997. [Google Scholar] [CrossRef]
- Elsner, A.; Kreikemeyer, B.; Braun-Kiewnick, A.; Spellerberg, B.; Buttaro, B.A.; Podbielski, A. Involvement of Lsp, a Member of the LraI-Lipoprotein Family in Streptococcus pyogenes, in Eukaryotic Cell Adhesion and Internalization. Infect. Immun. 2002, 70, 4859–4869. [Google Scholar] [CrossRef]
- Spellerberg, B.; Rozdzinski, E.; Martin, S.; Weber-Heynemann, J.; Schnitzler, N.; Lütticken, R.; Podbielski, A. Lmb, a Protein with Similarities to the LraI Adhesin Family, Mediates Attachment of Streptococcus agalactiae to Human Laminin. Infect. Immun. 1999, 67, 871–878. [Google Scholar] [CrossRef]
- Kallio, A.; Sepponen, K.; Hermand, P.; Denoël, P.; Godfroid, F.; Melin, M. Role of Pht Proteins in Attachment of Streptococcus pneumoniae to Respiratory Epithelial Cells. Infect. Immun. 2014, 82, 1683–1691. [Google Scholar] [CrossRef]
- Wu, C.; Labrie, J.; Tremblay, Y.; Haine, D.; Mourez, M.; Jacques, M. Zinc as an agent for the prevention of biofilm formation by pathogenic bacteria. J. Appl. Microbiol. 2013, 115, 30–40. [Google Scholar] [CrossRef]
- Danilova, T.A.; Danilina, G.A.; Adzhieva, A.A.; Vostrova, E.I.; Zhukhovitskii, V.G.; Cheknev, S.B. Inhibitory Effect of Copper and Zinc Ions on the Growth of Streptococcus pyogenes and Escherichia coli Biofilms. Bull. Exp. Biol. Med. 2020, 169, 648–652. [Google Scholar] [CrossRef]
- Martin, J.E.; Lisher, J.P.; Winkler, M.E.; Giedroc, D.P. Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017, 104, 334–348. [Google Scholar] [CrossRef]
- Brazel, E.B.; Tan, A.; Neville, S.L.; Iverson, A.R.; Udagedara, S.R.; Cunningham, B.A.; Sikanyika, M.; De Oliveira, D.M.; Keller, B.; Bohlmann, L.; et al. Dysregulation of Streptococcus pneumoniae zinc homeostasis breaks ampicillin resistance in a pneumonia infection model. Cell Rep. 2022, 38, 110202. [Google Scholar] [CrossRef]
- Martin, J.E.; Giedroc, D.P. Functional Determinants of Metal Ion Transport and Selectivity in Paralogous Cation Diffusion Facilitator Transporters CzcD and MntE in Streptococcus pneumoniae. J. Bacteriol. 2016, 198, 1066–1076. [Google Scholar] [CrossRef]
- Francis, J.D.; Guevara, M.A.; Lu, J.; Madhi, S.A.; Kwatra, G.; Aronoff, D.M.; Manning, S.D.; Gaddy, J.A. The antimicrobial activity of zinc against group B Streptococcus is strain-dependent across diverse sequence types, capsular serotypes, and invasive versus colonizing isolates. BMC Microbiol. 2022, 22, 23. [Google Scholar] [CrossRef]
- Bosma, E.F.; Rau, M.H.; A van Gijtenbeek, L.; Siedler, S. Regulation and distinct physiological roles of manganese in bacteria. FEMS Microbiol. Rev. 2021, 45, fuab028. [Google Scholar] [CrossRef]
- Ge, R.; Sun, X. Iron acquisition and regulation systems in Streptococcus species. Metallomics 2014, 6, 996–1003. [Google Scholar] [CrossRef]
- Kehres, D.G.; Zaharik, M.L.; Finlay, B.B.; Maguire, M.E. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000, 36, 1085–1100. [Google Scholar] [CrossRef]
- Whalan, R.H.; Funnell, S.G.P.; Bowler, L.D.; Hudson, M.J.; Robinson, A.; Dowson, C.G. Distribution and Genetic Diversity of the ABC Transporter Lipoproteins PiuA and PiaA within Streptococcus pneumoniae and Related Streptococci. J. Bacteriol. 2006, 188, 1031–1038. [Google Scholar] [CrossRef]
- Kuipers, K.; Gallay, C.; Martinek, V.; Rohde, M.; Martínková, M.; Van Der Beek, S.L.; Jong, W.; Venselaar, H.; Zomer, A.; Bootsma, H.; et al. Highly conserved nucleotide phosphatase essential for membrane lipid homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 2016, 101, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Puccio, T.; Kunka, K.S.; Zhu, B.; Xu, P.; Kitten, T. Manganese Depletion Leads to Multisystem Changes in the Transcriptome of the Opportunistic Pathogen Streptococcus sanguinis. Front. Microbiol. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Geno, K.A.; Hauser, J.R.; Gupta, K.; Yother, J. Streptococcus pneumoniae Phosphotyrosine Phosphatase CpsB and Alterations in Capsule Production Resulting from Changes in Oxygen Availability. J. Bacteriol. 2014, 196, 1992–2003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardy, G.G.; Magee, A.D.; Ventura, C.L.; Caimano, M.J.; Yother, J. Essential Role for Cellular Phosphoglucomutase in Virulence of Type 3 Streptococcus pneumoniae. Infect. Immun. 2001, 69, 2309–2317. [Google Scholar] [CrossRef]
- Ong, C.-L.Y.; Walker, M.J.; McEwan, A.G. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci. Rep. 2015, 5, 10799. [Google Scholar] [CrossRef]
- McFarland, A.L.; Bhattarai, N.; Joseph, M.; Winkler, M.E.; Martin, J.E. Cellular Mn/Zn ratio influences phosphoglucomutase activity and capsule production in Streptococcus pneumoniae D39. J. Bacteriol. 2021, 203, e00602-20. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The Neutrophil Lipocalin NGAL Is a Bacteriostatic Agent that Interferes with Siderophore-Mediated Iron Acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, T.; Li, N.; Yang, X.-Y.; Ding, J.; He, Q.-Y.; Sun, X. Identification and Tetramer Structure of Hemin-Binding Protein SPD_0310 Linked to Iron Homeostasis and Virulence of Streptococcus pneumoniae. mSystems 2022, 7, e00221-22. [Google Scholar] [CrossRef]
- Burcham, L.R.; Akbari, M.S.; Alhajjar, N.; Keogh, R.A.; Radin, J.N.; Kehl-Fie, T.E.; Belew, A.T.; El-Sayed, N.M.; McIver, K.S.; Doran, K.S. Genomic Analyses Identify Manganese Homeostasis as a Driver of Group B Streptococcal Vaginal Colonization. mBio 2022, 13, e00985-22. [Google Scholar] [CrossRef]
- VanderWal, A.R.; Makthal, N.; Pinochet-Barros, A.; Helmann, J.D.; Olsen, R.J.; Kumaraswami, M. Iron Efflux by PmtA Is Critical for Oxidative Stress Resistance and Contributes Significantly to Group A Streptococcus Virulence. Infect. Immun. 2017, 85, e00091-17. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular Defenses against Superoxide and Hydrogen Peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Yang, F.; Hu, Q.; Tong, H.; Dong, X. Molecular Insights into Hydrogen Peroxide-sensing Mechanism of the Metalloregulator MntR in Controlling Bacterial Resistance to Oxidative Stresses. J. Biol. Chem. 2017, 292, 5519–5531. [Google Scholar] [CrossRef]
- Anjem, A.; Varghese, S.; Imlay, J.A. Manganese import is a key element of the OxyR response to hydrogen peroxide inEscherichia coli. Mol. Microbiol. 2009, 72, 844–858. [Google Scholar] [CrossRef]
- Johnston, J.W.; Briles, D.E.; Myers, L.E.; Hollingshead, S.K. Mn2+ -Dependent Regulation of Multiple Genes in Streptococcus pneumoniae through PsaR and the Resultant Impact on Virulence. Infect. Immun. 2006, 74, 1171–1180. [Google Scholar] [CrossRef]
- Begg, S.L.; Eijkelkamp, B.; Luo, Z.; Couñago, R.; Morey, J.R.; Maher, M.; Ong, C.-L.; McEwan, A.G.; Kobe, B.; O’Mara, M.; et al. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae. Nat. Commun. 2015, 6, 6418. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Higuchi, M.; Poole, L.B.; Kamio, Y. Role of the dpr Product in Oxygen Tolerance in Streptococcus mutans. J. Bacteriol. 2000, 182, 3740–3747. [Google Scholar] [CrossRef][Green Version]
- Tsou, C.-C.; Chiang-Ni, C.; Lin, Y.-S.; Chuang, W.-J.; Lin, M.-T.; Liu, C.-C.; Wu, J.-J. An Iron-Binding Protein, Dpr, Decreases Hydrogen Peroxide Stress and Protects Streptococcus pyogenes against Multiple Stresses. Infect. Immun. 2008, 76, 4038–4045. [Google Scholar] [CrossRef][Green Version]
- Haikarainen, T.; Thanassoulas, A.; Stavros, P.; Nounesis, G.; Haataja, S.; Papageorgiou, A.C. Structural and Thermodynamic Characterization of Metal Ion Binding in Streptococcus suis Dpr. J. Mol. Biol. 2011, 405, 448–460. [Google Scholar] [CrossRef]
- Hua, C.-Z.; Howard, A.; Malley, R.; Lu, Y.-J. Effect of Nonheme Iron-Containing Ferritin Dpr in the Stress Response and Virulence of Pneumococci. Infect. Immun. 2014, 82, 3939–3947. [Google Scholar] [CrossRef]
- Kauko, A.; Haataja, S.; Pulliainen, A.T.; Finne, J.; Papageorgiou, A.C. Crystal Structure of Streptococcus suis Dps-like Peroxide Resistance Protein Dpr: Implications for Iron Incorporation. J. Mol. Biol. 2004, 338, 547–558. [Google Scholar] [CrossRef]
- Johnson, M.; Kehl-Fie, T.; Rosch, J.W. Copper intoxication inhibits aerobic nucleotide synthesis in Streptococcus pneumoniae. Metallomics 2015, 7, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.-I.; Daldal, F.; Koch, H.-G. Cu Homeostasis in Bacteria: The Ins and Outs. Membranes 2020, 10, 242. [Google Scholar] [CrossRef]
- Arguello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of copper homeostasis in bacteria. Front. Cell. Infect. Microbiol. 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef]
- Neubert, M.J.; Dahlmann, E.A.; Ambrose, A.; Johnson, M.D.L. Copper Chaperone CupA and Zinc Control CopY Regulation of the Pneumococcal cop Operon. mSphere 2017, 2, e00372-17. [Google Scholar] [CrossRef]
- Stewart, L.J.; Ong, C.-L.Y.; Zhang, M.M.; Brouwer, S.; McIntyre, L.; Davies, M.R.; Walker, M.J.; McEwan, A.G.; Waldron, K.J.; Djoko, K.Y. Role of Glutathione in Buffering Excess Intracellular Copper in Streptococcus pyogenes. mBio 2020, 11, e02804-20. [Google Scholar] [CrossRef]
- Johnson, M.D.L.; Kehl-Fie, T.E.; Klein, R.; Kelly, J.; Burnham, C.; Mann, B.; Rosch, J.W. Role of Copper Efflux in Pneumococcal Pathogenesis and Resistance to Macrophage-Mediated Immune Clearance. Infect. Immun. 2015, 83, 1684–1694. [Google Scholar] [CrossRef]
- Vats, N.; Lee, S.F. Active detachment of Streptococcus mutans cells adhered to epon–hydroxylapatite surfaces coated with salivary proteins in vitro. Arch. Oral Biol. 2000, 45, 305–314. [Google Scholar] [CrossRef]
- Goh, K.G.K.; Sullivan, M.J.; Ulett, G.C. The Copper Resistome of Group B Streptococcus Reveals Insight into the Genetic Basis of Cellular Survival during Metal Ion Stress. J. Bacteriol. 2022, 204, e00068-22. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.J.; Benoit, S.L. Role of Nickel in Microbial Pathogenesis. Inorganics 2019, 7, 80. [Google Scholar] [CrossRef]
- Mulrooney, S.B.; Hausinger, R. Nickel uptake and utilization by microorganisms. FEMS Microbiol. Rev. 2003, 27, 239–261. [Google Scholar] [CrossRef]
- Desguin, B.; Urdiain-Arraiza, J.; Da Costa, M.; Fellner, M.; Hu, J.; Hausinger, R.P.; Desmet, T.; Hols, P.; Soumillion, P. Uncovering a superfamily of nickel-dependent hydroxyacid racemases and epimerases. Sci. Rep. 2020, 10, 18123. [Google Scholar] [CrossRef]
- Burcham, L.R.; A Hill, R.; Caulkins, R.C.; Emerson, J.P.; Nanduri, B.; Rosch, J.W.; Fitzkee, N.C.; A Thornton, J. Streptococcus pneumoniae metal homeostasis alters cellular metabolism. Metallomics 2020, 12, 1416–1427. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Counago, R.M.; McDevitt, C.A.; Ween, M.P.; Kobe, B. Prokaryotic substrate-binding proteins as targets for antimicrobial therapies. Curr. Drug Targets 2012, 13, 1400–1410. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Birkett, M.; Dover, L.; Lukose, C.C.; Zia, A.W.; Tambuwala, M.M.; Serrano-Aroca, A. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef]
| Cluster | Types of Ligands | Average Size (kDa) | Binding Dynamics |
|---|---|---|---|
| A | Zinc, manganese, iron, heme, siderophores | 29–37 | Spring hammer |
| B | Carbohydrates, Leu, Lle, Val, Autoinducer-2, natriuretic peptide | 31–50 | One domain movement |
| C | Di- and oligopeptides, nickel, arginine, cellobiose | 59–70 | One or two domain movement |
| D | Iron, carbohydrates, putrescine, thiamine, tetrahedral oxyanions | 26–47 | One domain movement |
| E | Sialic acid, 2-keto acids, ectoine, pyroglutamic acid | 35–41 | One sub-domain movement |
| F | Trigonal planar anions, methionine, compatible solutes, amino acids | 24–60 | One domain movement |
| G | Alginate | 60 | One domain movement |
| H | Iron | 80 | One sub-domain movement |
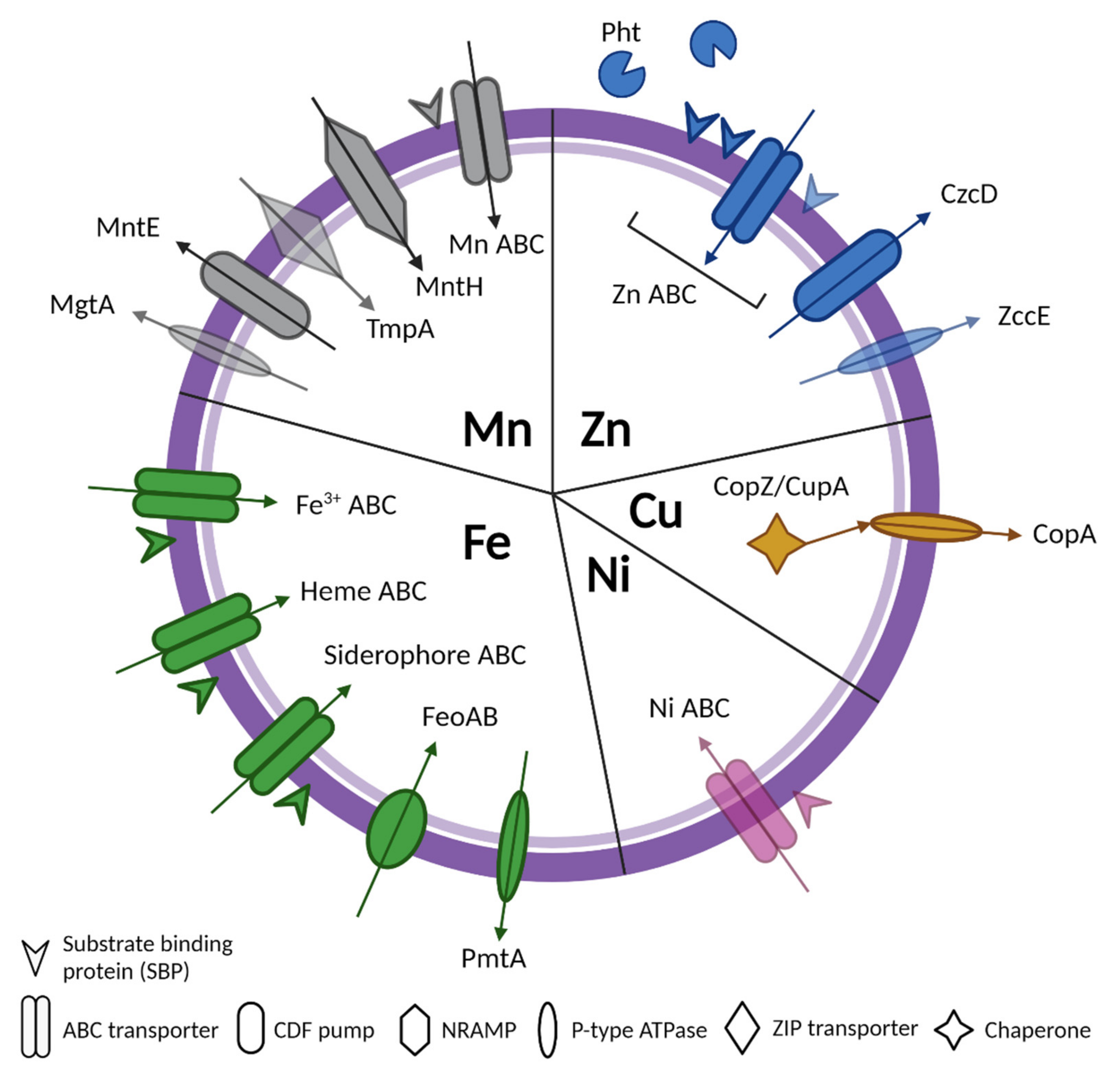
| Organism | Substrate | Transporter/Protein | Function | References |
|---|---|---|---|---|
| S. agalactiae | Zn | Sht, ShtII/Blr | Histidine triad proteins | [14,15,16] |
| AdcABC, AdcAII, Lmb | ABC transporter (import) | |||
| CzcD | CDF pump (export) | |||
| Mn, Fe | MtsABC | ABC transporter (import) | [17,18] | |
| MntH | NRAMP transporter (import) | |||
| Heme | PefAB, PefCD | ABC transporter (import) | [19] | |
| Siderophore | FhuCDBG | ABC transporter (import) | [20] | |
| Cu | CopA | P-type ATPase (export) | [21] | |
| CopZ | Chaperone | |||
| S. equi | Heme | SeShp | Cell surface protein | [22,23] |
| SeShr | Cell surface receptor | |||
| SeHtsABC | ABC transporter (import) | |||
| Siderophore | EqbHIJ | ABC transporter (import) | [24] | |
| S. gordonii | Mn | ScaABC | ABC transporter (import) | [25,26] |
| AdcABC | ABC transporter (import) | |||
| Cu | CopA | P-type ATPase (export) | [27] | |
| CopZ | Chaperone | |||
| S. mutans | Zn | AdcABC | ABC transporter (import) | [28,29,30] |
| ZccE | P-type ATPase (export) | |||
| Mn | MntE | CDF pump (export) | [31] | |
| Mn, Fe | SloABC | ABC transporter (import) | [32,33] | |
| MntH | NRAMP transporter (import) | |||
| Fe | FimA | ABC transporter element (import) | [34,35,36] | |
| FeoABC | Ferrous iron transport (import) | |||
| Smu995–998 | ABC transporter (import) | |||
| Cu | CopA | P-type ATPase (export) | [37] | |
| CopZ | Chaperone | |||
| S. oligofermentans | Mn | MntABC | ABC transporter (import) | [38] |
| MntH | NRAMP transporter (import) | |||
| S. parasanguinis | Mn, Fe | FimABC | ABC transporter (import) | [39] |
| S. pneumoniae | Zn | AdcABC AdcAII/Lmb | ABC transporter (import) | [40,41,42] |
| PhtABDE | Histidine triad proteins | |||
| CzcD | CDF pump (export) | |||
| Mn, Cd, Zn | PsaABC | ABC transporter (import) | [43,44] | |
| Mn | MgtA | P-type ATPase (export) | [45,46] | |
| MntE | CDF pump (export) | |||
| Fe | PitABCD | ABC transporter (import) | [47] | |
| Hemin | SPD_1590 | Hemin transporter (import) | [48,49,50] | |
| Siderophore | Pia/FhuDBGC | ABC transporter (import) | [48,51,52] | |
| PiuABCD | ABC transporter (import) | |||
| Cu | CopA | P-type ATPase (export) | [53,54] | |
| CupA | Chaperone | |||
| S. pyogenes | Zn | AdcABC, AdcAII/Lmb/Lsp | ABC transporter (import) | [55,56,57,58,59] |
| PhtD/HtpA, PhtY/Slr | Histidine triad proteins | |||
| CzcD | CDF pump (export) | |||
| Mn, Fe, Zn | MtsABC | ABC transporter (import) | [60,61,62] | |
| Mn | MntE | CDF pump (export) | [63] | |
| Fe | PmtA | P-type ATPase (export) | [64] | |
| Heme | SiuADBG/Spy383–386 | ABC transporter (import) | [65,66,67,68,69,70] | |
| SiaABC/HtsABC | ABC transporter (import) | |||
| Shp | Cell surface protein | |||
| Shr | Cell surface receptor | |||
| Siderophore | FtsABCD | ABC transporter (import) | [71] | |
| Cu | CopA | P-type ATPase (export) | [72] | |
| CupA | Chaperone | |||
| S. salivarius | Ni | UreMQO | ABC transporter (import) | [73] |
| S. sanguinis | Zn | SSA_0136–137, 260–261 | ABC transporter (import) | [74] |
| Mn | TmpA | ZIP transporter (import) | [13] | |
| Mn, Fe | SsaABC | ABC transporter (import) | [75,76] | |
| MntH | NRAMP transporter (import) | |||
| S. suis | Zn | AdcABC, AdcAII | ABC transporter (import) | [77,78,79,80] |
| Pht309/HtpsABC | Histidine triad protein | |||
| Mn | TroABCD | ABC transporter (import) | [81,82,83] | |
| MntE | CDF pump (export) | |||
| Fe | FeoAB | Ferrous iron transport (import) | [84] | |
| Fe, Co | PmtA | CDF pump (export) | [85] | |
| Cu | CopA | P-type ATPase (export) | [86] | |
| CopZ | Chaperone | |||
| S. uberis | Mn | MtuABC | ABC transporter (import) | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, M.S.; Doran, K.S.; Burcham, L.R. Metal Homeostasis in Pathogenic Streptococci. Microorganisms 2022, 10, 1501. https://doi.org/10.3390/microorganisms10081501
Akbari MS, Doran KS, Burcham LR. Metal Homeostasis in Pathogenic Streptococci. Microorganisms. 2022; 10(8):1501. https://doi.org/10.3390/microorganisms10081501
Chicago/Turabian StyleAkbari, Madeline S., Kelly S. Doran, and Lindsey R. Burcham. 2022. "Metal Homeostasis in Pathogenic Streptococci" Microorganisms 10, no. 8: 1501. https://doi.org/10.3390/microorganisms10081501
APA StyleAkbari, M. S., Doran, K. S., & Burcham, L. R. (2022). Metal Homeostasis in Pathogenic Streptococci. Microorganisms, 10(8), 1501. https://doi.org/10.3390/microorganisms10081501






