Effects of Mixed Decomposition of Pinus sylvestris var. mongolica and Morus alba Litter on Microbial Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Litter Sampling
2.3. Determination of Litter Characteristics
2.4. DNA Extraction and Amplification Sequencing
2.5. Statistical Analysis
3. Results
3.1. Chemical Properties of Leaves with Different Proportions of Leaf Litter
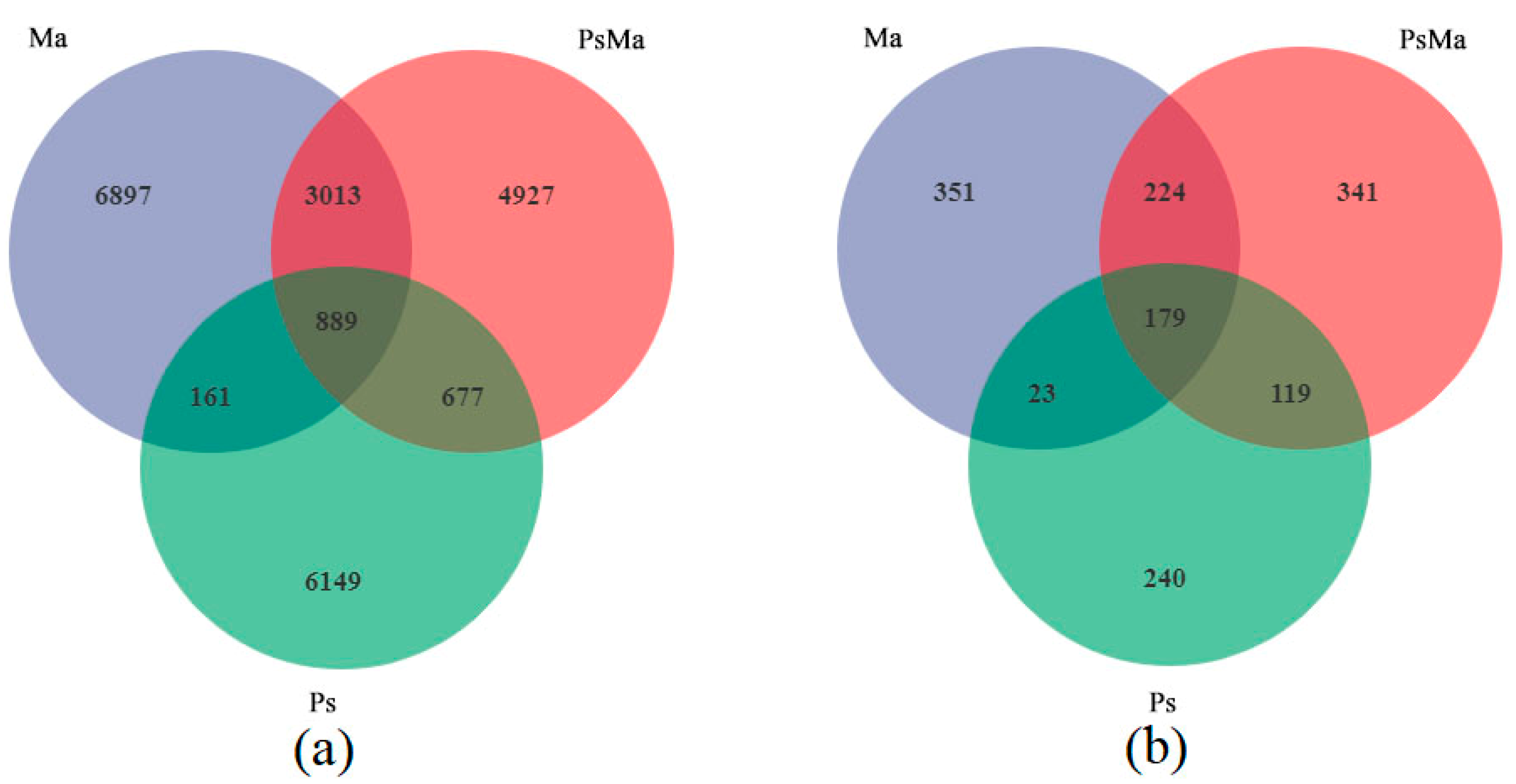
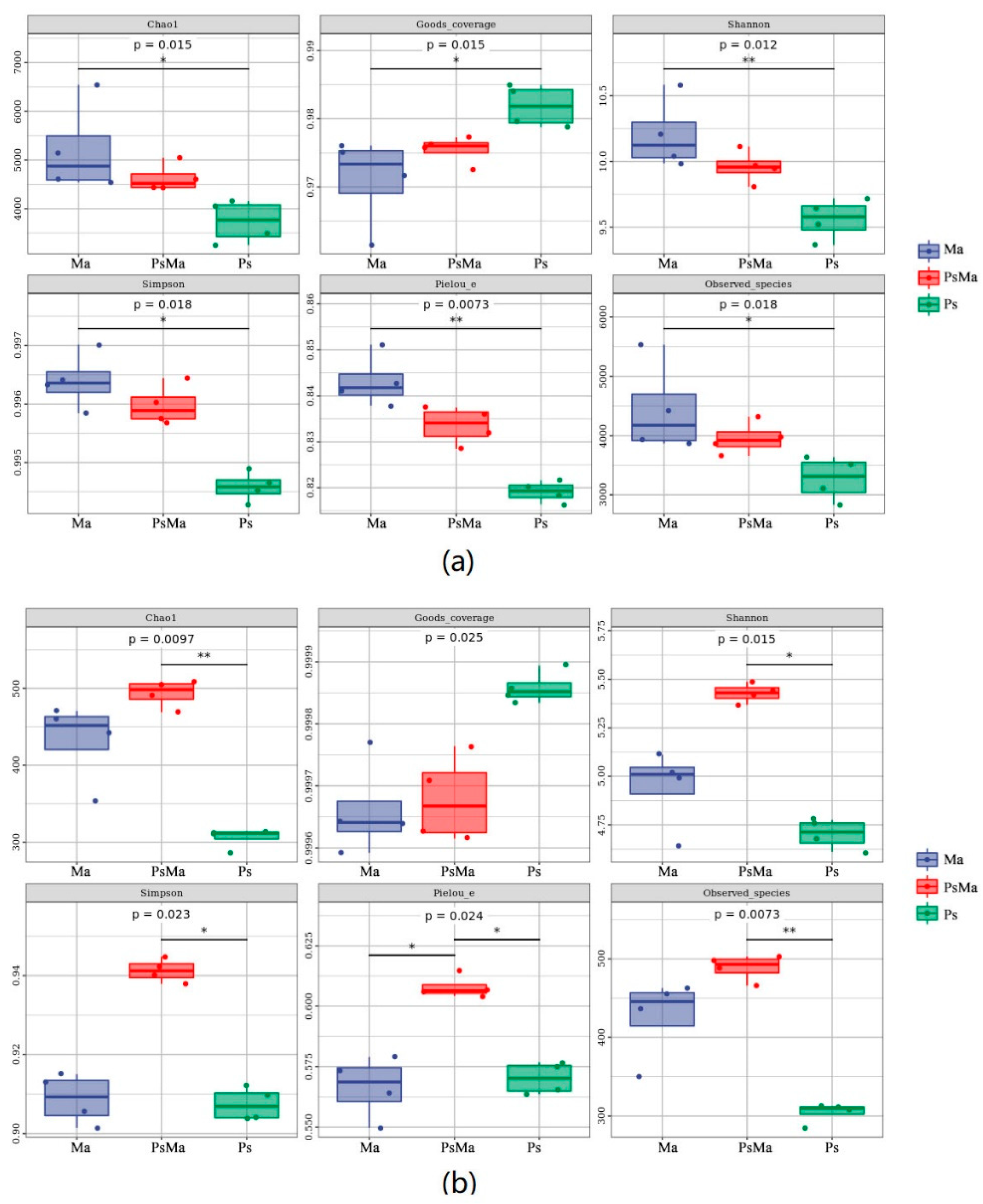
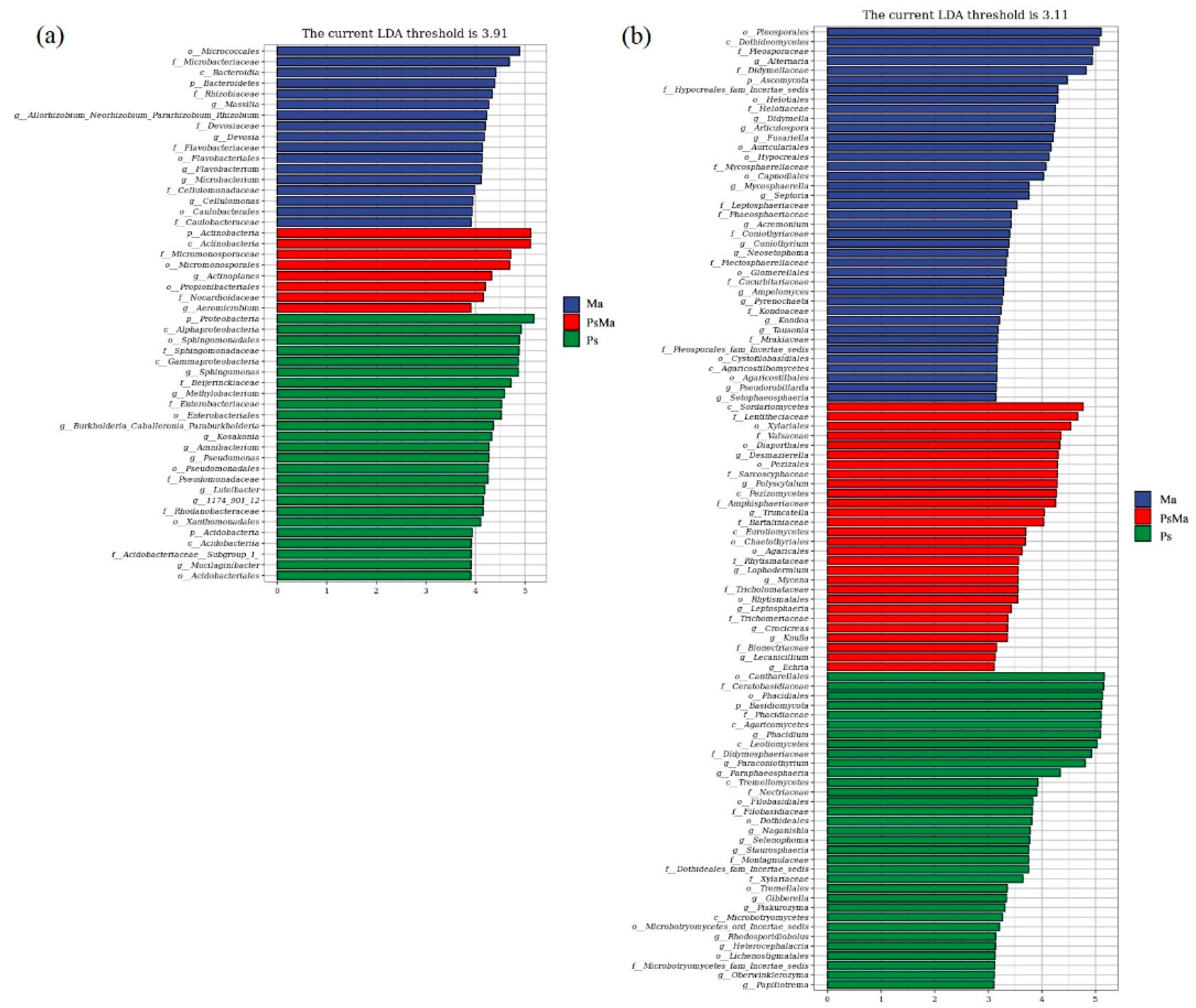
3.2. Microbial Community Compositions and Structural Characteristics of Different Leaf Litter Ratios
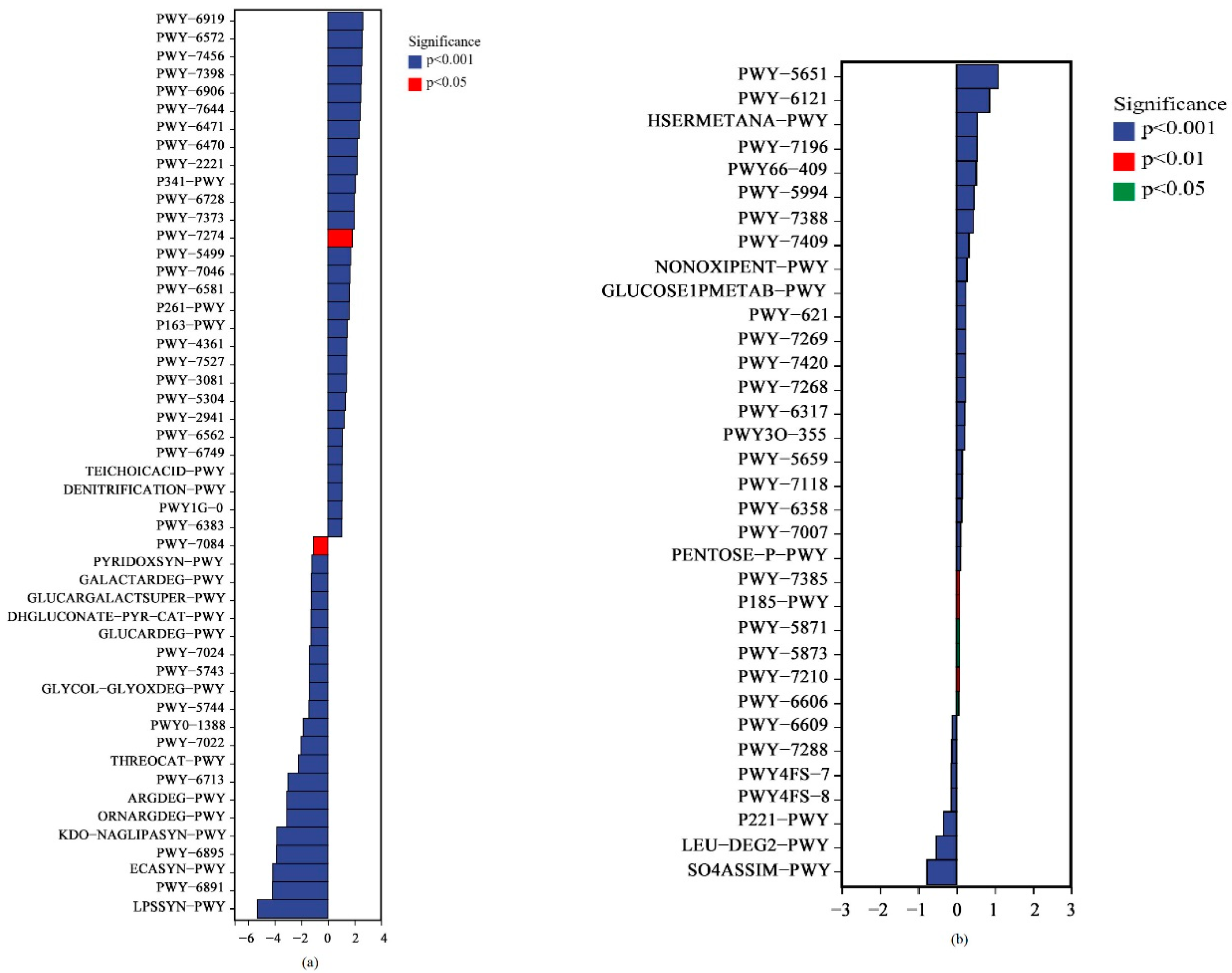
3.3. Prediction of Microbial Community Functions with Different Leaf Litter Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.-J.; Kang, H.Z.; Tan, H.; Xu, M.-L.; Wang, J. Natural regeneration characteristics ofPinus sylvestris var.mongolica forests on sandy land in Honghuaerji, China. J. For. Res. 2005, 16, 253–259. [Google Scholar] [CrossRef]
- Cao, Z.; Si, H.; Li, W.; Zhang, Y.; Yu, D.; Shao, Q. Natural regeneration characteristics of Pinus sylvestris var. mongolica litv. plantation in Nenjiang sand land. Prot. For. Sci. Technol. 2013, 09, 36–40. [Google Scholar] [CrossRef]
- Shu, M.; Wang, D.L.; Wang, K.; Lian, Z.; Tang, J.X.; Han, X.M.; Si, R.M.J. Ecological stoichiometric characteristics of needles, litter leaves and soils in Pinus sylvestris plantations of different ages. J. Soil Water Conserv. 2018, 32, 174–179. [Google Scholar] [CrossRef]
- Shu, M.; Jiang, T.; Wang, D.L.; Lian, Z.; Tang, J.X.; Kong, T.; Xu, Y.Y.; Han, X.M. Soil Ecological Stoichiometric Characteristics of Pinus sylvestris plantations of different forest ages in Horqin Sandy Land. Res. Arid. Areas 2018, 35, 789–795. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Wang, W. Variation of soil carbon pools in Pinus sylvestris plantations of different ages in north China. Acta Ecol. Sin. 2018, 38, 248–254. [Google Scholar] [CrossRef]
- Gao, G.-L.; Ding, G.-D.; Zhao, Y.-Y.; Wu, B.; Zhang, Y.-Q.; Guo, J.-B.; Qin, S.-G.; Bao, Y.-F.; Yu, M.-H.; Liu, Y.-D. Characterization of Soil Particle Size Distribution with a Fractal Model in the Desertified Regions of Northern China. Acta Geophys. 2016, 64, 1–14. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Zhan, H.; Shi, M. Can the Pinus sylvestris var. mongolica sand-fixing forest develop sustainably in a semi-arid region? Hydrol. Earth Syst. Sci. Discuss. 2019, 1–17. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Yang, K.; Yu, L.; Zhang, J. Nutrient removal under different harvesting scenarios for larch plantations in northeast China: Implications for nutrient conservation and management. For. Ecol. Manag. 2017, 400, 150–158. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, J.; Yan, Q.; Hu, Z.; Zheng, X. Changes in vegetation carbon stocks between 1978 and 2007 in central Loess Plateau, China. Environ. Earth Sci. 2016, 75, 312. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Li, M.; Zhang, J.; Zheng, X.; Wang, K. Canopy transpiration of Pinus sylvestris var. mongolica in a sparse wood grassland in the semiarid sandy region of Northeast China. Agric. For. Meteorol. 2018, 250–251, 192–201. [Google Scholar] [CrossRef]
- Zeng, D.H.; Hu, Y.L.; Chang, S.X.; Fan, Z.P. Land cover change effects on soil chemical and biological properties after planting mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 2008, 317, 121–133. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, J.; Yan, Q.; Song, L. Effects of land use changes on the groundwater table and the decline of Pinus sylvestris var. mongolica plantations in southern Horqin Sandy Land, Northeast China. Agric. Water Manag. 2012, 109, 94–106. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, M.; Gao, G.; Zhang, Y.; Ding, G.; Ren, Y.; Akhtar, M. Community structure and functional group of root-associated Fungi of Pinus sylvestris var. mongolica across stand ages in the Mu Us Desert. Ecol. Evol. 2020, 10, 3032–3042. [Google Scholar] [CrossRef]
- Zhu, J.; Kang, H.; Tan, H.; Xu, M. Effects of drought stresses induced by polyethylene glycol on germination of Pinus sylvestris var. mongolica seeds from natural and plantation forests on sandy land. J. For. Res. 2006, 11, 319–328. [Google Scholar] [CrossRef]
- Scott, D.; Welch, D.; Thurlow, M.; Elston, D.A. Regeneration of Pinus sylvestris in a natural pinewood in NE Scotland following reduction in grazing by Cervus elaphus. For. Ecol. Manag. 2000, 130, 199–211. [Google Scholar] [CrossRef]
- Wu, X.Y.; Jiang, F.; Li, X.D.; Xue, Y.; Qiu, S. The main characteristics of the decline of artificial sand-fixation forest of Pinus sylvestris. Chin. J. Appl. Ecol. 2004, 15, 2221–2224. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Guo, M.S.; Cao, H.Y.; Su, M. Stoichiometric characteristics of nitrogen and phosphorus in leaf-litter-soil system of Pinus sylvestris var. mongolica plantations. Ying Yong Sheng Tai Xue Bao (J. Appl. Ecol.) 2019, 30, 743–750. [Google Scholar]
- York, R.A.; Battles, J.J.; Heald, R.C. Gap-Based Silviculture in a Sierran Mixed-Conifer Forest: Effects of Gap Size on Early Survival and 7-Year Seedling Growth; General Technical Report PSW-GTR-203; USDA Forest Service: Albany, CA, USA, 2007. [Google Scholar]
- Groenendijk, M.; Dolman, A.; van der Molen, M.; Leuning, R.; Arneth, A.; Delpierre, N.; Gash, J.; Lindroth, A.; Richardson, A.; Verbeeck, H.; et al. Assessing parameter variability in a photosynthesis model within and between plant functional types using global Fluxnet eddy covariance data. Agric. For. Meteorol. 2011, 151, 22–38. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhu, B.; Bing, Y.; Luc, N.T.; Du, L.; Zhu, Z. Impacts of mixed litter decomposition from Robinia pseudoacacia and other tree species on C loss and nutrient release in the Loess Plateau of China. J. For. Res. 2015, 27, 525–532. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Ji, G. Responses of biomass allocation and photosynthesis in mulberry to Pb-contaminated soil. Acta Physiol. Plant. 2022, 44, 1–9. [Google Scholar] [CrossRef]
- Baruah, J.P.; Das, A.; Verma, R.; Narzary, P.R.; Linggi, B. Soil Nutrient Management in Mulberry Plant; Vital Biotech Publication: Rajasthan, India, 2022; ISBN 978-93-92953-01-9. [Google Scholar]
- Dhanyalakshmi, K.H.; Nataraja, K.N. Universal stress protein-like gene from mulberry enhances abiotic stress tolerance in Escherichia coli and transgenic tobacco cells. Plant Biol. 2021, 23, 1190–1194. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Gan, T.; Lin, Z.; Bao, L.; Hui, T.; Cui, X.; Huang, Y.; Wang, H.; Su, C.; Jiao, F.; Zhang, M.; et al. Comparative Proteomic Analysis of Tolerant and Sensitive Varieties Reveals That Phenylpropanoid Biosynthesis Contributes to Salt Tolerance in Mulberry. Int. J. Mol. Sci. 2021, 22, 9402. [Google Scholar] [CrossRef]
- Alam, I.; Ahmed, M.U.; Yeasmin, S.; Debrot, A.O.; Ahsan, N.; Verdegem, M. Effect of mixed leaf litter of four mangrove species on shrimp post larvae (Penaeus monodon, Fabricius, 1798) performance in tank and mesocosm conditions in Bangladesh. Aquaculture 2022, 551, 737968. [Google Scholar] [CrossRef]
- Tardif, A.; Shipley, B. The relationship between functional dispersion of mixed-species leaf litter mixtures and species’ interactions during decomposition. Oikos 2014, 124, 1050–1057. [Google Scholar] [CrossRef]
- Osburn, E.D.; Hoch, P.J.; Lucas, J.M.; McBride, S.G.; Strickland, M.S. Evaluating the roles of microbial functional breadth and home-field advantage in leaf litter decomposition. Funct. Ecol. 2022, 36, 1258–1267. [Google Scholar] [CrossRef]
- Hobbie, S. Interactions between Litter Lignin and Nitrogenitter Lignin and Soil Nitrogen Availability during Leaf Litter Decomposition in a Hawaiian Montane Forest. Ecosystems 2000, 3, 484–494. [Google Scholar] [CrossRef]
- Sanger, L.; Cox, P.; Splatt, P.; Whelan, M.; Anderson, J. Variability in the quality and potential decomposability of Pinus sylvestris litter from sites with different soil characteristics: Acid detergent fibre (ADF) and carbohydrate signatures. Soil Biol. Biochem. 1998, 30, 455–461. [Google Scholar] [CrossRef]
- Santos, F.M.; Balieiro, F.d.C.; Fontes, M.A.; Chaer, G.M. Understanding the enhanced litter decomposition of mixed-species plantations of Eucalyptus and Acacia mangium. Plant Soil 2017, 423, 141–155. [Google Scholar] [CrossRef]
- Song, F.; Fan, X.; Song, R. Review of mixed forest litter decomposition researches. Acta Ecol. Sin. 2010, 30, 221–225. [Google Scholar] [CrossRef]
- Guo, X.; Luo, Z.; Sun, O.J. Long-term litter type treatments alter soil carbon composition but not microbial carbon utilization in a mixed pine-oak forest. Biodegradation 2021, 152, 327–343. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Sariyildiz, T. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Teng, Z.; Fan, W.; Deng, P.; Wen, Z.; Zhou, K.; Xu, X. Nitrogen and Phosphorus Additions Impact Stability of Soil Organic Carbon and Nitrogen in Subtropical Evergreen Broad-Leaved Forest. Eurasian Soil Sci. 2022, 55, 425–436. [Google Scholar] [CrossRef]
- Del Río, M.; Sterba, H. Comparing volume growth in pure and mixed stands of Pinus sylvestris and Quercus pyrenaica. Ann. For. Sci. 2009, 66, 502. [Google Scholar] [CrossRef]
- Zhiyanski, M.; Glushkova, M.; Ferezliev, A.; Menichetti, L.; Leifeld, J. Carbon storage and soil property changes following afforestation in mountain ecosystems of the Western Rhodopes, Bulgaria. iFor. Biogeosci. For. 2016, 9, 626–634. [Google Scholar] [CrossRef]
- Song, P.; Ren, H.; Jia, Q.; Guo, J.; Zhang, N.; Ma, K. Effects of historical logging on soil microbial communities in a subtropical forest in southern China. Plant Soil 2015, 397, 115–126. [Google Scholar] [CrossRef]
- Ba, H.; Jiang, H.; Shen, P.; Cao, Y.; Song, Y.; Li, L. Screening of Phosphate-Resolving Bacteria in Rhizosphere of Cold Sunflower and Physiological and Biochemical Study. IOP Conf. Ser. Earth Environ. Sci. 2020, 526, 012038. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; De Vos, W.M.; Paul Ross, R.; O’Toole, P.W. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 315–322. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 407. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Chiani, M. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Good, I.J. The Population Frequencies of Species and the Estimation of Population Parameters. Biometrika 1953, 40, 237. [Google Scholar] [CrossRef]
- Ramette, A.N. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Bradshaw, C.; Kautsky, U.; Kumblad, L. Ecological Stoichiometry and Multi-element Transfer in a Coastal Ecosystem. Ecosystems 2012, 15, 591–603. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I. Stoichiometry and Nutrition of Plant Growth in Natural Communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J. Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biol. Biochem. 2014, 75, 102–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, D.; Qin, Y.; Zhou, Y.; Song, S.; Zhang, J. Characteristics of fungal community structure during the decomposition of mixed foliage litter from Pinus massoniana and broadleaved tree species in southwestern China. J. Plant Ecol. 2020, 13, 574–588. [Google Scholar] [CrossRef]
- Guo, K.; Liu, R.; Zhang, L.; Mao, Z.J. Decomposition characteristics and interaction of mixed leaf litter mixtures of Pinus koraiensis and Quercus mongolica in original broadleaved-Korean pine forest. Bull. Bot. Res. 2015, 35, 716–723. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Zhang, N.; Ma, K. The research of mixed litter effects on litter decomposition in terrestrial ecosystems. Acta Ecol. Sin. 2016, 36, 4977–4987. [Google Scholar] [CrossRef]
- Wardle, D.; Bonner, K.I.; Nicholson, K.S. Biodiversity and Plant Litter: Experimental Evidence Which Does Not Support the View That Enhanced Species Richness Improves Ecosystem Function. Oikos 1997, 79, 247–258. [Google Scholar] [CrossRef]
- Hu, Y.L.; Wang, S.L.; Zeng, D.H. Effects of Single Chinese Fir and Mixed Leaf Litters on Soil Chemical, Microbial Properties and Soil Enzyme Activities. Plant Soil 2006, 282, 379–386. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Macmillan Company: New York, NY, USA, 1960. [Google Scholar]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Aerts, R.; Callaghan, T.V.; Dorrepaal, E.; Van Logtestijn, R.S.P.; Cornelissen, J.H.C. Seasonal climate manipulations have only minor effects on litter decomposition rates and N dynamics but strong effects on litter P dynamics of sub-arctic bog species. Oecologia 2012, 170, 809–819. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P. Effects of enhanced atmospheric CO2 and nutrient supply on the quality and subsequent decomposition of fine roots of Betula pendula Roth. and Picea sitchensis (Bong.) Carr. Plant Soil 1995, 170, 267–277. [Google Scholar] [CrossRef]
- Xu, X.; Hirata, E. Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 2005, 273, 279–289. [Google Scholar] [CrossRef]
- Grace, J.; Rayment, M. Respiration in the balance. Nature 2000, 404, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, UK, 2002; pp. 1–42. [Google Scholar]
- Wang, Z.; Zheng, F. Impact of vegetation succession on leaf-litter-soil C:N:P stoichiometry and their intrinsic relationship in the Ziwuling Area of China’s Loess Plateau. J. For. Res. 2020, 32, 697–711. [Google Scholar] [CrossRef]
- Ya-Mei, C.; Run-Lian, H.; Chang-Chun, D.; Yang, L.; Wan-Qin, Y.; Jian, A.Z. Litter cellulolytic enzyme activities in alpine timberline ecotone of western Sichuan. Chin. J. Plant Ecol. 2014, 38, 334–342. [Google Scholar] [CrossRef]
- Liu, D.; Keiblinger, K.M.; Leitner, S.; Mentler, A.; Zechmeister-Boltenstern, S. Is there a convergence of deciduous leaf litter stoichiometry, biochemistry and microbial population during decay? Geoderma 2016, 272, 93–100. [Google Scholar] [CrossRef]
- Pan, F.J.; Zhang, W.; Wang, K.L.; He, X.Y.; Liang, S.C.; Wei, G.F. C:N:P ecological stoichiometric characteristics of litter from vegetation communities in typical karst peak-cluster depressions. Acta Ecol. Sin. 2011, 31, 335–343. [Google Scholar]
- Aneja, M.K.; Sharma, S.; Fleischmann, F.; Stich, S.; Heller, W.; Bahnweg, G.; Munch, J.C.; Schloter, M. Microbial Colonization of Beech and Spruce Litter—Influence of Decomposition Site and Plant Litter Species on the Diversity of Microbial Community. Microb. Ecol. 2006, 52, 127–135. [Google Scholar] [CrossRef]
- Chapman, S.K.; Newman, G.S. Biodiversity at the plant–soil interface: Microbial abundance and community structure respond to litter mixing. Oecologia 2009, 162, 763–769. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of Fungi, Bacteria, and Actinomycetes on Leaves Decomposing in a Stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Malosso, E. Use of 13C-labelled plant materials and ergosterol, PLFA and NLFA analyses to investigate organic matter decomposition in Antarctic soil. Soil Biol. Biochem. 2004, 36, 165–175. [Google Scholar] [CrossRef]
- Xu, W.; Shi, L.; Chan, O.; Li, J.; Casper, P.; Zou, X. Assessing the Effect of Litter Species on the Dynamic of Bacterial and Fungal Communities during Leaf Decomposition in Microcosm by Molecular Techniques. PLoS ONE 2013, 8, e84613. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Sun, X.; Zhang, Q.; Koide, R.T.; Insam, H.; Zhang, S. Impacts of mixed litter on the structure and functional pathway of microbial community in litter decomposition. Appl. Soil Ecol. 2019, 144, 72–82. [Google Scholar] [CrossRef]
- Li, Y.; Bezemer, M.; Yang, J.; Lü, X.; Li, X.; Liang, W.; Han, X.; Li, Q. Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biol. Biochem. 2018, 130, 33–42. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2012, 7, 477–486. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Z.; Yang, K.; Zhu, J. Impacts of conversion from secondary forests to larch plantations on the structure and function of microbial communities. Appl. Soil Ecol. 2016, 111, 73–83. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Fromin, N.; Barantal, S. Functional diversity of terrestrial microbial decomposers and their substrates. C. R. Biol. 2011, 334, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Vitousek, P.M. Effects of plant composition and diversity on nutrient cycling. Ecol. Monogr. 1998, 68, 121–149. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and Litter Decomposition in Terrestrial Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Q.-X.; Liu, N.; Li, L.; Zhang, C.-G.; Liu, Z.-B.; Zhang, Y.-Y. Effects of different leaf litters on the physicochemical properties and bacterial communities in Panax ginseng -growing soil. Appl. Soil Ecol. 2017, 111, 17–24. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.-J. Impact of tree litter decomposition on soil biochemical properties obtained from a temperate secondary forest in Northeast China. J. Soils Sediments 2014, 15, 13–23. [Google Scholar] [CrossRef]
- Cao, J.; He, X.; Chen, Y.; Chen, Y.; Zhang, Y.; Yu, S.; Zhou, L.; Liu, Z.; Zhang, C.; Fu, S. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2019, 704, 135341. [Google Scholar] [CrossRef]
- Frey, S.; Elliott, E.; Paustian, K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Guggenberger, G.; Frey, S.D.; Six, J.; Paustian, K.; Elliott, E.T. Bacterial and Fungal Cell-Wall Residues in Conventional and No-Tillage Agroecosystems. Soil Sci. Soc. Am. J. 1999, 63, 1188–1198. [Google Scholar] [CrossRef]
- Pascoal, C.; Cássio, F. Contribution of Fungi and Bacteria to Leaf Litter Decomposition in a Polluted River. Appl. Environ. Microbiol. 2004, 70, 5266–5273. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.; De Moraes Sa, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils—Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Pointing, S.B.; Hyde, K.D. Lignocellulose-degrading marine fungi. Biofouling 2000, 15, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, Y.; Zhang, X.; Feng, X.; Wu, J.; Wang, L.; Chen, G. Wheat straw: An inefficient substrate for rapid natural lignocellulosic composting. Bioresour. Technol. 2016, 209, 402–406. [Google Scholar] [CrossRef]
- Kazakov, A.E.; Rodionov, D.A.; Alm, E.; Arkin, A.P.; Dubchak, I.; Gelfand, M. Comparative Genomics of Regulation of Fatty Acid and Branched-Chain Amino Acid Utilization in Proteobacteria. J. Bacteriol. 2009, 191, 52–64. [Google Scholar] [CrossRef]
- Schweitzer, B.; Huber, I.; Amann, R.; Ludwig, W.; Simon, M. α- and β- Proteobacteria Control the Consumption and Release of Amino Acids on Lake Snow Aggregates. Appl. Environ. Microbiol. 2001, 67, 632–645. [Google Scholar] [CrossRef] [PubMed]

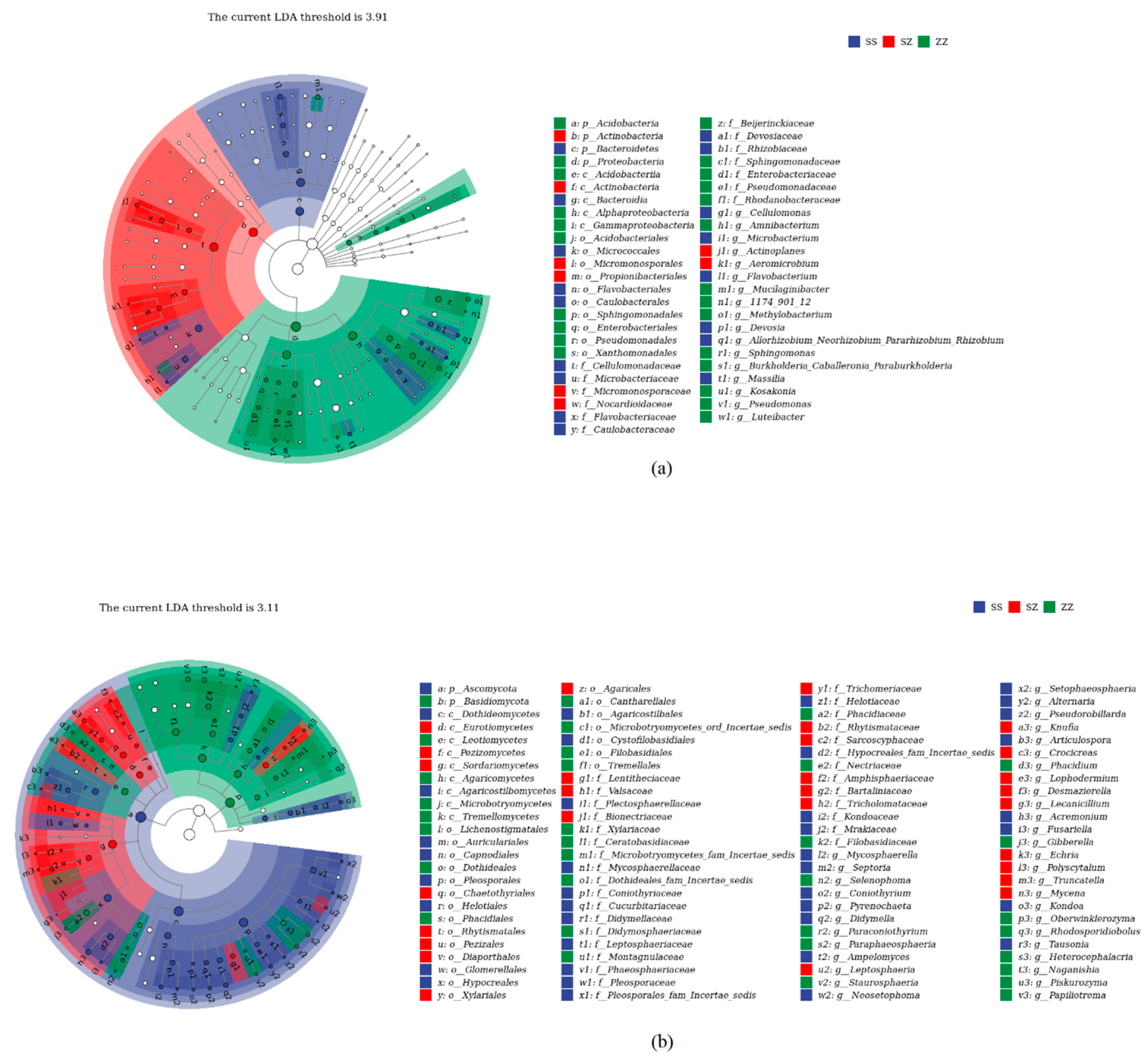

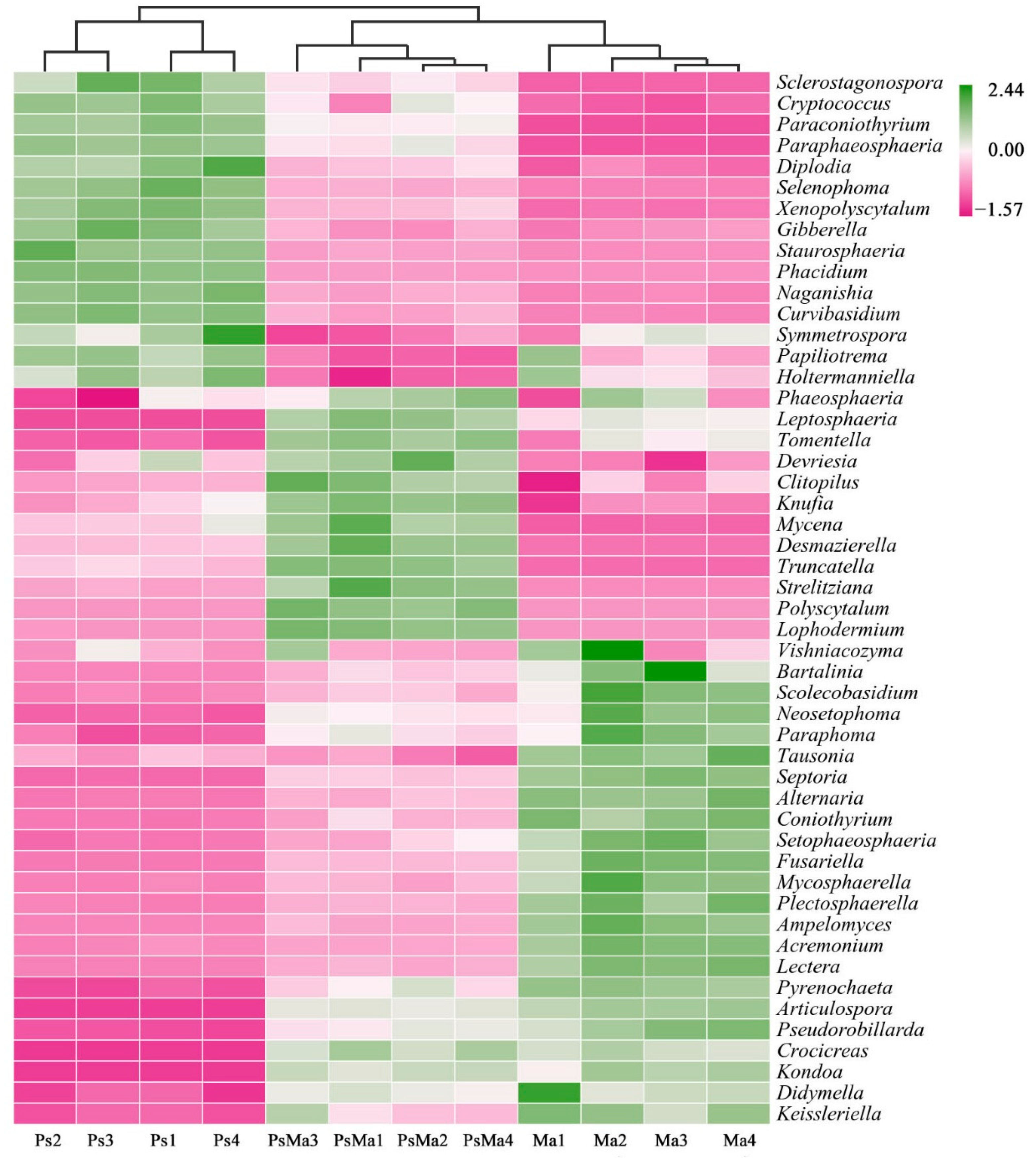
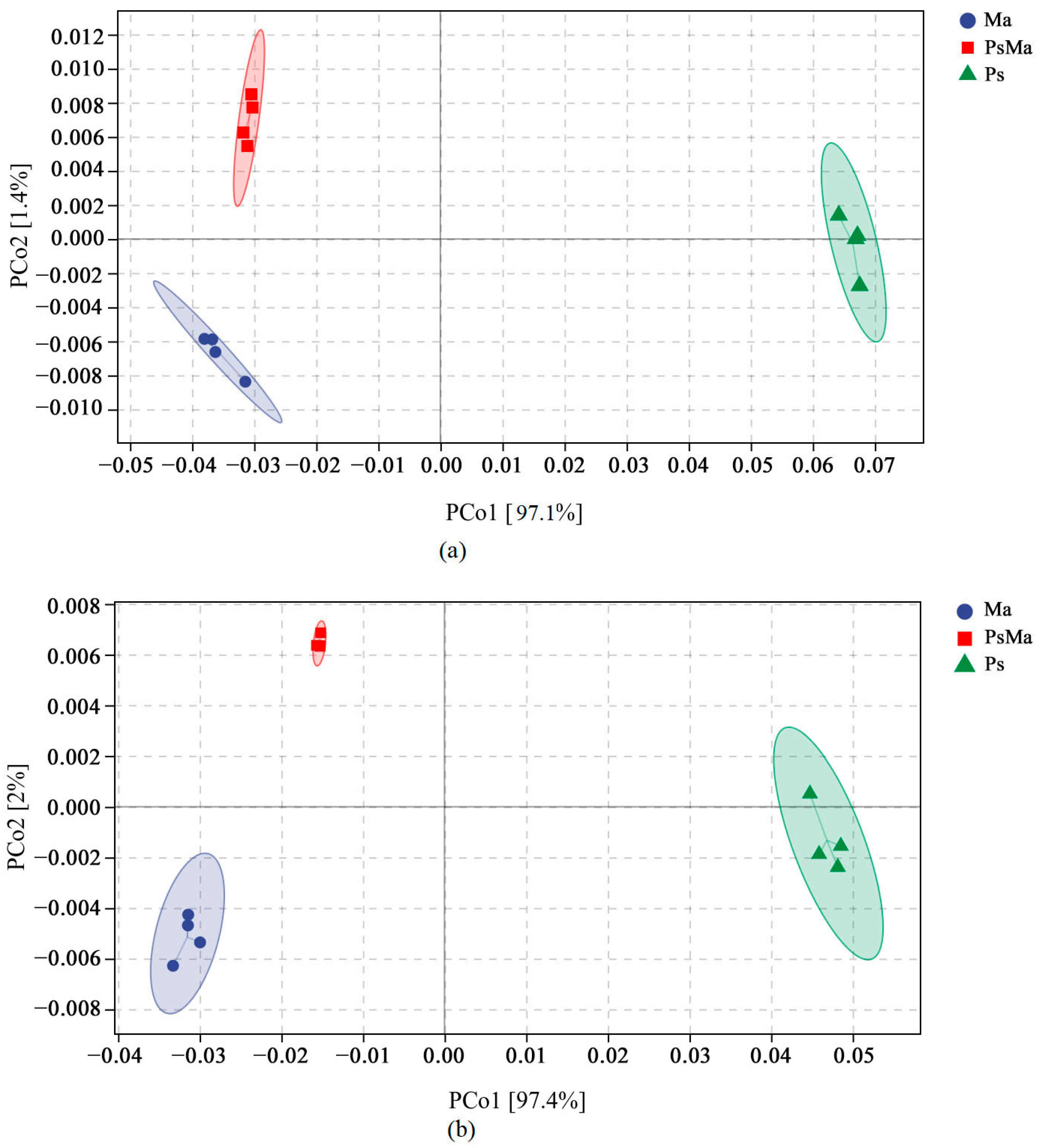
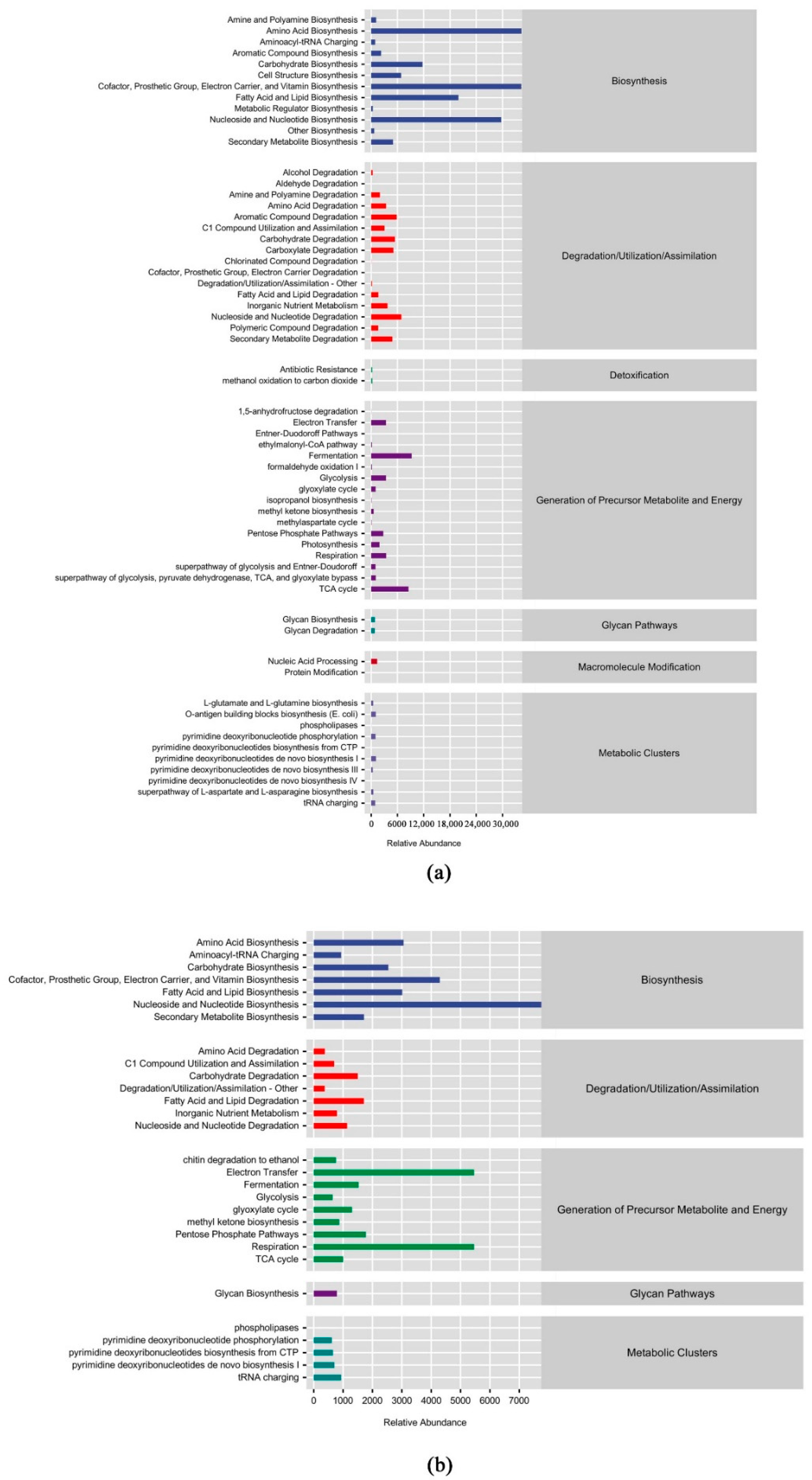
| Different Samples | Total C/g·kg−1 | Total N/g·kg−1 | Total P/g·kg−1 | C/N | N/P | C/P |
|---|---|---|---|---|---|---|
| Ps | 824.50 ± 12.87 bB | 54.00 ± 0.82 bB | 0.78 ± 0.15 cB | 15.27 ± 0.10 aA | 71.75 ± 14.37 aA | 1094.78 ± 214.60 aA |
| Ma | 923.50 ± 43.80 aA | 58.00 ± 0.82 aA | 3.73 ± 0.59 aA | 15.93 ± 0.81 aA | 15.87 ± 2.75 cB | 253.62 ± 50.76 cB |
| PsMa | 731.00 ± 25.53 cC | 49.25 ± 1.26 cC | 1.51 ± 0.49 bB | 14.85 ± 0.57 aA | 35.32 ± 11.07 bB | 525.95 ± 168.83 bB |
| F test | 40.64 | 78.94 | 46.18 | 3.60 | 28.68 | 28.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wei, Y.; Yin, Y.; Zhu, K.; Liu, Y.; Ding, H.; Lei, J.; Zhu, W.; Zhou, Y. Effects of Mixed Decomposition of Pinus sylvestris var. mongolica and Morus alba Litter on Microbial Diversity. Microorganisms 2022, 10, 1117. https://doi.org/10.3390/microorganisms10061117
Liu J, Wei Y, Yin Y, Zhu K, Liu Y, Ding H, Lei J, Zhu W, Zhou Y. Effects of Mixed Decomposition of Pinus sylvestris var. mongolica and Morus alba Litter on Microbial Diversity. Microorganisms. 2022; 10(6):1117. https://doi.org/10.3390/microorganisms10061117
Chicago/Turabian StyleLiu, Jiaying, Yawei Wei, You Yin, Keye Zhu, Yuting Liu, Hui Ding, Jiawei Lei, Wenxu Zhu, and Yongbin Zhou. 2022. "Effects of Mixed Decomposition of Pinus sylvestris var. mongolica and Morus alba Litter on Microbial Diversity" Microorganisms 10, no. 6: 1117. https://doi.org/10.3390/microorganisms10061117
APA StyleLiu, J., Wei, Y., Yin, Y., Zhu, K., Liu, Y., Ding, H., Lei, J., Zhu, W., & Zhou, Y. (2022). Effects of Mixed Decomposition of Pinus sylvestris var. mongolica and Morus alba Litter on Microbial Diversity. Microorganisms, 10(6), 1117. https://doi.org/10.3390/microorganisms10061117






