Evolutionary Adaptation by Repetitive Long-Term Cultivation with Gradual Increase in Temperature for Acquiring Multi-Stress Tolerance and High Ethanol Productivity in Kluyveromyces marxianus DMKU 3-1042
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Determination of Cell Growth and Viability
2.3. Determination of Fermentation Parameters
2.4. Evolutionary Adaptation by RLCGT
2.5. Characterization of Adapted Strains
2.6. Preparation of Genomic DNA, Genomic Sequencing, and Genome Mapping Analysis
2.7. RNA-Seq Analysis
3. Results
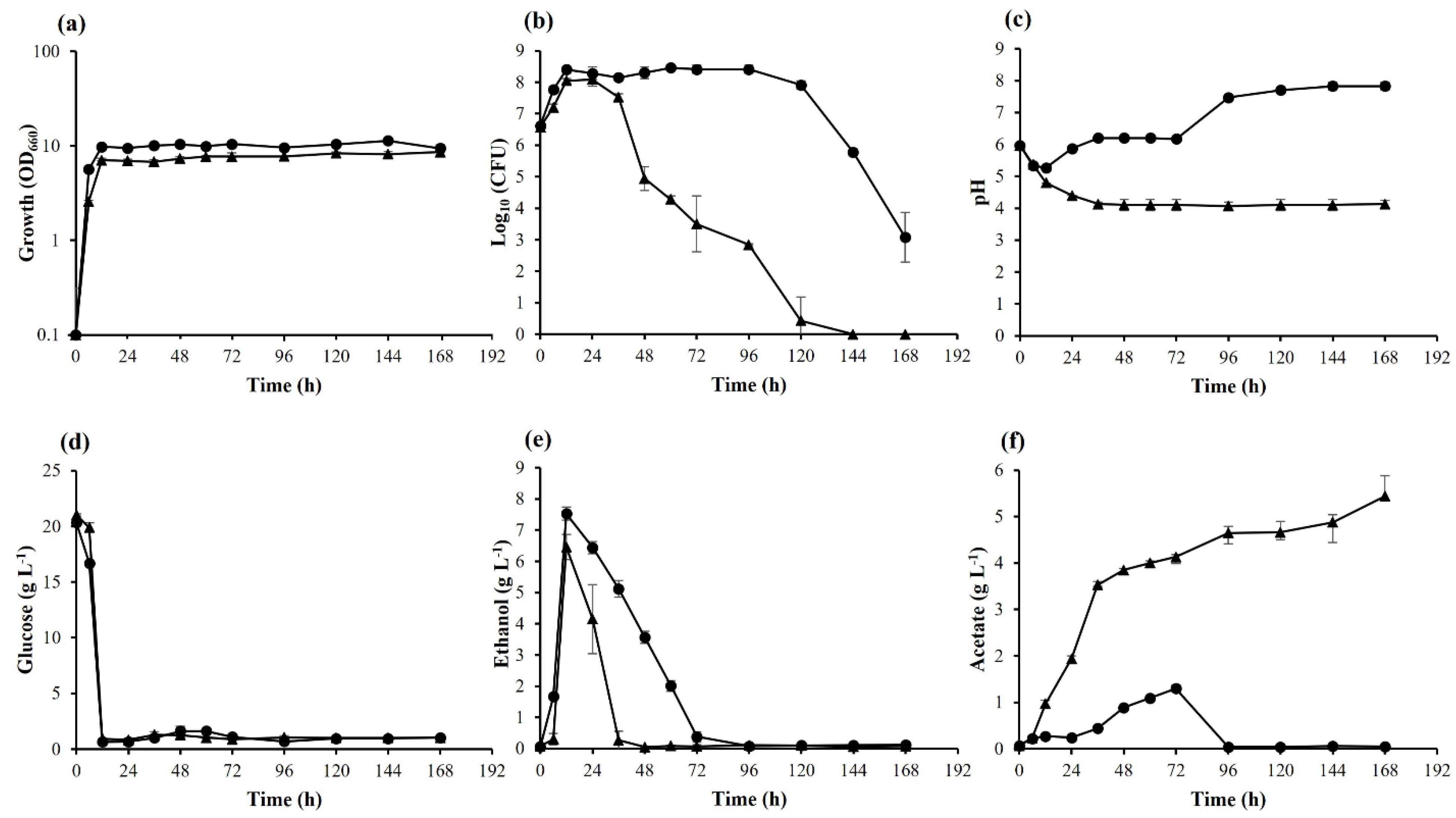
3.1. Effects of Long-Term Cultivation on K. marxianus DMKU 3-1042
3.2. Adaptive Laboratory Evolution of K. marxianus DMKU 3-1042 by RLCGT
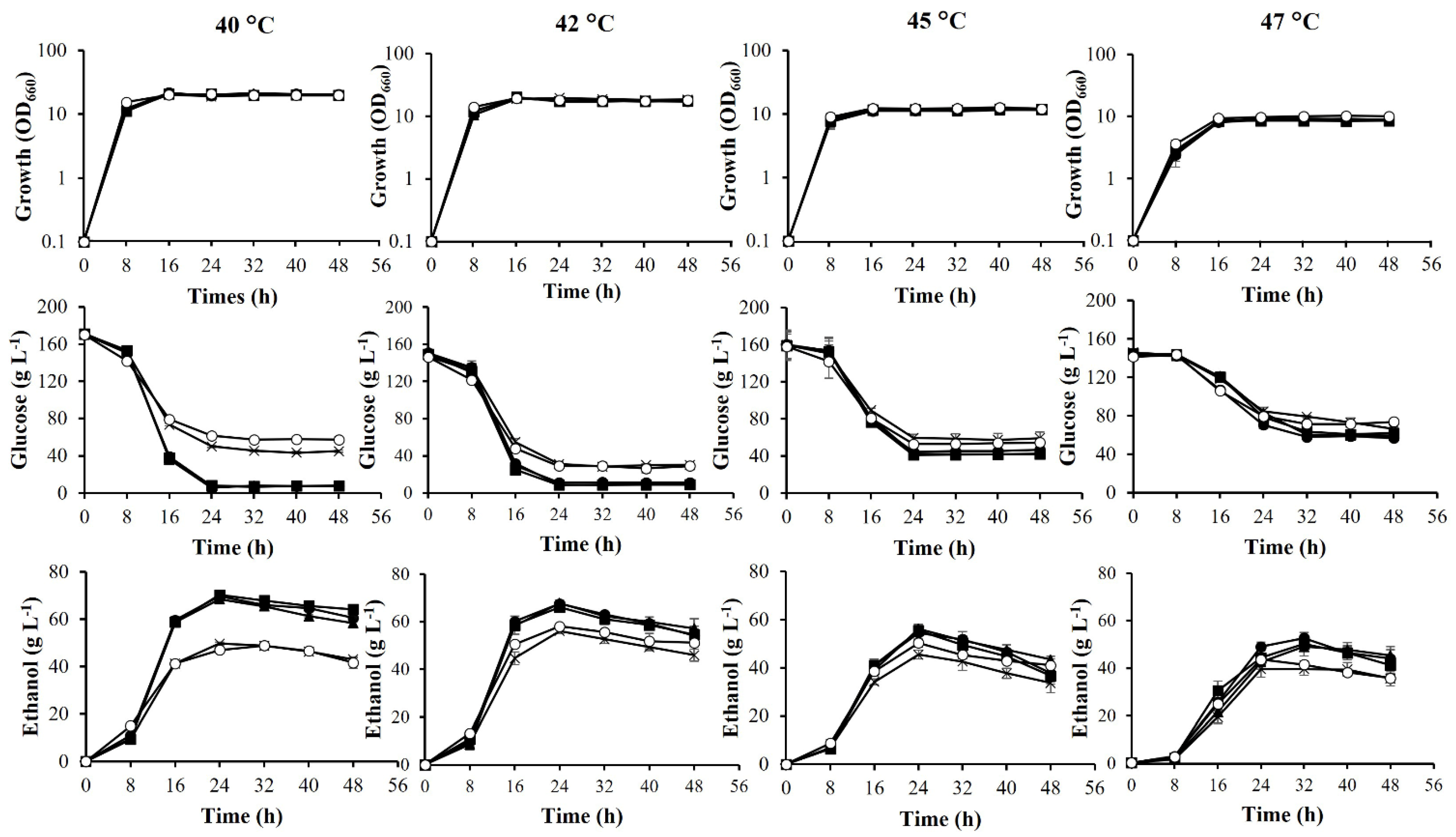
3.3. Fermentation Ability of Adapted Strains at High Temperatures
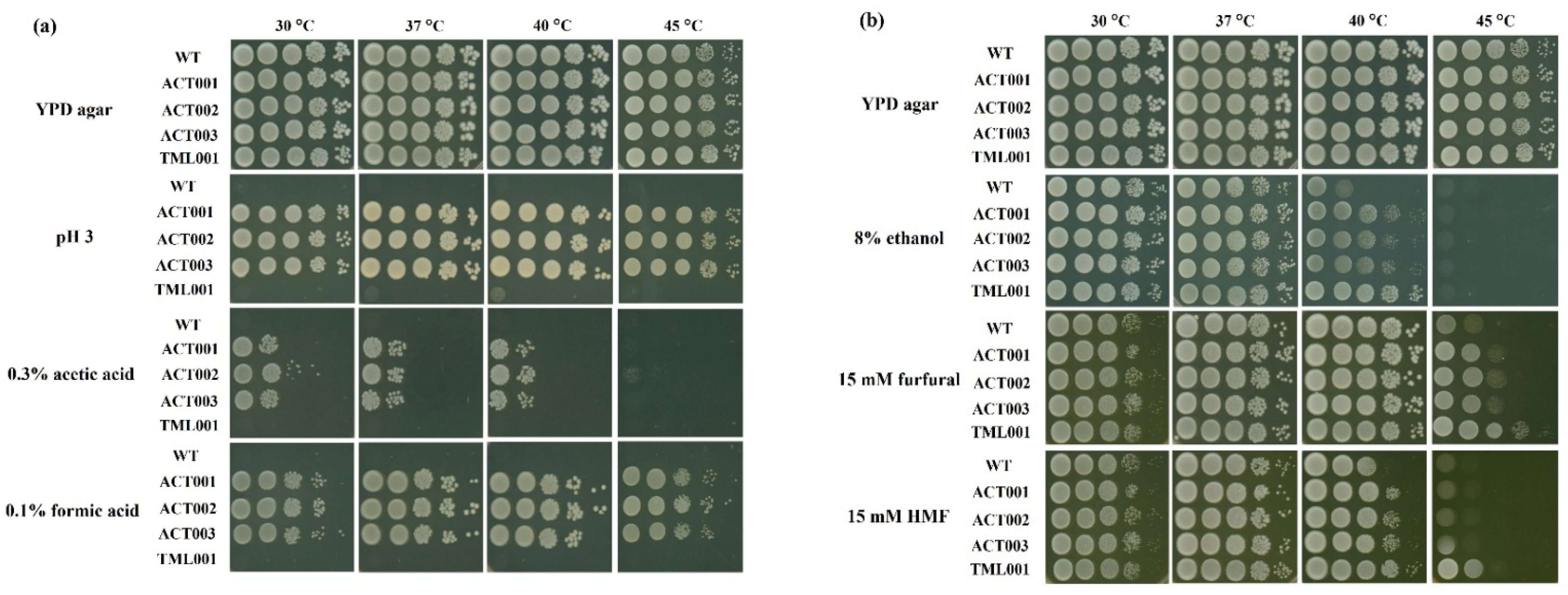
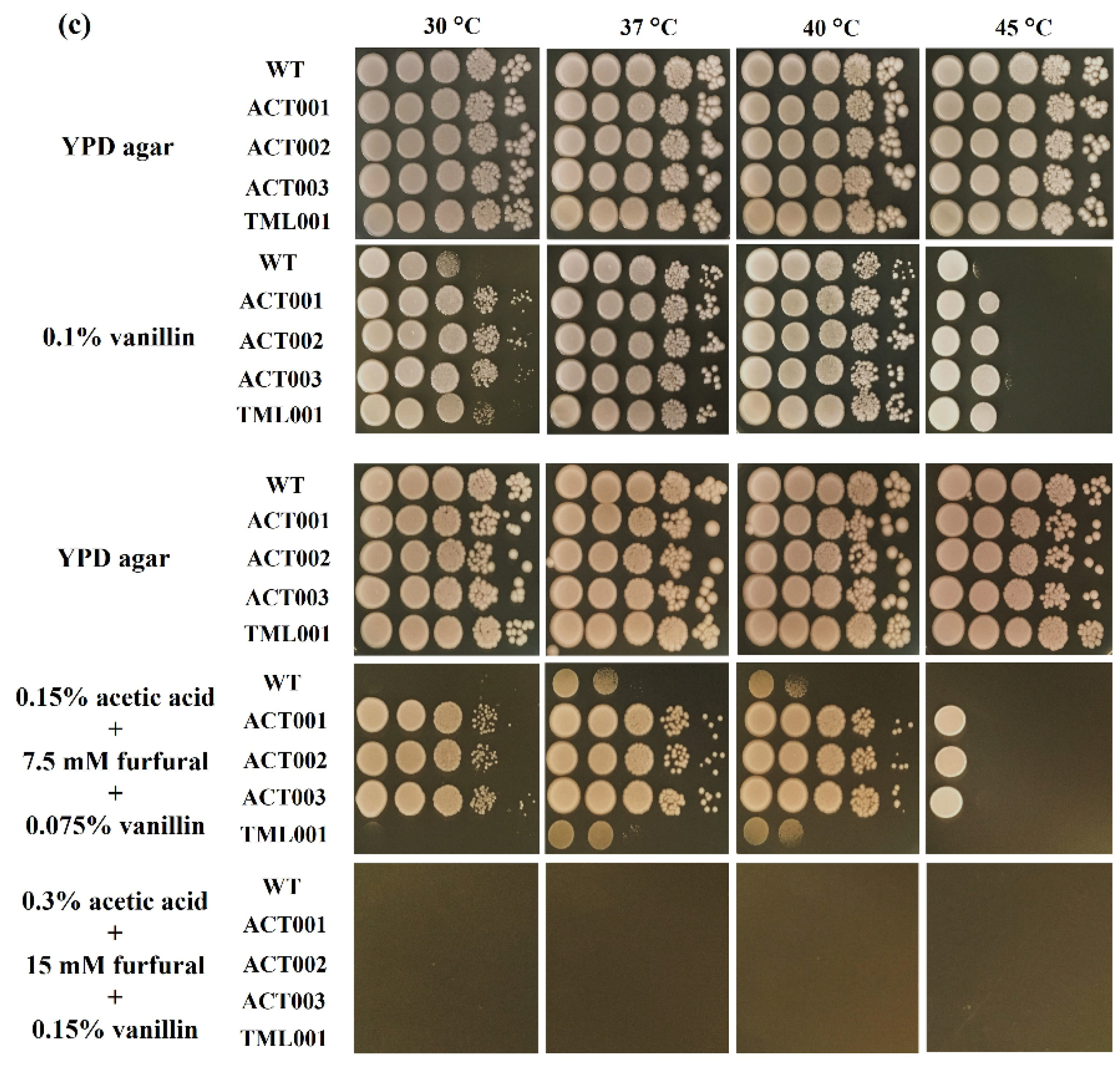
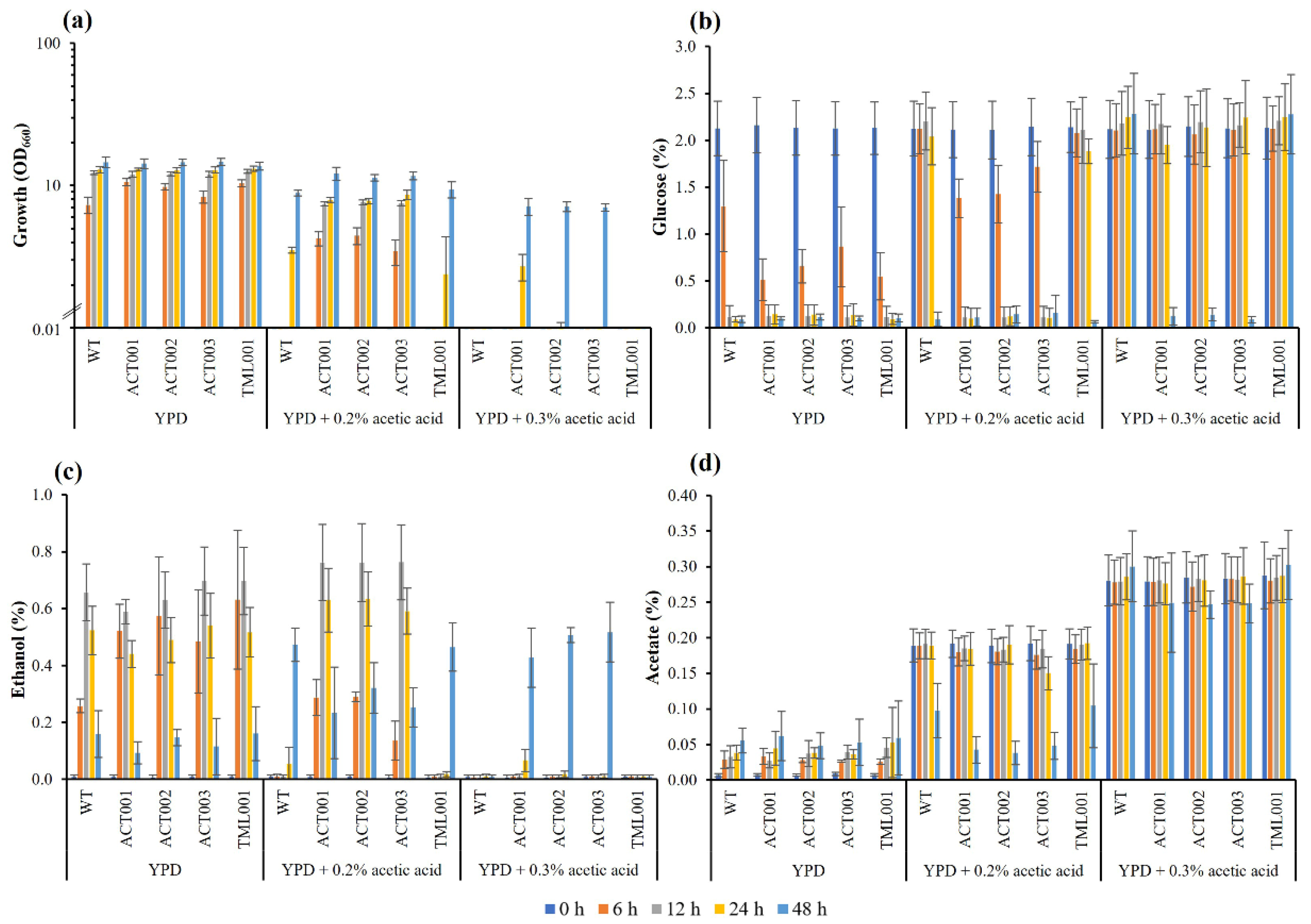
3.4. Characterization of the Adapted Strains
3.5. Mutation Points of Adapted Strains
3.6. Transcriptome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 17, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Goshima, T.; Tsuji, M.; Inoue, H.; Yano, S.; Hoshino, T.; Matsushika, A. Bioethanol production from lignocellulosic biomass by a novel Kluyveromyces marxianus strain. Biosci. Biotechnol. Biochem. 2013, 77, 1505–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitiyon, S.; Keo-oudone, C.; Murata, M.; Lertwattanasakul, N.; Limtong, S.; Kosaka, T.; Yamada, M. Efficient conversion of xylose to ethanol by stress tolerant Kluyveromyces marxianus BUNL-21. Springerplus 2016, 5, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Evolutionary adaptation of Kluyveromyces marxianus strain for efficient conversion of whey lactose to bioethanol. Process Biochem. 2017, 62, 69–79. [Google Scholar] [CrossRef]
- Kosaka, T.; Lertwattanasakul, N.; Rodrussamee, N.; Nurcholis, M.; Dung, N.T.P.; Keo-Oudone, C.; Murata, M.; Götz, P.; Theodoropoulos, C.; Suprayogi; et al. Potential of thermotolerant ethanologenic yeasts isolated from ASEAN countries and their application in high-temperature fermentation. In Fuel Ethanol Production from Sugarcane; Thalita Basso, P., Basso, L.C., Eds.; IntechOpen: London, UK, 2018; pp. 121–154. [Google Scholar]
- Nachaiwieng, W.; Lumyong, S.; Yoshioka, K.; Watanabe, T.; Khanongnuch, C. Bioethanol production from rice husk under elevated temperature simultaneous saccharification and fermentation using Kluyveromyces marxianus CK8. Biocatal. Agric. Biotechnol. 2015, 4, 543–549. [Google Scholar] [CrossRef]
- Nurcholis, M.; Nitiyon, S.; Suprayogi; Rodrussamee, N.; Lertwattanasakul, N.; Limtong, S.; Kosaka, T.; Yamada, M. Functional analysis of Mig1 and Rag5 as expressional regulators in thermotolerant yeast Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2019, 103, 395–410. [Google Scholar] [CrossRef]
- Kılmanoğlu, H.; Hoşoğlu, M.İ.; Güneşer, O.; Yüceer, Y.K. Optimization of pretreatment and enzymatic hydrolysis conditions of tomato pomace for production of alcohols and esters by Kluyveromyces marxianus. LWT 2020, 138, 110728. [Google Scholar] [CrossRef]
- Tinôco, D.; Genier, H.L.A.; da Silveira, W.B. Technology valuation of cellulosic ethanol production by Kluyveromyces marxianus CCT 7735 from sweet sorghum bagasse at elevated temperatures. Renew. Energy 2021, 173, 188–196. [Google Scholar] [CrossRef]
- Lertwattanasakul, N.; Nurcholis, M.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Kluyveromyces marxianus as a platform in synthetic biology for the production of useful materials. In Synthetic Biology of Yeasts; Darvishi Harzevili, F., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 293–335. [Google Scholar]
- Rodrussamee, N.; Lertwattanasakul, N.; Hirata, K.; Suprayogi; Limtong, S.; Kosaka, T.; Yamada, M. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2011, 90, 1573–1586. [Google Scholar] [CrossRef]
- Lertwattanasakul, N.; Kosaka, T.; Hosoyama, A.; Suzuki, Y.; Rodrussamee, N.; Matsutani, M.; Murata, M.; Fujimoto, N.; Suprayogi; Tsuchikane, K.; et al. Genetic basis of the highly efficient yeast Kluyveromyces marxianus: Complete genome sequence and transcriptome analyses. Biotechnol. Biofuels 2015, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, Y.L.; Fang, Y.; Zhao, H. Adaptive evolution and selection of stress resistant Saccharomyces cerevisiae for very high-gravity bioethanol fermentation. Electron. J. Biotechnol. 2019, 41, 88–94. [Google Scholar] [CrossRef]
- Taweecheep, P.; Naloka, K.; Matsutani, M.; Yakushi, T.; Matsushita, K.; Theeragool, G. In vitro thermal and ethanol adaptations to improve vinegar fermentation at high temperature of Komagataeibacter oboediens MSKU 3. Biotechnol. Appl. Biochem. 2019, 189, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Banat, B.A.; Hoshida, H.; Ano, A.; Nongklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef]
- Murata, M.; Nitiyon, S.; Lertwattanasakul, N.; Sootsuwan, K.; Kosaka, T.; Thanonkeo, P.; Limtong, S.; Yamada, M. High-temperature fermentation technology for low-cost bioethanol. J. Jpn. Inst. Energy 2015, 94, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shi, J.; Jiang, L. Modulation of mitochondrial membrane integrity and ROS formation by high temperature in Saccharomyces cerevisiae. Electron. J. Biotechnol. 2015, 18, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef]
- Bro, C.; Regenberg, B.; Forster, J.; Nielsen, J. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab. Eng. 2006, 8, 102–111. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe, I.; Yamamoto, M.; Ando, A.; Nakamura, T. A UV-induced mutant of Pichia stipitis with increased ethanol production from xylose and selection of a spontaneous mutant with increased ethanol tolerance. Bioresour. Technol. 2011, 102, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y. Adaptive evolution of Saccharomyces cerevisiae with enhanced ethanol tolerance for Chinese rice wine fermentation. Biotechnol. Appl. Biochem. 2014, 173, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Pattanakittivorakul, S.; Lertwattanasakul, N.; Yamada, M.; Limtong, S. Selection of thermotolerant Saccharomyces cerevisiae for high temperature ethanol production from molasses and increasing ethanol production by strain improvement. Antonie Van Leeuwenhoek 2019, 112, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.N. Development of xylose-fermenting yeast Pichia stipites for ethanol production through adaptation on hardwood hemicellulose acid prehydrolysate. J. Appl. Microbiol. 2001, 90, 208–215. [Google Scholar] [CrossRef]
- Çakar, Z.P.; Turanlı-Yıldız, B.; Alkım, C.; Yılmaz, Ü. Evolutionary engineering of Saccharomyces cerevisiae for improved industrially important properties. FEMS Yeast Res. 2012, 12, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhu, R.; Zhang, M.; Wang, S. Creation of an ethanol-tolerant Saccharomyces cerevisiae strain by 266 nm laser radiation and repetitive cultivation. J. Biosci. Bioeng. 2014, 118, 508–513. [Google Scholar] [CrossRef]
- Gurdo, N.; Novelli Poisson, G.F.; Juárez, Á.B.; Rios de Molina, M.C.; Galvagno, M.A. Improved robustness of an ethanologenic yeast strain through adaptive evolution in acetic acid is associated with its enzymatic antioxidant ability. J. Appl. Microbiol. 2018, 125, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Russell, D.W. Preparation and analysis of eukaryotic genomic DNA. In Molecular Cloning a Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; pp. 1–62. [Google Scholar]
- Akuzawa, S.; Nagaoka, J.; Kanekatsu, M.; Kanesaki, Y.; Suzuki, T. Draft genome sequence of Oceanobacillus picturae Heshi-B3, isolated from fermented rice bran in a traditional Japanese seafood dish. Genome Announc. 2016, 4, e01621-15. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, Y.; Kanesaki, Y.; Arai, S.; Saruta, F.; Hayashihara, K.; Murakami, A.; Shimizu, K.; Honda, H.; Yoshikawa, H.; Hanai, T. Mutations responsible for alcohol tolerance in the mutant of Synechococcus elongatus PCC 7942 (SY1043) obtained by single-cell screening system. J. Biosci. Bioeng. 2018, 125, 572–577. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1997, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Kim, Y.S.; Kim, H.; Jin, I.; Yoon, H.S. Saccharomyces cerevisiae KNU5377 stress response during high-temperature ethanol fermentation. Mol. Cells 2013, 35, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural and alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Hemansi; Himanshu; Patel, A.K.; Saini, J.K.; Singhania, R.R. Development of multiple inhibitor tolerant yeast via adaptive laboratory evolution for sustainable bioethanol production. Bioresour. Technol. 2022, 344, 126247. [Google Scholar] [CrossRef]
- Ingley, E.; Hemmings, B.A. Pleckstrin homology (PH) domains in signal transduction. J. Cell. Biochem. 1994, 56, 436–443. [Google Scholar] [CrossRef]
- Saraste, M.; Hyvönen, M. Pleckstrin homology domains: A fact file. Curr. Opin. Struct. Biol. 1995, 5, 403–408. [Google Scholar] [CrossRef]
- Breslow, D.; Cameron, D.; Collins, S.; Schuldiner, M.; Stewart-Ornstein, J.; Newman, H.; Madhani, H.; Krogan, N.; Weissman, J. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat. Methods 2008, 5, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.; Malik, A.; Zeng, F. Identification of YPL014W (Cip1) as a novel negative regulator of cyclin-dependent kinase in Saccharomyces cerevisiae. Genes Cells 2016, 21, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.M.; Kim, J.; Jin, E.; Jeon, N.L. Overproduction of recombinant E. coli malate synthase enhances Chlamydomonas reinhardtii biomass by upregulating heterotrophic metabolism. Bioresour. Technol. 2019, 272, 594–598. [Google Scholar] [CrossRef]
- Graybill, E.R.; Rouhier, M.F.; Kirby, C.E.; Hawes, J.W. Functional comparison of citrate synthase isoforms from S. cerevisiae. Arch. Biochem. Biophys. 2007, 465, 26–37. [Google Scholar] [CrossRef]
- Paiva, S.; Devaux, F.; Barbosa, S.; Jacq, C.; Casal, M. Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 2004, 21, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.K.; Behera, S.; Arora, R.; Kumar, S. Evolutionary adaptation of Kluyveromyces marxianus NIRE-K3 for enhanced xylose utilization. Front. Energy Res. 2017, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Cuny, C.; Lesbats, M.; Dukan, S. Induction of a global stress response during the first step of Escherichia coli plate growth. Appl. Environ. Microbiol. 2007, 73, 885–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phommachan, K.; Keo-oudone, C.; Nurcholis, M.; Vongvilaisak, N.; Chanhming, M.; Savanhnaly, V.; Bounphanmy, S.; Matsutani, M.; Kosaka, T.; Limtong, S.; et al. Adaptive laboratory evolution for multistress tolerance, including fermentability at high glucose concentrations in thermotolerant Candida tropicalis. Energies 2022, 15, 561. [Google Scholar] [CrossRef]
- Kim, S.B.; Kwon, D.H.; Park, J.B.; Ha, S.J. Alleviation of catabolite repression in Kluyveromyces marxianus: The thermotolerant SBK1 mutant simultaneously coferments glucose and xylose. Biotechnol. Biofuels 2019, 12, 90. [Google Scholar] [CrossRef]
- Pereira, T.; Vilaprinyo, E.; Belli, G.; Sorribas, A.; Alte, G.; Pereira, T.; Vilaprinyo, E.; Belli, G.; Herrero, E.; Salvado, B.; et al. Quantitative operating principles of yeast metabolism during adaptation to heat stress. Cell Rep. 2018, 22, 2421–2430. [Google Scholar] [CrossRef]
- Helsen, J.; Voordeckers, K.; Vanderwaeren, L.; Santermans, T.; Tsontaki, M.; Verstrepen, K.J.; Jelier, R. Gene loss predictably drives evolutionary adaptation. Mol. Biol. Evol. 2020, 37, 2989–3002. [Google Scholar] [CrossRef]
- Dhar, R.; Sägesser, R.; Weikert, C.; Yuan, J.; Wagner, A. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 2011, 24, 1135–1153. [Google Scholar] [CrossRef] [Green Version]
- Berry, D.B.; Guan, Q.; Hose, J.; Haroon, S.; Gebbia, M.; Heisler, L.E.; Nislow, C.; Giaever, G.; Gasch, A.P. Multiple means to the same end: The genetic basis of acquired stress resistance in yeast. PLoS Genet. 2011, 7, e1002353. [Google Scholar] [CrossRef] [Green Version]
- Brandt, B.A.; García-Aparicio, M.D.; Görgens, J.F.; Zyl, W.H.V. Rational engineering of Saccharomyces cerevisiae towards improved tolerance to multiple inhibitors in lignocellulose fermentations. Biotechnol. Biofuels 2021, 14, 173. [Google Scholar] [CrossRef]
- Yu, J.W.; Mendrola, J.M.; Audhya, A.; Singh, S.; Keleti, D.; DeWald, D.B.; Murray, D.; Emr, S.D.; Lemmon, M.A. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 2004, 13, 677–688. [Google Scholar] [CrossRef]
| Strains | Temp. (°C) | Medium | Cultivation Time (h) | Growth (OD660) | Remaining Sugar (g L−1) | Acetic Acid Accumulation (g L−1) | Ethanol Production (g L−1) | Increased Ethanol (%) | Ethanol Productivity (g L−1 h−1) | Ethanol Yield (g g−1) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K. marxianus DMKU 3-1042 | |||||||||||
| Wild-type | 40 | YP + 160 g L−1 glucose | 24 | 19.0 ± 0.8 | 50.1 ± 1.2 | 1.7 ± 0.1 | 49.6 ± 0.7 | - | 2.1 ± 0.0 | 0.3 ± 0.0 | This study |
| ACT001 | 40 | YP + 160 g L−1 glucose | 24 | 19.4 ± 0.7 | 7.3 ± 2.4 | 1.5 ± 0.1 | 69.7 ± 0.7 | 40 | 2.9 ± 0.0 | 0.4 ± 0.0 | This study |
| ACT002 | 40 | YP + 160 g L−1 glucose | 24 | 20.3 ± 1.2 | 5.8 ± 2.0 | 1.5 ± 0.0 | 68.5 ± 0.7 | 38 | 2.9 ± 0.0 | 0.4 ± 0.0 | This study |
| ACT003 | 40 | YP + 160 g L−1 glucose | 24 | 20.7 ± 1.0 | 8.1 ± 0.4 | 1.5 ± 0.0 | 70.3 ± 0.7 | 42 | 2.9 ± 0.0 | 0.4 ± 0.0 | This study |
| TML001 | 40 | YP + 160 g L−1 glucose | 24 | 20.7 ± 0.9 | 61.5 ± 0.4 | 1.3 ± 0.1 | 46.9 ± 0.7 | 0 | 2.0 ± 0.1 | 0.3 ± 0.0 | This study |
| Wild-type | 42 | YP + 160 g L−1 glucose | 24 | 19.6 ± 1.1 | 31.2 ± 2.2 | 1.7 ± 0.1 | 56.1 ± 1.1 | - | 2.3 ± 0.1 | 0.4 ± 0.0 | This study |
| ACT001 | 42 | YP + 160 g L−1 glucose | 24 | 17.5 ± 1.3 | 11.3 ± 1.1 | 1.4 ± 0.0 | 67.6 ± 0.3 | 20 | 2.8 ± 0.0 | 0.4 ± 0.0 | This study |
| ACT002 | 42 | YP + 160 g L−1 glucose | 24 | 17.5 ± 0.9 | 8.7 ± 1.4 | 1.5 ± 0.0 | 67.9 ± 1.2 | 21 | 2.8 ± 0.0 | 0.4 ± 0.0 | This study |
| ACT003 | 42 | YP + 160 g L−1 glucose | 24 | 17.0 ± 0.8 | 8.8 ± 1.4 | 1.5 ± 0.0 | 66.2 ± 1.0 | 18 | 2.8 ± 0.0 | 0.4 ± 0.0 | This study |
| TML001 | 42 | YP + 160 g L−1 glucose | 24 | 17.9 ± 1.2 | 29.0 ± 0.8 | 1.3 ± 0.0 | 58.1 ± 0.4 | 3 | 2.4 ± 0.0 | 0.4 ± 0.0 | This study |
| Wild-type | 45 | YP + 160 g L−1 glucose | 24 | 12.1 ± 0.6 | 59.5 ± 4.0 | 1.6 ± 0.0 | 45.5 ± 1.8 | - | 1.9 ± 0.1 | 0.3 ± 0.0 | This study |
| ACT001 | 45 | YP + 160 g L−1 glucose | 24 | 11.3 ± 0.5 | 44.5 ± 2.0 | 1.4 ± 0.0 | 54.5 ± 3.0 | 20 | 2.3 ± 0.1 | 0.3 ± 0.0 | This study |
| ACT002 | 45 | YP + 160 g L−1 glucose | 24 | 11.9 ± 1.0 | 42.8 ± 1.6 | 1.4 ± 0.0 | 56.2 ± 1.8 | 23 | 2.3 ± 0.1 | 0.4 ± 0.0 | This study |
| ACT003 | 45 | YP + 160 g L−1 glucose | 24 | 11.6 ± 0.6 | 41.4 ± 1.6 | 1.4 ± 0.1 | 55.4 ± 2.0 | 22 | 2.3 ± 0.1 | 0.4 ± 0.0 | This study |
| TML001 | 45 | YP + 160 g L−1 glucose | 24 | 12.2 ± 0.7 | 52.9 ± 2.3 | 1.4 ± 0.0 | 50.3 ± 1.7 | 11 | 2.1 ± 0.1 | 0.3 ± 0.0 | This study |
| Wild-type | 47 | YP + 160 g L−1 glucose | 32 | 9.3 ± 1.1 | 78.9 ± 1.6 | 1.9 ± 0.1 | 39.5 ± 2.8 | - | 1.2 ± 0.1 | 0.3 ± 0.0 | This study |
| ACT001 | 47 | YP + 160 g L−1 glucose | 32 | 8.9 ± 0.9 | 57.8 ± 1.4 | 1.8 ± 0.2 | 52.6 ± 2.5 | 33 | 1.7 ± 0.1 | 0.3 ± 0.0 | This study |
| ACT002 | 47 | YP + 160 g L−1 glucose | 32 | 9.0 ± 0.7 | 60.2 ± 3.2 | 1.8 ± 0.1 | 49.0 ± 3.8 | 24 | 1.5 ± 0.1 | 0.3 ± 0.0 | This study |
| ACT003 | 47 | YP + 160 g L−1 glucose | 32 | 8.5 ± 0.5 | 63.5 ± 1.3 | 1.7 ± 0.1 | 50.2 ± 1.8 | 27 | 1.6 ± 0.1 | 0.3 ± 0.0 | This study |
| TML001 | 47 | YP + 160 g L−1 glucose | 32 | 10.0 ± 0.8 | 71.4 ± 2.0 | 1.8 ± 0.1 | 41.5 ± 1.7 | 5 | 1.3 ± 0.1 | 0.3 ± 0.0 | This study |
| S. cerevisiae G85 | 28 | Sugar juice (207.25 g L−1 sugar) | 480 | NR | 10.4 ± 0.8 | NR | 126.6 ± 5.6 | - | 0.3 | 0.6 | [24] |
| S. cerevisiae G85X-8 | 28 | Sugar juice (207.25 g L−1 sugar) | 480 | NR | 7.0 ± 1.0 | NR | 130.0 ± 4.5 | 3 | 0.3 | 0.6 | [24] |
| S. cerevisiae YE0 | 34 | YP + 300 g L−1 glucose + 50 g L−1 ethanol | 72 | NR | 31.5 | NR | 106.8 | - | 1.5 | 0.4 | [28] |
| S. cerevisiae SM4 | 34 | YP + 300 g L−1 glucose + 50 g L−1 ethanol | 72 | NR | 2.1 | NR | 138.1 | 29 | 1.9 | 0.5 | [28] |
| K. marxianus MTCC1389 (Wild-type) | 37 | Whey permeate (200 g L−1 lactose) | 50 | NR | 0.7 ± 0.0 | NR | 66.8 ± 0.9 | - | 1.3 ± 0.0 | 0.3 ± 0.0 | [4] |
| K. marxianus MTCC1389 (Adapted strain) | 37 | Whey permeate (200 g L−1 lactose) | 42 | NR | 0.8 ± 0.0 | NR | 79.3 ± 0.8 | 19 | 1.7 ± 0.1 | 0.4 ± 0.1 | [4] |
| S. cerevisiae Y-1 | 30 | Fermentation medium (350 g L−1 glucose) | 60 | NR | 75.7 | NR | 125.0 | - | 2.1 ± 0.0 | 0.3 | [14] |
| S. cerevisiae YF10-5 | 30 | Fermentation medium (350 g L−1 glucose) | 60 | NR | 5.5 | NR | 145.8 | 16.6 | 2.43 ± 0.1 | 0.4 | [14] |
| Adapted Strain | Gene/Locus_Tag | Product | Region | Ref | Allele | Type | Amino Acid Change |
|---|---|---|---|---|---|---|---|
| ACT001 | KLMA_10738 | PH domain-containing protein Orthologue of YHR131C | 1552906^1552907 | - | G | Insertion | Lle812fs |
| 47658 | T | - | Deletion | Non-coding region | |||
| ACT002 | 1161817 | G | A | SNP | Non-coding region | ||
| GAL1 | Galactokinase | 712698 | G | T | SNP | Lue391Phe | |
| ALY2 | UPF0675 protein Orthologue of YJL084C | 385614 | G | A | SNP | synonymous | |
| KLMA_40563 | ATP-dependent protease La | 1269738 | T | C | SNP | synonymous | |
| ACT003 | 1107715 | T | C | SNP | Non-coding region | ||
| TML001 | FSH3 | Family of serine hydrolase 3 | 613476 | C | G | SNP | Lle253Met |
| PMA1 | Plasma membrane ATPase | 968552^968553 | - | GCT | Insertion | Ala255_Leu256insAla | |
| TNA1 | High-affinity nicotinic acid transporter | 1010140 | C | G | SNP | Phe467Leu | |
| 1714543 | G | A | SNP | Non-coding region | |||
| KLMA_40326 | Hypothetical protein Orthologue of YPL014W | 767036 | G | T | SNP | Glu106 * | |
| 724738 | T | G | SNP | Non-coding region | |||
| SVL3 | Styryl dye vacuolar localization protein 3 | 815396 | C | A | SNP | Ser629 * |
| Locus_Tag | Gene | Log2 Fold Change | Product |
|---|---|---|---|
| Up-regulated | |||
| KLMA_70179 | ICL1 | 1.90 | Isocitrate lyase |
| KLMA_70444 | CIT3 | 1.43 | Citrate synthase 3 |
| KLMA_30101 | SPG4 | 1.24 | stationary phase protein 4 |
| KLMA_20819 | KLMA_20819 | 1.20 | hypothetical protein |
| KLMA_60471 | RRT12 | 1.12 | putative subtilase-type proteinase YCR045C |
| KLMA_60452 | FBP1 | 1.08 | fructose-1,6-bisphosphatase |
| KLMA_20009 | ADY2_1 | 1.04 | acetate transporter |
| Down-regulated | |||
| KLMA_50379 | HAK1 | −1.99 | high-affinity potassium transporter |
| KLMA_50489 | ZRT2 | −1.39 | zinc-regulated transporter 2 |
| KLMA_10655 | CDR4 | −1.37 | ATPase-coupled transporter |
| KLMA_50332 | SEO1 | −1.33 | probable transporter SEO1 |
| KLMA_10677 | MET17 | −1.18 | protein MET17 |
| KLMA_30339 | KLMA_30339 | −1.17 | ATP synthase subunit b |
| KLMA_30724 | SNZ3 | −1.17 | pyridoxal-5′-phosphate synthase |
| KLMA_30338 | KLMA_30338 | −1.08 | protein ICY2 |
| KLMA_60029 | ACAD11 | −1.01 | acyl-CoA dehydrogenase family member 11 |
| KLMA_30726 | SNO3 | −1.00 | probable pyridoxal-5′-phosphate synthase subunit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanakittivorakul, S.; Tsuzuno, T.; Kosaka, T.; Murata, M.; Kanesaki, Y.; Yoshikawa, H.; Limtong, S.; Yamada, M. Evolutionary Adaptation by Repetitive Long-Term Cultivation with Gradual Increase in Temperature for Acquiring Multi-Stress Tolerance and High Ethanol Productivity in Kluyveromyces marxianus DMKU 3-1042. Microorganisms 2022, 10, 798. https://doi.org/10.3390/microorganisms10040798
Pattanakittivorakul S, Tsuzuno T, Kosaka T, Murata M, Kanesaki Y, Yoshikawa H, Limtong S, Yamada M. Evolutionary Adaptation by Repetitive Long-Term Cultivation with Gradual Increase in Temperature for Acquiring Multi-Stress Tolerance and High Ethanol Productivity in Kluyveromyces marxianus DMKU 3-1042. Microorganisms. 2022; 10(4):798. https://doi.org/10.3390/microorganisms10040798
Chicago/Turabian StylePattanakittivorakul, Sornsiri, Tatsuya Tsuzuno, Tomoyuki Kosaka, Masayuki Murata, Yu Kanesaki, Hirofumi Yoshikawa, Savitree Limtong, and Mamoru Yamada. 2022. "Evolutionary Adaptation by Repetitive Long-Term Cultivation with Gradual Increase in Temperature for Acquiring Multi-Stress Tolerance and High Ethanol Productivity in Kluyveromyces marxianus DMKU 3-1042" Microorganisms 10, no. 4: 798. https://doi.org/10.3390/microorganisms10040798
APA StylePattanakittivorakul, S., Tsuzuno, T., Kosaka, T., Murata, M., Kanesaki, Y., Yoshikawa, H., Limtong, S., & Yamada, M. (2022). Evolutionary Adaptation by Repetitive Long-Term Cultivation with Gradual Increase in Temperature for Acquiring Multi-Stress Tolerance and High Ethanol Productivity in Kluyveromyces marxianus DMKU 3-1042. Microorganisms, 10(4), 798. https://doi.org/10.3390/microorganisms10040798







