Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease
Abstract
:1. Introduction
2. Methods
2.1. Recruitment
2.2. Statistical Analysis
3. Results
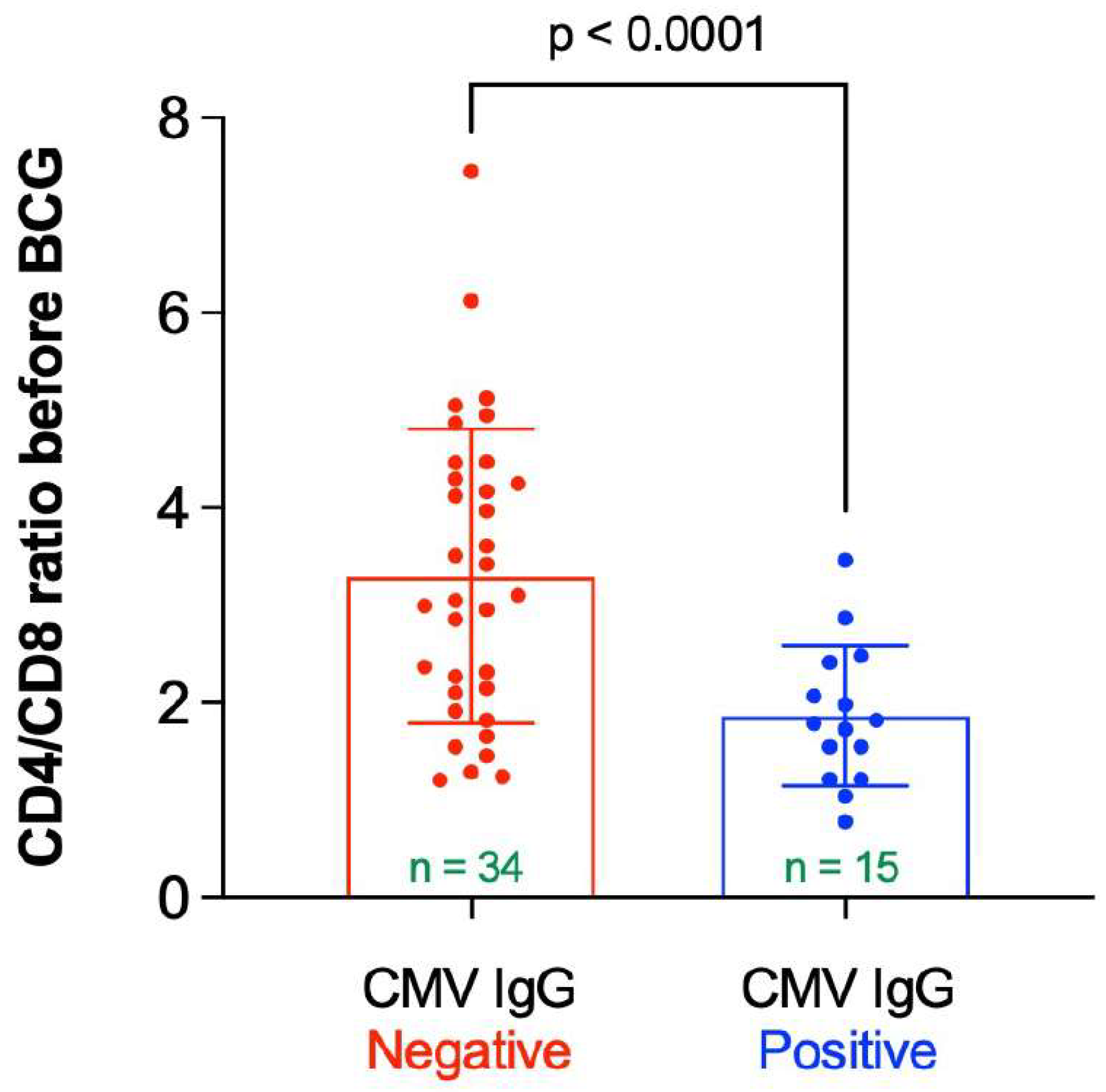
3.1. Demographics, CMV Antibody Titer, and ApoE4 Allele
3.2. BCG Significantly Increased the Aβ42/40 Ratio
3.3. Younger Participants Had More of a “Favorable” Increase in Their Aβ42/40 Ratio Than Older Participants
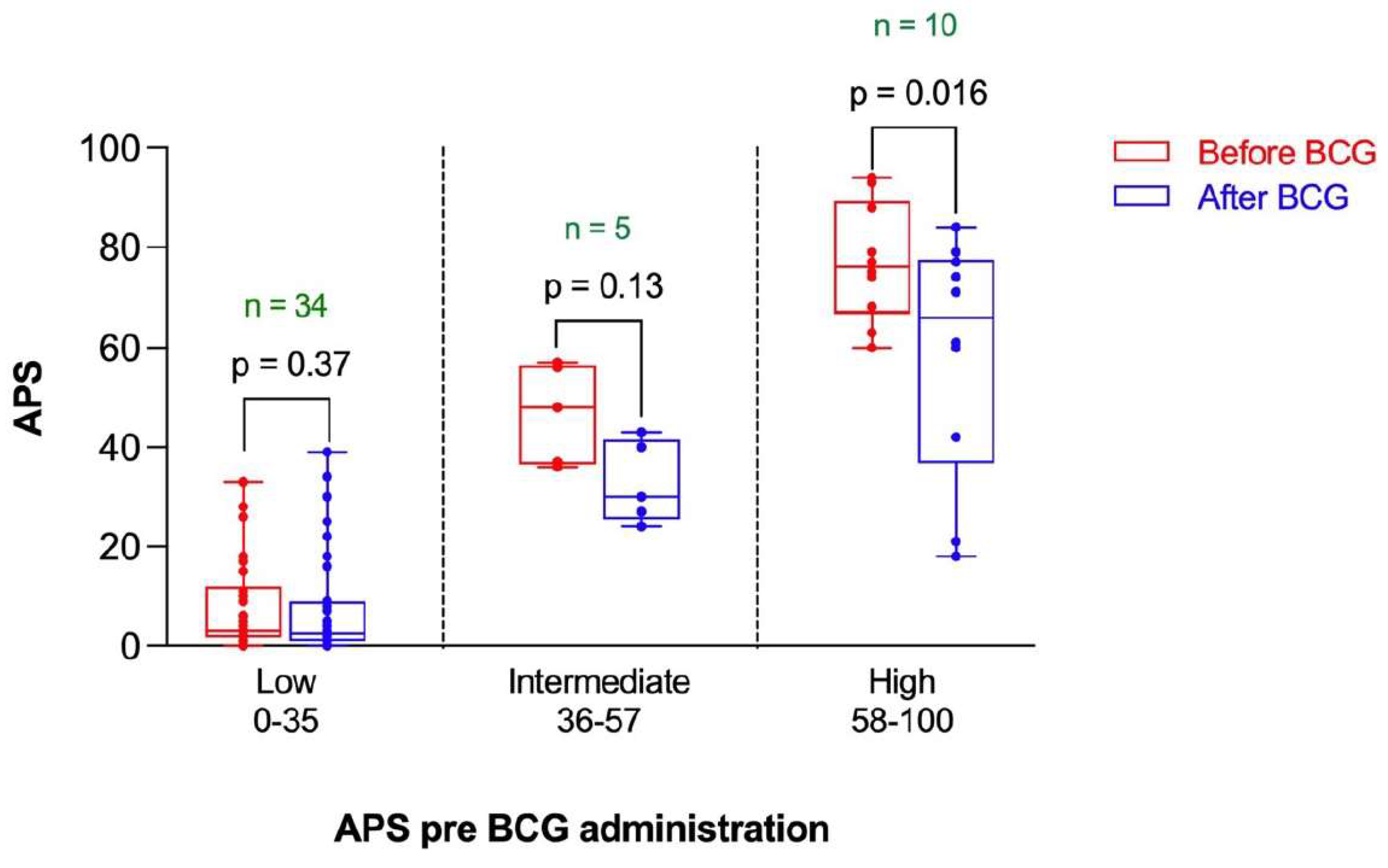
3.4. High Risk Participants Had More of a “Favorable” Decrease in Their APS Than Intermediate Risk and Low Risk Participants
3.5. Participants without Latent CMV Infection Had a “Favorable” Decrease in Their APS with BCG Compared to Participants Latently Infected with CMV
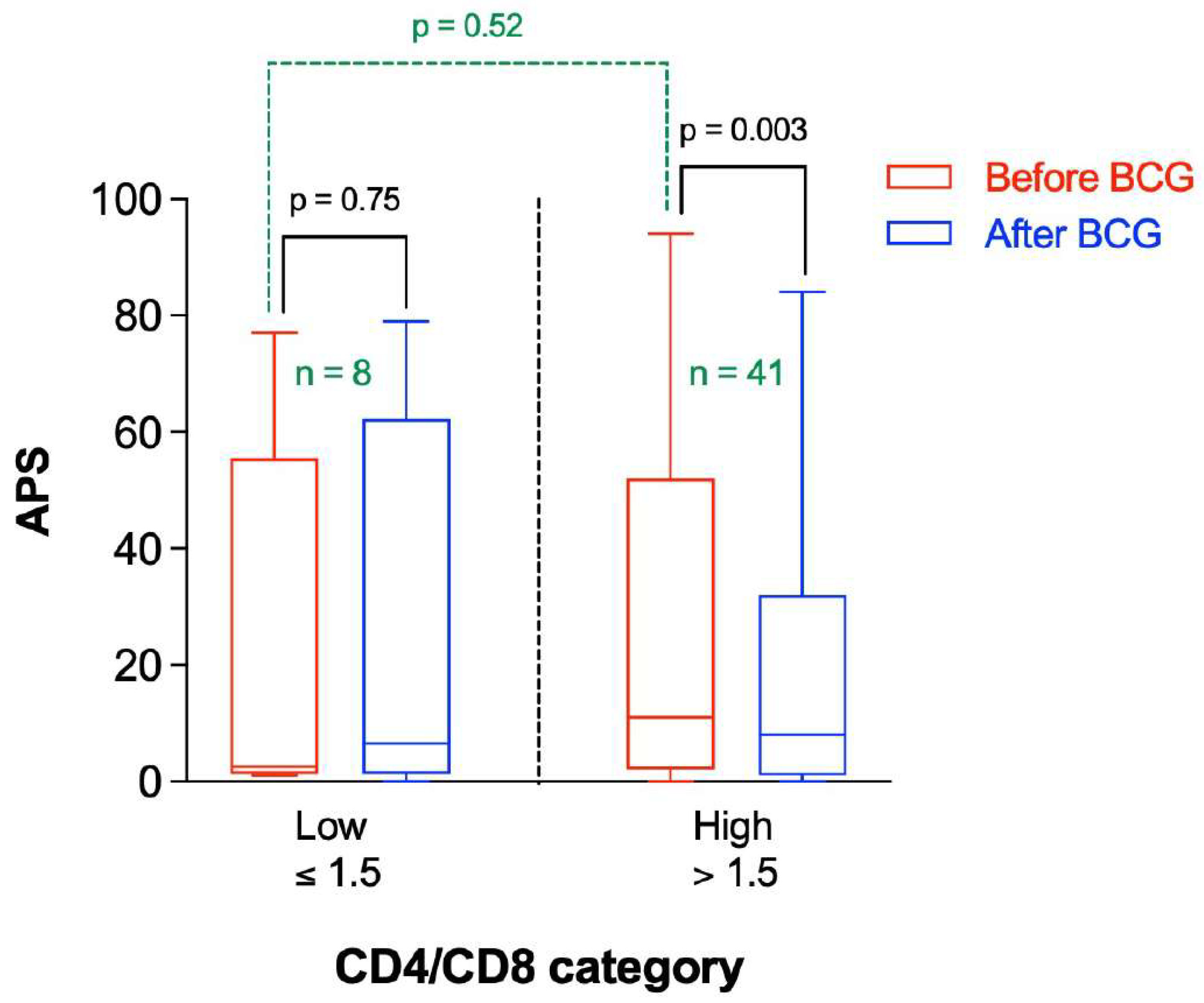
3.6. Participants with a Higher CD4/CD8 Ratio Had a Greater Decrease in APS following BCG Vaccination Compared to Those with Lower Baseline CD4/CD8 Ratio
3.7. Participants without Latent CMV Infection Had a More “Favorable” “Immune Risk Profile” Than Those with Latent CMV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calmette, A. Preventive Vaccination Against Tuberculosis with BCG. Proc. R. Soc. Med. 1931, 24, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.ciil.fr/bcg-symposium/scientific-program (accessed on 10 February 2022).
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gofrit, O.N.; Bercovier, H.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med. Hypotheses 2019, 123, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Qi, F.; Yang, J.; Wang, X.; Wu, Y.; Wen, Y.; Yuan, Q.; Zou, J.; Guo, K.; Bin Yao, Z. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol. Dis. 2017, 101, 27–39. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Jack, C.R.; Therneau, T.M.; Weigand, S.D.; Wiste, H.J.; Knopman, D.S.; Vemuri, P.; Lowe, V.J.; Mielke, M.M.; Roberts, R.O.; Machulda, M.M.; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging–Alzheimer’s Association Research Framework. JAMA Neurol. 2019, 76, 1174–1183. [Google Scholar] [CrossRef] [Green Version]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef]
- Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Jackson, E.N.; Harpstrite, S.E.; Verghese, P.B.; West, T.; Fogelman, I.; et al. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin. Chim. Acta 2021, 519, 267–275. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Maier, A.; Hähnel, K.; Beck, R.; De Craen, A.J.M.; Slagboom, P.; Westendorp, R.G.J.; Pawelec, G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J. Gen. Virol. 2011, 92, 2746–2756. [Google Scholar] [CrossRef]
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179. [Google Scholar] [CrossRef]
- Lurain, N.S.; Hanson, B.A.; Martinson, J.; Leurgans, S.E.; Landay, A.L.; Bennett, D.A.; Schneider, J.A. Virological and Immunological Characteristics of Human Cytomegalovirus Infection Associated With Alzheimer Disease. J. Infect. Dis. 2013, 208, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westman, G.; Berglund, D.; Widén, J.; Ingelsson, M.; Korsgren, O.; Lannfelt, L.; Sehlin, D.; Lidehall, A.-K.; Eriksson, B.-M. Increased Inflammatory Response in Cytomegalovirus Seropositive Patients with Alzheimer’s Disease. PLoS ONE 2014, 9, e96779. [Google Scholar] [CrossRef] [Green Version]
- Lövheim, H.; Olsson, J.; Weidung, B.; Johansson, A.; Eriksson, S.; Hallmans, G.; Elgh, F. Interaction between Cytomegalovirus and Herpes Simplex Virus Type 1 Associated with the Risk of Alzheimer’s Disease Development. J. Alzheimers Dis. 2018, 61, 939–945. [Google Scholar] [CrossRef]
- Larbi, A.; Pawelec, G.; Witkowski, J.M.; Schipper, H.M.; Derhovanessian, E.; Goldeck, D.; Fulop, T. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 91–103. [Google Scholar] [CrossRef]
- Pawelec, G.; Koch, S.; Griesemann, H.; Rehbein, A.; Hähnel, K.; Gouttefangeas, C. Immunosenescence, suppression and tumour progression. Cancer Immunol. Immunother. 2006, 55, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Wagner, W.M.; Wikby, A.; Walter, S.; Aubert, G.; Dodi, A.I.; Travers, P.; Pawelec, G. Large Numbers of Dysfunctional CD8+ T Lymphocytes Bearing Receptors for a Single Dominant CMV Epitope in the Very Old. J. Clin. Immunol. 2003, 23, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Dage, J.L.; Wennberg, A.M.; Airey, D.C.; Hagen, C.E.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R.; Petersen, R.C.; Mielke, M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016, 12, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, J.D.; Syrjanen, J.A.; Graff-Radford, J.; Petersen, R.C.; Machulda, M.M.; Campbell, M.R.; Algeciras-Schimnich, A.; Lowe, V.; Knopman, D.S.; Jack, C.R.; et al. Comparison of plasma neurofilament light and total tau as neurodegeneration markers: Associations with cognitive and neuroimaging outcomes. Alzheimers Res. Ther. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Ahmed, S.S.; Curtis, N.; Kollmann, T.; Levy, O.; Netea, M.G.; Pollard, A.J.; Van Crevel, R.; Wilson, C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016, 16, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Faustman, D.L.; Wang, L.; Okubo, Y.; Burger, U.; Ban, L.; Man, G.; Zheng, H.; Schoenfeld, D.; Pompei, R.; Avruch, J.; et al. Proof-of-Concept, Randomized, Controlled Clinical Trial of Bacillus-Calmette-Guerin for Treatment of Long-Term Type 1 Diabetes. PLoS ONE 2012, 7, e41756. [Google Scholar] [CrossRef] [PubMed]
- Ristori, G.; Faustman, D.; Matarese, G.; Romano, S.; Salvetti, M. Bridging the gap between vaccination with Bacille Calmette-Guérin (BCG) and immunological tolerance: The cases of type 1 diabetes and multiple sclerosis. Curr. Opin. Immunol. 2018, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.; Chinn, D.; Rodgers, C.; Mackintosh, C. Optimal models to evaluate the protective efficacy of tuberculosis vaccines. Tuberculosis 2001, 81, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Hamzabegovic, F.; Eickhoff, C.S.; Hoft, D.F. BCG Vaccination Induces, M. avium and M. abscessus Cross-Protective Immunity. Front. Immunol. 2019, 10, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontturi, A.; Soini, H.; Ollgren, J.; Salo, E. Increase in Childhood Nontuberculous Mycobacterial Infections After Bacille Calmette-Guérin Coverage Drop: A Nationwide, Population-Based Retrospective Study, Finland, 1995–2016. Clin. Infect. Dis. 2018, 67, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Trnka, L.; Daňková, D.; Švandová, E. Six years’ experience with the discontinuation of BCG vaccination: 4. Protective effect of BCG vaccination against the Mycobacterium avium intracellulare complex. Tuber. Lung Dis. 1994, 75, 348–352. [Google Scholar] [CrossRef]
- Setia, M.S.; Steinmaus, C.; Ho, C.S.; Rutherford, G.W. The role of BCG in prevention of leprosy: A meta-analysis. Lancet Infect. Dis. 2006, 6, 162–170. [Google Scholar] [CrossRef]
- Smith, P.; Revill, W.; Lukwago, E.; Rykushin, Y. The protective effect of BCG against Mycobacterium ulcerans disease: A controlled trial in an endemic area of Uganda. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 449–457. [Google Scholar] [CrossRef]
- Zimmermann, P.; Finn, A.; Curtis, N. Does BCG Vaccination Protect Against Nontuberculous Mycobacterial Infection? A Systematic Review and Meta-Analysis. J. Infect. Dis. 2018, 218, 679–687. [Google Scholar] [CrossRef]
- Dow, C.T. Warm, Sweetened Milk at the Twilight of Immunity-Alzheimer’s Disease-Inflammaging, Insulin Resistance, M. paratuberculosis and Immunosenescence. Front. Immunol. 2021, 12, 714179. [Google Scholar] [CrossRef]
- Dow, C.T. Proposing BCG Vaccination for Mycobacterium avium ss. paratuberculosis (MAP) Associated Autoimmune Diseases. Microorganisms 2020, 8, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorlag, S.J.C.F.M.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martinez, E.; Falfán-Valencia, R.; Pérez-Rubio, G.; Andrade, W.A.; Rojas-Serrano, J.; Ambrocio-Ortiz, E.; Galicia-Álvarez, D.S.; Bárcenas-Montiel, I.; Velasco-Medina, A.; Velázquez-Sámano, G. Effect of BCG Revaccination on Occupationally Exposed Medical Personnel Vaccinated against SARS-CoV-2. Cells 2021, 10, 3179. [Google Scholar] [CrossRef]
- Counoupas, C.; Johansen, M.D.; Stella, A.O.; Nguyen, D.H.; Ferguson, A.L.; Aggarwal, A.; Bhattacharyya, N.D.; Grey, A.; Hutchings, O.; Patel, K.; et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines 2021, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Hilligan, K.L.; Namasivayam, S.; Clancy, C.S.; O’Mard, D.; Oland, S.D.; Robertson, S.J.; Baker, P.J.; Castro, E.; Garza, N.L.; Lafont, B.A.P.; et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022, 219, e20211862. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2010, 121, 171–181. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B. Alzheimer’s disease: Innate immunity gone awry? Metabolism 2017, 69, S41–S49. [Google Scholar] [CrossRef]
- Baek, H.; Ye, M.; Kang, G.H.; Lee, C.; Lee, G.; Choi, D.B.; Jung, J.; Kim, H.; Lee, S.; Kim, J.S.; et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 2016, 7, 69347–69357. [Google Scholar] [CrossRef] [Green Version]
- Ristori, G.; Romano, S.; Cannoni, S.; Visconti, A.; Tinelli, E.; Mendozzi, L.; Cecconi, P.; Lanzillo, R.; Quarantelli, M.; Buttinelli, C.; et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology 2014, 82, 41–48. [Google Scholar] [CrossRef]
- Lee, J.; Reinke, E.; Zozulya, A.L.; Sandor, M.; Fabry, Z. Mycobacterium bovisBacille Calmette-Guérin Infection in the CNS Suppresses Experimental Autoimmune Encephalomyelitis and Th17 Responses in an IFN-γ-Independent Manner. J. Immunol. 2008, 181, 6201–6212. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Jacobs, C.; E Morley, J.; Hoft, D.F. Lower Risk for Dementia Following Adult Tetanus, Diphtheria, and Pertussis (Tdap) Vaccination. J. Gerontol. Ser. A 2021, 76, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Hoft, D.F.; Jacobs, C.; Morley, J.E. Impact of herpes zoster vaccination on incident dementia: A retrospective study in two patient cohorts. PLoS ONE 2021, 16, e0257405. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Salas, J.; Morley, J.E.; Hoft, D.F.; Jacobs, C.; Scherrer, J.F. Comparison of rates of dementia among older adult recipients of two, one, or no vaccinations. J. Am. Geriatr. Soc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuizen, P.J.; Ng, B.H.; Chang, J.; Fell, A.L.; Cheong, R.M.Y.; Wong, W.Y.; Gan, P.Y.; Holdsworth, S.R.; Ooi, J.D. BCG Vaccine Derived Peptides Induce SARS-CoV-2 T Cell Cross-Reactivity. Front. Immunol. 2021, 12, 692729. [Google Scholar] [CrossRef]
- Urbán, S.; Paragi, G.; Burián, K.; McLean, G.R.; Virok, D.P. Identification of similar epitopes between severe acute respiratory syndrome coronavirus-2 and Bacillus Calmette–Guérin: Potential for cross-reactive adaptive immunity. Clin. Transl. Immunol. 2020, 9, 1227. [Google Scholar] [CrossRef]
- Koster, K.J.; Webb, H.L.; Cirillo, J.D. COVID-19 and Beyond: Exploring Public Health Benefits from Non-Specific Effects of BCG Vaccination. Microorganisms 2021, 9, 2120. [Google Scholar] [CrossRef]




| Females | Males | p-Value | |
|---|---|---|---|
| n(%) | 28 (57.1) | 21 (42.9) | |
| Age, years | |||
| Mean (SD) | 64.3 (6.7) | 65.7 (8.4) | 0.52 a |
| Length of education, years | |||
| Median (IQR) | 16 (14–18) | 16 (16–18) | 0.47 b |
| CMV infection, n (%) | |||
| Negative | 19 (67.9%) | 15 (71.4%) | 0.79 c |
| Positive | 9 (32.1%) | 6 (28.6%) | |
| ApoE4 allele, n (%) | |||
| Negative | 18 (64.3%) | 9 (42.9%) | 0.14 c |
| Positive | 10 (35.7%) | 12 (57.1%) | |
| SAGE | |||
| Before BCG | |||
| Median (IQR) | 22 (21–22) | 22 (21.5–22) | 0.70 b |
| After BCG | |||
| Median (IQR) | 22 (22–22) | 22 (21–22) | 0.37 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dow, C.T.; Greenblatt, C.L.; Chan, E.D.; Dow, J.F. Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease. Microorganisms 2022, 10, 424. https://doi.org/10.3390/microorganisms10020424
Dow CT, Greenblatt CL, Chan ED, Dow JF. Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease. Microorganisms. 2022; 10(2):424. https://doi.org/10.3390/microorganisms10020424
Chicago/Turabian StyleDow, Coad Thomas, Charles L. Greenblatt, Edward D. Chan, and Jordan F. Dow. 2022. "Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease" Microorganisms 10, no. 2: 424. https://doi.org/10.3390/microorganisms10020424
APA StyleDow, C. T., Greenblatt, C. L., Chan, E. D., & Dow, J. F. (2022). Evaluation of BCG Vaccination and Plasma Amyloid: A Prospective, Pilot Study with Implications for Alzheimer’s Disease. Microorganisms, 10(2), 424. https://doi.org/10.3390/microorganisms10020424






