BCG Vaccine—The Road Not Taken
Abstract
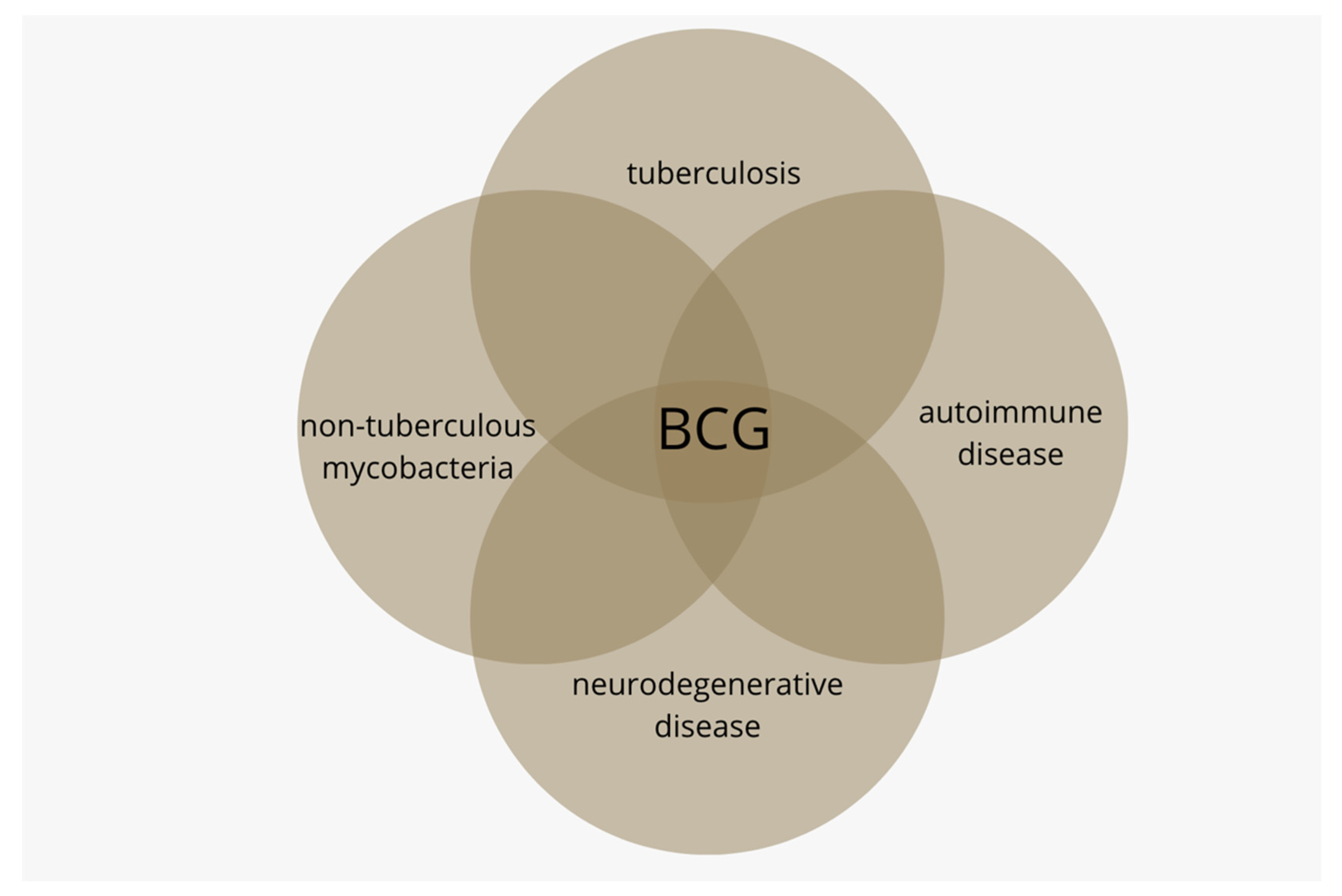
1. Introduction
2. Tuberculosis after BCG Discontinuation
3. BCG and Non-Tuberculous Mycobacteria
4. BCG and Autoimmune Disease
4.1. BCG and Type One Diabetes (T1D)
4.2. BCG and Multiple Sclerosis (MS)
5. BCG and Neurodegenerative Disease
5.1. BCG and Alzheimer’s Disease (AD)
5.2. BCG and Parkinson’s Disease (PD)
6. Discussion
“… despite the epidemiological evidence for heterologous protective effects of BCG vaccination, the perceived lack of biological plausibility has been a major obstacle in recognizing and in investigating these effects.”[92]
6.1. T1D
6.2. MS
“Multiple sclerosis is not common but is a potentially severe cause of neurological disability throughout adult life. Prevalence has increased substantially in many regions since 1990.”[96]
6.3. AD
“We estimated that the number of people with dementia would increase from 57.4 million cases globally in 2019 to 152.8 million cases in 2050.”[97]
6.4. PD
“Over the past generation, the global burden of Parkinson’s disease has more than doubled as a result of increasing numbers of older people, with potential contributions from longer disease duration and environmental factors. Demographic and potentially other factors are poised to increase the future burden of Parkinson’s disease substantially.”[98]
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hershkovitz, I.; Donoghue, H.D.; Minnikin, D.E.; Besra, G.S.; Lee, O.Y.; Gernaey, A.M.; Galili, E.; Eshed, V.; Greenblatt, C.L.; Lemma, E.; et al. Detection and molecular characterization of 9000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS ONE 2008, 3, e3426. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Winau, F. From bacteriology to immunology: The dualism of specificity. Nat. Immunol. 2005, 6, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef]
- World Health Organization. BCG Immunization Coverage Estimates by WHO Region. Available online: https://apps.who.int/gho/data/view.main.81500?lang=en (accessed on 27 July 2022).
- Andersen, P.; Kaufmann, S.H. Novel vaccination strategies against tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 4, a018523. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. The TB vaccine development pipeline: Present and future priorities and challenges for research and 909 innovation. In Essential Tuberculosis; Migliori, G.B., Raviglione, M.C., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 395–405. [Google Scholar]
- Calmette, A. Preventive vaccination against tuberculosis with BCG. Proc. R. Soc. Med. 1931, 24, 1481–1490. [Google Scholar] [CrossRef]
- Fox, G.J.; Orlova, M.; Schurr, E. Tuberculosis in newborns: The lessons of the “Lübeck Disaster” (1929–1933). PLoS Pathog. 2016, 12, e1005271. [Google Scholar] [CrossRef]
- Luca, S.; Mihaescu, T. History of BCG vaccine. Maedica 2013, 8, 53–58. [Google Scholar]
- Milstien, J.B.; Gibson, J.J. Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bull. World Health Organ. 1990, 68, 93–108. [Google Scholar]
- Palmer, C.E.; Long, M.W. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 1966, 94, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin. Exp. Immunol. 2010, 160, 70–79. [Google Scholar] [CrossRef]
- Kowalewicz-Kulbat, M.; Locht, C. BCG for the prevention and treatment of allergic asthma. Vaccine 2021, 39, 7341–7352. [Google Scholar] [CrossRef]
- Moulson, A.J.; Av-Gay, Y. BCG immunomodulation: From the ‘hygiene hypothesis’ to COVID-19. Immunobiology 2021, 226, 152052. [Google Scholar] [CrossRef]
- World Health Organization. BCG vaccines: WHO position paper—February 2018. Wkly. Epidemiol. Rec. 2018, 93, 73–96. [Google Scholar]
- Aronson, N.E.; Santosham, M.; Comstock, G.W.; Howard, R.S.; Moulton, L.H.; Rhoades, E.R.; Harrison, L.H. Long-term efficacy of BCG vaccine in American Indians and Alaska natives: A 60-year follow-up study. JAMA 2004, 291, 2086–2091. [Google Scholar] [CrossRef]
- Usher, N.T.; Chang, S.; Howard, R.S.; Martinez, A.; Harrison, L.H.; Santosham, M.; Aronson, N.E. Association of BCG vaccination in childhood with subsequent cancer diagnoses: A 60-year follow-up of a clinical trial. JAMA Netw. Open 2019, 2, e1912014. [Google Scholar] [CrossRef]
- Kremenovic, M.; Schenk, M.; Lee, D.J. Clinical and molecular insights into BCG immunotherapy for melanoma. J. Intern. Med. 2020, 288, 625–640. [Google Scholar] [CrossRef]
- Taye, H.; Alemu, K.; Mihret, A.; Wood, J.L.N.; Shkedy, Z.; Berg, S.; Aseffa, A. Global prevalence of Mycobacterium bovis infections among human tuberculosis cases: Systematic review and meta-analysis. Zoonoses Public Health 2021, 68, 704–718. [Google Scholar] [CrossRef]
- Suazo, F.M.; Escalera, A.M.; Torres, R.M. A review of M. bovis BCG protection against TB in cattle and other animals species. Prev. Vet. Med. 2003, 58, 1–13. [Google Scholar] [CrossRef]
- Buddle, B.M.; Vordermeier, H.M.; Chambers, M.A.; de Klerk-Lorist, L.M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Front. Vet. Sci. 2018, 5, 259. [Google Scholar] [CrossRef]
- Anonymous. Criteria for discontinuation of vaccination programmes using Bacille Calmette-Guerin (BCG) in countries with a low prevalence of tuberculosis. A statement of the International Union Against Tuberculosis and Lung Disease. Tuber. Lung Dis. 1994, 75, 179–180. [Google Scholar] [CrossRef]
- World Health Organization. WHO Releases New Global Lists of High-Burden Countries for TB, HIV-Associated TB and Drug-Resistant TB. Available online: https://www.who.int/news/item/17-06-2021-who-releases-new-global-lists-of-high-burden-countries-for-tb-hiv-associated-tb-and-drug-resistant-tb (accessed on 27 July 2022).
- Fu, H.; Lin, H.H.; Hallett, T.B.; Arinaminpathy, N. Modelling the effect of discontinuing universal Bacillus Calmette-Guérin vaccination in an intermediate tuberculosis burden setting. Vaccine 2018, 36, 5902–5909. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yoshiyama, T.; Uchimura, K.; Hamaguchi, Y.; Kato, S. Epidemiology of childhood tuberculosis after ceasing universal Bacillus Calmette-Guérin vaccination. Sci. Rep. 2021, 11, 15902. [Google Scholar] [CrossRef]
- Zimmermann, P.; Finn, A.; Curtis, N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J. Infect. Dis. 2018, 218, 679–687. [Google Scholar] [CrossRef]
- Donohue, M.J. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect. Dis. 2018, 18, 163. [Google Scholar] [CrossRef]
- Henkle, E.; Hedberg, K.; Schafer, S.; Novosad, S.; Winthrop, K.L. Population-based Incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann. Am. Thorac. Soc. 2015, 12, 642–647. [Google Scholar] [CrossRef]
- Schildkraut, J.A.; Gallagher, J.; Morimoto, K.; Lange, C.; Haworth, C.; Floto, R.A.; Hoefsloot, W.; Griffith, D.E.; Wagner, D.; Ingen, J.V.; et al. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir. Med. 2020, 173, 106164. [Google Scholar] [CrossRef]
- Shah, N.M.; Davidson, J.A.; Anderson, L.F.; Lalor, M.K.; Kim, J.; Thomas, H.L.; Lipman, M.; Abubakar, I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect. Dis. 2016, 16, 195. [Google Scholar] [CrossRef]
- Lacroix, A.; Piau, C.; Lanotte, P.; Carricajo, A.; Guillouzouic, A.; Peuchant, O.; Cady, A.; Dupin, C.; Fangous, M.-S.; Martin, C.; et al. Emergence of nontuberculous mycobacterial lymphadenitis in children after the discontinuation of mandatory Bacillus Calmette and GuÉrin immunization in France. Pediatr. Infect. Dis. J. 2018, 37, e257–e260. [Google Scholar] [CrossRef]
- Trnka, L.; Danková, D.; Svandová, E. Six years’ experience with the discontinuation of BCG vaccination. 4. Protective effect of BCG vaccination against the Mycobacterium avium intracellulare complex. Tuber. Lung Dis. 1994, 75, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Romanus, V.; Hallander, H.O.; Wåhlén, P.; Olinder-Nielsen, A.M.; Magnusson, P.H.; Juhlin, I. Atypical mycobacteria in extrapulmonary disease among children. Incidence in Sweden from 1969 to 1990, related to changing BCG-vaccination coverage. Tuber. Lung Dis. 1995, 76, 300–310. [Google Scholar] [CrossRef]
- Katila, M.L.; Brander, E.; Backman, A. Neonatal BCG vaccination and mycobacterial cervical adenitis in childhood. Tubercle 1987, 68, 291–296. [Google Scholar] [CrossRef]
- Available online: http://www.decide-collaboration.eu/WP5/Strategies/Framework (accessed on 20 September 2022).
- World Health Organization. Leprosy. Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 27 July 2022).
- Setia, M.S.; Steinmaus, C.; Ho, C.S.; Rutherford, G.W. The role of BCG in prevention of leprosy: A meta-analysis. Lancet Infect. Dis. 2006, 6, 162–170. [Google Scholar] [CrossRef]
- Anonymous. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996, 348, 17–24. [Google Scholar] [CrossRef]
- Merle, C.S.; Cunha, S.S.; Rodrigues, L.C. BCG vaccination and leprosy protection: Review of current evidence and status of BCG in leprosy control. Expert Rev. Vaccines 2010, 9, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yotsu, R.R.; Suzuki, K.; Simmonds, R.E.; Bedimo, R.; Ablordey, A.; Yeboah-Manu, D.; Phillips, R.; Asiedu, K. Buruli Ulcer: A review of the current knowledge. Curr. Trop. Med. Rep. 2018, 5, 247–256. [Google Scholar] [CrossRef]
- MacCallum, P.; Tolhurst, J.C.; Buckle, G.; Sissons, H.A. A new mycobacterial infection in man. J. Pathol. Bacteriol. 1948, 60, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.G.; Quertinmont, M.J.; Sieniawski, J.; Gatti, F. Necrotic tropical ulcers and mycobacterial causative agents. Trop. Geogr. Med. 1959, 11, 293–312. [Google Scholar]
- Clancey, J.K.; Dodge, O.G.; Lunn, H.F.; Oduori, M.L. Mycobacterial skin ulcers in Uganda. Lancet 1961, 2, 951–954. [Google Scholar] [CrossRef]
- Asiedu, K.; Hayman, J. Epidemiology. In Buruli Ulcer: Mycobacterium Ulcerans Infection; Asiedu, K., Scherpbier, R., Raviglione, M., Eds.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization. WHO Joins Battle against a New Emerging Disease, Buruli Ulcer; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- World Health Organization. Buruli ulcer disease: Mycobacterium ulcerans infection: Background = Ulcère de Buruli: Infection à Mycobacterium ulcerans: Généralités. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2003, 78, 163–168. [Google Scholar]
- Portaels, F.; Aguiar, J.; Debacker, M.; Guédénon, A.; Steunou, C.; Zinsou, C.; Meyers, W.M. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect. Immun. 2004, 72, 62–65. [Google Scholar] [CrossRef]
- Smith, P.G.; Revill, W.D.; Lukwago, E.; Rykushin, Y.P. The protective effect of BCG against Mycobacterium ulcerans disease: A controlled trial in an endemic area of Uganda. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 449–457. [Google Scholar] [CrossRef]
- Anonymous. BCG vaccination against mycobacterium ulcerans infection (Buruli ulcer). First results of a trial in Uganda. Lancet 1969, 1, 111–115. [Google Scholar]
- Dow, C.T. Proposing BCG Vaccination for Mycobacterium avium ss. paratuberculosis (MAP) associated autoimmune diseases. Microorganisms 2020, 8, 212. [Google Scholar] [CrossRef]
- Dow, C.T.; Alvarez, B.L. Mycobacterium paratuberculosis zoonosis is a one health emergency. Ecohealth 2022, 19, 164–174. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Okoh, A.I. Systematic assessment of mycobacterium avium subspecies paratuberculosis infections from 1911-2019: A growth analysis of association with human autoimmune diseases. Microorganisms 2020, 8, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ekundayo, T.C.; Olasehinde, T.A.; Falade, A.O.; Adewoyin, M.A.; Iwu, C.D.; Igere, B.E.; Ijabadeniyi, O.A. Systematic review and meta-analysis of Mycobacterium avium subsp. paratuberculosis as environmental trigger of multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 59, 103671. [Google Scholar] [CrossRef]
- Dow, C.T. Warm, sweetened milk at the twilight of immunity—Alzheimer’s Disease—Inflammaging, insulin resistance, M. paratuberculosis and immunosenescence. Front. Immunol. 2021, 12, 714179. [Google Scholar] [CrossRef]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef]
- Lemke, G.; Huang, Y. The dense-core plaques of Alzheimer’s disease are granulomas. J. Exp. Med. 2022, 219, e20212477. [Google Scholar] [CrossRef]
- Frothingham, R. Evolutionary bottlenecks in the agents of tuberculosis, leprosy, and paratuberculosis. Med. Hypotheses 1999, 52, 95–99. [Google Scholar] [CrossRef]
- Ristori, G.; Faustman, D.; Matarese, G.; Romano, S.; Salvetti, M. Bridging the gap between vaccination with Bacille Calmette-Guérin (BCG) and immunological tolerance: The cases of type 1 diabetes and multiple sclerosis. Curr. Opin. Immunol. 2018, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Chan, E.D. What is the evidence that mycobacteria are associated with the pathogenesis of Sjogren’s syndrome? J. Transl. Autoimmun. 2021, 4, 100085. [Google Scholar] [CrossRef] [PubMed]
- Kühtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef]
- Ryu, S.; Kodama, S.; Ryu, K.; Schoenfeld, D.A.; Faustman, D.L. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J. Clin. Investig. 2001, 108, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kühtreiber, W.M.; Faustman, D.L. BCG therapy for type 1 diabetes: Restoration of balanced immunity and metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Hattori, N. Bacteria-host interactions in multiple sclerosis. Front. Microbiol. 2018, 9, 2966. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; Rocca, N.L.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T cells driving multiple sclerosis: Identity, mechanisms and potential triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Sato, S.; Noda, S.; Sechi, L.A.; Hattori, N. PARKIN modifies peripheral immune response and increases neuroinflammation in active experimental autoimmune encephalomyelitis (EAE). J. Neuroimmunol. 2021, 359, 577694. [Google Scholar] [CrossRef]
- Nakken, O.; Holmøy, T.; Stigum, H.; Myhr, K.M.; Dahl, J.; Heldal, E.; Meyer, H.E. Strong tuberculin response after BCG vaccination is associated with low multiple sclerosis risk: A population-based cohort study. Int. J. Epidemiol. 2022, dyac039. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, A.; Buzzi, M.G.; Giugni, E.; Sabatini, U.; Bastianello, S.; Pozzilli, C.; Salvetti, M.; Ristori, G. The effect of Bacille Calmette-Guérin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS. J. Neurol. 2003, 250, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Ristori, G.; Romano, S.; Cannoni, S.; Visconti, A.; Tinelli, E.; Mendozzi, L.; Cecconi, P.; Lanzillo, R.; Quarantelli, M.; Buttinelli, C.; et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology 2014, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Handy, A.; Lord, J.; Green, R.; Xu, J.; Aarsland, D.; Velayudhan, L.; Hye, A.; Dobson, R.; Proitsi, P.; Alzheimer’s Disease Neuroimaging Initiative; et al. Assessing genetic overlap and causality between blood plasma proteins and Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Hill, B.L.; Barda, N.; Halperin, E.; Gofrit, O.N.; Greenblatt, C.L.; Rappoport, N.; Linial, M.; Bercovier, H. Bladder cancer immunotherapy by BCG is associated with a significantly reduced risk of Alzheimer’s Disease and Parkinson’s Disease. Vaccines 2021, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Bercovier, H.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med. Hypotheses 2019, 123, 95–97. [Google Scholar] [CrossRef]
- Zuo, Z.; Qi, F.; Yang, J.; Wang, X.; Wu, Y.; Wen, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z.B. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol. Dis. 2017, 101, 27–39. [Google Scholar] [CrossRef]
- Peyton, C.C.; Chipollini, J.; Azizi, M.; Kamat, A.M.; Gilbert, S.M.; Spiess, P.E. Updates on the use of intravesical therapies for non-muscle invasive bladder cancer: How, when and what. World J. Urol. 2019, 37, 2017–2029. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433. [Google Scholar] [CrossRef]
- Dow, C.T.; Greenblatt, C.L.; Chan, E.D.; Dow, J.F. Evaluation of BCG vaccination and plasma amyloid: A prospective, pilot study with implications for Alzheimer’s Disease. Microorganisms 2022, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Verreault, R.; Laurin, D.; Lindsay, J.; De Serres, G. Past exposure to vaccines and subsequent risk of Alzheimer’s disease. CMAJ 2001, 165, 1495–1498. [Google Scholar] [PubMed]
- Wu, X.; Yang, H.; He, S.; Xia, T.; Chen, D.; Zhou, Y.; Liu, J.; Liu, M.; Sun, Z. Adult vaccination as a protective factor for dementia: A meta-analysis and systematic review of population-based observational studies. Front. Immunol. 2022, 13, 872542. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Salas, J.; Morley, J.E.; Hoft, D.F.; Jacobs, C.; Scherrer, J.F. Comparison of rates of dementia among older adult recipients of two, one, or no vaccinations. J. Am. Geriatr. Soc. 2022, 70, 1157–1168. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Salas, J.; Hoft, D.F.; Jacobs, C.; Morley, J.E.; Scherrer, J.F. Dementia risk following influenza vaccination in a large veteran cohort. Vaccine 2021, 39, 5524–5531. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Halliday, G.M.; Holton, J.L.; Revesz, T.; Dickson, D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011, 122, 187–204. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia response during Parkinson’s Disease: Alpha-synuclein intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef]
- Laćan, G.; Dang, H.; Middleton, B.; Horwitz, M.A.; Tian, J.; Melega, W.P.; Kaufman, D.L. Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 1292–1302. [Google Scholar] [CrossRef]
- Yong, J.; Lacan, G.; Dang, H.; Hsieh, T.; Middleton, B.; Wasserfall, C.; Tian, J.; Melega, W.P.; Kaufman, D.L. BCG vaccine-induced neuroprotection in a mouse model of Parkinson’s disease. PLoS ONE 2011, 6, e16610. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/results?cond=Parkinson+Disease&term=BCG (accessed on 27 July 2022).
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of neuron-derived pathological α-synuclein in blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A small jab—A big effect: Nonspecific immunomodulation by vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.-E.; Damoraki, G.; et al. Activate: Randomized clinical trial of BCG vaccination against infection in the elderly. Cell 2020, 183, 315–323.e9. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; van Crevel, R. BCG-induced protection: Effects on innate immune memory. Semin. Immunol. 2014, 26, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Smolen, K.K.; van Haren, S.D.; Dowling, D.J.; Husson, R.N.; Levy, O. BCG as a case study for precision vaccine development: Lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front. Microbiol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef]
- Available online: https://www.idf.org/component/attachments/attachments.html?id=275&task=download (accessed on 27 July 2022).
- Available online: https://www.healthdata.org/research-article/global-regional-and-national-burden-multiple-sclerosis-1990%E2%80%932016-systematic (accessed on 27 July 2022).
- Available online: https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(21)00249-8/fulltext (accessed on 27 July 2022).
- Available online: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(18)30295-3/fulltext (accessed on 27 July 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dow, C.T.; Kidess, L. BCG Vaccine—The Road Not Taken. Microorganisms 2022, 10, 1919. https://doi.org/10.3390/microorganisms10101919
Dow CT, Kidess L. BCG Vaccine—The Road Not Taken. Microorganisms. 2022; 10(10):1919. https://doi.org/10.3390/microorganisms10101919
Chicago/Turabian StyleDow, Coad Thomas, and Laith Kidess. 2022. "BCG Vaccine—The Road Not Taken" Microorganisms 10, no. 10: 1919. https://doi.org/10.3390/microorganisms10101919
APA StyleDow, C. T., & Kidess, L. (2022). BCG Vaccine—The Road Not Taken. Microorganisms, 10(10), 1919. https://doi.org/10.3390/microorganisms10101919





