Abstract
Legionella pneumophila is defined as a bacterium that can cause severe pneumonia. It is found in the natural environment and in water, and is often found in water tanks. It can be an integral part of biofilms in nature, and the protozoa in which it can live provide it with food and protect it from harmful influences; therefore, it has the ability to move into a sustainable but uncultured state (VBNC). L. pneumophila has been shown to cause infections in dental practices. The most common transmission route is aerosol generated in dental office water systems, which can negatively affect patients and healthcare professionals. The most common way of becoming infected with L. pneumophila in a dental office is through water from dental instruments, and the dental unit. In addition to these bacteria, patients and the dental team may be exposed to other harmful bacteria and viruses. Therefore, it is vital that the dental team regularly maintains and decontaminates the dental unit, and sterilizes all accessories that come with it. In addition, regular water control in dental offices is necessary.
1. Introduction
Members of the Legionellaceae family are small, Gram-negative, aerobic bacilli that do not form spores, are capsule-free, and possess the enzymes catalase and oxidase (Table 1) [1]. Amino acids are their primary carbon and energy sources for bacterial growth in the intracellular environment [2]. Amino acids are catabolized by the Krebs cycle, and gluconeogenetic enzymes synthesize sugars from the Embden–Meyerhof–Parnas pathway. Legionella is non-saccharolytic and possesses the enzyme protease. The guanine and cytosine content in its DNA ranges from 38% to 52% [3].
Of the known 15 serogroups of Legionella pneumophila (L. pneumophila), serogroup 1 is present in 84% of cases worldwide (Table 2) [4,5,6]. Serogroups 2 to 15 account for 16 to 20% of Legionella pneumonia cases [7]. Patients with L. pneumophila of serogroups 2 to 15 showed typical symptoms of Legionella pneumonia, although Legionella urinary antigen detection tests were negative [8,9,10]. In addition, specific differences in the virulence of different serogroups were observed; for instance, Buse et al. showed a significant difference in the mobility of L. pneumophila between serogroups [11]. A characteristic of the L. pneumophila genome is the presence of many different eukaryotic-like proteins and protein domains that are probably acquired by horizontal gene transfer [12,13,14].

Table 2.
Legionella species, serogroup details, and their ability to cause human infection and mortality rate [7,17,20,21].

Table 1.
Classification of Legionella pneumophila.
Table 1.
Classification of Legionella pneumophila.
| Characteristics | Legionella pneumophila | References |
|---|---|---|
| Family | Legionellaceae | [15] |
| Form | bacillus | [1] |
| Coloring per gram | Gram (-) | [1] |
| Metabolism | Aerobic | [1] |
| pH | 5–8.5 | [16] |
| Habitat | Aquatic habitats (biofilm, within multicellular organisms) | [17] |
| Reproduction temperature | 25–37 °C | [18] |
| Survival temperature | 0–63 °C | [16] |
| Nutrients | Amino acids (L-cysteine), iron | [17] |
| Sensitivity | Drying, chlorine, UV radiation | [19] |
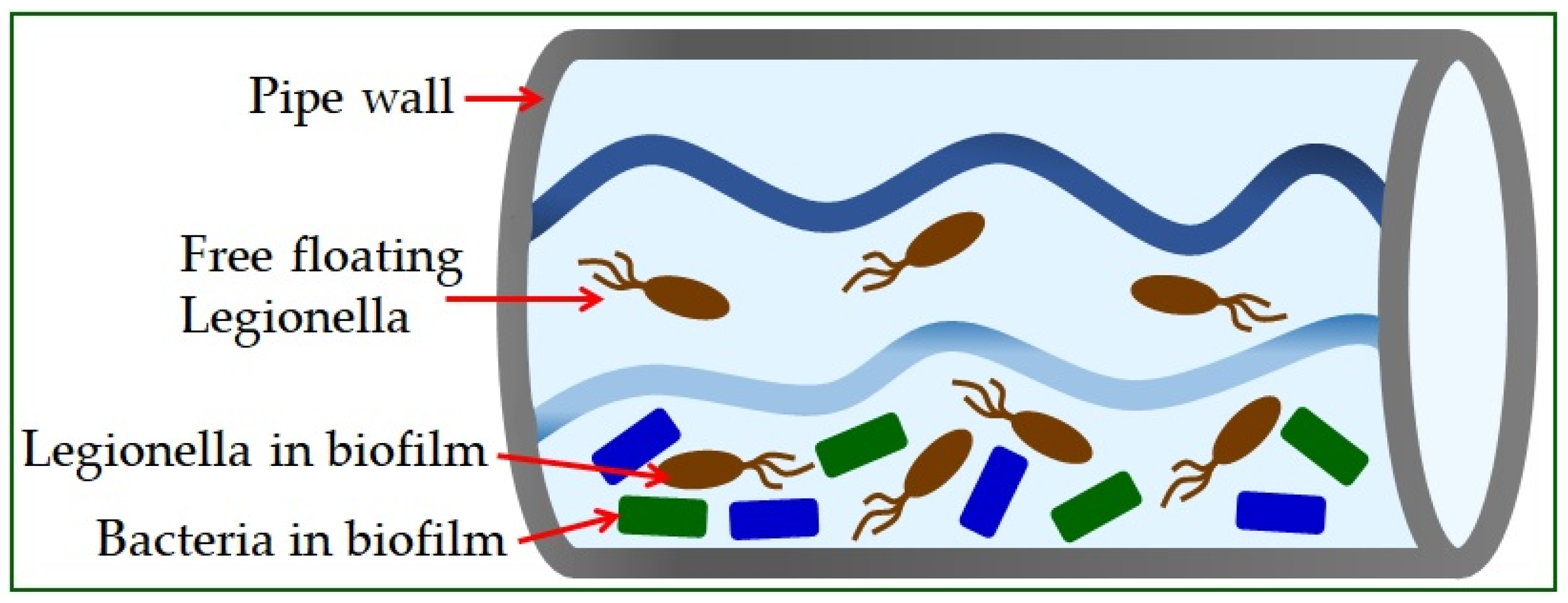
Legionella is found in the natural or artificial aquatic environment [22]. It occurs in planktonic form or as part of biofilms [23] and has the ability to move into a sustainable but uncultured state (VBNC) [19]. Elevated temperature, inorganic and organic water content, and the presence of protozoa play a crucial role in their growth and spread [24]. The most significant number of Legionella is found in water samples with temperatures from 30 °C to 40 °C [25]. Infections in humans occur exclusively by inhalation of contaminated aerosols, which can occur in air conditioning systems, cooling towers, spas, fountains, ice machines, plant sprayers, dental appliances, and showerheads [26]. The material from which the pipeline system is built significantly influences the appearance of high concentrations of bacteria (Figure 1) [5]. The use of copper as a plumbing material has been shown to help reduce the risk of Legionnaires’ disease, while plastic materials support many L. pneumophila bacteria [27].
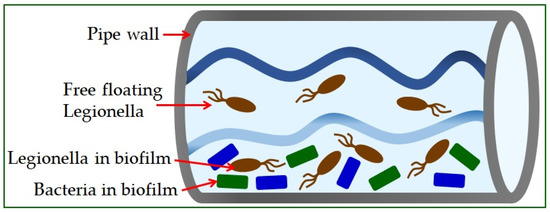
Figure 1.
Legionella in water supply systems.
Legionella can be found in water distribution cooling towers, where it can replicate within protozoa. It is often Vermamoeba vermiformis that protects Legionella pneumophila from the effects of heat and disinfectants, which can result in nosocomial infections [28]. Coevolution with multiple protozoan species has resulted in the development of mechanisms that allow L. pneumophila to occupy different hosts, and the possibility of human cell infection [2,29,30].
Legionella pneumophila is responsible for most cases of legionellosis and is one of the major causes of community-acquired and nosocomial-acquired atypical cases of pneumonia, with a mortality rate between 7% and 25% [20,31]. In comparison, the mortality rate of non-Legionella pneumophila species is 5% [31]. Legionnaires’ disease is a pulmonary form of legionellosis with an incubation period of two to fourteen days, and involves severe pneumonia and systemic infection [32,33]. A benign flu-like condition is called Pontiac fever [34]. It is a non-pneumonic disease with unclear pathogenesis requiring no antimicrobial treatment [32]. The mortality rate of adequately treated patients with Legionnaires’ disease varies from 7% to 24%, with immunocompromised and elderly patients being the most susceptible [34]. It is estimated that 25,000 to 100,000 people are diagnosed with legionellosis each year in the United States [35]. In North America and Western Europe, 1–13% of all types of pneumonia were associated with this pathogen [36].
2. History of Legionellosis
The two principal clinical forms of bacterial infection caused by inhalation or aspiration of the genus Legionella, together with the aerosol in which they are contained, are Pontiac fever and Legionnaires’ disease [26]. Infections obtain their names from the events after which they were described. The identification of Legionnaires’ disease was preceded by a conference of American veterans from the Second World War, the so-called Legionnaire, in 1976, which, as it turned out, became the source of a previously unknown disease [37]; the tragedy was blamed on the ventilation and air conditioning system of a luxury hotel in Philadelphia. Participants of the gathering fell ill and suffered from pneumonia, and there were also fatalities. After this event, the causative agent was isolated in 1977 and named Legionella [38,39]. Now, it is known that the 1974 event from the same hotel is also linked to a disease that broke out two years later [40].
This type of bacterium was also responsible for infecting employees and patients of the Health Department in Pontiac, Michigan, in 1968. Although respiratory symptoms were present in both cases, the disease here was milder, with no pneumonia or death; it was called Pontiac fever. A weaker form of the disease in Pontiac could indicate a low infectious dose, but the infection rate among healthcare workers was 95%. This high infection rate suggested that the pathogen was spread through the air [3,37,41].
Among the first discoveries related to Legionella spp. was an isolate in 1947 that was described as a “rickettsia-like” organism, and in 1977, it was identified as the same bacterial species and serogroup responsible for the disease in Philadelphia [39,42]. Outbreaks of this disease attract much attention, but it still occurs mainly in individual cases. Its occurrence has been reported in Europe, the USA, Canada, New Zealand, Japan, Singapore, and Australia. The registered case number of infections is increasing despite numerous guidelines on preventing the spread of Legionella spp. [43]. Today, over 60 species of Legionella are known. Legionella pneumophila is the most common pathogenic species and includes 15 serogroups, although most human diseases are caused by the L. pneumophila serogroup 1 [44]. Other species that are clinically significant for human infection, in addition to those shown in Table 2, are Legionella feeleii, Legionella micdadei, Legionella longbeachae, Legionella anisa, Legionella dumoffii, and Legionella bozemanii [21,45,46].
3. Virulence Factors
The development and clinical form of the disease depend on the number of bacteria in the aerosol, the serogroup to which they belong, the virulence factors, and the person’s immunity. Therefore, in addition to preventing the colonization of water bodies, it is also essential to determine the virulence factors of Legionella spp. [47]. Initial adhesion to cell surface receptors is associated with the bacterium’s surface structures, namely pili (fimbriae), lipopolysaccharides, and proteins [48]. Potential invasiveness is increased by flagellar motility and toxin production [46]. Microbial pathogenicity enhances the possibility of long-term intracellular survival and replication in alveolar macrophages, thereby increasing infectivity while bypassing the patient’s immune system [49].
3.1. Surface Virulence Factors
Surface virulence factors that affect the virulence of Legionella spp. and enhance infectivity are lipopolysaccharide (LPS), flagella, pili, and outer membrane proteins [46,47,49,50,51].
The LPS is located on the outer envelope of the outer membrane and is crucial in interacting with various host immune cells, as well as in intracellular traffic modulating [22]. The LPS molecule consists of an O-specific chain, a nucleus, and a lipid A component, combined with an endotoxin with a relatively low toxic potential. LPS contains many extended, branched fatty acids and O- and N-acetyl groups. It is highly hydrophobic and differs from the lipopolysaccharides of other Gram-negative bacteria [51,52].
Legionella spp. move, in most cases, by means of a polar and/or lateral flagella consisting of a basal body, a hooked structure, and a filament [53]. Motility may be crucial for dispersing in the lungs of patients, as such forms of L. pneumophila have been detected in alveolar parts [46,51,52,54]. The gene responsible for expression depends on the temperature, availability of nutrients, and viscosity of the medium in which they reside. Although flagella are not a condition for intracellular proliferation, they enhance host cell invasion regardless of attachment to it [51,52]. Pili can be divided into two forms: the long form, measuring 0.8 to 1.5 µm, and the short form, measuring 0.1 to 0.6 µm. The PilE protein is an integral part of the long form of type IV pili. It is involved in binding and adhesion to host cells. Prepilin peptidase (PilD) is another protein liable for the production of type IV pili. It is crucial for successful intracellular proliferation. This protein is also involved in secretion type II [51,52].
Legionella spp. outer membrane proteins are essential for phagocyte entry and survival. The Macrophage Infectivity Potentiator (MIP) displays peptidyl–prolyl cis/trans isomerase activity and is necessary for the early stages of intracellular infection and survival in macrophages, protozoa, and pulmonary epithelium [55]. Due to the MIP gene’s characteristics not being found in other Legionella genes, it is possible to identify clinical and environmental strains of L. pneumophila and other Legionella spp. by MIP sequencing [51,56].
The major Outer Membrane Protein (MOMP) has porin properties, and is required for bacterial interaction with CR1 and CR3 receptors on monocytes and other phagocytes [57]. Factors of both the host and bacteria that facilitate initial adherence and entry of Legionella spp. into the cell, are essential [57].
3.2. Secreted Factors
Legionella spp. secretes various pigments, toxins, and enzymes [58]. Moreover, Legionella spp. secretes more than 18,000 proteins containing eukaryotic-like domains, called effector proteins, through secretion systems [13]. Many effector proteins are secreted into the host cell, facilitating Legionella intracellular replication [13]. These secretion systems are essential virulence factors in L. pneumophila.
The type I secretion system known as Lss consists of the ABC transporter (ATP-binding cassette), a membrane-fusion protein, and an outer-membrane protein [59]. The lss gene cluster, lssXYZABD, which includes the ABC transporter and membrane fusion protein, was found in all L. pneumophila strains [50].
Secretion system type II, termed Lsp and often referred to as the general secretory pathway, contains numerous degradation enzymes, including RNase, two acid phosphatases, zinc metalloprotease, etc. [60,61]. The L. pneumophila type II secretion system has 12 components. Some components are the prepilin peptidase pilD, the outer membrane secretin and ATPase lspDE, and the pseudopilins lspFGHIJK, lspC, and lspLM [57]. The Lsp secretion system is essential for L. pneumophila survival at low temperatures [50].
The type III secretion system is a protein-transport mechanism that translocates cytoplasmic substrates directly into the host cytoplasm [62]. It contains flagellum-encoding genes [59] and secretins as part of a type II secretion system, homologous to the DotD protein in a type IVB secretion system [63].
Another important secretory system is the type IV secretory system. There are two subclasses of the type IV system—IVA (called Lvh) and IVB (called Dot (defect in organelle trafficking) /Icm (intracellular multiplication)) [59]. The Dot/Icm secretory system is encoded by the Dot/Icm genes [63]. The Dot/Icm secretion system secretes more than 300 different effector proteins into the host cell and is crucial for the virulence of L. pneumophila [13]. Therefore, the Dot/Icm secretion system constitutes around 10% of the L. pneumophila proteome, suggesting that the effectors include a significant determinant of L. pneumophila survival [50]. The Dot/Icm system is vital for establishing a replicator niche and avoiding lysosomal/endocytic fusion [64]. The action of bacterial degradation enzymes ultimately leads to the death and lysis of host cells and damage to lung tissue [65,66]. In addition, the Dot/Icm effector proteins are required to translocate the Legionella-containing vacuole across the membrane [23]. The Lvh secretion system contains genes encoding mobility factors and enzymes [59]. It may have a role during intracellular replication of L. pneumophila and thus complement Dot/Icm function [50]. In addition, the Lvh can functionally replace defective Dot/Icm [67].
Type II and IVB secretion systems are found in all Legionella strains, while the type I secretion system is exclusive for L. pneumophila [65]. In addition, type II and IVA secretion systems are found in some Legionella strains [59]. However, some effector proteins of type II, III, and IVA secretion systems are homologous to the components of the type IVB secretion system, and are therefore not found in all Legionella strains [63]. Secretory systems are essential for virulence, and Legionella species that have additional secretory systems have increased pathogenicity [59].
The brown pigment, or pyomelanin, is one of the frequently studied secreted factors. It is a polymer formed with homogenetic acid (HGA), produced by bacteria with oxygen [68]. The lly gene is necessary for its formation [69]. Mutagenesis of Lly gene does not affect intracellular replication within amoebae or macrophage-like host cells [69]. Secreted HGA becomes toxic in the presence of oxygen [70]. Pyomelanin protects Legionella from possible light-induced damage and helps it to obtain iron, an essential micronutrient for its survival [51,58].
3.3. Biofilm
Nowadays, the increased use of drugs (especially antibiotics) has led to bacterial resistance, a significant problem in patient treatment [71]. The biofilm is one of the protective mechanisms that increases the resistance of bacteria to specific external agents [72,73]. Legionella spp. is characterized by the ability to survive in a biofilm and achieve high virulence and resistance, even after severe physical and chemical treatments [74]. In addition, many biological factors influence the persistence of the biofilm [75]. As such, the biofilm allows more excellent adhesion of specific bacterial species through various stages, from initiation to maturation of the biofilm and the formation of an extracellular matrix [74,76,77,78]. Survival of Legionella spp. under oligotrophic conditions with a lower content of organic matter and nutrients requires the incorporation of Legionella into the microbial community with other bacteria [79,80]. On this basis, it is possible to explain the increased numbers of Legionella spp. in artificial habitats such as hot water systems [81]. Bacteria in these communities participate in interactions such as the food chain formation or the congregation continuation. The microorganisms in the biofilm may also repel other microbes that are unlikely to contribute to the community [58,80].
For Legionella spp., biofilm formation is essential for survival [25]. Like many other microorganisms, it responds to environmental factors that significantly affect biofilm formation and/or colonization. Temperature is an influential agent affecting biofilm colonization and can affect the biofilm determinants produced by Legionella spp. In vitro, monotypic biofilms at 37–42 °C consist of filamentous bacteria, but at 25 °C, they are thinner and dominated by rod-shaped bacteria. Legionella cell length is associated with ppGpp signaling. A biofilm at 37 °C is much more solid than at 25 °C, and interestingly, at 25 °C, it is more prone to better adhesion potential [29,82]. Specific genes may also regulate biofilm formation, such as the putative twin-arginine translocation pathway involving the tatB and tatC genes [83]. In the same case, expression of the MIP gene has been found to promote biofilm creation at an early stage, when Legionella spp. does not require a host for growth [83,84].
Quorum sensing (QS) in Gram-negative bacteria regulates the gene expression of various bacterial processes, including biofilm formation [85]. Bacteria that show QS signaling are usually found in artificial aquatic systems and may regulate biofilm production in the environment [86]. The QS autoinductor used by Legionella pneumophila is LAI-1 (3-hydroxypentadecan-4-one), which produces and detects the Lqs system and contains the autoinductive synthase LqsA, homologous sensory kinase LqsS, and the LqsR response regulator [87]. The quorum sensing autoinducer (3-oxo-C12-HSL) possessing P. aeruginosa inhibits L. pneumophila biofilm formation [88]. This effect is associated with a decrease in LqsR. This suggests that QS could play a role in the dispersion of L. pneumophila during the later stages of biofilm development [89]. Several biological factors such as microbial communities, temperature, and specific genes regulate L. pneumophila biofilm production. These factors should suppress biofilm formation to reduce colonization in aqueous systems [74].
3.4. Legionella and Protozoan Interactions
Certain species of protozoa are crucial for the growth of Legionella in natural and artificial environments [90]. Accordingly, the presence of Legionella in these environments depends on the spectrum of protozoa present in the host [91]. Acanthamoeba, Hartmannella, and Naegleria are most commonly isolated from Legionella-contaminated water systems [92]. Other protozoa associated with Legionella are Saccamoeba, Vexillifera, and Platyamoeba [93].
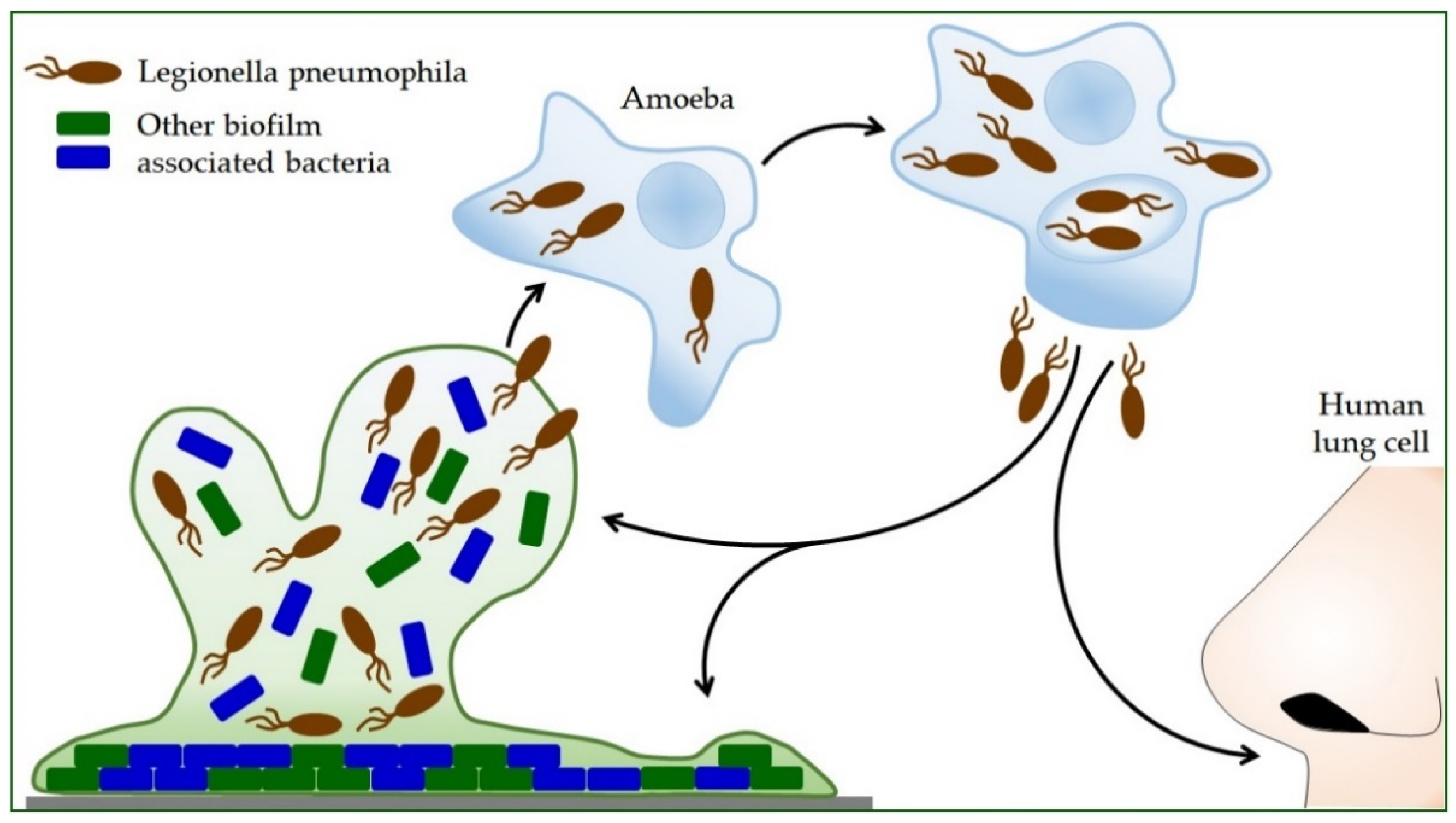
Propagation within the amoeba L. pneumophila increases the ability to produce polysaccharides, which increases its ability to form a biofilm (Figure 2) [74]. Protozoa provide nutrients for intracellular Legionella and protect them from adverse environmental influences. Bacteria survive high temperatures, disinfection procedures, and drying inside the Acanthamoeba cyst [19]. L. pneumophila can use protozoa to colonize new habitats, so inhaled protozoa are an effective mode of transmission to humans [94]. Thus, the symbiosis of Legionella and protozoa contributes to the infection process itself [22]. After intracellular replication within the protozoan, L. pneumophila shows more excellent resistance to stress [30,95]. During coevolution with protozoan cells, L. pneumophila acquires highly sophisticated and diverse strategies for taking over the host cell process [96,97]. It secretes hundreds of effectors into the host cell, controlling host signaling pathways and key cellular processes [57,98,99]. L. pneumophila can also alter host transcription and translation processes and utilize epigenetic mechanisms in the cells in which it is found to counteract host responses [100].
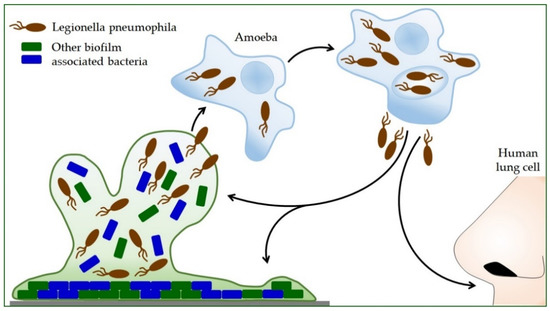
Figure 2.
The life cycle of Legionella pneumophila. Legionella reproduces only inside other cells. Bacterial-feeding amoebae also live in the environment where Legionella is found. After the Legionella is eaten by the amoeba, it is encapsulated inside the amoeba, where it continues to grow and multiply. By releasing Legionella bacteria from the amoeba, they can disperse into the environment and form a new biofilm with other bacteria, or humans can inhale them. In humans, this cycle is repeated, but in this case, the human lung cells are infected.
Upon internalization, intracellular bacteria reprogram the endosomal–lysosomal pathway of host degradation [101]. The multiplication of Legionella bacteria within a maturation-blocked vacuole, which fails to acidify and fuse with lysosomes, shows many similarities to human phagocytic cell infection [102]. It includes the recruitment of the rough endoplasmic reticulum surrounding the membrane-bound vacuole. Interaction with protozoa is thought to be the driving force in the evolution of Legionella pathogenicity. In recent years, tremendous progress has been made in unraveling the mechanisms by which intracellular pathogens attack host cells and establish intracellular infections [103].
4. Legionella pneumophila in Dental Practice
Dental staff may be at high risk of Legionella infection, and therefore, an occupational risk assessment is required. In addition to many dentists, other healthcare professionals in the dental clinic, such as dental assistants and hygienists, are also exposed to the occupational risk of Legionella infection. It is estimated that the occupational risk of Legionella infection may affect 1 to 2 million healthcare professionals worldwide [104].
Based on research in dental offices conducted in 1986 in Austria, the presence of Legionella pneumophila serogroup 1 was determined in 10% of water supply systems. The first death of a dentist due to Legionnaires’ disease was in 1995, and Legionella was discovered in the plumbing system of his office [104]. In 2012, an 83-year-old patient from Italy died of Legionnaires’ disease, and the source of the infection was contaminated water in the dental office she visited [105]. In addition, in the same year, an elderly, immunocompromised man in Sweden died due to Legionella in the cup filler outlet used for rinsing at the dental ward [106]. Legionella pneumophila is fatal in many cases [20]. However, the cause of the fatal outcome related to the dental practice was determined only in the patients shown. According to a study by Kevorkyan et al., antibodies to Legionella were significantly higher in medical and dental professionals than in non-professionally exposed subjects [107]. The possibility of contamination in a dental unit water system with microorganisms has been discussed since the beginning of dental chair use. Due to the constant exposure of patients and staff in the dental team to the aerosol produced during operations, the microbial quality of the water is critical. Water supply systems might contain opportunistic and pathogenic bacteria, mostly Gram-negative species that pose a particular risk in immunocompromised individuals [108,109]. The water systems of the dental unit can be initially contaminated through water coming into the system or by pulling and sucking saliva and other fluids from the patient’s mouth. Dental chairs can receive water through a public water supply network or special tanks built into the chair to pour liquid. Other factors that might influence this are the work unit’s model and the time of its construction, whether there is a built-in system against the return flow of patient fluids, infection prevention measures, used disinfection methods, etc. [109,110].
The guidelines for the allowable number of bacteria in the dental unit’s water are different and mostly coincide with the number of microorganisms allowed in the drinking water. In Europe, this number is up to 100 colony-forming units per milliliter of water (CFU/mL). The American Dental Association (ADA) set the allowable number of microorganisms in the water for dental supply at ≤200 CFU/mL, while the Centers for Disease Control and Prevention (CDC) recommended that the number be ≤500 CFU/mL [109,110,111,112,113,114]. The risk of infection arises because most instruments that are necessary for work in dentistry, such as micromotors, turbines, sonic and ultrasonic scalers, water/air syringes, etc., produce an aerosol in the inhalation zone. The presence of Legionella spp. in saliva and dental plaque biofilm has also been shown [115,116].
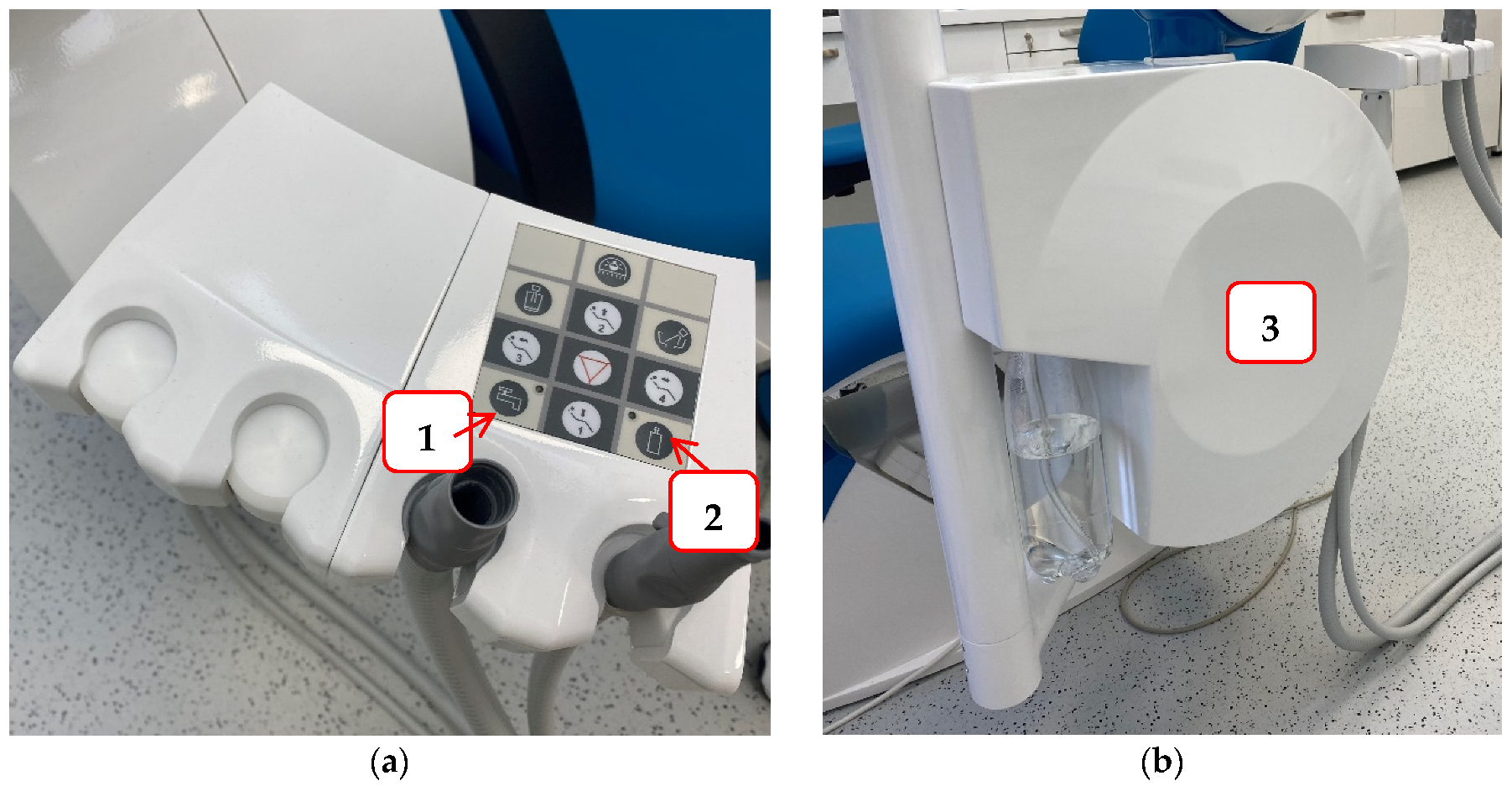
To reduce the possibility of infection and protect staff and patients in the dental office, it is necessary to try to prevent contamination of the hydraulic system of the dental unit. Some of the measures that can be achieved are: a dental unit that can be connected to sterile or distilled water (Figure 3); flushing water through the instruments at the beginning and end of the working day, and also between each patient to prevent cross-contamination and water stagnation; continuous disinfection; procurement of thick filters, etc. [117]. For this reason, dentists are required to conduct a legal risk assessment of their hydraulic systems, identify and assess the sources of risk, and prepare guidelines for the prevention and control of the risk of Legionella infection. Furthermore, they must monitor the quality of their hydraulic systems once a year to ensure that the hydraulic systems are free of Legionella [117].
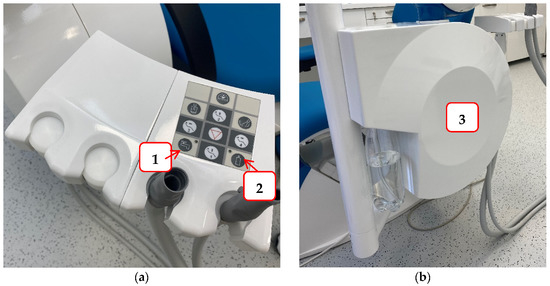
Figure 3.
An example of a dental unit with a choice of water supply: Panel (a) the buttons that allow the selection of water supply either from the public network (1) or a bottle of distilled water (2); Panel (b) water tank for distilled water.
Particular attention should be paid to prevention measures in these times of the COVID-19 pandemic. COVID patients are more susceptible to secondary infections for several months during recovery. Lock-down and government measures such as staying at home and delaying the procedures also result in prolonged water standing in the dental unit’s supply tanks. Hence, the biofilm accumulation in the system is more likely [116,118,119].
4.1. Resistance of L. pneumophila Biofilms to Biocides
L. pneumophila poses a constant threat to human health in anthropogenic water sources [21]. Due to the intracellular lifestyle within protozoa, it is difficult to assess whether the resistance of L. pneumophila in environmental biofilms is due to the structure of the biofilm, its association with amoebae, or both [120]. However, the fact is that L. pneumophila, which is found in biofilms, is highly resistant to the action of biocides [74].
Numerous disinfection methods were used to limit the growth of L. pneumophila, but none succeeded in complete eradication; namely, recolonization occurred very soon after treatment [120,121]. In addition, some studies showed that the biocide action on the L. pneumophila biofilm could lead to the transition of the bacterium to the VBNC state [74]. The most common biocides used in L. pneumophila water disinfection protocols are chlorine and chlorine derivatives. However, they show efficacy only on planktonic cells, but not on biofilm [122].
Two reasons for this are the resistance of L. pneumophila to disinfectants; one is due to its ability to survive within the biofilm, and the other is that it possesses an intra-amoebic lifestyle [29]. Namely, vesicles containing intracellular L. pneumophila released by the amoeba are resistant to biocidal treatments. It is important to note that these vesicles remain viable for several months [74]. Chlorine dioxide, unlike chlorine, can penetrate the biofilm and can also inactivate free-living amoebae which L. pneumophila inhabits. Therefore, it is concluded that chlorine dioxide can be used as a secondary disinfectant to reduce the risk of Legionnaires’ disease in hospital systems [19].
The use of phages in the treatment of biofilm infections is known in many bacterial pathogens [123], so the addition of specific phages can be used to control the growth of L. pneumophila. However, the phage can degrade polysaccharides and destabilize the biofilm [124].
The antimicrobial activity of silver has long been known, and silver is an increasingly frequent target of research to find new antimicrobial agents [125]. When it comes to a significant reduction in the volume of L. pneumophila biofilm, silver nanoparticles have shown outstanding results [126].
Natural compounds that have demonstrated antimicrobial efficacy on Legionella strains are antimicrobial peptides, biosurfactants, and essential oils [19]. Different filtration methods are possible, but as filters have a certain lifespan, this could significantly increase the cost of maintenance in the hospital system [127].
4.2. Antimicrobial Therapy
Legionella possesses the enzyme ß-lactamase. Therefore, beta-lactam antibiotics are ineffective in treating legionellosis [128]. For that reason, azithromycin and fluoroquinolones, including levofloxacin and moxifloxacin, are recommended for Legionella pneumonia in some guidelines [129,130]. In addition, treatment of legionellosis is long-term, from 7 to 14 days, while the symptoms themselves are not present for too long [131,132].
5. Conclusions
In dental offices, along with many other potential causes of infections for both staff and patients, there is a possibility of exposure to the bacterium Legionella pneumophila, which can cause severe pneumonia—Legionnaires’ disease. Vulnerable patients are the immunocompromised and elderly, and chronic disease patients such as those with chronic obstructive pulmonary disease, cardiovascular disease, and diabetes. Legionella infection could be fatal for patients on hemodialysis and with kidney transplants. Smoking and alcoholism are risk factors for Legionnaires’ disease. The most common reservoir of Legionella in dental practices are water tanks, and the route of spread is through contaminated aerosol generated during the use of dental instruments. Understanding the molecular mechanisms responsible for intra-amoebic related resistance is necessary, and would result in the development of new strategies for eradicating L. pneumophila. It is essential to know the breeding characteristics of L. pneumophila, as well as its virulence factors, and spread methods. Knowing the hygienic measures and that disinfectants can be used to prevent the spread of L. pneumophila is imperative. It is necessary to carry out water control, appropriate sampling in dental offices, and microbiological processing of samples.
Author Contributions
Conceptualization: J.T. and E.F.; writing the manuscript: J.T., E.F., M.J. (Martina Juzbašić) and M.T.; updating the text: J.T. and I.Š.; literature searches: J.T., E.F., M.J. (Martina Juzbašić), M.T., S.M., M.J. (Melita Jukić), M.S. and I.Š.; figure drawings: I.Š.; critical reviewing of the manuscript: J.T. and I.Š.; organization and editing of the manuscript: J.T. and I.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Brady, M.F.; Sundareshan, V. Legionnaires’ Disease. StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430807/ (accessed on 12 November 2021).
- Best, A.; Price, C.; Ozanic, M.; Santic, M.; Jones, S.; Kwaik, Y.A. A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae. Sci. Rep. 2018, 8, 6340. [Google Scholar] [CrossRef] [PubMed]
- Winn, W.C., Jr. Legionnaires disease: Historical perspective. Clin. Microbiol. Rev. 1988, 1, 60. [Google Scholar] [CrossRef]
- Chaabna, Z.; Forey, F.; Reyrolle, M.; Jarraud, S.; Atlan, D.; Fontvieille, D.; Gilbert, C. Molecular diversity and high virulence of Legionella pneumophila strains isolated from biofilms developed within a warm spring of a thermal spa. BMC Microbiol. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Cullom, A.C.; Martin, R.L.; Song, Y.; Williams, K.; Williams, A.; Pruden, A.; Edwards, M.A. Critical Review: Propensity of Premise Plumbing Pipe Materials to Enhance or Diminish Growth of Legionella and Other Opportunistic Pathogens. Pathogens 2020, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Keše, D.; Obreza, A.; Rojko, T.; Kišek, T.C. Legionella pneumophila-Epidemiology and Characterization of Clinical Isolates, Slovenia, 2006–2020. Diagnostics 2021, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Ditommaso, S.; Giacomuzzi, M.; Rivera, S.R.A.; Raso, R.; Ferrero, P.; Zotti, C.M. Virulence of Legionella pneumophila strains isolated from hospital water system and healthcare-associated Legionnaires’ disease in Northern Italy between 2004 and 2009. BMC Infect. Dis. 2014, 14, 483. [Google Scholar] [CrossRef]
- Furugen, M.; Koide, M.; Teruya, H.; Naha, Y.; Tamayose, M.; Akamine, M.; Uchihara, T.; Atsumi, E.; Haranaga, S.; Yara, S.; et al. Legionella pneumonia caused by Legionella pneumophila serogroup 2: Second case report in Japan. J. Infect. Chemother. 2008, 14, 161–165. [Google Scholar] [CrossRef]
- Kawanami, T.; Yatera, K.; Fukuda, K.; Yamasaki, K.; Kunimoto, M.; Nagata, S.; Nishida, C.; Ishimoto, H.; Ogawa, M.; Taniguchi, H.; et al. Diagnosis of fulminant pneumonia caused by Legionella pneumophila serogroup 8 with the sequence analysis of the 16S rRNA gene. Tohoku J. Exp. Med. 2011, 225, 65–69. [Google Scholar] [CrossRef][Green Version]
- Ito, A.; Ishida, T.; Tachibana, H.; Ito, Y.; Takaiwa, T.; Fujii, H.; Hashimoto, T.; Nakajima, H.; Amemura-Maekawa, J. A Case of Community-Acquired Pneumonia Due to Legionella pneumophila Serogroup 9 Wherein Initial Treatment with Single-Dose Oral Azithromycin Appeared Useful. Jpn. J. Infect. Dis. 2017, 70, 660–662. [Google Scholar] [CrossRef]
- Buse, H.Y.; Hoelle, J.M.; Muhlen, C.; Lytle, D.A. Electrophoretic mobility of Legionella pneumophila serogroups 1 to 14. FEMS Microbiol. Lett. 2018, 365, 67. [Google Scholar] [CrossRef] [PubMed]
- Burstein, D.; Amaro, F.; Zusman, T.; Lifshitz, Z.; Cohen, O.; Gilbert, J.A.; Pupko, T.; Shuman, H.A.; Segal, G. Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat. Genet. 2016, 48, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Valero, L.; Rusniok, C.; Carson, D.; Mondino, S.; Pérez-Cobas, A.E.; Rolando, M.; Pasricha, S.; Reuter, S.; Demirtas, J.; Crumbach, J.; et al. More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells. Proc. Natl. Acad. Sci. USA 2019, 116, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Mondino, S.; Schmidt, S.; Buchrieser, C. Molecular mimicry: A paradigm of host-microbe coevolution illustrated by Legionella. MBio 2020, 11, e01201-20. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, S.; Mohammadi, M.; Tabatabaiepour, S.; Tabatabaiepour, S.; Hosseini-Nave, H.; Soltani, M.; Alizadeh, H.; Hadizadeh, M. A Systematic In Silico Analysis of the Legionellaceae Family for Identification of Novel Drug Target Candidates. Microb. Drug Resist. 2019, 25, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rakić, A.; Štambuk-Giljanović, N. Physical and chemical parameter correlations with technical and technological characteristics of heating systems and the presence of Legionella spp. in the hot water supply. Environ. Monit. Assess. 2016, 188, 73. [Google Scholar] [CrossRef]
- Winn, W.J. Legionella. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; pp. 1611–1625. ISBN 9780123971692. [Google Scholar]
- Wadowsky, R.M.; Wolford, R.; McNamara, A.M.; Yee, R.B. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 1985, 49, 1197–1205. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Laganà, P.; Filice, S.; Scalese, S.; Libertino, S.; Corso, D.; Faro, G.; Coniglio, M.A. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms 2021, 9, 577. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease in Europe; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2016.
- Lesnik, R.; Brettar, I.; Höfle, M.G. Legionella species diversity and dynamics from surface reservoir to tap water: From cold adaptation to thermophily. ISME J. 2016, 10, 1064–1080. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Chmiel, E.; Palusinska-Szysz, M. The Role of Lipids in Legionella-Host Interaction. Int. J. Mol. Sci. 2021, 22, 1487. [Google Scholar] [CrossRef]
- Manske, C.; Hilbi, H. Metabolism of the vacuolar pathogen Legionella and implications for virulence. Front. Cell. Infect. Microbiol. 2014, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Legionella pneumophila and Protozoan Hosts: Implications for the Control of Hospital and Potable Water Systems. Pathogens 2020, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Silva, A.R.; Melo, L.F. Legionella and Biofilms—Integrated Surveillance to Bridge Science and Real-Field Demands. Microorganisms 2021, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Schwake, D.O.; Marr, L.C. Ten questions concerning the aerosolization and transmission of Legionella in the built environment. Build. Environ. 2017, 123, 684–695. [Google Scholar] [CrossRef]
- Martin, R.L.; Strom, O.R.; Pruden, A.; Edwards, M.A. Interactive Effects of Copper Pipe, Stagnation, Corrosion Control, and Disinfectant Residual Influenced Reduction of Legionella pneumophila during Simulations of the Flint Water Crisis. Pathogens 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Saoud, J.; Mani, T.; Faucher, S.P. The Tail-Specific Protease Is Important for Legionella pneumophila To Survive Thermal Stress in Water and inside Amoebae. Appl. Environ. Microbiol. 2021, 87, e02975-20. [Google Scholar] [CrossRef]
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The stronghold of Legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Valero, L.; Buchrieser, C. Intracellular parasitism, the driving force of evolution of Legionella pneumophila and the genus Legionella. Genes Immun. 2019, 20, 394–402. [Google Scholar] [CrossRef]
- Chambers, S.T.; Slow, S.; Scott-thomas, A.; Murdoch, D.R. Legionellosis caused by non-legionella pneumophila species, with a focus on legionella longbeachae. Microorganisms 2021, 9, 291. [Google Scholar] [CrossRef]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Wang, C.; Chuai, X.; Liang, M. Legionella feeleii: Pneumonia or Pontiac fever? Bacterial virulence traits and host immune response. Med. Microbiol. Immunol. 2019, 208, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Takahashi, K.; Crump, A. Legionellosis in Japan: A Self-inflicted Wound? Intern. Med. 2021, 60, 173. [Google Scholar] [CrossRef] [PubMed]
- Alarcon Falconi, T.M.; Cruz, M.S.; Naumova, E.N. The Shift in Seasonality of Legionellosis in the U.S. Epidemiol. Infect. 2018, 146, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.K.; Vail, A.; Jeans, A.R.; Chamorro, A.; Di Napoli, M.; Kalra, L.; Langhorne, P.; Roffe, C.; Westendorp, W.; Nederkoorn, P.J.; et al. Microbiological Etiologies of Pneumonia Complicating Stroke: A Systematic Review. Stroke 2018, 49, 1602–1609. [Google Scholar] [CrossRef]
- Graham, F.F. The mysterious illness that drove them to their knees—Ah, that Legionnaires’ disease—A historical reflection of the work in Legionnaires’ disease in New Zealand (1978 to mid-1990s) and the ‘One Health’ paradigm. One Health 2020, 10, 100149. [Google Scholar] [CrossRef]
- Mcdade, J.E.; Shepard, C.C.; Fraser, D.W.; Tsai, T.R.; Redus, M.A.; Dowdle, W.R. Legionnaires’ disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 1977, 297, 1197–1203. [Google Scholar] [CrossRef]
- Mercante, J.W.; Morrison, S.S.; Raphael, B.H.; Winchell, J.M. Complete Genome Sequences of the Historical Legionella pneumophila Strains OLDA and Pontiac. Genome Announc. 2016, 4, e00866-16. [Google Scholar] [CrossRef]
- Terranova, W.; Cohen, M.L.; Fraser, D.W. 1974 outbreak of Legionnaires’ Disease diagnosed in 1977. Clinical and epidemiological features. Lancet 1978, 2, 122–124. [Google Scholar] [CrossRef]
- Glick, T.H.; Gregg, M.B.; Berman, B.; Mallison, G.; Rhodes, W.W.; Kassanoff, I. Pontiac fever. An epidemic of unknown etiology in a health department: I. Clinical and epidemiologic aspects. Am. J. Epidemiol. 1978, 107, 149–160. [Google Scholar] [CrossRef]
- McDade, J.E.; Brenner, D.J.; Bozeman, F.M. Legionnaires’ disease bacterium isolated in 1947. Ann. Intern. Med. 1979, 90, 659–661. [Google Scholar] [CrossRef]
- Hamilton, K.A.; Prussin, A.J.; Ahmed, W.; Haas, C.N. Outbreaks of Legionnaires’ Disease and Pontiac Fever 2006–2017. Curr. Environ. Health Rep. 2018, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Principe, L.; Tomao, P.; Visca, P. Legionellosis in the occupational setting. Environ. Res. 2017, 152, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Misch, E.A. Legionella: Virulence factors and host response. Curr. Opin. Infect. Dis. 2016, 29, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Appelt, S.; Heuner, K. The flagellar regulon of Legionella—A review. Front. Cell. Infect. Microbiol. 2017, 7, 454. [Google Scholar] [CrossRef]
- Arslan-Aydoğdu, E.Ö.; Kimiran, A. An investigation of virulence factors of Legionella pneumophila environmental isolates. Braz. J. Microbiol. 2018, 49, 189–199. [Google Scholar] [CrossRef]
- Rehman, S.; Grigoryeva, L.S.; Richardson, K.H.; Corsini, P.; White, R.C.; Shaw, R.; Portlock, T.J.; Dorgan, B.; Zanjani, Z.S.; Fornili, A.; et al. Structure and functional analysis of the Legionella pneumophila chitinase ChiA reveals a novel mechanism of metal-dependent mucin degradation. PLoS Pathog. 2020, 16, e1008342. [Google Scholar] [CrossRef]
- Rodgers, F. The role of structure and invasiveness on the pathogenicity of Legionella. Zent. Bakteriol. Mikrobiol. Hyg. A 1983, 255, 138–144. [Google Scholar] [CrossRef]
- Newton, H.J.; Ang, D.K.Y.; Van Driel, I.R.; Hartland, E.L. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 2010, 23, 274–298. [Google Scholar] [CrossRef]
- Cianciotto, N.P. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 2001, 291, 331–343. [Google Scholar] [CrossRef]
- Shevchuk, O.; Jäger, J.; Steinert, M. Virulence properties of the Legionella pneumophila cell envelope. Front. Microbiol. 2011, 2, 74. [Google Scholar] [CrossRef]
- Garcia, L.S.; Arrowood, M.; Kokoskin, E.; Paltridge, G.P.; Pillai, D.R.; Procop, G.W.; Ryan, N.; Shimizu, R.Y.; Visvesvara, G. Laboratory diagnosis of parasites from the gastrointestinal tract. Clin. Microbiol. Rev. 2018, 31, e00025-17. [Google Scholar] [CrossRef] [PubMed]
- Jäger, J.; Marwitz, S.; Tiefenau, J.; Rasch, J.; Shevchuk, O.; Kugler, C.; Goldmann, T.; Steinert, M. Human Lung Tissue Explants Reveal Novel Interactions during Legionella pneumophila Infections. Infect. Immun. 2014, 82, 275. [Google Scholar] [CrossRef] [PubMed]
- Van Kenhove, E.; Dinne, K.; Janssens, A.; Laverge, J. Overview and comparison of Legionella regulations worldwide. Am. J. Infect. Control 2019, 47, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Haroon, A.; Koide, M.; Higa, F.; Tateyama, M.; Fujita, J. Identification of Legionella pneumophila serogroups and other Legionella species by mip gene sequencing. J. Infect. Chemother. 2012, 18, 276–281. [Google Scholar] [CrossRef]
- Chauhan, D.; Shames, S.R. Pathogenicity and Virulence of Legionella: Intracellular replication and host response. Virulence 2021, 12, 1122–1144. [Google Scholar] [CrossRef]
- Hughes, E.D.; Swanson, M.S. How Legionella defend their turf. eLife 2019, 8, e48695. [Google Scholar] [CrossRef]
- Qin, T.; Zhou, H.; Ren, H.; Liu, W. Distribution of secretion systems in the genus Legionella and its correlation with pathogenicity. Front. Microbiol. 2017, 8, 388. [Google Scholar] [CrossRef][Green Version]
- Tyson, J.Y.; Vargas, P.; Cianciotto, N.P. The novel Legionella pneumophila type II secretion substrate NttC contributes to infection of amoebae Hartmannella vermiformis and Willaertia magna. Microbiology 2014, 160, 2732. [Google Scholar] [CrossRef]
- Portlock, T.J.; Tyson, J.Y.; Dantu, S.C.; Rehman, S.; White, R.C.; McIntire, I.E.; Sewell, L.; Richardson, K.; Shaw, R.; Pandini, A.; et al. Structure, Dynamics and Cellular Insight Into Novel Substrates of the Legionella pneumophila Type II Secretion System. Front. Mol. Biosci. 2020, 7, 112. [Google Scholar] [CrossRef]
- Nakano, N.; Kubori, T.; Kinoshita, M.; Imada, K.; Nagai, H. Crystal Structure of Legionella DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems. PLoS Pathog. 2010, 6, e1001129. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.; Du, D.; Li, S.; Yan, W. Advances in the Assembly Model of Bacterial Type IVB Secretion Systems. Appl. Sci. 2018, 8, 2368. [Google Scholar] [CrossRef]
- Liu, X.; Shin, S. Viewing Legionella pneumophila Pathogenesis through an Immunological Lens. J. Mol. Biol. 2019, 431, 4321–4344. [Google Scholar] [CrossRef] [PubMed]
- Lammertyn, E.; Anné, J. Protein secretion in Legionella pneumophila and its relation to virulence. FEMS Microbiol. Lett. 2004, 238, 273–279. [Google Scholar] [CrossRef]
- Bitar, D.M.; Molmeret, M.; Abu Kwaik, Y. Molecular and cell biology of Legionella pneumophila. Int. J. Med. Microbiol. 2004, 293, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Liu, S.; Gabbai, C.B.; Venitelli, Z.; Steinman, H.M. Environmental Mimics and the Lvh Type IVA Secretion System Contribute to Virulence-Related Phenotypes of Legionella pneumophila. Infect. Immun. 2007, 75, 723. [Google Scholar] [CrossRef]
- Lorquin, F.; Ziarelli, F.; Amouric, A.; Di Giorgio, C.; Robin, M.; Piccerelle, P.; Lorquin, J. Production and properties of non-cytotoxic pyomelanin by laccase and comparison to bacterial and synthetic pigments. Sci. Rep. 2021, 11, 8538. [Google Scholar] [CrossRef]
- Steinert, M.; Flugel, M.; Schuppler, M.; Helbig, J.H.; Supriyono, A.; Proksch, P.; Luck, P.C. The Lly protein is essential for p-hydroxyphenylpyruvate dioxygenase activity in Legionella pneumophila. FEMS Microbiol. Lett. 2001, 203, 41–47. [Google Scholar] [CrossRef]
- Brigmon, R.L.; Turick, C.E.; Knox, A.S.; Burckhalter, C.E. The Impact of Storms on Legionella pneumophila in Cooling Tower Water, Implications for Human Health. Front. Microbiol. 2020, 11, 2979. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 16. [Google Scholar] [CrossRef]
- Patini, R.; Mangino, G.; Martellacci, L.; Quaranta, G.; Masucci, L.; Gallenzi, P. The Effect of Different Antibiotic Regimens on Bacterial Resistance: A Systematic Review. Antibiotics 2020, 9, 22. [Google Scholar] [CrossRef]
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Abu Khweek, A.; Amer, A.O. Factors Mediating Environmental Biofilm Formation by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Cont, A.; Rossy, T.; Al-Mayyah, Z.; Persat, A. Biofilms deform soft surfaces and disrupt epithelia. eLife 2020, 9, e56533. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Mader, J.T.; Camper, A.K. Molecular interactions in biofilms. Chem. Biol. 2002, 9, 859–871. [Google Scholar] [CrossRef]
- Mampel, J.; Spirig, T.; Weber, S.S.; Haagensen, J.A.J.; Molin, S.; Hilbi, H. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 2006, 72, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef]
- Stewart, C.R.; Muthye, V.; Cianciotto, N.P. Legionella pneumophila Persists within Biofilms Formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under Dynamic Flow Conditions. PLoS ONE 2012, 7, e50560. [Google Scholar] [CrossRef]
- Cebrián, F.; Montero, J.C.; Fernández, P.J. New approach to environmental investigation of an explosive legionnaireś disease outbreak in Spain: Early identification of potential risk sources by rapid Legionella spp. immunosensing technique. BMC Infect. Dis. 2018, 18, 696. [Google Scholar] [CrossRef]
- Declerck, P. Biofilms: The environmental playground of Legionella pneumophila. Environ. Microbiol. 2010, 12, 557–566. [Google Scholar] [CrossRef]
- De Buck, E.; Maes, L.; Meyen, E.; Van Mellaert, L.; Geukens, N.; Anné, J.; Lammertyn, E. Legionella pneumophila Philadelphia-1 tatB and tatC affect intracellular replication and biofilm formation. Biochem. Biophys. Res. Commun. 2005, 331, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Di Cesare, A.; Sabatini, L.; Chessa, E.; Sisti, D.; Rocchi, M.; Citterio, B. Role of biofilm in protection of the replicative form of Legionella pneumophila. Curr. Microbiol. 2014, 69, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Tiaden, A.; Spirig, T.; Sahr, T.; Wälti, M.A.; Boucke, K.; Buchrieser, C.; Hilbi, H. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ. Microbiol. 2010, 12, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Sousa, P.; Gaspar, A.; Vilar, S.; Borges, F.; Simões, M. Furvina inhibits the 3-oxo-C12-HSL-based quorum sensing system of Pseudomonas aeruginosa and QS-dependent phenotypes. Biofouling 2017, 33, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Seesuay, W.; Mahasongkram, K.; Sookrung, N.; Pumirat, P.; Ampawong, S.; Reamtong, O.; Chongsa-Nguan, M.; Chaicumpa, W.; Indrawattana, N. Human Single-chain Variable Fragments Neutralize Pseudomonas aeruginosa Quorum Sensing Molecule, 3O-C12-HSL, and Prevent Cells From the HSL-mediated Apoptosis. Front. Microbiol. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Boamah, D.K.; Zhou, G.; Ensminger, A.W.; O’Connor, T.J. From Many Hosts, One Accidental Pathogen: The Diverse Protozoan Hosts of Legionella. Front. Cell. Infect. Microbiol. 2017, 7, 477. [Google Scholar] [CrossRef]
- Valster, R.M.; Wullings, B.A.; Van Der Kooij, D. Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl. Environ. Microbiol. 2010, 76, 7144–7153. [Google Scholar] [CrossRef]
- Dobrowsky, P.H.; Khan, S.; Cloete, T.E.; Khan, W. Molecular detection of Acanthamoeba spp., Naegleria fowleri and Vermamoeba (Hartmannella) vermiformis as vectors for Legionella spp. in untreated and solar pasteurized harvested rainwater. Parasit. Vectors 2016, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.M.; Von Dwingelo, J.E.; Price, C.T.; Kwaik, Y.A. Cellular microbiology and molecular ecology of Legionella-amoeba interaction. Virulence 2013, 4, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mraz, A.L.; Weir, M.H. Knowledge to Predict Pathogens: Legionella pneumophila Lifecycle Critical Review Part I Uptake into Host Cells. Water 2018, 10, 132. [Google Scholar] [CrossRef]
- Alli, O.A.T.; Gao, L.Y.; Pedersen, L.L.; Zink, S.; Radulic, M.; Doric, M.; Abu Kwaik, Y. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 2000, 68, 6431–6440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliva, G.; Sahr, T.; Buchrieser, C. The Life Cycle of L. pneumophila: Cellular Differentiation Is Linked to Virulence and Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 3. [Google Scholar] [CrossRef]
- Price, C.T.D.; Kwaik, Y.A. Evolution and Adaptation of Legionella pneumophila to Manipulate the Ubiquitination Machinery of Its Amoebae and Mammalian Hosts. Biomolecules 2021, 11, 112. [Google Scholar] [CrossRef]
- Lee, P.C.; Machner, M.P. The Legionella Effector Kinase LegK7 Hijacks the Host Hippo Pathway to Promote Infection. Cell Host Microbe 2018, 24, 429–438.e6. [Google Scholar] [CrossRef]
- Jeng, E.E.; Bhadkamkar, V.; Ibe, N.U.; Gause, H.; Jiang, L.; Chan, J.; Jian, R.; Jimenez-Morales, D.; Stevenson, E.; Krogan, N.J.; et al. Systematic Identification of Host Cell Regulators of Legionella pneumophila Pathogenesis Using a Genome-wide CRISPR Screen. Cell Host Microbe 2019, 26, 551–563.e6. [Google Scholar] [CrossRef]
- Hanford, H.E.; von Dwingelo, J.; Kwaik, Y.A. Bacterial nucleomodulins: A coevolutionary adaptation to the eukaryotic command center. PLoS Pathog. 2021, 17, e1009184. [Google Scholar] [CrossRef]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016972. [Google Scholar] [CrossRef]
- Xu, L.; Luo, L.Z. Cell biology of infection by Legionella pneumophila. Microbes Infect. 2013, 15, 157–167. [Google Scholar] [CrossRef]
- Krause, K.; Amer, A.O. Caspase Exploitation by Legionella pneumophila. Front. Microbiol. 2016, 7, 515. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Vitali, M. Occupational risk for Legionella infection among dental healthcare workers: Meta-analysis in occupational epidemiology. BMJ Open 2017, 7, e015374. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C. Legionella: What is the risk? BDJ Team 2014, 1, 18. [Google Scholar] [CrossRef][Green Version]
- Schönning, C.; Jernberg, C.; Klingenberg, D.; Andersson, S.; Pääjärvi, A.; Alm, E.; Tano, E.; Lytsy, B. Legionellosis acquired through a dental unit: A case study. J. Hosp. Infect. 2017, 96, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kevorkyan, A.; Tomova, I.; Raycheva, R.; Stoeva, V.; Stoilova, Y.; Lalabonova, H.; Kondeva, V. Legionella pneumophila antibodies in serum samples from medical and dental personnel: A seroepidemiological survey. Biotechnol. Biotechnol. Equip. 2017, 31, 588–593. [Google Scholar] [CrossRef][Green Version]
- Sedlata Juraskova, E.; Sedlackova, H.; Janska, J.; Holy, O.; Lalova, I.; Matouskova, I. Legionella spp. in dental unit waterlines. Bratisl. Lek. Listy 2017, 118, 310–314. [Google Scholar] [CrossRef]
- Uzel, A.; Cogulu, D.; Oncag, O. Microbiological evaluation and antibiotic susceptibility of dental unit water systems in general dental practice. Int. J. Dent. Hyg. 2008, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.M.; Sartini, M.; Di Cave, D.; Casini, B.; Tuvo, B.; Cristina, M.L. Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines. Int. J. Environ. Res. Public Health 2019, 16, 2648. [Google Scholar] [CrossRef]
- Pankhurst, C.L.; Coulter, W.A. Do contaminated dental unit waterlines pose a risk of infection? J. Dent. 2007, 35, 712–720. [Google Scholar] [CrossRef]
- Tuvo, B.; Totaro, M.; Cristina, M.L.; Spagnolo, A.M.; Di Cave, D.; Profeti, S.; Baggiani, A.; Privitera, G.; Casini, B. Prevention and Control of Legionella and Pseudomonas spp. Colonization in Dental Units. Pathogens 2020, 9, 305. [Google Scholar] [CrossRef]
- Dental unit waterlines: Approaching the year 2000. ADA Council on Scientific Affairs. J. Am. Dent. Assoc. 1999, 130, 1653–1664.
- Kohn, W.; Collins, A.; Cleveland, J.; Harte, J.; Eklund, K.; Malvitz, D. Centers for Disease Control and Prevention (CDC) Guidelines for infection control in dental health-care settings—2003. MMWR Recomm. Rep. 2003, 52, 1–61. [Google Scholar] [PubMed]
- Tesauro, M.; Petrelli, F.; Lizioli, A.; Pregliasco, F.; Masia, C.; Cossellu, G.; Farronato, G.; Consonni, M.; Sisto, F. Presence of Legionella spp. in human dental plaque. Ann. Ig. 2018, 30, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.A.; Kuppravalli, A.; Heida, A.; Joshi, S.; Haas, C.N.; Verhougstraete, M.; Gerrity, D. Legionnaires’ disease in dental offices: Quantifying aerosol risks to dental workers and patients. J. Occup. Environ. Hyg. 2021, 18, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Nardone, M.; Gaudio, R.M.; Candotto, V.; Carinci, F. Risk assessment of colonization of Legionella spp. in dental unit waterlines. Oral Implantol. 2017, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Surman-Lee, S.; Chalker, V.; Crespi, S.; de Jong, B.; Kusnetsov, J.; Lee, J.V.; Ricci, M.L.; van der Lugt, W.; Moran-Gilad, J.; Walker, J.T.; et al. ESGLI Guidance for Managing Legionella in Dental Practises during the COVID-19 Pandemic. 2020, 5. Available online: https://www.escmid.org/fileadmin/src/media/PDFs/3Research_Projects/ESGLI/ESGLI_GUIDANCE_FOR_MANAGING_LEGIONELLA_IN_DENTAL_WATER_SYSTEMS_DURING_THE_COVID-19_PANDEMIC_22042024_v01.01.pdf (accessed on 12 December 2021).
- Verhasselt, H.L.; Buer, J.; Dedy, J.; Ziegler, R.; Steinmann, J.; Herbstreit, F.; Brenner, T.; Rath, P.M. COVID-19 Co-infection with Legionella pneumophila in 2 Tertiary-Care Hospitals, Germany. Emerg. Infect. Dis. 2021, 27, 1535. [Google Scholar] [CrossRef]
- Berjeaud, J.M.; Chevalier, S.; Schlusselhuber, M.; Portier, E.; Loiseau, C.; Aucher, W.; Lesouhaitier, O.; Verdon, J. Legionella pneumophila: The Paradox of a Highly Sensitive Opportunistic Waterborne Pathogen Able to Persist in the Environment. Front. Microbiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Orsi, G.B.; Vitali, M.; Marinelli, L.; Ciorba, V.; Tufi, D.; Del Cimmuto, A.; Ursillo, P.; Fabiani, M.; De Santis, S.; Protano, C.; et al. Legionella control in the water system of antiquated hospital buildings by shock and continuous hyperchlorination: 5 years experience. BMC Infect. Dis. 2014, 14, 394. [Google Scholar] [CrossRef]
- Di Pippo, F.; Di Gregorio, L.; Congestri, R.; Tandoi, V.; Rossetti, S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol. Ecol. 2018, 94, fiy044. [Google Scholar] [CrossRef]
- Talapko, J.; Škrlec, I.; Alebić, T.; Bekić, S.; Včev, A. From Bacteriophage to Antibiotics and Back. Coll. Antropol. 2018, 42, 131–138. [Google Scholar]
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Matijević, T.; Juzbašić, M.; Antolović-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Janczak, K.; Kosmalska, D.; Kaczor, D.; Raszkowska-kaczor, A.; Wedderburn, L.; Malinowski, R. Bactericidal and Fungistatic Properties of LDPE Modified with a Biocide Containing Metal Nanoparticles. Materials 2021, 14, 4228. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Management of Legionella in Water Systems; National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Fu, K.P.; Neu, H.C. Inactivation of beta-lactam antibiotics by Legionella pneumophila. Antimicrob. Agents Chemother. 1979, 16, 561–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44, S27. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45. [Google Scholar] [CrossRef]
- Viasus, D.; Di Yacovo, S.; Garcia-Vidal, C.; Verdaguer, R.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Community-Acquired Legionella pneumophila Pneumonia: A Single-Center Experience With 214 Hospitalized Sporadic Cases Over 15 Years. Medicine 2013, 92, 51–60. [Google Scholar] [CrossRef]
- Velazco, J.F. Legionnaires’ Disease Treatment. In Hospital Acquired Infection and Legionnaires’ Disease; Surani, S., Ed.; IntechOpen: London, UK, 2020; pp. 1–10. ISBN 978-1-78985-970-6. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).