Contrasting Community Composition and Co-Occurrence Relationships of the Active Pico-Sized Haptophytes in the Surface and Subsurface Chlorophyll Maximum Layers of the Arctic Ocean in Summer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Measurement of Environmental Parameters
2.2. RNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.3. Data Analysis
2.4. The Co-Occurrence Network Analyses
3. Results
3.1. Environmental Parameters
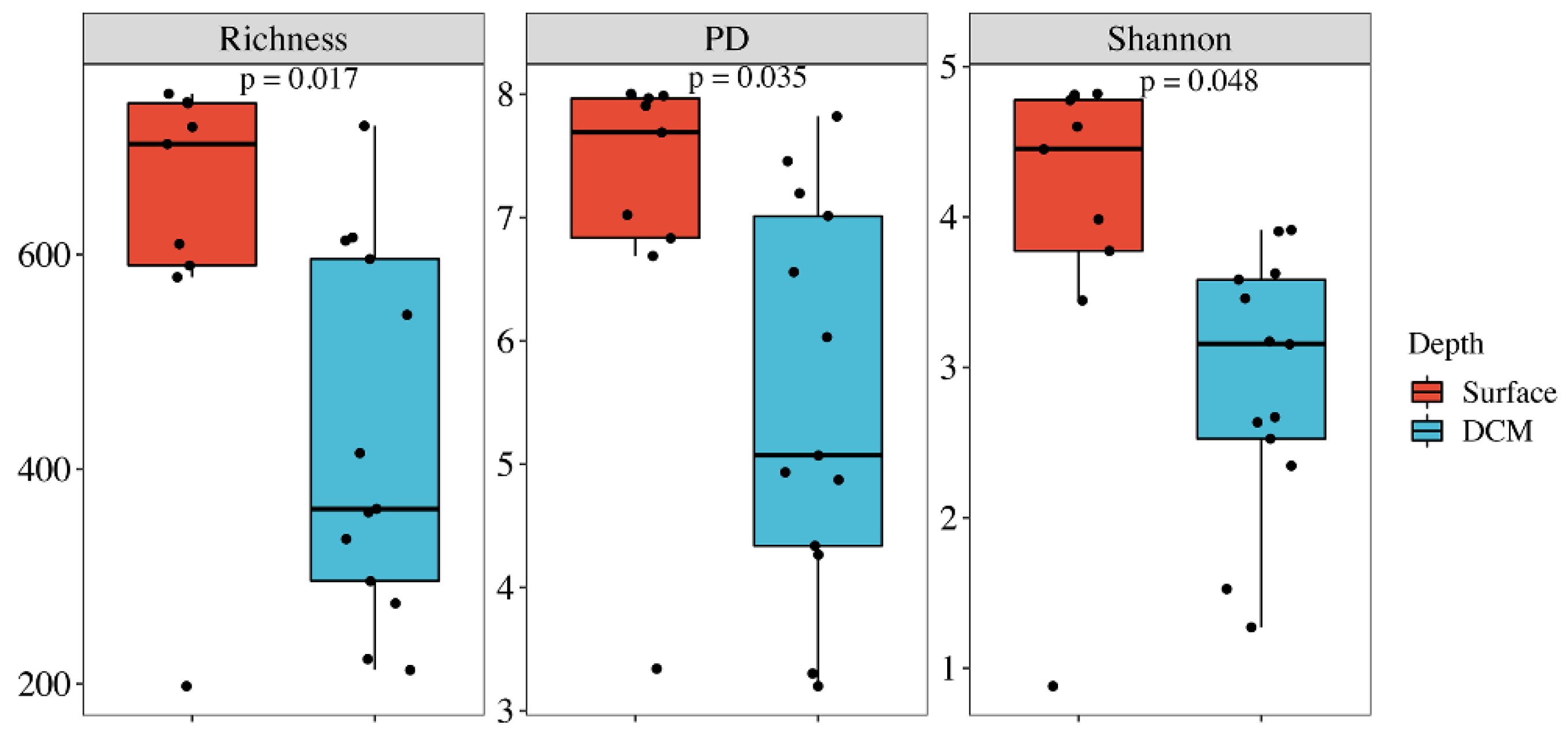
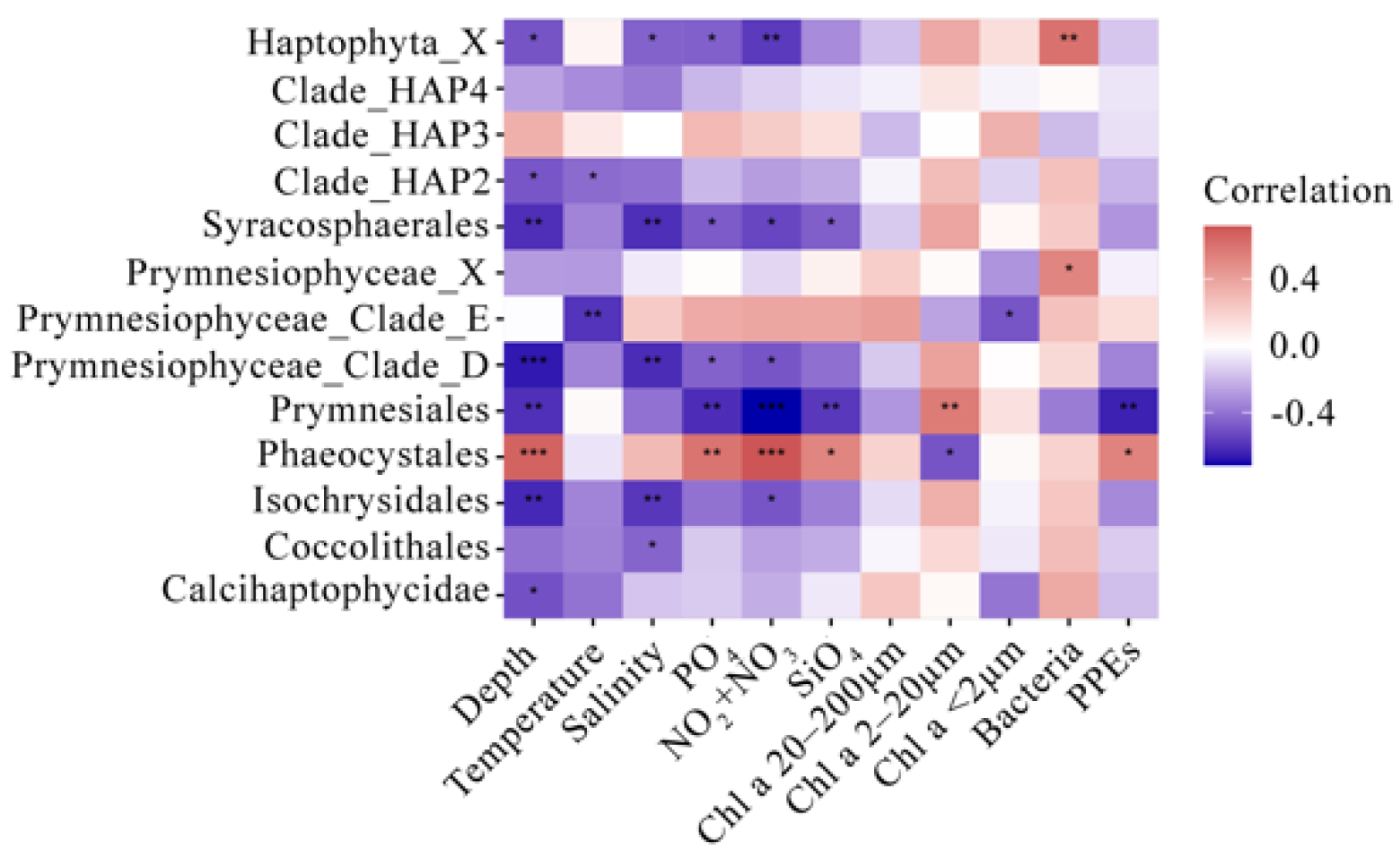
3.2. Alpha Diversity and Correlations with Environmental Parameters
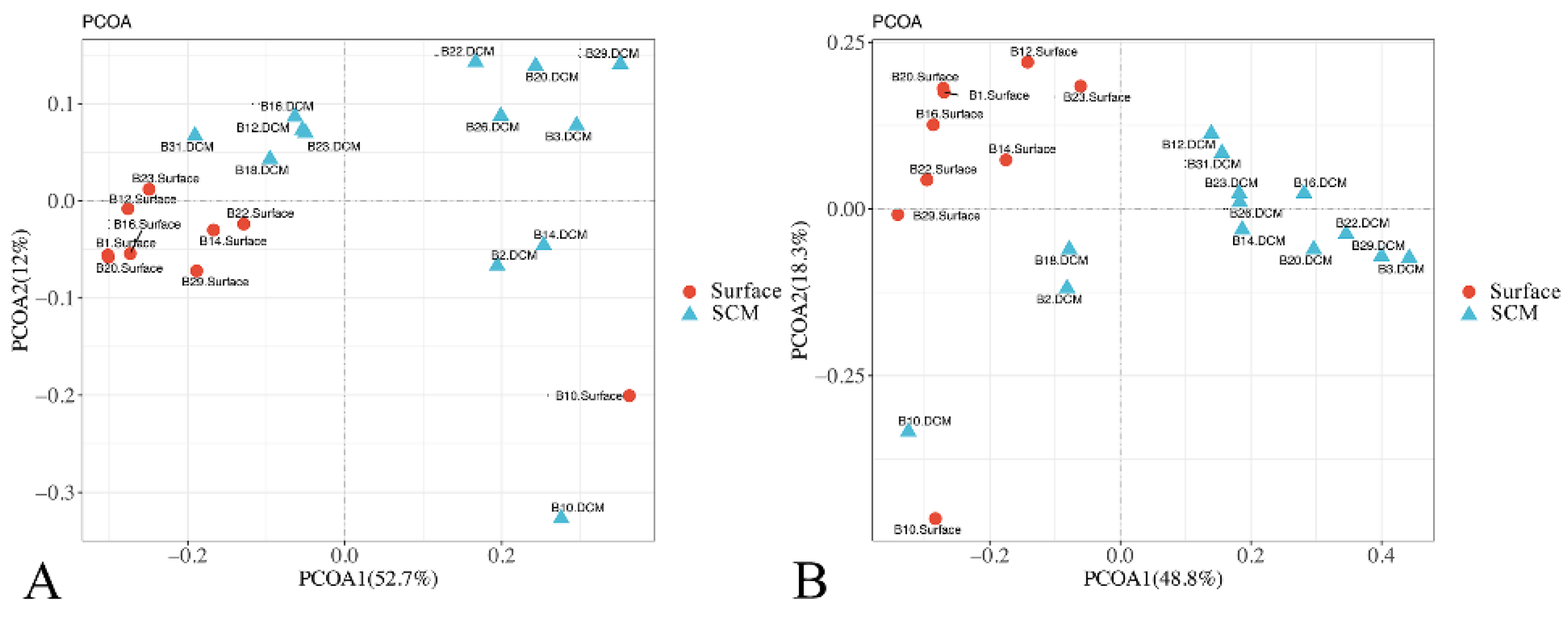
3.3. Beta Diversity and Its Driving Factors
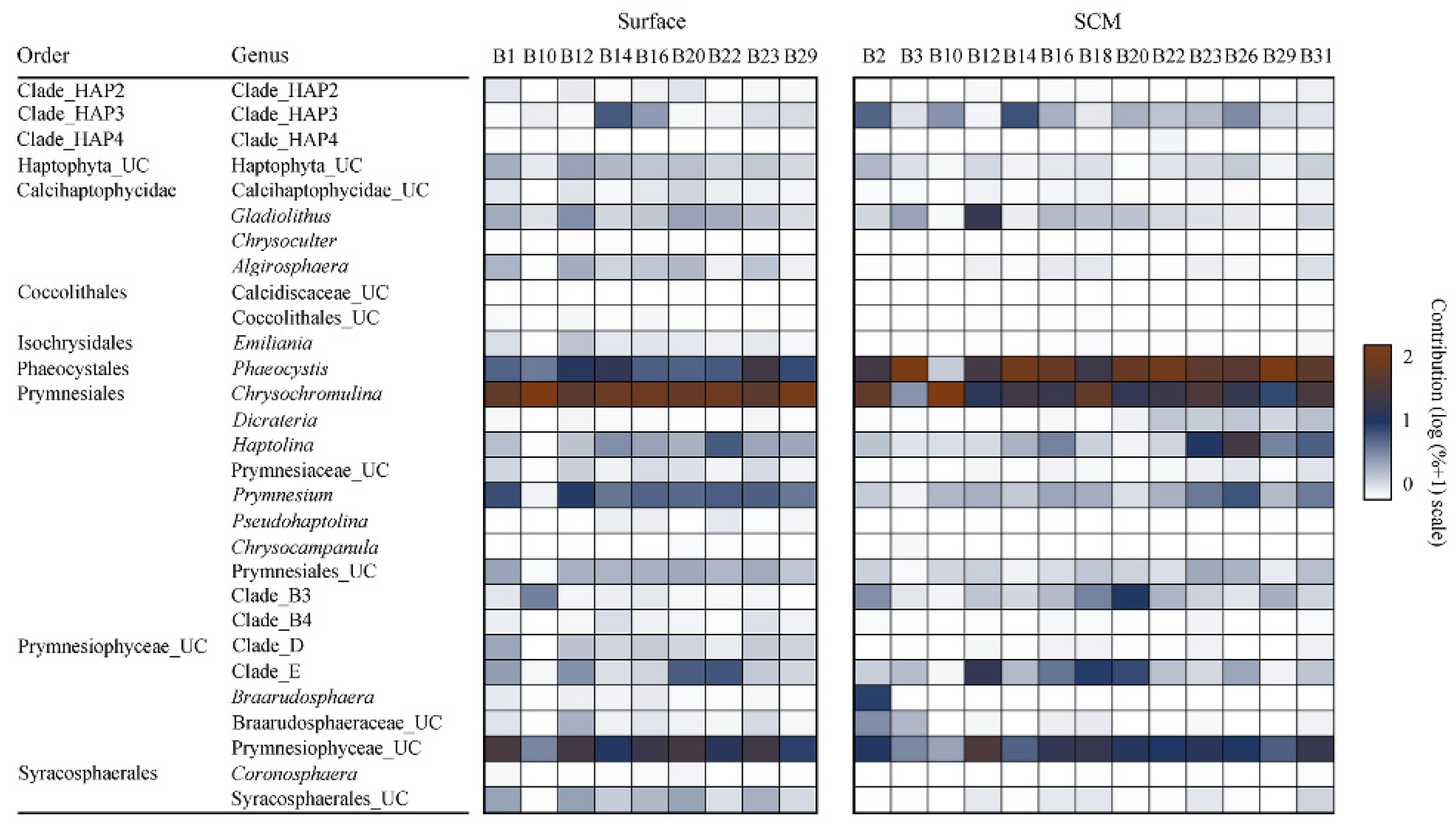
3.4. Community Composition
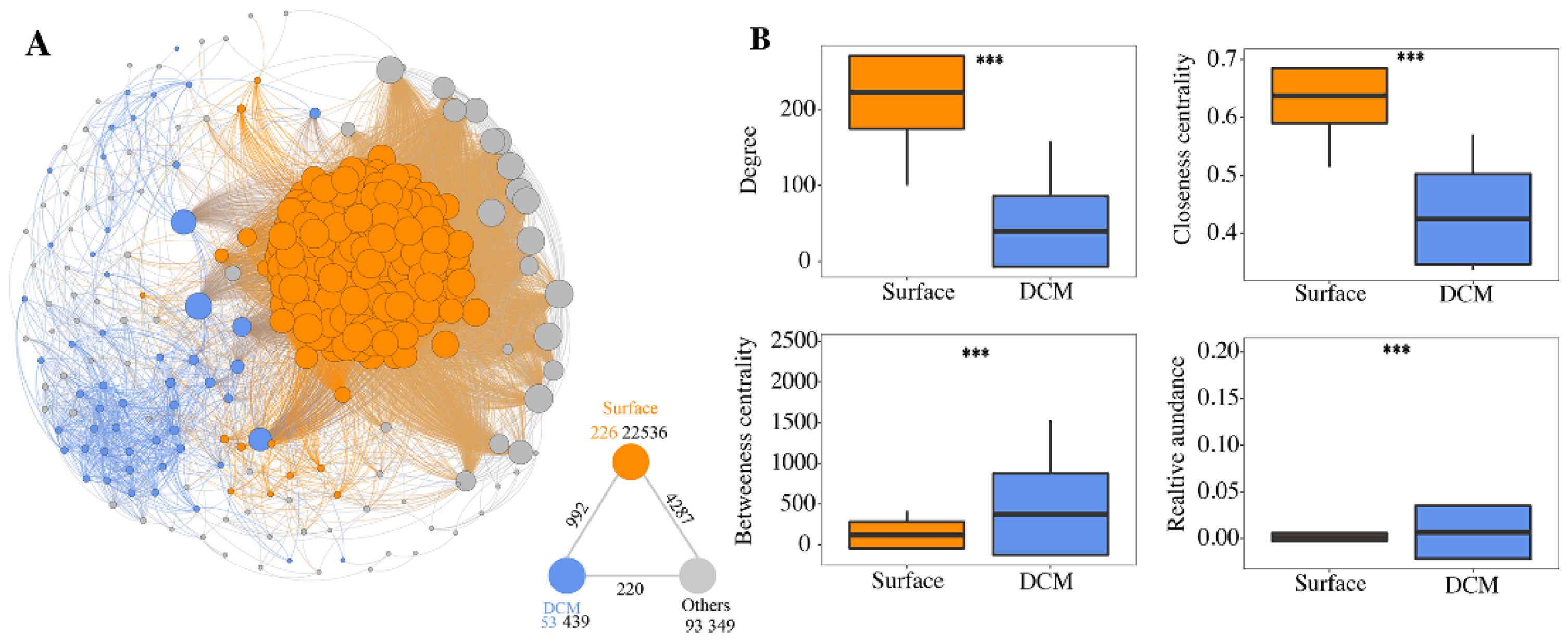
3.5. Co-Occurrence Networks
4. Discussion
4.1. Beta Diversity, Taxonomic Composition, and Environmental Driving Factors
4.2. Co-Occurrence Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Probert, I.; Uitz, J.; Claustre, H.; Aris-Brosou, S.; Frada, M.; Not, F.; de Vargas, C. Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. Proc. Natl. Acad. Sci. USA 2009, 106, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Jardillier, L.; Zubkov, M.V.; Pearman, J.; Scanlan, D.J. Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J. 2010, 4, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Frias-Lopez, J.; Thompson, A.; Waldbaurer, J.; Chisholm, S.W. Use of stable isotope-labelled cells to identify active grazers of picocyanobacgeria in ocean surface waters. Environ. Microbiol. 2009, 11, 512–525. [Google Scholar] [CrossRef]
- Unrein, F.; Gasol, J.M.; Not, F.; Forn, I.; Massana, R. Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters. ISME J. 2014, 8, 164–176. [Google Scholar] [CrossRef]
- Chan, Y.F.; Chiang, K.P.; Ku, Y.; Gong, G.C. Abiotic and Biotic Factors Affecting the Ingestion Rates of Mixotrophic Nanoflagellates (Haptophyta). Microb. Ecol. 2019, 77, 607–615. [Google Scholar] [CrossRef]
- Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; et al. Phytoplankton calcification in a high-CO2 world. Science 2008, 320, 336–340. [Google Scholar] [CrossRef]
- Aanesen, R.T.; Eilertsen, H.C.; Stabell, O.B. Light-induced toxic properties of the marine alga Phaeocystis pouchetii towards cod larvae. Aquat. Toxicol. 1998, 40, 109–121. [Google Scholar] [CrossRef]
- Hansen, E.; Eilertsen, H.C.; Ernstsen, A.; Genevière, A. Anti-mitotic activity towards sea urchin embryos in extracts from the marine haptophycean Phaeocystis pouchetii (Hariot) Lagerheim collected along the coast of northern Norway. Toxicon 2003, 41, 803–812. [Google Scholar] [CrossRef]
- Oduro, H.; Van Alstyne, K.L.; Farquhar, J. Sulfur isotope variability of oceanic DMSP generation and its contributions to marine biogenic sulfur emissions. Proc. Natl. Acad. Sci. USA 2012, 109, 9012–9016. [Google Scholar] [CrossRef]
- Cabello, A.M.; Cornejo-Castillo, F.M.; Raho, N.; Blasco, D.; Vidal, M.; Audic, S.; de Vargas, C.; Latasa, M.; Acinas, S.G.; Massana, R. Global distribution and vertical patterns of a prymnesiophyte-cyanobacteria obligate symbiosis. ISME J. 2015, 10, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Zehr, J.P.; Shilova, I.N.; Farnelid, H.M.; Muñoz-Marín, M.D.; Turk-Kubo, K.A. Unusual marine unicellular symbiosis with the nitrogen-fixing cyanobacterium UCYN-A. Nat. Microbiol. 2016, 2, 16214. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Turk-Kubo, K.A.; Sipler, R.E.; Mills, M.M.; Bronk, D.A.; Zehr, J.P. Symbiotic unicellular cyanobacteria fix nitrogen in the Arctic Ocean. Proc. Natl. Acad. Sci. USA 2018, 115, 13371–13375. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, Y.; Jiao, N.; Tian, J.; Kao, S.; Zhang, Y. Biogeographic drivers of diazotrophs in the western Pacific Ocean. Limnol. Oceanogr. 2019, 9999, 1–19. [Google Scholar] [CrossRef]
- Edvardsen, B.; Egge, E.S.; Vaulot, D. Diversity and distribution of haptophytes revealed by environmental sequencing and metabarcoding—A review. Perspect Phycol. 2016, 3, 77–91. [Google Scholar] [CrossRef]
- Vaulot, D.; Eikrem, W.; Viprey, M.; Moreau, H. The diversity of small eukaryotic phytoplankton (≤3 um) in marine ecosystems. FEMS Microbiol. Rev. 2008, 32, 795–820. [Google Scholar] [CrossRef]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef]
- Not, F.; Latasa, M.; Scharek, R.; Viprey, M.; Karleskind, P.; Balagué, V.; Ontoria-Oviedo, I.; Cumino, A.; Goetze, E.; Vaulot, D.; et al. Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep Sea Res. 2008, 55, 1456–1573. [Google Scholar] [CrossRef][Green Version]
- Xu, D.; Sun, P.; Zhang, Y.; Li, R.; Huang, B.; Jiao, N.; Warren, A.; Wang, L. Pigmented microbial eukaryotes fuel the deep sea carbon pool in the tropical Western Pacific Ocean. Environ. Microbiol. 2018, 20, 3811–3824. [Google Scholar] [CrossRef]
- Bittner, L.; Gobet, A.; Audic, S.; Romac, S.; Egge, E.S.; Santini, S.; Ogata, H.; Probert, I.; Edvardsen, B.; de Vargas, C. Diversity patterns of uncultured Haptophytes unravelled by pyrosequencing in Naples Bay. Mol. Ecol. 2013, 22, 87–101. [Google Scholar] [CrossRef]
- Egge, E.; Bittner, L.; Andersen, T.; Audic, S.; de Vargas, C.; Edvardsen, B. 454 pyrosequencing to describe microbial eukaryotic community composition, diversity and relative abundance: A test for marine haptophytes. PLoS ONE 2013, 8, e74371. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.J.; Bachy, C.; Jaeger, G.S.; Poirier, C.; Sudek, L.; Sarma, V.; Mahadevan, A.; Giovannoni, S.J.; Worden, A.Z. Newly discovered deep-branching marine plastid lineages are numerically rare but globally distributed. Curr. Biol. 2017, 27, R15–R16. [Google Scholar] [CrossRef] [PubMed]
- Screen, J.A.; Simmonds, I. The central role of diminishing sea ice in recent Arctic temperature amplification. Nature 2010, 464, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.L.; Labe, Z. NOAA Arctic Report Card 2020: Sea Surface Temperature. 2020. Available online: https://arctic.noaa.gov/Report-Card/Report-Card-2020/ArtMID/7975/ArticleID/885/Sea-Surface-Temperature#:~:text=August%20mean%20sea%20surface%20temperatures,are%20ice%2Dfree%20in%20August (accessed on 15 October 2021).
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.; Heinze, C.; Ilyina, T.; Seferian, R.; et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Toseland, A.; Daines, S.J.; Clark, J.R.; Kirkham, A.; Strauss, J.; Uhlig, C.; Lenton, T.M.; Valentin, K.; Pearson, G.A.; Moulton, V.; et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 2013, 3, 979. [Google Scholar] [CrossRef]
- Levinsen, H.; Nielsen, T.G. The trophic role of marine pelagic ciliates and heterotrophic dinoflagellates in arctic and temperate coastal ecosystems: A cross-latitude comparison. Limnol. Oceanogr. 2002, 47, 427–439. [Google Scholar] [CrossRef]
- López-García, P.; Rodríguez-Valera, F.; Pedrós-Alió, C.; Moreira, D. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 2001, 409, 603–607. [Google Scholar] [CrossRef]
- Logares, R.; Audic, S.; Bass, D.; Bittner, L.; Boutte, C.; Christen, R.; Claverie, J.-M.; Decelle, J.; Dolan, J.R.; Dunthorn, M.; et al. Patterns of rare and abundant marine microbial eukaryotes. Curr. Biol. 2014, 24, 813–821. [Google Scholar] [CrossRef]
- Schoenle, A.; Hohlfeld, M.; Hermanns, K.; Mahé, F.; de Vargas, C.; Nitsche, F.; Arndt, H. High and specific diversity of protists in the deep-sea basins dominated by diplonemids, kinetoplastids, ciliates and foraminiferans. Commun. Biol. 2021, 4, 501. [Google Scholar] [CrossRef]
- Stoeck, T.; Zuendorf, A.; Breiner, H.-W.; Behnke, A. A molecular approach to identify active microbes in environmental eukaryote clone libraries. Microb. Ecol. 2007, 53, 328–339. [Google Scholar] [CrossRef]
- Not, F.; del Campo, J.; Balagué, V.; de Vargas, C.; Massana, R. New insights into the diversity of marine picoeukaryotes. PLoS ONE 2009, 4, e7143. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.K.; Campbell, V.; Connell, P.; Gellene, A.G.; Liu, Z.; Terrado, R.; Caron, D.A. Protistan diversity and activity inferred from RNA and DNA at a coastal site in the eastern North Pacific. FEMS Microbiol. Ecol. 2016, 92, fiw050. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, R.; Hu, C.; Sun, P.; Jiao, N.; Warren, A. Microbial eukaryote diversity and activity in the water column of the South China Sea based on DNA and RNA high-throughput sequencing. Front. Microbiol. 2017, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, Y.; Laws, E.; Huang, B. Water mass-driven spatial effects and environmental heterogeneity shape microeukaryote biogeography in a subtropical, hydrographically complex ocean systemA case study of ciliates. Sci. Total Environ. 2020, 706, 135753. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, S.; Wang, Y.; Huang, B. Biogeographic role of the Kuroshio Current intrusion in the microzooplankton community in the boundary zone of the northern South China Sea. Microorganisms 2021, 9, 1104. [Google Scholar] [CrossRef]
- Sun, P.; Huang, X.; Wang, Y.; Huang, B. Protistan-bacterial microbiota exhibit stronger species sorting and greater network connectivity offshore than nearshore across a coast-to-basin continuum. mSystems 2021, 6, e00100-21. [Google Scholar] [CrossRef]
- Li, R.; Hu, C.; Wang, J.; Sun, J.; Wang, Y.; Jiao, N.; Xu, D. Biogeographical distribution and community assembly of active protistan assemblages along an estuary to a basin transect of the northern South China Sea. Microorganisms 2021, 9, 351. [Google Scholar] [CrossRef]
- Charvet, S.; Vincent, W.F.; Lovejoy, C. Effects of light and prey availability on Arctic freshwater protist communities examined by high-throughput DNA and RNA sequencing. FEMS Microbiol. Ecol. 2014, 88, 550–564. [Google Scholar] [CrossRef]
- Lovejoy, C.; Massana, R.; Pedrós-Alió, C. Diversity and distribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas. Appl. Environ. Microbiol. 2006, 72, 3085–3095. [Google Scholar] [CrossRef]
- Bachy, C.; López-García, P.; Vereshchaka, A.; Moreira, D. Diversity and vertical distribution of microbial eukaryotes in the snow, sea ice and seawater near the North Pole at the end of the polar night. Front. Microbiol. 2011, 2, 106. [Google Scholar] [CrossRef]
- Kilias, E.S.; Kattner, G.; Wolf, C.; Frickenhaus, S.; Metfies, K. A molecular survey of protist diversity through the central Arctic Ocean. Polar Biol. 2014, 37, 1271–1287. [Google Scholar] [CrossRef]
- Hardge, K.; Peeken, I.; Neuhaus, S.; Lange, B.A.; Stock, A.; Stoeck, T.; Weinisch, L.; Metfies, K. The importance of sea ice for exchange of habitat-specific protist communities in the Central Arctic Ocean. J. Mar. Syst. 2017, 165, 124–138. [Google Scholar] [CrossRef]
- de Sousa, A.G.G.; Tomasino, M.P.; Duarte, P.; Fernández-Méndez, M.; Assmy, P.; Ribeiro, H.; Surkont, J.; Leite, R.B.; Pereira-Leal, J.B.; Torgo, L. Diversity and composition of pelagic prokaryotic and protist communities in a thin Arctic sea-ice regime. Microb. Ecol. 2018, 78, 388–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Kong, H.; Yang, E.-J.; Li, X.; Jiao, N.; Warren, A.; Wang, Y.; Lee, Y.; Jung, J.; Kang, S.-H. Contrasting community composition of active microeukaryotes in melt ponds and sea water of the Arctic Ocean revealed by high-throughput sequencing. Front. Microbiol. 2020, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Kong, H.; Yang, E.; Wang, Y.; Li, X.; Sun, P.; Jiao, N.; Lee, Y.; Jung, J.; Cho, K. Spatial dynamics of active microeukaryotes along a latitudinal gradient: Diversity, assembly process, and co-occurrence relationship. Environ. Res. 2022; In press. [Google Scholar]
- Freyria, N.J.; Joli, N.; Lovejoy, C. A decadal perspective on north water microbial eukaryotes as Arctic Ocean sentinels. Sci. Rep. 2021, 11, 8413. [Google Scholar] [CrossRef]
- Ardyna, M.; Babin, M.; Devred, E.; Forest, A.; Grosselin, M.; Raimbault, P.; Tremblay, J.-É. Shelf-basin gradients shape ecological phytoplankton niches and community composition in the coastal Arctic Ocean (Beaufort Sea). Limnol. Oceanogr. 2017, 62, 2113–2132. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016. [Google Scholar] [CrossRef]
- Edgar, R.C. SINTAX: A simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 2016. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2012, 41, D597–D604. [Google Scholar] [CrossRef]
- Gran-Stadniczeñko, S.; Supraha, L.; Egge, E.D.; Edvardsen, B. Haptophyte diversity and vertical distribution explored by 18S and 28S ribosomal RNA gene metabarcoding and scanning electron microscopy. J. Eukaryot. Microbiol. 2017, 64, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Moon-van der Staay, S.Y.; van der Staay, G.W.M.; Guillou, L.; Vaulot, D.; Claustre, H.; Medlin, L.K. Abundance and diversity of prymnesiophytes in the picoplankton community from the equatorial Pacific Ocean inferred from 18S rDNA sequences. Limnol. Oceanogr. 2000, 45, 98–109. [Google Scholar] [CrossRef]
- Simon, M.; López-García, P.; Moreira, D.; Jardillier, L. New haptophyte lineages and multiple independent colonizations of freshwater ecosystems. Environ. Microbiol. Rep. 2013, 5, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Egge, E.S.; Eikrem, W.; Edvardsen, B. Deep-branching novel lineages and high diversity of haptophytes in the Skagerrak (Norway) uncovered by 454 pyrosequencing. J. Eukaryot. Microbiol. 2015, 62, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Marie, D.; Jardillier, L.; Scanlan, D.J.; Vaulot, D. Groups without cultured representatives dominate eukaryotic picophytoplankton in the oligotrophic South East Pacific Ocean. PLoS ONE 2009, 4, 7657. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER–E: Plymouth, UK, 2009; Available online: https://www.researchgate.net/publication/285668711_PRIMER_v6_user_manualtutorial_PRIMER-E_Plymouth (accessed on 15 October 2021).
- Fields, A.; Miles, J.; Field, Z. Discovering Statistics Using R; Sage Publications: London, UK, 2012. [Google Scholar]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Harrell, F. Hmisc: Harrell Miscellaneous. 2008. Available online: http://cranrprojectorg/web/packages/Hmisc/indexhtml (accessed on 15 October 2021).
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Ju, F.; Xia, Y.; Guo, F.; Wang, Z.; Zhang, T. Taxonomic relatedness shapes bacterial assembly in activated sludge of globally distributed wastewater treatment plants. Environ. Microbiol. 2014, 16, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.; Cornejo-Castillo, F.M.; Borrull, E.; Díez-Vives, C.; Lara, E.; Vaqué, D. Arrieta, J.M.; Duarte, C.M.; Gasol, J.M.; Acinas, S.G. Particle-association lifestyle is a phylogenetically conserved trait in bathypelagic prokaryotes. Mol. Ecol. 2015, 24, 5692–5706. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, Menlo Park, CA, USA, 17–20 May 2009. [Google Scholar]
- Peng, G.; Wu, J. Optimal network topology for structural robustness based on natural connectivity. Physica A 2016, 443, 212–220. [Google Scholar] [CrossRef]
- Cullen, J.J. Subsurface chlorophyll maximum layers: Enduring enigma or mystery solved? Annu. Rev. Mar. Sci. 2015, 7, 207–239. [Google Scholar] [CrossRef] [PubMed]
- Carmack, E.; McLaughlin, F. Towards recognition of physical and geochemical change in Subarctic and Arctic Seas. Prog. Oceanogr. 2011, 90, 90–104. [Google Scholar] [CrossRef]
- Lovejoy, C. Polar marine microbiology. In Polar Microbiology: Life in a Deep Freeze; Miller, R.V., Whyte, L.G., Eds.; ASM Press: Washington, DC, USA, 2012; pp. 1–17. [Google Scholar]
- Martin, J.; Tremblay, J.E.; Gagnon, J.; Tremblay, G.; Lapoussiere, A.; Jose, C.; Poulin, M.; Gosselin, M.; Gratton, Y.; Michel, C. Prevalence, structure and properties of subsurface chlorophyll maxima in Canadian Arctic waters. Mar. Ecol. Prog. Ser. 2010, 412, 69–84. [Google Scholar] [CrossRef]
- Ardyna, M.; Gosselin, M.; Michel, C.; Poulin, M.; Tremblay, J.-É. Environmental forcing of phytoplankton community structure and function in the Canadian High Arctic: Contrasting oligotrophic and eutrophic regions. Mar. Ecol. Prog. Ser. 2011, 442, 37–57. [Google Scholar] [CrossRef]
- Onda, D.F.L.; Medrinal, E.; Comeau, A.M.; Thaler, M.; Babin, M.; Lovejoy, C. Seasonal and interannual changes in ciliate and dinoflagellate species assemblages in the Arctic Ocean (Amundsen Gulf, Beaufort Sea, Canada). Front. Mar. Sci. 2017, 4, 16. [Google Scholar] [CrossRef]
- Crawford, D.W.; Cefarelli, A.O.; Wrohan, I.A.; Wyatt, S.N.; Varela, D.E. Spatial patterns in abundance, taxonomic composition and carbon biomass of nano- and microphytoplankton in Subarctic and Arctic Seas. Prog. Oceanogr. 2018, 162, 132–159. [Google Scholar] [CrossRef]
- Man-Aharonovich, D.; Philosof, A.; Kirkup, B.C.; Gall, F.L.; Yogev, T.; Berman-Frank, I.; Polz, M.F.; Vaulot, D.; Béjà, O. Diversity of active marine picoeukaryotes in the eastern Mediterranean Sea unveiled using photosystem-II psbA transcripts. ISME J. 2010, 4, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Ogata, H.; Suzuki, K. Contrasting biogeography and diversity patterns between diatoms and haptophytes in the central Pacific Ocean. Sci. Rep. 2018, 8, 10916. [Google Scholar] [CrossRef] [PubMed]
- García, F.C.; Bestion, E.; Warfield, R.; Yvon-Durocher, G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning. P. Natl. Acad. Sci. USA 2018, 115, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, M.; Inouye, I.; Maeda, O.; Chihara, M. The haptonema as a food–capturing device: Observations on Chrysochromulina hirta (Prymnesiophyceae). Phycologia 1991, 30, 563–573. [Google Scholar] [CrossRef]
- Tillmann, U. Phagotrophy of a plastidic Haptyphyte, Prymnesium patelliferum. Aquat. Microb. Ecol. 1998, 14, 155–160. [Google Scholar] [CrossRef]
- Legrand, C.; Johansson, N.; Johnsen, G.; Børsheim, Y.; Granéli, E. Phagotrophy and toxicity variation in the mixotrophic Prymnesium patelliferum (Haptophyta). Limnol. Oceanogr. 2001, 46, 1208–1214. [Google Scholar] [CrossRef]
- Hansen, P.J.; Hjorth, M. Growth and grazing responses of Chrysochromulina ericina (Prymnesiophyceae): The role of irradiance, prey concentration and pH. Mar. Biol. 2002, 141, 975–983. [Google Scholar] [CrossRef]
- Carpenter, K.J.; Bose, M.; Polerecky, L.; Lie, A.A.Y.; Heidelberg, K.B.; Caron, D.A. Single-cell view of carbon and nitrogen acquisition in the mixotrophic alga Prymnesium parvum (Haptophyta) inferred from stable isotope tracers and NanoSIMS. Front. Mar. Sci. 2018, 5, 157. [Google Scholar] [CrossRef]
- Wassmann, P.; Vernet, M.; Mitchell, B.G.; Rey, F. Mass sedimentation of Phaeocystis pouchelii the Barents Sea. Mar. Ecol. Prog. Ser. 1990, 66, 183–195. [Google Scholar] [CrossRef]
- Saiz, E.; Calbet, A.; Isari, S.; Antó, M.; Velasco, E.M.; Almeda, R.; Movilla, J.; Alcaraz, M. Zooplankton distribution and feeding in the Arctic Ocean during a Phaeocystis pouchetii bloom. Deep Sea Res I 2013, 72, 17–33. [Google Scholar] [CrossRef]
- Pavlov, A.K.; Taskjelle, H.M.; Kauko, B.; Hamre, S.R.; Hudson, P.; Assmy, P.; Duarte, M.; Fernández-Méndez, C.; Mundy, J.; Granskog, M.A. Altered inherent optical properties and estimates of the underwater light field during an Arctic under-ice bloom of Phaeocystis pouchetii. J. Geophys. Res. Oceans 2017, 122, 4939–4961. [Google Scholar] [CrossRef]
- Alexander, H.; Rouco, M.; Haley, S.T.; Wilson, S.T.; Karl, D.M.; Dyhrman, S.T. Functional group-specific traits drive phytoplankton dynamics in the oligotrophic ocean. P. Natl. Acad. Sci. USA 2015, 112, E5972–E5979. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; Countway, P.D.; Xia, L.; Vigil, P.D.; Beman, J.M.; Kim, D.Y.; Chow, C.T.; Sachdeva, R.; Jones, A.C.; Schwalbach, M.S.; et al. Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J. 2011, 5, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Jeroen, R. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Zakharova, Y.R.; Bukin, Y.S.; Galachyants, Y.P.; Petrova, D.P.; Sakirko, M.V.; Likhoshway, Y.V. Co-occurrence networks among bacteria and microbial eukaryotes of Lake Baikal during a spring phytoplankton bloom. Microb. Ecol. 2019, 77, 96–109. [Google Scholar] [CrossRef]
- Hiraoka, S.; Hirai, M.; Matsui, Y.; Makabe, A.; Minegishi, H.; Tsuda, M.; Rastelli, E.; Danovaro, R.; Corinaldesi, C.; Kitahashi, T.; et al. Microbial community and geochemical analyses of trans-trench sediments for understanding the roles of hadal environments. ISME J. 2020, 14, 740–756. [Google Scholar] [CrossRef]
- Gu, R.; Sun, P.; Wang, Y.; Yu, F.; Jiao, N.; Xu, D. Genetic Diversity, Community Assembly, and Shaping Factors of Benthic Microbial Eukaryotes in Dongshan Bay, Southeast China. Front. Microbiol. 2020, 11, 592489. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Zhang, C.; Wang, M.; Kan, J. Estuarine gradients dictate spatiotemporal variations of microbiome networks in the Chesapeake Bay. Environ. Microbiome 2021, 16, 22. [Google Scholar] [CrossRef]
- Monier, A.; Comte, J.; Babin, M.; Forest, A.; Matsuoka, A.; Lovejoy, C. Oceanographic structure drives the assembly processes of microbial eukaryotic communities. ISME J. 2015, 9, 990–1002. [Google Scholar] [CrossRef]
- Czárán, T.L.; Hoekstra, R.F.; Pagie, L. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. USA 2002, 99, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Mouquet, N.; Gonzalez, A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. USA 2003, 100, 12765–12770. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Singh, M.; Soni, S.K.; Singh, R.; Kalra, A. Biodiversity acts as insurance of productivity of bacterial communities under abiotic perturbations. ISME J. 2014, 8, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Scheu, S.; Jousset, A. Bacterial diversity stabilizes community productivity. PLoS ONE 2012, 7, e34517. [Google Scholar] [CrossRef]

| Environmental Parameters | R2 | p |
|---|---|---|
| Geographic distance | 0.006 | 0.415 |
| Depth | 0.180 | 0.055 |
| Temperature | 0.448 | 0.002 |
| Salinity | 0.098 | 0.149 |
| PO4 | 0.096 | 0.194 |
| NO2 + NO3 | 0.040 | 0.313 |
| SiO2 | 0.072 | 0.216 |
| Chl a (>20 µm) | 0.086 | 0.111 |
| Chl a (2–20 µm) | 0.162 | 0.046 |
| Chl a (<2 µm) | 0.151 | 0.081 |
| HPs, abundance | 0.029 | 0.365 |
| PPEs, abundance | 0.108 | 0.167 |
| Variable | Cumulative % |
|---|---|
| Temperature | 12.9 |
| Chl a (<2 μm) | 4.3 |
| Chl a (2–20 μm) | 2.6 |
| PPEs | 1.5 |
| Chl a (>20 μm) | 1.4 |
| Heterotrophic bacteria | 1.3 |
| Salinity | 1.3 |
| Geographic distance | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Liao, Y.; Wang, Y.; Yang, E.-J.; Jiao, N.; Lee, Y.; Jung, J.; Cho, K.-H.; Moon, J.-K.; Xu, D. Contrasting Community Composition and Co-Occurrence Relationships of the Active Pico-Sized Haptophytes in the Surface and Subsurface Chlorophyll Maximum Layers of the Arctic Ocean in Summer. Microorganisms 2022, 10, 248. https://doi.org/10.3390/microorganisms10020248
Sun P, Liao Y, Wang Y, Yang E-J, Jiao N, Lee Y, Jung J, Cho K-H, Moon J-K, Xu D. Contrasting Community Composition and Co-Occurrence Relationships of the Active Pico-Sized Haptophytes in the Surface and Subsurface Chlorophyll Maximum Layers of the Arctic Ocean in Summer. Microorganisms. 2022; 10(2):248. https://doi.org/10.3390/microorganisms10020248
Chicago/Turabian StyleSun, Ping, Yuyu Liao, Ying Wang, Eun-Jin Yang, Nianzhi Jiao, Youngju Lee, Jinyoung Jung, Kyoung-Ho Cho, Jong-Kuk Moon, and Dapeng Xu. 2022. "Contrasting Community Composition and Co-Occurrence Relationships of the Active Pico-Sized Haptophytes in the Surface and Subsurface Chlorophyll Maximum Layers of the Arctic Ocean in Summer" Microorganisms 10, no. 2: 248. https://doi.org/10.3390/microorganisms10020248
APA StyleSun, P., Liao, Y., Wang, Y., Yang, E.-J., Jiao, N., Lee, Y., Jung, J., Cho, K.-H., Moon, J.-K., & Xu, D. (2022). Contrasting Community Composition and Co-Occurrence Relationships of the Active Pico-Sized Haptophytes in the Surface and Subsurface Chlorophyll Maximum Layers of the Arctic Ocean in Summer. Microorganisms, 10(2), 248. https://doi.org/10.3390/microorganisms10020248







