Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed
Abstract
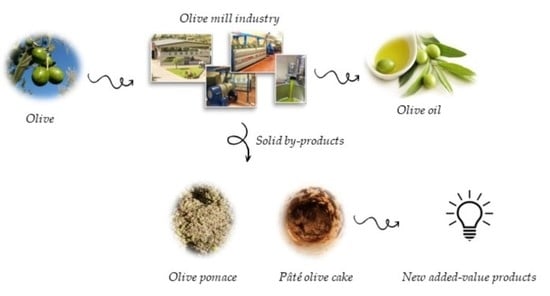
:1. Introduction
2. Physico-Chemical and Microbiological Characteristics of Olive Solid Waste
2.1. Physico-Chemical Traits of OP
2.2. Microbiological Traits of OP
2.3. Physico-Chemical Traits of POC
2.4. Microbiological Traits of POC
3. Bioactive Compounds of Solid Oil Waste Products
4. Exploitation of OP
5. Use of Solid Olive By-Products in the Food and Feed Sectors
5.1. Use of OP in Food
5.2. Use of OP in Feed
5.3. Use of DMF POC for New Functional Food
| Sector | Aim | POC-Form | Amount | Results | References |
|---|---|---|---|---|---|
| Livestock | Feed fortification | Fresh | 35% | Reduction in oxidation of cholesterol and fatty acids in lamb | [49] |
| Feed fortification | Fresh | 82.5/165.0 g/Kg | Enhancement of meat oxidative stability at higher POC concentrations | [50] | |
| Natural additives for food application | Increase the nutritional value of spaghetti | POC endured air-dried at low temperature | 10 and 15% (w/w) | Increase in the content of flavonoids and total phenols | [19] |
| Increase the quality and nutritional value of taralli | POC fermented with S. cerevisiae and Leuc. mesenteroides | 20% | Increase in the bioactive compounds and saturated fatty acids maintained at a low level | [58] | |
| Food | New fermented product | Fermented POC with S. cerevisiae and Leuc. mesenteroides | - | Increase in hydroxytyrosol content and improved sensory notes by production of alcohols and esters | [59] |
| Formulation of new functional food | Fresh, fermented, or extracted POC | Carboncella, Tortiglione and Leccino cv. show different total phenol contents The metabolic activity, tested on CaCo2 and HCT116, suggest beneficial effects related to modulation of gene expression | [9] | ||
| Nutraceutical | Formulation of new functional ingredients | Fresh | - | The Frantoio cv show high antioxidant activity with a phenol content of 26.66 gGAE/kg The POC obtained from Ascolana cv show higher tyrosinase and amylase inhibition than those obtained from Frantoio cv. | [61] |
| Pharmaceutical | Evaluation of antiaging effect of phenolic extract | Fresh and dried | - | Dried POC contains high levels of hydroxytyrosol, oleuropein derivatives. and exhibited a long stability, unlike fresh POC, where a breakdown of hydroxytyrosol occurred The diluted hydroalcoholic extract shows an in vitro antiaging effect | [59] |
| Evaluation of effect on cardiovascular and metabolic diseases | Tablet | 4 tablets/day of POC (corresponding to 30 mg/day of hydroxytyrosol for 2 months) | Reduction in total cholesterol, LDL-cholesterol, and urea, and significant increase in plasma calcium levels Decrease in the proinflammatory protein MCP-1 in plasma | [63] | |
| Evaluation of interaction between POC and intestinal microbiota through SHIME® | Powder | 4 g/L of POC | No antimicrobial effect on the colonic microbial community, as SCFA production is not reduced Reduction in Fusobacteriaceae and increase in Lactobacillaceae and Bifidobacteriaceae. | [64] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Producing 69% of the World’s Production, the EU is the Largest Producer of Olive Oil. 2020. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil_en (accessed on 23 December 2021).
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial application to improve olive mill wastewater phenolic extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbá, C.; Moschetta, A. Extra virgin olive oil: Lesson from nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Pisante, M.; Inglese, P.; Lercker, G. L’Ulivo e L’Olio; Cultura&Cultura; Bayer Crop Science: Bologna, Italy, 2009; pp. 691–692. [Google Scholar]
- Lozano-Sánchez, J.; Bendini, A.; Di Lecce, G.; Valli, E.; Gallina Toschi, T.; Segura-Carretero, A. Macro-and micro functional components of a spreadable olive by-product (pâté) generated by new concept of two-phase decanter. Eur. J. Lipid Sci. Technol. 2017, 119, 1600096. [Google Scholar] [CrossRef]
- Lanza, B.; Cellini, M.; Di Marco, S.; D’Amico, E.; Simone, N.; Giansante, L.; Pompilio, A.; Di Loreto, G.; Bacelli, M.; Del Re, P.; et al. Olive pâté by multi-phase decanter as potential source of bioactive compounds of both nutraceutical and anticancer effects. Molecules 2020, 25, 5967. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid-and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and sustainable valorisation of olive pomace using a fractionation approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Sierra, J.; Martí, E.; Garau, M.A.; Cruañas, R. Effects of the agronomic use of olive oil mill wastewater: Field experiment. Sci. Total Environ. 2007, 378, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Cegarra, J.; Roig, A.; Sanchez-Monedero, M.A.; Bernal, M.P. Characterization of olive-mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; Garcia, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Vivas, A.; Moreno, B.; Garcia-Rodriguez, S.; Benitez, E. Assessing the impact of composting and vermicomposting on bacterial community size and structure, and microbial functional diversity of an olive-mill waste. Bioresour. Technol. 2009, 100, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Jmeii, L.; Soufi, L.; Abid, N.; Mahjoubi, M.; Roussos, S.; Ouzari, H.I.; Cherif, A.; Garna, H. Assessment of biotechnological potentials of strains isolated from repasso olive pomace in Tunisia. Ann. Microbiol. 2019, 69, 1177–1190. [Google Scholar] [CrossRef]
- Padalino, L.; D’Antuono, I.; Durante, M.; Conte, A.; Cardinali, A.; Linsalata, V.; Mita, G.; Logrieco, A.F.; Del Nobile, M.A. Use of olive oil industrial by-product for pasta enrichment. Antioxidants 2018, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health Part B 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Kumar, S.; Kushwaha, R.; Verma, M.L. Recovery and utilization of bioactives from food processing waste. In Biotechnological Production of Bioactive Compounds; Verma, M.L., Chandel, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 37–68. [Google Scholar]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods–A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Savournin, C.; Baghdikian, B.; Elias, R.; Dargouth-Kesraoui, F.; Boukef, K.; Balansard, G. Rapid high-performance liquid chromatography analysis for the quantitative determination of oleuropein in Olea Europaea leaves. J. Agric. Food Chem. 2001, 49, 618–621. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Priego-Capote, F.; Luque de Castro, M.D. Characterization of refined edible oils enriched with phenolic extracts from olive leaves and pomace. J. Agric. Food Chem. 2012, 60, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Innangi, M.; Niro, E.; D’Ascoli, R.; Danise, T.; Proietti, P.; Nasini, L.; Regni, L.; Castaldi, S.; Fioretto, A. Effects of olive pomace amendment on soil enzyme activities. Appl. Soil Ecol. 2017, 119, 242–249. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Sánchez-Moreno, L.; Peña, A. Assessment of olive cake as soil amendment for the controlled release of triazine herbicides. Sci. Total Environ. 2007, 378, 119–123. [Google Scholar] [CrossRef] [PubMed]
- MIPAF, Ministero delle Politiche Agricole e Forestali. Decreto 6 luglio 2005. Criteri e Norme Tecniche Generali per la Disciplina Regionale Dell’Utilizzazione Agronomica delle Acque di Vegetazione e degli Scarichi dei Frantoi Oleari, di cui All’articolo 38 del Decreto Legislativo 11 Maggio 1999, n. 152; Gazzetta Ufficiale: Rome, Italy, 2005. [Google Scholar]
- Marano, V.; De Francesco, P.; Maraglino, A. Verso la Sostenibilità della Filiera Olivicola: Trattamento, Recupero e Valorizzazione dei Sottoprodotti Oleari. L’Officina GBS—UNASCO. 2004. Available online: www.tirsavplus.eu/documenti/divulgazione/2007/VERSO.pdf (accessed on 4 October 2021).
- Abu Tayeh, H.; Najami, N.; Dosoretz, C.; Tafesh, A.; Azaizeh, H. Potential of bioethanol production from olive mill solid wastes. Bioresour. Technol. 2014, 152, 24–30. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- Marinelli, V.; Padalino, L.; Nardiello, D.; Del Nobile, M.A.; Conte, A. New approach to enrich pasta with polyphenols from grape marc. J. Chem. 2015, 2015, 734578. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT—Food Sci. Technol. 2019, 114, 108368. [Google Scholar] [CrossRef]
- Lin, S.; Chi, W.; Hu, J.; Pan, Q.; Zheng, B.; Zeng, S. Sensory and nutritional properties of chinese olive pomace based high fibre biscuit. Emir. J. Food Agric. 2017, 29, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catry, E.; Bindels, L.; Tailleux, A.; Lestave, S.; Neyrinck, A.M.; Goossens, J.F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.K. Impact of westernized diet on gut microbiota in children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Costa, C.M.; Bonifácio-Lopes, T.; Silva, S.; Veiga, M.; Monforte, A.R.; Nunes, J.; Vincente, A.A.; Pintado, M. Prebiotic effects of olive pomace powders in the gut: In vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. 2021, 112, 106312. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casale, M.; Paini, M.; Casazza, A.A.; Lanteri, S.; Perego, P. Production of a novel fermented milk fortified with natural antioxidants and its analysis by NIR spectroscopy. LWT—Food Sci. Technol. 2015, 62, 376–383. [Google Scholar] [CrossRef]
- Mikdame, H.; Kharmach, E.; Mtarfi, N.E.; Alaoui, K.; Ben Abbou, M.; Rokni, Y.; Majbar, Z.; Taleb, M.; Rais, Z. By-products of olive oil in the service of the deficiency of food antioxidants: The case of butter. J. Food Qual. 2020, 2020, 6382942. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Fernandez-Bolanos, J.G.; Lopez, O.; Fernandez-Bolanos, J.; Rodriguez-Gutierrez, G. Hydroxytyrosol and derivatives: Isolation, synthesis, and biological properties. Curr. Org. Chem. 2008, 12, 442–463. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nasopoulou, C.; Gogaki, V.; Stamatakis, G.; Papaharisis, L.; Demopoulos, C.A.; Zabetakis, I. Evaluation of the in vitro anti-atherogenic properties of lipid fractions of olive pomace, olive pomace enriched fish feed and gilthead sea bream (Sparus aurata) fed with olive pomace enriched fish feed. Mar. Drugs 2013, 11, 3676–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem. 2014, 145, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- Luciano, G.; Pauselli, M.; Servili, M.; Mourvaki, E.; Serra, A.; Monahan, F.J.; Lanza, M.; Priolo, A.; Zinnai, A.; Mele, M. Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 2013, 93, 703–714. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef] [Green Version]
- Doyle, S.P.; Harrison, K.R.; Daley, C.A.; Hamilton, P.C.; Sinnott, D.K. Effects of Feeding Olive Pomace on the Fatty Acid Profile of Pork. In Proceedings of the American Society of Animal Science Western Section, Logan, UT, USA, 21–23 June 2006; pp. 216–218. [Google Scholar]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Mannelli, F.; Cappucci, A.; Pini, F.; Pastorelli, R.; Decorosi, F.; Giovannetti, L.; Mele, M.; Minieri, S.; Conte, G.; Pauselli, M.; et al. Effect of different types of olive oil pomace dietary supplementation on the rumen microbial community profile in Comisana ewes. Sci. Rep. 2018, 8, 1–11. [Google Scholar]
- Vargas-Bello-Pérez, E.; Vera, R.R.; Aguilar, C.; Lira, R.; Peña, I.; Fernández, J. Feeding olive cake to ewes improves fatty acid profile of milk and cheese. Anim. Feed Sci. Technol. 2013, 184, 94–99. [Google Scholar] [CrossRef]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of olive cake for ewe feeding: Effect on milk yield and composition. Small Rumin. Res. 2004, 55, 169–176. [Google Scholar] [CrossRef]
- Iannaccone, M.; Ianni, A.; Ramazzotti, S.; Grotta, L.; Marone, E.; Cichelli, A.; Martino, G. Whole blood transcriptome analysis reveals positive effects of dried olive pomace-supplemented diet on inflammation and cholesterol in laying hens. Animals 2019, 9, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive compounds and stability of a typical Italian bakery products “Taralli” enriched with fermented olive paste. Molecules 2019, 24, 3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchi, L.; Bellumori, M.; Cipriani, C.; Mocali, A.; Innocenti, M.; Mulinacci, N.; Giovannelli, L. A two-phase olive mill by-product (pâté) as a convenient source of phenolic compounds: Content, stability, and antiaging properties in cultured human fibroblasts. J. Funct. Foods 2018, 40, 751–759. [Google Scholar] [CrossRef]
- Tufariello, M.; Durante, M.; Veneziani, G.; Taticchi, A.; Servili, M.; Bleve, G.; Mita, G. Pâté olive cake: Possible exploitation of a by-product for food applications. Front. Nutr. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Peršurić, Ž.; Martinović, L.S.; Zengin, G.; Šarolić, M.; Pavelić, S.K. Characterization of phenolic and triacylglycerol compounds in the olive oil by-product pâté and assay of its antioxidant and enzyme inhibition activity. LWT—Food Sci. Technol. 2020, 125, 109225. [Google Scholar] [CrossRef]
- Rauf, A.; Noor, J. Natural Products as a Potential Enzyme Inhibitors from Medicinal Plants. In Enzyme Inhibitors and Activators; InTech: Rijeka, Croatia, 2017; pp. 165–177. [Google Scholar]
- Dinu, M.; Pagliai, G.; Scavone, F.; Bellumori, M.; Cecchi, L.; Nediani, C.; Maggini, N.; Sofi, F.; Giovannelli, L.; Mulinacci, N. Effects of an olive by-product called pâté on cardiovascular risk factors. J. Am. Coll. Nutr. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- Giuliani, C.; Marzorati, M.; Innocenti, M.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Van De Wiele, T.; Mulinacci, N. Dietary supplement based on stilbenes: A focus on gut microbial metabolism by the In vitro simulator M-SHIME®. Food Funct. 2016, 7, 4564–4575. [Google Scholar] [CrossRef]
| Extraction System | Olive Oil (kg/100 kg olive) | Added Water (%) | Olive Mill Wastewater | Olive Pomace (kg/100 kg olive) | Pomace Moisture (%) |
|---|---|---|---|---|---|
| Traditional | - | 40 | 40 | 20 | |
| Three phases | 20 | 50 | 80–110 | 55–57 | 48–54 |
| Two phases | 20 | 0–10 | 8–10 | 75–80 | 58–62 |
| Two phases-DMF | 20 | - | - | 45–55 | 75–90 |
| Three phases to savings | 20 | 10–20 | 33–35 | 56–60 | 50–52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.; Pino, A.; Romeo, F.V.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed. Microorganisms 2022, 10, 237. https://doi.org/10.3390/microorganisms10020237
Foti P, Pino A, Romeo FV, Vaccalluzzo A, Caggia C, Randazzo CL. Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed. Microorganisms. 2022; 10(2):237. https://doi.org/10.3390/microorganisms10020237
Chicago/Turabian StyleFoti, Paola, Alessandra Pino, Flora V. Romeo, Amanda Vaccalluzzo, Cinzia Caggia, and Cinzia L. Randazzo. 2022. "Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed" Microorganisms 10, no. 2: 237. https://doi.org/10.3390/microorganisms10020237
APA StyleFoti, P., Pino, A., Romeo, F. V., Vaccalluzzo, A., Caggia, C., & Randazzo, C. L. (2022). Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed. Microorganisms, 10(2), 237. https://doi.org/10.3390/microorganisms10020237