Abstract
Antioxidants protect against oxidative stress that can damage proteins, the cellular immune system, and DNA. In recent studies, probiotics have been shown to impart a microbial balance to the gastrointestinal tract, demonstrating significant antioxidant capacity. In this study, the probiotic properties and antioxidant mechanism of probiotics were evaluated in HepG2 cells and in an animal model. The characteristics of Lactococcus lactis MG5125, Bifidobacterium bifidum MG731, and Bifidobacterium animalis subsp. lactis MG741, which were used as lactic acid bacteria in this study, were analyzed. The results revealed the safety and stability of these probiotics in the gastrointestinal tract because they did not cause hemolysis and had excellent intestinal adhesion (75–84%). In HepG2 cells, the three probiotics alleviated H2O2-induced oxidative stress by mediating lipid peroxidation and glutathione levels and upregulating antioxidant enzymes, including catalase, superoxide dismutase, and glutathione peroxidase. In the tBHP-induced mouse model, administration of the three probiotics reduced hepatic aspartate transaminase, alanine transaminase, and lipid peroxidation levels. In conclusion, Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 showed considerable antioxidant activity both in vitro and in vivo.
1. Introduction
Oxidative stress attacks living tissues and damages cells. In particular, reactive oxygen species (ROS), including superoxide anion (O2−), hydroxyl radical, and hydrogen peroxide (H2O2), contain oxygen with strong oxidizing power [1]. The most important organ for the oxidative energy system is the liver, which is responsible for most metabolic processes occurring in the human body [2]. Mitochondria are organelles present in hepatocytes; they decompose the consumed nutrients and generate constituent units of the substances needed by living organisms and the energy required for metabolism [3]. The electron transport system present in the mitochondrial inner membrane is a major site of ROS production; oxidative stress causes the overexpression of ROS that bind to unsaturated fatty acids in the cell membrane, causing oxidative damage to hepatocytes and inducing lipid peroxidation [2]. Therefore, a large amount of ROS leads to the breakdown of the antioxidant system, which can promote aging and cause a variety of diseases [4]. The human body has an endogenous defense system that includes Cu/Zn superoxide dismutase (SOD1), Mn superoxide dismutase (SOD2), catalase (CAT), and glutathione peroxidase (GPx), which protect cells from active oxygen by converting O2 to H2O [5]. However, the antioxidant enzymes present in the body are not sufficient to prevent oxidative stress-induced damage [6].
Probiotics, especially lactic acid bacteria, are live microorganisms that balance the microflora in the gut when administered in appropriate amounts [7]. Among them, probiotics belonging to the genera Lactobacillus and Bifidobacterium have various biological effects [8]. It is well known that the basic health-promoting function of probiotics is to reduce intestinal-related diseases by regulating and improving the intestinal microbial balance and strengthening the intestinal wall in humans [9]. Recently, probiotics have been found to benefit animal health in addition to human health [10]. Probiotics not only have beneficial effects in the colon but also have various bioactive effects, such as the alleviation of hypersensitive immune responses, the prevention of liver disease, and antioxidant and other effects [11,12,13]. In particular, the antioxidant mechanisms of Lactococcus lactis include the scavenging of oxidant compounds, reducing activity, chelation of metal ions, and prevention of intestinal ROS formation [6,14]. Bifidobacterium bifidum, which produces exopolysaccharides, exerts antioxidant activity by reducing oxidant radicals [15]. In addition, Bifidobacterium lactis has been reported to exhibit antioxidant effects by producing metabolites such as folic acid, glutathione (GSH), and butyrate [6].
Therefore, in this study, we investigated the probiotic properties of L. lactis MG5125, B. bifidum MG731, and B. lactis MG741 and assessed their antioxidant effects by evaluating toxicity, lipid peroxidation, and antioxidant enzymes (CAT, SOD, and GPx) in hepatocytes and liver tissues with induced oxidative stress.
2. Materials and Methods
2.1. Chemicals
Antibiotic strips were purchased from BioMérieux (Marcy-l’Étoile, Lyon, France). Palcam agar was purchased from Oxoid (CM0877; Hampshire, UK). Sheep blood was obtained from MBCell (Seoul, Korea). De Man, Rogosa, and Sharpe (MRS) agar and broth were purchased from Difco (Detroit, MN, USA). HepG2 and HT-29 cells were obtained from the Korea Cell Line Bank (Seoul, Korea). Minimum essential medium (MEM), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin (PS) were purchased from Gibco (Grand Island, NY, USA). Thiobarbituric-acid-reactive substance (TBARS) and GSH assay kits were purchased from Cayman (Ann Arbor, MI, USA). Mouse malondialdehyde (MDA) ELISA kit was obtained from LSBio (Seattle, DC, USA). GSH detection assay kit and CAT, SOD, and GPx assay kits were purchased from Abcam (Cambridge, UK). NuceloZOL for mRNA extraction was obtained from Macherey-Nagel GmbH & Co. (Dueren, Germany). The reverse transcriptase premix was purchased from Intron (Seongnam-si, Korea). The iQ™ SYBR® Green Supermix was purchased from Bio-Rad (Hercules, CA, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Bacterial Strain Culture and Sample Preparation
The probiotic strains (Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741) were provided by MEDIOGEN Co., Ltd. (Jechon, Korea). The strains were identified by 16S rRNA gene sequencing using universal rRNA gene primers (27F and 1492R) (SolGent Co., Ltd., Daejeon, Korea). Each strain was cultured in MRS broth at 37 °C in an anaerobic chamber.
Cell-free supernatant (CFS) was prepared following the procedure described by Escamilla et al. [16]. The probiotic strains grown in MRS broth were diluted to an optical density of 0.9–1.0 (108–109 CFU/mL) at 600 nm and then inoculated at 2% (v/v) in fresh MRS broth. After 24 h, the probiotic strains were centrifuged at 4000× g at 4 °C for 10 min. The supernatants were filtered through a 0.2 μm polytetrafluoroethylene membrane syringe filter (Advantec, Tokyo, Japan) and stored at −80 °C until used for the in vitro study.
For the animal study, the harvested probiotic bacterial pellets were freeze-dried. The powdered cells were harvested, mixed with maltodextrin for dilution to a cell density of 5 × 1010 CFU/g, and stored at 4 °C until further use.
2.3. Cell Culture
HepG2 cells, frequently used to model hepatocytes, were cultured in MEM; HT-29 cells, intestinal epithelial cells, were cultured in DMEM at 37 °C in a 5% CO2 incubator. All media used for cell culture contained 10% FBS and 1% PS. The cells were subcultured until 70–80% confluent.
2.4. Probiotic Properties
2.4.1. Hemolytic Activity
Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 were streaked on CM0877 palcam agar plates containing 5% sheep blood and incubated for 48 h at 37 °C. Hemolytic activity was determined as α (partial hemolysis), β (toxin), or γ (no hemolysis) [17].
2.4.2. Antimicrobial Susceptibility Test
An antimicrobial susceptibility test was performed using antibiotic minimum inhibitory concentration (MIC) strips. The bacteria were grown for 24 h at 37 °C in MRS agar. The colonies were harvested and resuspended in phosphate-buffered saline (PBS) to 0.5 McFarland turbidity. The suspensions were smeared on brain heart infusion agar using a cotton swab. The MIC test strips were placed on the agar surface according to the manufacturer’s instructions. The plates were incubated at 37 °C, and the results were assessed after 24 h according to the European Food Safety Authority (EFSA) (Parma, Italy) guidelines [18].
2.4.3. Adhesion Assay on Intestinal Epithelial Cells
Adhesion of probiotics to HT-29, the colonic epithelial cell line, was performed as described by Chen et al. with slight modifications [19]. Briefly, HT-29 cells were seeded at a density of 4 × 105 cells/mL in 12-well plates under 5% CO2 at 37 °C until a monolayer was formed. Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 (each 1 × 108 CFU/mL) in DMEM without FBS and PS were added to each well and incubated for 2 h. HT-29 cells were washed with PBS to remove the nonadherent bacterial cells. The adhesion ratio (%) was calculated by comparing the number of adherent cells with the initial number of viable cells determined by plate counting.
2.5. Cytotoxicity Assay
Cytotoxicity was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [20]. Briefly, HepG2 cells were seeded at 4 × 105 cell/mL in a 96-well plate and pretreated with the CFS of probiotics (2%) for 24 h followed by treatment with H2O2 (1 mM) for an additional 24 h. The hepatocytes were treated with the MTT solution (0.2 mg/mL) and incubated for 2–4 h at 37 °C. The formazan product produced by MTT was dissolved in dimethyl sulfoxide (150 μL), and absorbance was measured at 550 nm using a microplate reader (EPOCH2, BioTek, Winooski, VT, USA).
2.6. Measurement of Lipid Peroxidation and GSH
Lipid peroxidation based on the TBARS assay and GSH were determined according to the manufacturer’s instructions [11]. Briefly, after probiotic treatment, the cells were washed with PBS, resuspended in assay buffer, and sonicated. Lipid peroxidation, determined by MDA content, was estimated using the whole lysate, and absorbance was measured at 540 nm using a microplate reader. For GSH, cell lysate was centrifuged at 2000× g for 10 min at 4 °C; the supernatant was collected, and absorbance was measured at 405 nm using a microplate reader (EPOCH2, BioTek).
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from hepatocytes using NuceloZOL, and mRNA (1 μg) was reverse-transcribed to cDNA using reverse transcriptase premix. qRT-PCR was performed using CFX Connect Real-Time PCR Detection System (Bio-Rad) and iQ™ SYBR® Green Supermix along with primers. The following primer sequences were used: CAT forward, 5-AACTGTCCCTACCGTGCTCGA-3 and reverse, 5-CCAGAATATTGGATGCTGTGCTCCAGG-3; SOD forward, 5-AATGGACCAGTGAAGGTGTGGGG-3 and reverse, 5-CACATTGCCCAAGTCTCCAACATGC-3; GPx forward, 5- CGGCCCAGTCGGTGTATGC-3 and reverse, 5-CGTGGTGCCTCAGAGGGAC-3; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5-ACCCACTCCTCCACCTTTG-3 and reverse, 5-CTCTTGTGCTCTTGCTGGG-3′. Relative gene expression was normalized to that of GAPDH. Expression levels were analyzed using the 2−ΔΔCT method [21].
2.8. Animal Treatment
C57BL/6 female mice (5-weeks old) were purchased from Orient Bio Inc. (Gyeonggi-do, Korea) and housed under a 12/12 h light–dark cycle (7 a.m. to 7 p.m.) in a room under controlled conditions (22 ± 3 °C, 50 ± 20% relative humidity, 150–300 Lux). The mice were fed a commercial laboratory diet (D11112201; Research Diets Inc., New Brunswick, NJ, USA) and water ad libitum. All animal experimental protocols used in this study were approved (Approval No. P214054) by the Institutional Animal Care and Use Committee at the NDIC, Gyeonggi-do, Korea.
The treatments have been described previously [22]. All animals were randomly divided into five groups (n = 7 in each group) and orally administered (p.o.): (1) saline (normal control, NOR), (2) tert-butyl hydroperoxide (t-BHP), (3) Lc. lactis MG5125 (1 × 109 CFU/g/day in saline), (4) B. bifidum MG731 (1 × 109 CFU/g/day in saline), and (5) B. lactis MG741 (1 × 109 CFU/g/day in saline). On day 15, all animals except the NOR group were intraperitoneally (i.p.) injected with t-BHP (0.5 mmol/kg body weight), and the mice were sacrificed after 24 h. Blood was collected to obtain serum samples and stored at 80 °C until further experiments.
2.9. Biochemical Parameters
Serum samples were analyzed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels using a chemistry analyzer (AU480; Beckman Coulter, CA, USA). To measure MDA, GSH, CAT, SOD, and GPx activity, the liver tissues were lysed using TissueLyser II (Qiagen, Hilden, Germany), and their levels were evaluated according to the manufacturer’s instructions. Absorbance was measured using a microplate reader (SpectraMax M2; Molecular Devices, San Jose, CA, USA).
2.10. Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed by multiple comparison test using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using the IBM SPSS Statistics 21 software program (SPSS Inc., Chicago, IL, USA). The results were considered statistically significant at p values less than 0.05.
3. Results
3.1. Safety Test of Probiotics
Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 showed white colonies with no hemolytic activity (Figure 1).

Figure 1.
Hemolytic activity of (a) Lc. lactis MG5125, (b) B. bifidum MG731, and (c) B. lactis MG741.
In the antimicrobial susceptibility test, Lc. lactis MG5125 was susceptible; however, B. bifidum MG731 and B. lactis MG741 showed resistance to gentamicin, and B. lactis MG741 showed resistance to streptomycin (Table 1).

Table 1.
Determination of the MIC values of the antibiotics tested for probiotics.
3.2. Adhesion of Probiotics to HT-29 Cells
Probiotics must attach to intestinal epithelial cells and form colonies. Therefore, adhesion rates of Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 were determined in HT-29 cells. Lc. lactis MG5125 (84.09 ± 0.96%), B. bifidum MG731 (75.37 ± 0.37%), and B. lactis MG741 (79.55 ± 0.32%) exhibited adhesion rates ≥75%.
3.3. Cytoprotective Effect of Probiotics on H2O2-Exposed Hepatocytes
The viability of hepatocytes treated with probiotics was investigated using the MTT assay (Figure 2a). A cytotoxic effect on hepatocytes was not observed after incubation with the CFS of probiotics (2%) or N-acetylcysteine (NAC), a positive control, for 24 h. The cytoprotective effect on hepatocytes treated with H2O2 (1 mM) after pretreatment with the CFS of probiotics is shown in Figure 2b. A reduction in viability was observed in cells treated with H2O2 (1 mM). In cells treated with the CFS of probiotics and H2O2, viability was significantly increased by 1.26–1.40-fold compared to H2O2 treatment (p < 0.05). However, all CFSs of probiotics showed a lower protective effect than that of NAC. Based on these results, 2% CFS of probiotics, which exerted a cytoprotective effect, was chosen for subsequent experiments.
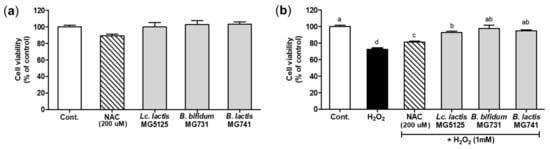
Figure 2.
Effect of probiotics on viability of hepatocytes measured by the MTT assay. (a) Effect on viability of hepatocytes treated with Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 (2%). (b) Cytoprotective effect of Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 on H2O2-exposed hepatocytes. The cells were pretreated with the CFS of probiotics (2%) for 24 h and then exposed to H2O2 (1 mM) for 24 h. The data represent the mean ± SEM (n = 3). Different letters among columns indicate significance at p < 0.05, as determined by Duncan’s test. Cont., control; NAC, N-acetylcysteine.
3.4. Probiotics Elevated Antioxidant Activity in H2O2-Exposed Hepatocytes
The MDA and GSH levels were measured to assess antioxidant activity after treating H2O2-exposed hepatocytes with the CFS of probiotics (Figure 3a). The H2O2-exposed control group showed the highest MDA level (4.87 ± 0.36 nmol/protein mg, p < 0.05). Conversely, in H2O2-exposed hepatocytes treated with Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741, the MDA level was similar or significantly lower (2.07–2.72 nmol/protein mg) than in those treated with NAC, a positive control (3.10 ± 0.11 nmol/protein mg). Moreover, compared with the control, the GSH level was reduced in hepatocytes after exposure to H2O2 (20.15 ± 0.45 nmol/protein mg). H2O2-exposed hepatocytes treated with probiotics (Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741) showed increased GSH levels of 25.30–27.29 nmol/protein mg (p < 0.05), similar to that of the positive control (25.41 ± 0.95 nmol/protein mg, p < 0.05).

Figure 3.
Antioxidant effect of the CFS of probiotics against oxidative stress induced by H2O2 in hepatocytes. (a) MDA and GSH levels in H2O2-exposed hepatocytes pretreated with the CFS of probiotics. (b) mRNA levels of antioxidant enzymes (CAT, SOD, and GPx) in H2O2-exposed hepatocytes pretreated with the CFS of probiotics. The cells were pretreated with the CFS of probiotics for 24 h and then exposed to H2O2 (1 mM) for 24 h. Each mRNA level was normalized to the GAPDH. The data represent the mean ± SEM (n = 3). Different letters among columns indicate significance at p < 0.05, as determined by Duncan’s test. Cont., control; NAC, N-acetylcysteine.
To confirm the antioxidant activity of the CFS of probiotics in H2O2-exposed hepatocytes, the expression levels of CAT, SOD, and GPx were measured using qRT-PCR (Figure 3b). CAT, SOD, and GPx mRNA levels in cells treated with H2O2 were significantly lower than those in the control (p < 0.05). In H2O2-exposed hepatocytes, CAT, SOD, and GPx mRNA levels were increased after treatment with the CFS of Lc. lactis MG5125 (1.30-, 1.31-, and 1.41-fold higher than the H2O2 control, respectively, p < 0.05) and B. lactis MG741 (1.30-, 1.25-, and 1.60-fold higher than the H2O2 control, respectively, p < 0.05). However, the CFS of B. bifidum MG731 had no effect on antioxidant enzyme activity. These results suggest that probiotics protect hepatocytes from oxidative stress by upregulating the levels of antioxidant enzymes.
3.5. Probiotics Reduced Hepatic Injury in t-BHP-Induced Mice
To evaluate the hepatic damage caused by oxidative stress, ALT and AST levels were measured in the serum of mice administered probiotics and t-BHP (Figure 4). Due to oxidative stress induced by t-BHP in mice, there was a significant increase in ALT and AST levels (59.57 and 89.29 U/L), but only a similar or significant increase was observed in the probiotic administration groups (42.71–49.14 and 73.14–92.14 U/L). Lc. lactis MG5125 and B. lactis MG741 administration markedly alleviated hepatic injury by reducing ALT and AST levels in the serum of t-BHP-induced mice.

Figure 4.
(a) ALT and (b) AST levels in the serum of t-BHP-induced C57BL/6 mice pretreated with probiotics for 14 days. The data represent the mean ± SEM (n = 7). Different letters among columns indicate significance at p < 0.05, as determined by Duncan’s test.
3.6. Probiotics Increased Antioxidant Activity in the Liver Tissues of t-BHP-Induced Mice
The t-BHP-injected animal model is mainly used to observe the antioxidant effects of many compounds and extracts. To investigate the antioxidant effect of the three probiotics, MDA and GSH levels were evaluated (Table 2). Probiotics (Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741) suppressed the MDA level (0.61–0.68-fold of the t-BHP-injected group), which was similar to that in the normal group (0.66-fold of the t-BHP-injected group). The GSH level of t-BHP-injected mice was 0.87-fold that of the normal control; however, the probiotics used in this study slightly increased the GSH level (0.88–0.91-fold of the normal control). In addition, we confirmed that CAT, SOD, and GPx were affected by the administration of Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 in vitro (Table 2). The probiotics used in this study restored the CAT level, lowered by t-BHP injection, to the same extent as in the normal group, but no change in SOD and GPx levels was observed in any group.

Table 2.
Effect of probiotics on hepatic malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels in t-BHP-induced C57BL/6 mice.
4. Discussion
Although strains of the genera Lactobacillus and Bifidobacterium are generally considered safe based on their long-term use as probiotics in humans, it is important to conduct safety assessments on specific strains because it has not been confirmed that all members can be used as probiotics [24]. To confirm Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 as probiotics, a hemolytic activity test, antibiotic susceptibility test, and adhesion assay were conducted on intestinal epithermal cells. The three strains were found to be safe, as they did not cause hemolysis, showed a ≥70% adhesion rate in HT-29 intestinal cells, and were found to be stable in the colon (Figure 1). Probiotics reduce oxidative stress either by using their own antioxidant enzymes or by producing antioxidant metabolites [6]. In this study, to confirm the oxidative stress-relieving effect of probiotics, Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 were evaluated based on toxicity, lipid peroxide and glutathione levels, and antioxidant enzyme mRNA expression studies conducted in cells and animal models induced by oxidative stress.
The liver is one of the most important organs for metabolism. Overexpressed ROS bind to unsaturated fatty acids of the cell membrane to induce lipid peroxidation, which is the main mechanism of oxidative damage in hepatocytes [2]. In addition, HepG2 cells are sensitive to oxidative stress because NADPH oxidase (NOX)-generated ROS mediate N-ethylmaleimide-induced K+-Cl− cotransport activation [25]. When H2O2 treatment increased oxidative stress in hepatocytes, it damaged cells and DNA, causing cytotoxicity [26]. Additionally, t-BHP induced toxicity due to oxidative stress in vivo [27]. Serum AST and ALT enzyme levels are used as indicators of toxicity, and t-BHP injection has been reported to increase these levels [28]. These results suggest that toxicity induces apoptosis due to oxidative stress. In this study, we confirmed that treatment with probiotics (Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741) has protective effects on hepatocytes and hepatic tissues by preventing oxidative stress-induced toxicity (Figure 2 and Figure 4). The antioxidant effect of Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 might have resulted from short-chain fatty acid production by lactic acid bacteria [29].
To protect against oxidative stress, organelles have enzymatic and nonenzymatic activities, including glutathione, thioredoxin, vitamin C, and other metabolites, and antioxidant defense systems such as SOD, CAT, and GPx [30]. Excessive oxidative stress leads to the breakdown of the antioxidant system, which can promote aging and cause a variety of diseases [26]. In this study, Lc. lactis MG5125 and B. lactis MG741 reduced MDA levels and elevated GSH levels and SOD, CAT, and GPx enzyme activity in hepatocytes. B. bifidum MG731 modulated MDA and GSH levels; however, no increase in antioxidant enzyme activity was observed in hepatocytes. Oxidative stress is also mediated by other antioxidant signaling pathways [6]. It has been reported that Lactiplantibacillus plantarum CAI6 and Lactiplantibacillus plantarum SC4 showed antioxidant effects by regulating the NF-E2-related factor 2 (Nrf2)–Kelch-like ECH-associated protein 1 (Keap1) pathway in the liver [31]. Lacticaseibacillus rhamnosus GG exerted an antioxidant effect by mediating mitogen-activated protein kinases (MAPKs) [32]. Our study suggests that Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 exert an antioxidant effect in animal studies only by reducing MDA but not by affecting SOD, CAT, and GPx enzymes (Table 2); however, further studies based on other oxidation-related metabolism components are needed. Nonetheless, the strength of our study is that the ingestion of Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 is inherent to the axis of the antioxidant effect of the intestine and liver.
5. Conclusions
In conclusion, our study suggests that Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 are effective in ameliorating oxidative stress in H2O2-exposed HepG2 cells and t-BHP-induced mice. Treatment with the three probiotics enhanced the levels of antioxidant enzymes, including SOD, CAT, GPx, and GSH, to prevent cytotoxicity in H2O2-exposed HepG2 cells. Furthermore, it was observed that administration of the three probiotics lowered serum AST and ALT levels to control toxicity and lowered the MDA level to relieve oxidative stress caused by t-BHP, thereby exhibiting an antioxidant effect (Figure 5). These effects suggest that changes in intestinal microflora caused by Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 intake indirectly increase antioxidant effects in vivo. Therefore, the probiotics Lc. lactis MG5125, B. bifidum MG731, and B. lactis MG741 could serve as functional foods and therapeutic agents for preventing oxidative stress.
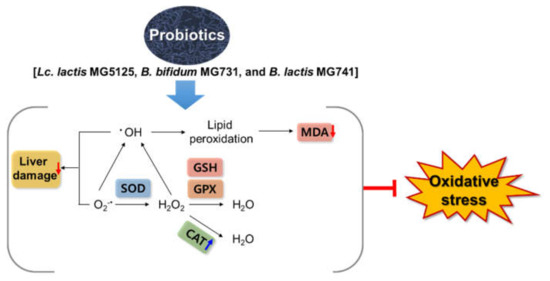
Figure 5.
Probiotics exert a hepatoprotective effect by preventing oxidative stress in HepG2 cells and t-BHP-induced mice.
Author Contributions
Conceptualization, C.-H.K.; methodology, J.Y.L.; investigation, J.Y.L.; resources, C.-H.K.; data curation, J.Y.L.; writing—original draft preparation, J.Y.L.; writing—review and editing, J.Y.L. and C.-H.K.; supervision, C.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee at the NDIC (No. P214054; Gyeonggi-do, Korea).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rhim, T.-J.; Choi, M.-Y. The antioxidative effects of Rhododendron brachycarpum extracts. Korean J. Plant Resour. 2011, 24, 456–460. [Google Scholar] [CrossRef]
- Jang, M.; Seo, H.L.; Kim, S.C.; Kim, Y.W. Effect of Prunellae Spica on oxidative stress and mitochondrial dysfunction in the hepatocyte. J. Physiol. Pathol. Korean Med. 2016, 30, 20–26. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.K.; Park, B.R.; Jeon, G.S.; Lee, S.B. Extraction of flavonoid components from persimmon leaf, thistle and new green. Appl. Chem. Eng. 2016, 27, 276–279. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, S.-H.; Oh, H.-T.; Chung, M.-J.; Cui, C.-B.; Ham, S.-S. Effects of Adenophora triphylla ethylacetate extract on mRNA levels of antioxidant enzymes in human HepG2 cells. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1238–1243. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kang, C.-H. In vivo confirmation of the antimicrobial effect of probiotic candidates against gardnerella vaginalis. Microorganisms 2021, 9, 1690. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, H.; Lee, J.Y.; Won, G.; Choi, S.-I.; Kim, G.-H.; Kang, C.-H. The antioxidant, anti-diabetic, and anti-adipogenesis potential and probiotic properties of lactic acid bacteria isolated from human and fermented foods. Fermentation 2021, 7, 123. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.d.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Zommiti, M.; Chikindas, M.L.; Ferchichi, M. Probiotics—Live biotherapeutics: A story of success, limitations, and future prospects—not only for humans. Probiotics Antimicrob. Proteins 2020, 12, 1266–1289. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Jeong, Y.; Kang, C.-H. Lactic acid bacteria exert a hepatoprotective effect against ethanol-Induced liver injury in HepG2 cells. Microorganisms 2021, 9, 1844. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, J.-K.; Kim, J.-Y.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr. Res. 2019, 67, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-G.; Kang, G.-D.; Kim, H.; Han, M.; Kim, D.-H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R. Blood agar plates and hemolysis protocols. Am. Soc. Microbiol. 2005, 30, 1–9. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; De Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar]
- Chen, Z.-Y.; Hsieh, Y.-M.; Huang, C.-C.; Tsai, C.-C. Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules 2017, 22, 107. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. In Protocols in In Vitro Hepatocyte Research; Springer: Berlin/Heidelberg, Germany, 2015; pp. 333–348. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.; Oh, S.B.; Lee, K.M.; Kim, S.M.; Nho, C.W.; Yoon, W.B.; Kang, K.; Pan, C.H. The protective effect of antioxidant enriched fractions from colored potatoes against hepatotoxic oxidative stress in cultured hepatocytes and mice. J. Food Biochem. 2017, 41, e12315. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-S.; Kim, Y.; Jeong, Y.; Kim, J.-E.; Paek, N.-S.; Kang, C.-H. Antioxidant and probiotic properties of Lactobacilli and Bifidobacteria of human origins. Biotechnol. Bioprocess Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Shi, L.H.; Balakrishnan, K.; Thiagarajah, K.; Ismail, N.I.M.; Yin, O.S. Beneficial properties of probiotics. Trop. Life Sci. Res. 2016, 27, 73. [Google Scholar]
- Kim, J.-A.; Lee, Y.S. Role of reactive oxygen species generated by NADPH oxidase in the mechanism of activation of K+-Cl--cotransport by N-ethylmaleimide in HepG2 human hepatoma cells. Free Radic. Res. 2001, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Woodside, J. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Martínez, R.; Navarro, R.; Ruiz-Sanz, J.I.; Lacort, M.; Ruiz-Larrea, M.B. tert-Butyl hydroperoxide-induced lipid signaling in hepatocytes: Involvement of glutathione and free radicals. Biochem. Pharmacol. 2001, 62, 705–712. [Google Scholar] [CrossRef]
- Oh, J.M.; Jung, Y.S.; Jeon, B.S.; Yoon, B.I.; Lee, K.S.; Kim, B.H.; Oh, S.J.; Kim, S.K. Evaluation of hepatotoxicity and oxidative stress in rats treated with tert-butyl hydroperoxide. Food Chem. Toxicol. 2012, 50, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kim, J.-S.; Park, H.M.; Kim, S.; Paek, N.-S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Wang, L.-X.; Liu, K.; Gao, D.-W.; Hao, J.-K. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J. Gastroenterol. WJG 2013, 19, 3150–3156. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).