Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Survey Strategy and Sampling
2.3. Sample Collection and Serological Test
2.4. Statistical Analysis
2.5. Ethical Clearance
3. Results
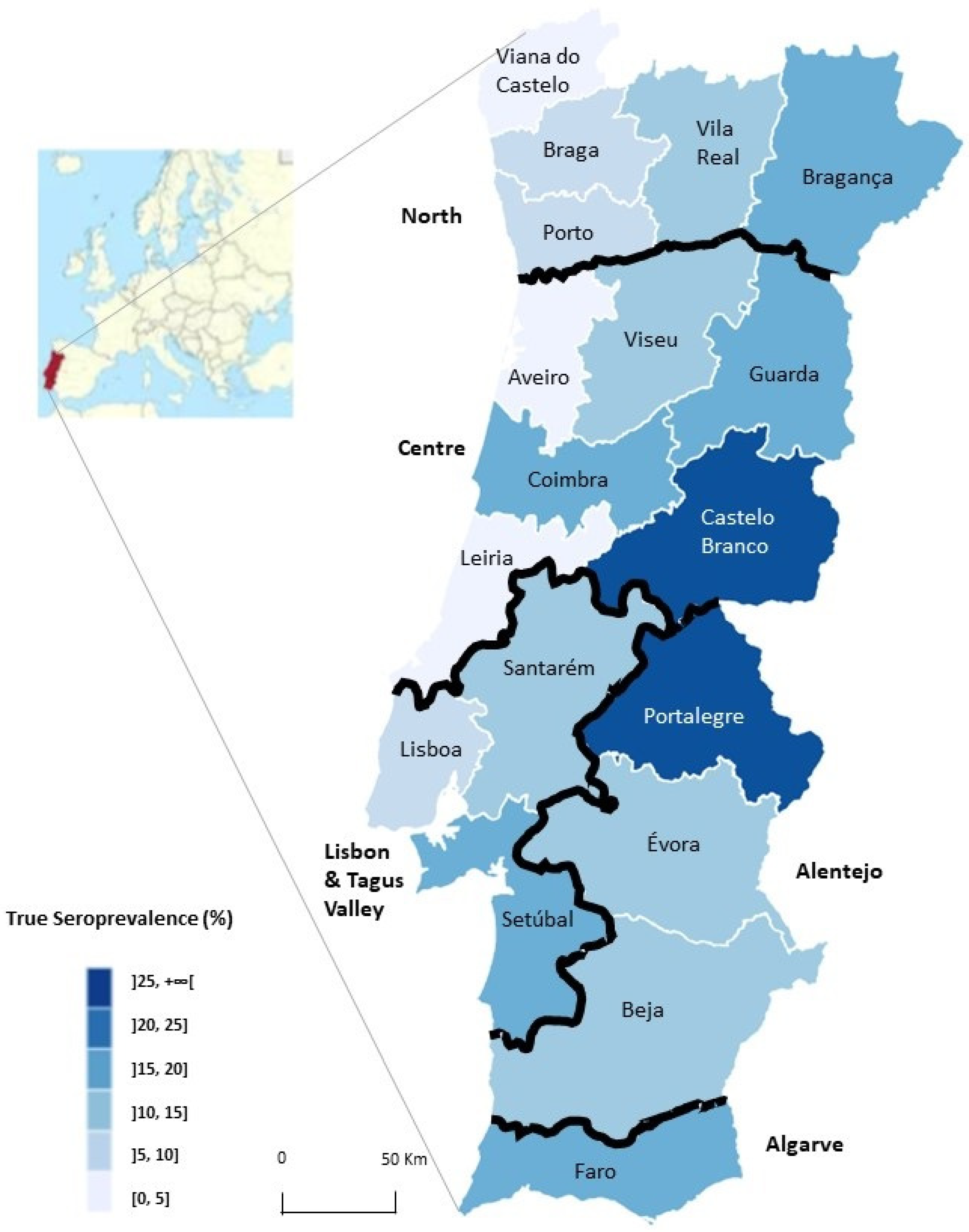
3.1. Seroprevalence According to Geographic Regions
3.2. Seroprevalence According to Dogs’ Characteristics
3.3. Associated Risk Factors for Leishmania Infection in Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Campino, L.; Maia, C. The Role of Reservoirs: Canine Leishmaniasis. In Drug Resistance in Leishmania Parasites: Consequences, Molecular Mechanisms and Possible Treatments, 2nd ed.; Ponte-Sucre, A., Padrón-Nieves, M., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 59–83. [Google Scholar] [CrossRef]
- Berriatua, E.; Jumakanova, Z.; Muñoz, C.; Ortuño, M.; Pérez-Cutillas, P.; Monge-Maillo, B.; Conceição, C.; Maia, C.; Pereira, A.; Rocha, R.; et al. Surveillance, Prevention, and Control of Leishmaniases in the European Union and Its Neighboring Countries; ECDC: Stockholm, Sweden, 2022; ISBN 978-92-9498-572-9. [Google Scholar]
- Bourdeau, P.; Saridomichelakis, M.N.; Oliveira, A.; Oliva, G.; Kotnik, T.; Gálvez, R.; Foglia Manzillo, V.; Koutinas, A.F.; Pereira da Fonseca, I.; Miró, G. Management of canine leishmaniosis in endemic SW European regions: A questionnaire-based multinational survey. Parasit. Vectors 2014, 7, 110. [Google Scholar] [CrossRef]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest trends in Leishmania infantum infection in dogs in Spain, Part I: Mapped seroprevalence and sand fly distributions. Parasit. Vectors 2020, 13, 204. [Google Scholar] [CrossRef]
- Gradoni, L.; Ferroglio, E.; Zanet, S.; Mignone, W.; Venco, L.; Bongiorno, G.; Fiorentino, E.; Cassini, R.; Grillini, M.; Simonato, G.; et al. Monitoring and detection of new endemic foci of canine leishmaniosis in northern continental Italy: An update from a study involving five regions (2018–2019). Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100676. [Google Scholar] [CrossRef]
- Le Rutte, E.A.; van der Wilt, L.S.; Bulstra, C.A.; Nieboer, D.; Kontoroupis, P.; de Vlas, S.J.; Richardus, J.H. Incidence and geographical distribution of canine leishmaniosis in 2016–2017 in Spain and France. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100613. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of visceral leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef]
- Maia, C.; Cardoso, L. Spread of Leishmania infantum in Europe with dog travelling. Vet. Parasitol. 2015, 213, 2–11. [Google Scholar] [CrossRef]
- Chalghaf, B.; Chemkhi, J.; Mayala, B.; Harrabi, M.; Benie, G.B.; Michael, E.; ben Salah, A. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: Impact of climate change. Parasit. Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; da Silva, M.E.; dos Santos, C.C.P.; Frézard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. BioMed Res. Int. 2018, 29, 3296893. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística. Doenças de Declaração Obrigatória em Portugal. 2017. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0009142&contexto=bd&selTab=tab2&xlang=pt (accessed on 14 August 2022).
- Cortes, S.; Vaz, Y.; Neves, R.; Maia, C.; Cardoso, L.; Campino, L. Risk factors for canine leishmaniasis in an endemic Mediterranean region. Vet. Parasitol. 2012, 189, 189–196. [Google Scholar] [CrossRef]
- Pires, H.; Martins, M.; Matos, A.C.; Cardoso, L.; Monteiro, F.; Roque, N.; Nunes, T.; Gottstein, B.; Cortes, H. Geospatial analysis applied to seroepidemiological survey of canine leishmaniosis in east-central Portugal. Vet. Parasitol. 2019, 274, 108930. [Google Scholar] [CrossRef]
- Maia, C.; Coimbra, M.; Ramos, C.; Cristóvão, J.M.; Cardoso, L.; Campino, L. Serological investigation of Leishmania infantum, Dirofilaria immitis and Angiostrongylus vasorum in dogs from southern Portugal. Parasit. Vectors 2015, 8, 158. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Diaz, S.; Santos, C.; Bourdeau, P.; Pereira, I. Geographical Distribution, Clinical Presentation, Treatment and Prevention of Canine Leishmaniosis in Portugal: A 2007 Field Survey. Rev. Port. Ciências Veterinárias 2010, 109, 21–29. [Google Scholar]
- Solano-Gallego, L.; Villanueva-Saz, S.; Carbonell, M.; Trotta, M.; Furlanello, T.; Natale, A. Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan®, ID Screen® and Leishmania 96®), a rapid test (Speed Leish K®) and an in-house IFAT. Parasit. Vectors 2014, 7, 111. [Google Scholar] [CrossRef]
- Monteiro, M.; Prata, S.; Cardoso, L.; Pereira da Fonseca, I.; Leal, R.O. Diagnosis and clinical management of canine leishmaniosis by general veterinary practitioners: A questionnaire-based survey in Portugal. Parasit. Vectors 2021, 14, 306. [Google Scholar] [CrossRef]
- Oskam, L.; Slappendel, R.J.; Beijer, E.G.M.; Kroon, N.C.M.; van Ingen, C.W.; Özensoy, S.; Özbel, Y.; Terpstra, W.J. Dog-DAT: A direct agglutination test using stabilized, freeze-dried antigen for the serodiagnosis of canine visceral leishmaniasis. FEMS Immunol. Med. Microbiol. 1996, 16, 235–239. [Google Scholar] [CrossRef][Green Version]
- Ferreira, E.C.; Lana, M.; Carneiro, M.; Reis, A.B.; Paes, D.V.; Silva, E.S.; Schallig, H.; Gontijo, C.M.F. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet. Parasitol. 2007, 146, 235–241. [Google Scholar] [CrossRef]
- Mendonça, I.L.; Batista, J.F.; Schallig, H.; Cruz, M.D.S.P.; Alonso, D.P.; Ribolla, P.E.M.; Costa, D.L.; Costa, C.H.N. The Performance of Serological Tests for Leishmania Infantum Infection Screening in Dogs Depends on the Prevalence of the Disease. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e39. [Google Scholar] [CrossRef][Green Version]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic Challenges in the Era of Canine Leishmania infantum Vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef]
- Velez, R.; Domenech, E.; Cairó, J.; Gállego, M. The impact of canine leishmaniosis vaccination with CaniLeish® in Leishmania infantum infection seroprevalence studies. Acta Trop. 2020, 202, 105259. [Google Scholar] [CrossRef]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Sargent, E. Epitools Epidemiological Calculators. Ausvet. 2018. Available online: https://epitools.ausvet.com.au/ (accessed on 5 June 2022).
- Humphry, R.W.; Cameron, A.; Gunn, G.J. A practical approach to calculate sample size for herd prevalence surveys. Prev. Vet. Med. 2004, 65, 173–188. [Google Scholar] [CrossRef]
- Cardoso, L.; Schallig, H.D.F.H.; Neto, F.; Kroon, N.; Rodrigues, M. Serological survey of Leishmania infection in dogs from the municipality of Peso da Régua (Alto Douro, Portugal) using the direct agglutination test (DAT) and fast agglutination screening test (FAST). Acta Trop. 2004, 91, 95–100. [Google Scholar] [CrossRef]
- Fagerland, M.W.; Hosmer, D.W. A generalized Hosmer-Lemeshow goodness-of-fit test for multinomial logistic regression models. Stata J. 2012, 12, 447–453. Available online: https://journals.sagepub.com/doi/pdf/10.1177/1536867X1201200307 (accessed on 5 June 2022).
- Greiner, M.; Gardner, I.A. Application of diagnostic tests in veterinary epidemiologic studies. Prev. Vet. Med. 2000, 45, 43–59. [Google Scholar] [CrossRef]
- ESCCAP. ESCCAP Guidelines. Control of Vector-Borne Diseases in Dogs and Cats, 3rd ed.; ESCCAP: Worcestershire, UK, 2019; ISBN 978-1-907259-69-2. [Google Scholar]
- Campino, L.; Maia, C. Epidemiologia das leishmanioses em Portugal. Acta Med. Port. 2010, 23, 859–864. [Google Scholar]
- Branco, S.; Alves-Pires, C.; Maia, C.; Cortes, S.; Cristovão, J.M.S.; Gonçalves, L.; Campino, L.; Afonso, M.O. Entomological and ecological studies in a new potential zoonotic leishmaniasis focus in Torres Novas municipality, Central Region, Portugal. Acta Trop. 2013, 125, 339–348. [Google Scholar] [CrossRef]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal Dynamics of Phlebotomine Sand Fly Species Proven Vectors of Mediterranean Leishmaniasis Caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef]
- Maia, C.; Altet, L.; Serrano, L.; Cristóvão, J.M.; Tabar, M.D.; Francino, O.; Cardoso, L.; Campino, L.; Roura, X. Molecular detection of Leishmania infantum, filariae and Wolbachia spp. in dogs from southern Portugal. Parasit. Vectors 2016, 9, 170. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Sousa, S.; Lopes, A.P.; Cardoso, L.; Silvestre, R.; Schallig, H.; Reed, S.G.; Cordeiro da Silva, A. Seroepidemiological survey of Leishmania infantum infection in dogs from northeastern Portugal. Acta Trop. 2011, 120, 82–87. [Google Scholar] [CrossRef]
- Velez, R.; Ballart, C.; Domenech, E.; Abras, A.; Fernández-Arévalo, A.; Gómez, S.A.; Tebar, S.; Muñoz, C.; Cairó, J.; Gállego, M. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: The example of North-Eastern and Pyrenean areas of Spain. Prev. Vet. Med. 2019, 162, 67–75. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Llull, J.; Ramos, G.; Riera, C.; Arboix, M.; Alberola, J.; Ferrer, L. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet. Parasitol. 2000, 90, 37–45. [Google Scholar] [CrossRef]
- Tamponi, C.; Scarpa, F.; Carta, S.; Knoll, S.; Sanna, D.; Gai, C.; Pipia, A.P.; Dessì, G.; Casu, M.; Varcasia, A.; et al. Seroprevalence and risk factors associated with Leishmania infantum in dogs in Sardinia (Italy), an endemic island for leishmaniasis. Parasitol. Res. 2021, 120, 289. [Google Scholar] [CrossRef]
- Selim, A.; Shoulah, S.; Abdelhady, A.; Alouffi, A.; Alraey, Y.; Al-Salem, W.S. Seroprevalence and Risk Factors Associated with Canine Leishmaniasis in Egypt. Vet. Sci. 2021, 8, 236. [Google Scholar] [CrossRef]
- Rombolà, P.; Barlozzari, G.; Carvelli, A.; Scarpulla, M.; Iacoponi, F.; Macrì, G. Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic Mediterranean region. PLoS ONE 2021, 16, e0244923. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef]
- Symeonidou, I.; Angelou, A.; Theodoridis, A.; Sioutas, G.; Papadopoulos, E. Canine Leishmaniosis in Greece: An Updated Countrywide Serological Study and Associated Risk Factors. Pathogens 2021, 10, 1129. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Marques, M.J.; Veloso, V.M.; Roatt, B.M.; Oliveira Aguiar-Soares, R.D.; Reis, L.E.S.; Braga, S.L.; Morais, M.H.F.; Reis, A.B.; Carneiro, M. Prevalence and factors associated with Leishmania infantum infection of dogs from an urban area of Brazil as identified by molecular methods. PLoS Negl. Trop. Dis. 2011, 5, e1291. [Google Scholar] [CrossRef]
- Gálvez, R.; Miró, G.; Descalzo, M.A.; Nieto, J.; Dado, D.; Martín, O.; Cubero, E.; Molina, R. Emerging trends in the seroprevalence of canine leishmaniasis in the Madrid region (central Spain). Vet. Parasitol. 2010, 169, 327–334. [Google Scholar] [CrossRef]
- Baxarias, M.; Homedes, J.; Mateu, C.; Attipa, C.; Solano-Gallego, L. Use of preventive measures and serological screening tools for Leishmania infantum infection in dogs from Europe. Parasit. Vectors 2022, 15, 134. [Google Scholar] [CrossRef]
- Le Rutte, E.A.; van Straten, R.; Overgaauw, P.A.M. Awareness and control of canine leishmaniosis: A survey among Spanish and French veterinarians. Vet. Parasitol. 2018, 253, 87–93. [Google Scholar] [CrossRef]
- Fernandez Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Maranon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Fernandez, M.; Tabar, M.D.; Arcas, A.; Mateu, C.; Homedes, J.; Roura, X. Comparison of efficacy and safety of preventive measures used against canine leishmaniasis in southern European countries: Longitudinal retrospective study in 1647 client-owned dogs (2012–2016). Vet. Parasitol. 2018, 263, 10–17. [Google Scholar] [CrossRef]
- Montoya, A.; Checa, R.; Marino, V.; Gálvez, R.; Portero, M.; de Mari, K.; Navarro, C.; Miró, G. Antibodies elicited by the CaniLeish® vaccine: Long-term clinical follow-up study of dogs in Spain. Parasitol. Res. 2021, 120, 1471–1479. [Google Scholar] [CrossRef]
- Mohebali, M.; Keshavarz, H.; Shirmohammad, S.; Akhoundi, B.; Borjian, A.; Hassanpour, G.; Mamishi, S.; Mahmoudi, S. The diagnostic accuracy of direct agglutination test for serodiagnosis of human visceral leishmaniasis: A systematic review with meta-analysis. BMC Infect. Dis. 2020, 20, 946. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef]
| Region/District | No. Veterinary Clinics | No. Dog Samples | No. Seropositive Dogs (%) | % True Seroprevalence | 95% CI |
|---|---|---|---|---|---|
| Littoral/Interior | |||||
| Littoral | 59 | 1076 | 99 (9.2) | 9.7 | 8.0–11.7 |
| Interior | 39 | 784 | 118 (15.1) * | 15.8 | 13.4–18.7 |
| North | 26 | 458 | 42 (9.2) | 9.6 | 7.2–12.8 |
| Braga 1 | 6 | 93 | 6 (6.5) | 6.9 | 3.2–14.4 |
| Bragança 2 | 5 | 82 | 12 (14.6) | 15.7 | 9.2–25.9 |
| Porto 1 | 7 | 128 | 11 (8.6) | 9.2 | 5.2–15.8 |
| Viana do Castelo 1 | 3 | 51 | 0 (0) | 0 | 0.0–7.5 |
| Vila Real 2 | 5 | 104 | 13 (12.5) | 13.4 | 8.0–21.7 |
| Centre | 31 | 529 | 63 (11.9) | 12.5 | 9.9–15.7 |
| Aveiro 1 | 5 | 89 | 1 (1.1) | 1.2 | 0.0–6.4 |
| Castelo Branco 2 | 3 | 72 | 20 (27.8) | 29.9 | 20.1–42.0 |
| Coimbra 1 | 8 | 131 | 20 (15.3) | 16.4 | 10.9–24.1 |
| Guarda 2 | 2 | 40 | 7 (17.9) | 19.3 | 9.6–35.1 |
| Leira 1 | 7 | 110 | 4 (3.6) | 3.9 | 1.5–9.6 |
| Viseu 2 | 6 | 87 | 11 (12.8) | 13.8 | 7.8–23.1 |
| Lisbon and Tagus Valley | 25 | 496 | 53 (10.7) | 11.2 | 8.7–14.4 |
| Lisboa 1 | 12 | 210 | 18 (8.6) | 9.2 | 5.9–14.1 |
| Santarém 2 | 7 | 161 | 17 (10.6) | 11.4 | 7.2–17.5 |
| Setúbal 1 | 6 | 125 | 18 (14.4) | 15.5 | 10.0–23.2 |
| Alentejo | 11 | 247 | 37 (15.3) | 16.1 | 11.9–21.4 |
| Beja 2 | 4 | 60 | 8 (13.3) | 14.3 | 7.4–26.0 |
| Évora 2 | 5 | 122 | 12 (9.8) | 10.6 | 6.2–17.6 |
| Portalegre 2 | 2 | 60 | 17 (28.3) | 30.5 | 19.9–43.8 |
| Algarve | 5 | 135 | 22 (16.3) | 17.2 | 11.8–24.7 |
| Faro 1 | 5 | 135 | 22 (16.3) | 17.2 | 11.8–25.2 |
| Total | 98 | 1860 | 217 (11.7) | 12.5 | 10.3–13.2 |
| Variables | No. Samples (%) | No. Seropositive Dogs (%) | 95% CI |
|---|---|---|---|
| Sex 1 | 1834 | ||
| Female | 944 (51.5) | 105 (11.1) | 9.1–13.1 |
| Male | 890 (48.5) | 111 (12.5) | 10.3–14.7 |
| Age group (years) 2 | 1731 | * | |
| (0.5–2) | 506 (29.2) | 36 (7.1) | 0.0–15.4 |
| (3–5) | 465 (26.9) | 64 (13.8) | 5.3–22.2 |
| (6–8) | 387 (22.4) | 51 (13.2) | 3.9–22.5 |
| (9–11) | 243 (14.0) | 27 (11.1) | 0.0–22.9 |
| (12–17) | 130 (7.5) | 20 (15.4) | 0.0–31.2 |
| Breed Non-autochthonous pure breed Autochthonous pure breed Crossbreed Mongrel dogs | 1860 | ||
| 819 (43.9) | 104 (12.7) | 6.3–19.1 | |
| 104 (5.9) | 15 (14.4) | 0.0–32.2 | |
| 165 (8.9) 772 (41.6) | 12 (7.3) 86 (11.1) | 0.0–22.0 4.4–17.7 | |
| Fur size 3 | 1841 | ||
| Short | 1109 (60.2) | 135 (12.2) | 6.7–17.7 |
| Medium | 505 (27.4) | 57 (11.3) | 3.1–19.5 |
| Long | 227 (12.3) | 24 (10.6) | 0.0–22.9 |
| Dog’s housing 4 | 1835 | ||
| Exclusively outdoors | 227 (12.4) | 22 (9.7) | 0.0–22.1 |
| Mostly outdoors | 637 (34.7) | 73 (11.5) | 4.2–18.8 |
| Equally indoors and outdoors | 323 (17.6) | 33 (10.2) | 0.0–20.5 |
| Mostly indoors | 252 (13.7) | 31 (12.3) | 0.1–23.9 |
| Exclusively indoors | 396 (21.6) | 58 (14.6) | 5.5–23.7 |
| Repellents/ insecticides 5 | 1825 | ||
| Effective | 732 (40.1) | 90 (12.3) | 5.5–19.1 |
| Noneffective | 295 (16.2) | 26 (8.8) | 0.0–19.7 |
| Use but unknown | 242 (13.3) | 25 (10.3) | 0.0–22.2 |
| Nonuse | 556 (30.4) | 73 (13.1) | 5.3–20.8 |
| Vaccination status 6 | 1824 | * | |
| Vaccinated with LetiFend® | 142 (7.8) | 26 (18.3) | 3.4–33.2 |
| Vaccinated with CaniLeish® | 63 (3.5) | 30 (47.6) | 29.7–65.5 |
| Vaccinated (unknown vaccine) | 66 (3.6) | 16 (24.2) | 3.2–45.2 |
| Nonvaccinated | 1553 (85.1) | 142 (9.1) | 4.3–13.8 |
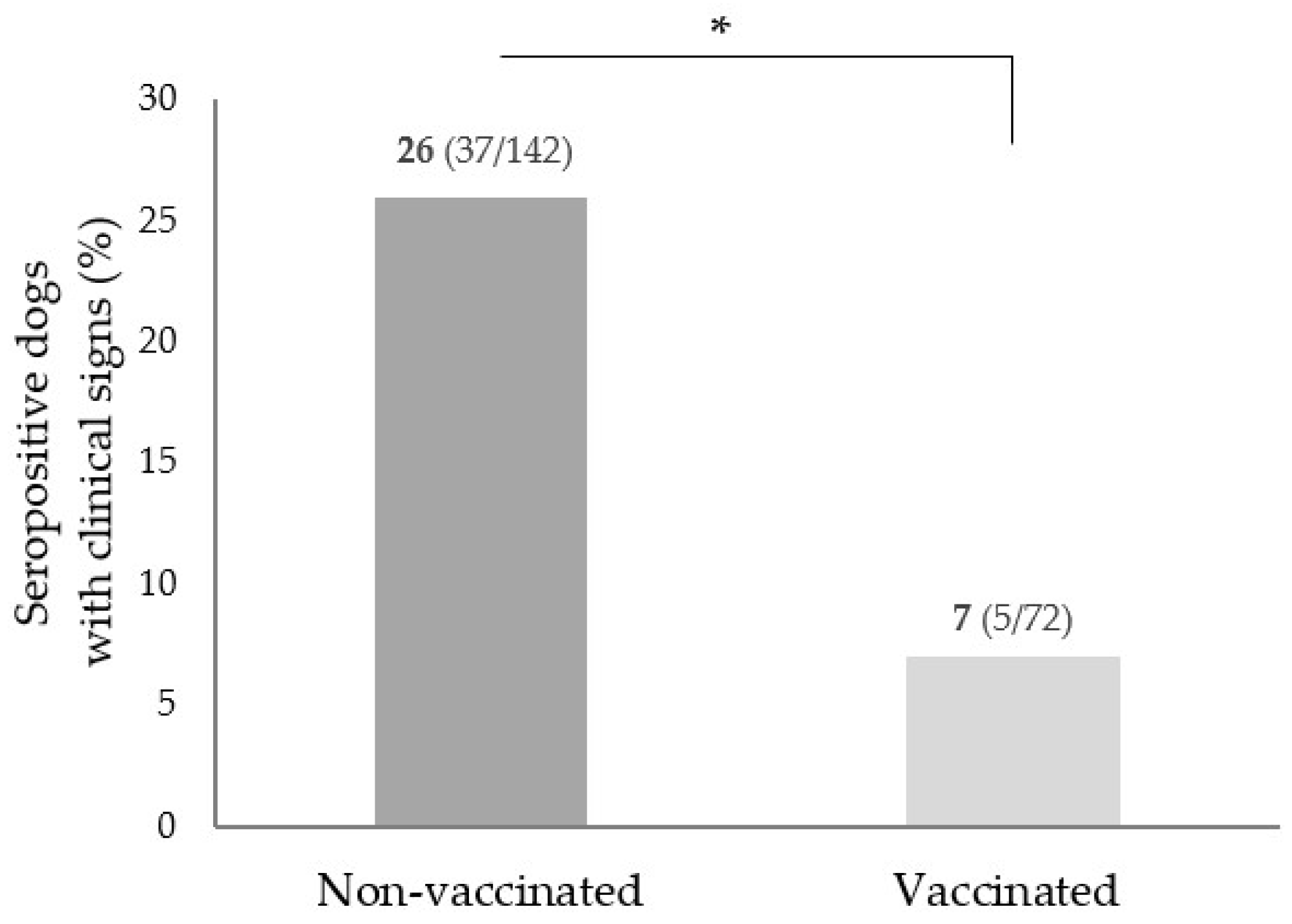
| CanL clinical signs 7 | 1804 | * | |
| Yes | 112 (6.2) | 42 (37.5) | 22.8–52.1 |
| No | 1692 (93.8) | 175 (10.3) | 5.8–14.8 |
| Risk Factor * | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| % in Sample | Crude OR | 95% CI | Adjusted OR | 95% CI | p-Value | |
| Older than 2 years | 30.1 | 1.60 | 1.0–2.5 | 1.68 | 1.1–2.6 | 0.02 |
| Residing in the Interior | 42.5 | 2.21 | 1.6–3.1 | 1.92 | 1.3–2.9 | 0.002 |
| Residing in Alentejo | 11.5 | 2.01 | 1.3–3.1 | -- | -- | -- |
| Nonuse of repellents/insecticides | 30.9 | 1.60 | 1.1–2.3 | 1.75 | 1.2–2.5 | 0.003 |
| Constant | 0.050 | <0.001 | ||||
| Hosmer and Lemeshow Test | Sig. = 0.811 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.; Maia, C.; Cristóvão, J.M.; Morgado, C.; Barbosa, I.; Ibars, R.F.; Campino, L.; Gonçalves, L.; Cortes, S. Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms 2022, 10, 2262. https://doi.org/10.3390/microorganisms10112262
Almeida M, Maia C, Cristóvão JM, Morgado C, Barbosa I, Ibars RF, Campino L, Gonçalves L, Cortes S. Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms. 2022; 10(11):2262. https://doi.org/10.3390/microorganisms10112262
Chicago/Turabian StyleAlmeida, Maria, Carla Maia, José M. Cristóvão, Cátia Morgado, Inês Barbosa, Ruben Foj Ibars, Lenea Campino, Luzia Gonçalves, and Sofia Cortes. 2022. "Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal" Microorganisms 10, no. 11: 2262. https://doi.org/10.3390/microorganisms10112262
APA StyleAlmeida, M., Maia, C., Cristóvão, J. M., Morgado, C., Barbosa, I., Ibars, R. F., Campino, L., Gonçalves, L., & Cortes, S. (2022). Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms, 10(11), 2262. https://doi.org/10.3390/microorganisms10112262







