First Report of Autochthonous Canine Leishmaniasis in Hong Kong
Abstract
1. Introduction
2. Materials and Methods
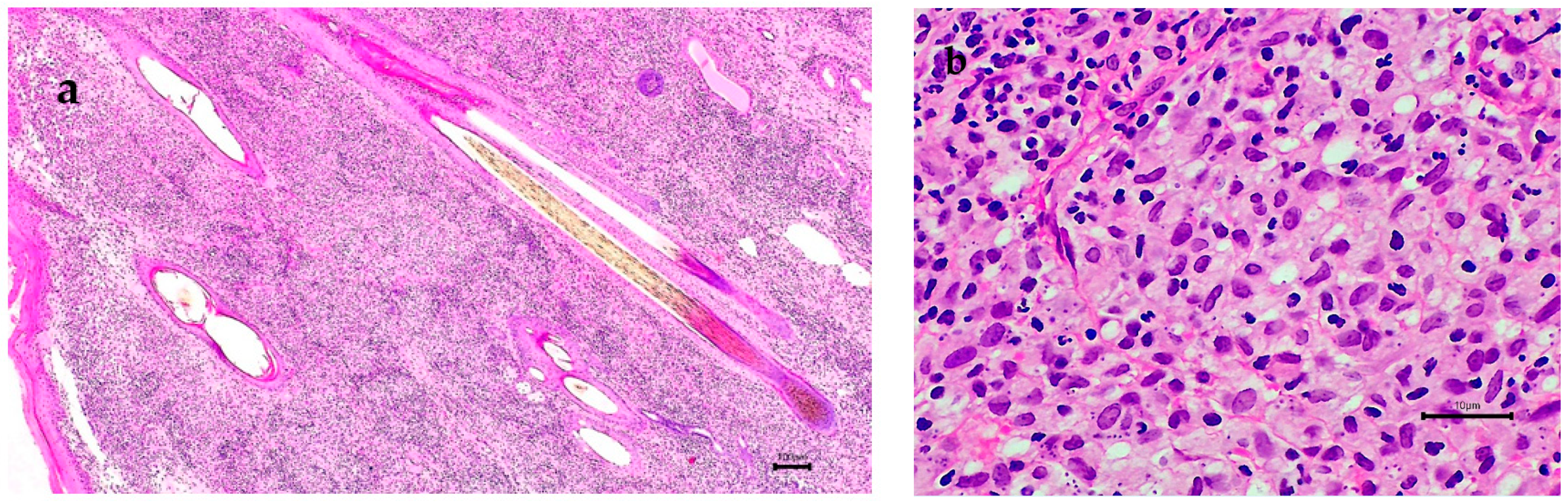
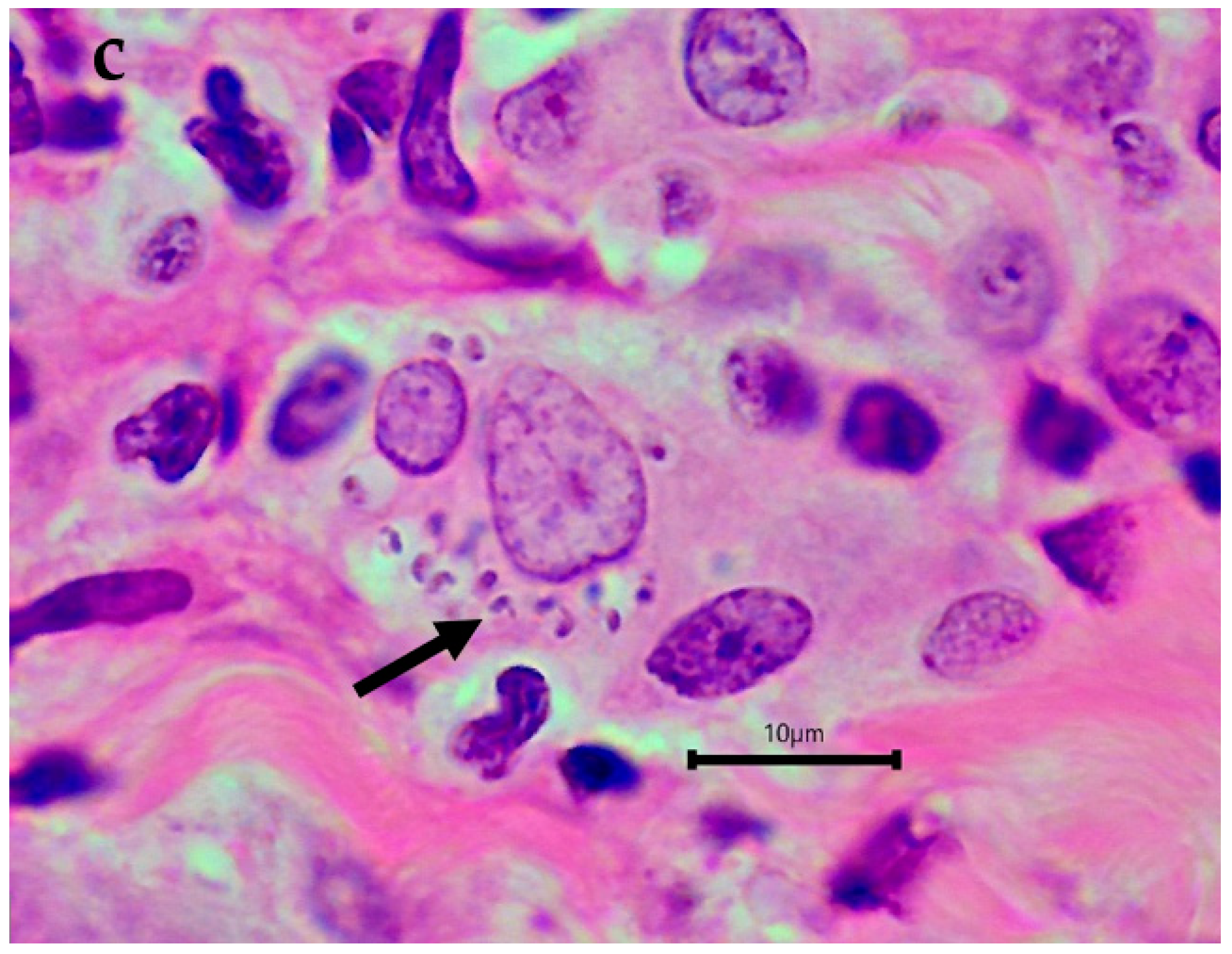
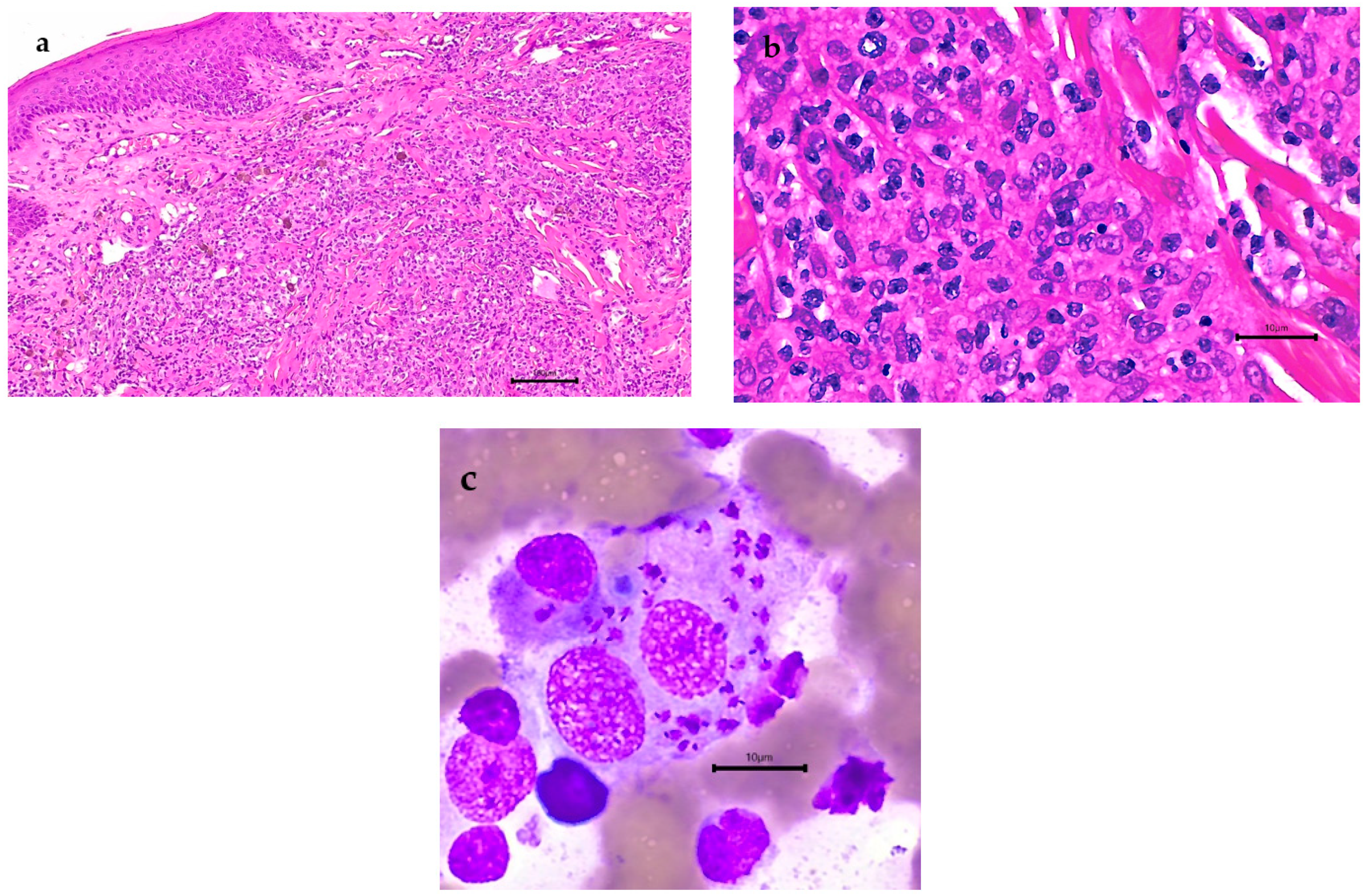
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- José, A.R.-P.; Saurabh, J.; Alexei, M.; Ana, N.M.-E.; Samantha, V.; Supriya, W.; Mona, O.; Zaw, L.; Abate, B.; Aya, Y.; et al. Global Leishmaniasis Surveillance: 2019–2020, a Baseline for the 2030 Roadmap. Available online: https://www.who.int/publications/i/item/who-wer9635-401-419 (accessed on 2 September 2022).
- Lun, Z.R.; Wu, M.S.; Chen, Y.F.; Wang, J.Y.; Zhou, X.N.; Liao, L.F.; Chen, J.P.; Chow, L.M.; Chang, K.P. Visceral Leishmaniasis in China: An Endemic Disease under Control. Clin. Microbiol. Rev. 2015, 28, 987–1004. [Google Scholar] [CrossRef]
- Nicolas, L.; Milon, G.; Prina, E. Rapid differentiation of Old World Leishmania species by LightCycler polymerase chain reaction and melting curve analysis. J. Microbiol. Methods 2002, 51, 295–299. [Google Scholar] [CrossRef]
- Talmi-Frank, D.; Nasereddin, A.; Schnur, L.F.; Schönian, G.; Töz, S.O.; Jaffe, C.L.; Baneth, G. Detection and identification of old world Leishmania by high resolution melt analysis. PLoS Negl. Trop. Dis. 2010, 4, e581. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Nachum-Biala, Y.; Zuberi, A.; Zipori-Barki, N.; Orshan, L.; Kleinerman, G.; Shmueli-Goldin, A.; Bellaiche, M.; Leszkowicz-Mazuz, M.; Salant, H.; et al. Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasit. Vectors 2020, 13, 115. [Google Scholar] [CrossRef]
- Michalsky, E.M.; Rocha, M.F.; da Rocha Lima, A.C.; França-Silva, J.C.; Pires, M.Q.; Oliveira, F.S.; Pacheco, R.S.; dos Santos, S.L.; Barata, R.A.; Romanha, A.J.; et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet. Parasitol. 2007, 147, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef]
- Marcondes, M.; Day, M.J. Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci. 2019, 123, 261–272. [Google Scholar] [CrossRef]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef]
- Laurenti, M.D.; Rossi, C.N.; da Matta, V.L.; Tomokane, T.Y.; Corbett, C.E.; Secundino, N.F.; Pimenta, P.F.; Marcondes, M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet. Parasitol. 2013, 196, 296–300. [Google Scholar] [CrossRef]
- Magalhães-Junior, J.T.; Mota, T.F.; Porfirio-Passos, G.; Larangeira, D.F.; Franke, C.R.; Barrouin-Melo, S.M. Xenodiagnosis on dogs with visceral leishmaniasis: Canine and sand fly aspects related to the parasite transmission. Vet. Parasitol. 2016, 223, 120–126. [Google Scholar] [CrossRef]
- Serafim, T.D.; Iniguez, E.; Oliveira, F. Leishmania infantum. Trends Parasitol. 2020, 36, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Young, C.W.; Hertig, M. The development of flagellates in Chinese sandflies (phlebotomus) fed on hamsters infected with Leishmania donovani. Proc. Soc. Exp. Biol. Med. 1926, 23, 611–615. [Google Scholar] [CrossRef]
- Duprey, Z.H.; Steurer, F.J.; Rooney, J.A.; Kirchhoff, L.V.; Jackson, J.E.; Rowton, E.D.; Schantz, P.M. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg. Infect. Dis. 2006, 12, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.A. Leishmaniasis, an emerging disease found in companion animals in the United States. Top. Companion Anim. Med. 2009, 24, 182–188. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Gibson-Corley, K.N.; Metz, K.; Gallup, J.M.; Hostetter, J.M.; Mullin, K.; Petersen, C.A. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl. Trop. Dis. 2011, 5, e1019. [Google Scholar] [CrossRef]
- de Almeida, M.E.; Spann, D.R.; Bradbury, R.S. Leishmania infantum in US-Born Dog. Emerg. Infect. Dis. 2020, 26, 1882–1884. [Google Scholar] [CrossRef]
- Alonso, F.H.; Vasilatis, D.M.; Veluvolu, S.M.; Willcox, J.L.; Scorza, B.M.; Petersen, C.A.; Kol, A. Canine leishmaniasis in Northern California-A case report. Vet. Clin. Pathol. 2021, 50, 71–75. [Google Scholar] [CrossRef]
- Fondevila, D.; Vilafranca, M.; Ferrer, L. Epidermal immunocompetence in canine leishmaniasis. Vet. Immunol. Immunopathol. 1997, 56, 319–327. [Google Scholar] [CrossRef]
- Ferrer, L.; Rabanal, R.; Fondevila, D.; Ramos, J.; Domingo, M. Skin lesions in canine leishmaniasis. J. Small Anim. Pract. 1988, 29, 381–388. [Google Scholar] [CrossRef]
- Ordeix, L.; Dalmau, A.; Osso, M.; Llull, J.; Montserrat-Sangrà, S.; Solano-Gallego, L. Histological and parasitological distinctive findings in clinically-lesioned and normal-looking skin of dogs with different clinical stages of leishmaniasis. Parasit. Vectors 2017, 10, 121. [Google Scholar] [CrossRef]
- Toepp, A.J.; Bennett, C.; Scott, B.; Senesac, R.; Oleson, J.J.; Petersen, C.A. Maternal Leishmania infantum infection status has significant impact on leishmaniasis in offspring. PLoS Negl. Trop. Dis. 2019, 13, e0007058. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, F.; Sozzi, S. Isolation of Leishmania from a newborn puppy. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 402. [Google Scholar] [CrossRef]
- Díaz-Espiñeira, M.M.; Slappendel, R.J. A case of autochthonous canine leishmaniasis in The Netherlands. Vet. Q. 1997, 19, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, A.A.; Schantz, P.; Jackson, J.; Birkenheuer, A.; Tomlinson, L.; Gramiccia, M.; Levy, M.; Steurer, F.; Kollmar, E.; Hegarty, B.C.; et al. Visceral leishmaniasis in a New York foxhound kennel. J. Vet. Intern. Med. 2002, 16, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Salant, H.; Nachum-Biala, Y.; Feinmesser, B.; Perelmutter, M.; Baneth, G. Early onset of clinical leishmaniosis in a litter of pups with evidence of in utero transmission. Parasit. Vectors. 2021, 14, 326. [Google Scholar] [CrossRef]
- Owens, S.D.; Oakley, D.A.; Marryott, K.; Hatchett, W.; Walton, R.; Nolan, T.J.; Newton, A.; Steurer, F.; Schantz, P.; Giger, U. Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J. Am. Vet. Med. Assoc. 2001, 219, 1076–1083. [Google Scholar] [CrossRef]
- Svobodova, V.; Svoboda, M.; Friedlaenderova, L.; Drahotsky, P.; Bohacova, E.; Baneth, G. Canine leishmaniosis in three consecutive generations of dogs in Czech Republic. Vet. Parasitol. 2017, 237, 122–124. [Google Scholar] [CrossRef]
- Karkamo, V.; Kaistinen, A.; Näreaho, A.; Dillard, K.; Vainio-Siukola, K.; Vidgrén, G.; Tuoresmäki, N.; Anttila, M. The first report of autochthonous non-vector-borne transmission of canine leishmaniosis in the Nordic countries. Acta Vet. Scand. 2014, 56, 84. [Google Scholar] [CrossRef]
- Naucke, T.J.; Amelung, S.; Lorentz, S. First report of transmission of canine leishmaniosis through bite wounds from a naturally infected dog in Germany. Parasit. Vectors 2016, 9, 256. [Google Scholar] [CrossRef]
- Petanides, T.A.; Koutinas, A.F.; Mylonakis, M.E.; Day, M.J.; Saridomichelakis, M.N.; Leontides, L.S.; Mischke, R.; Diniz, P.; Breitschwerdt, E.B.; Kritsepi, M.; et al. Factors associated with the occurrence of epistaxis in natural canine leishmaniasis (Leishmania infantum). J. Vet. Intern. Med. 2008, 22, 866–872. [Google Scholar] [CrossRef]
- Foglia Manzillo, V.; Di Muccio, T.; Cappiello, S.; Scalone, A.; Paparcone, R.; Fiorentino, E.; Gizzarelli, M.; Gramiccia, M.; Gradoni, L.; Oliva, G. Prospective study on the incidence and progression of clinical signs in naïve dogs naturally infected by Leishmania infantum. PLoS Negl. Trop. Dis. 2013, 7, e2225. [Google Scholar] [CrossRef] [PubMed]
- Saridomichelakis, M.N.; Koutinas, A.F. Cutaneous involvement in canine leishmaniasis due to Leishmania infantum (syn. L. chagasi). Vet. Dermatol. 2014, 25, 61-e22–71-e22. [Google Scholar] [CrossRef] [PubMed]
- Squarre, D.; Chambaro, H.M.; Hayashida, K.; Moonga, L.C.; Qiu, Y.; Goto, Y.; Oparaocha, E.; Mumba, C.; Muleya, W.; Bwalya, P.; et al. Autochthonous Leishmania infantum in Dogs, Zambia, 2021. Emerg. Infect. Dis. 2022, 28, 888–890. [Google Scholar] [CrossRef]
- Levy, E.; Mylonakis, M.E.; Saridomichelakis, M.N.; Polizopoulou, Z.S.; Psychogios, V.; Koutinas, A.F. Nasal and oral masses in a dog. Vet. Clin. Pathol. 2006, 35, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Shaw, J.J.; Dye, C. Infectiousness in a cohort of brazilian dogs: Why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 2002, 186, 1314–1320. [Google Scholar] [CrossRef]
- Costa, F.A.; Goto, H.; Saldanha, L.C.; Silva, S.M.; Sinhorini, I.L.; Silva, T.C.; Guerra, J.L. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet. Pathol. 2003, 40, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Solcà Mda, S.; Bastos, L.A.; Guedes, C.E.; Bordoni, M.; Borja, L.S.; Larangeira, D.F.; da Silva Estrela Tuy, P.G.; Amorim, L.D.; Nascimento, E.G.; de Sá Oliveira, G.G.; et al. Evaluating the accuracy of molecular diagnostic testing for canine visceral leishmaniasis using latent class analysis. PLoS ONE 2014, 9, e103635. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.; Silveira, P.; Melo-Júnior, O.A.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed. Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Miró, G.; Cardoso, L.; Pennisi, M.G.; Oliva, G.; Baneth, G. Canine leishmaniosis--new concepts and insights on an expanding zoonosis: Part two. Trends Parasitol. 2008, 24, 371–377. [Google Scholar] [CrossRef] [PubMed]
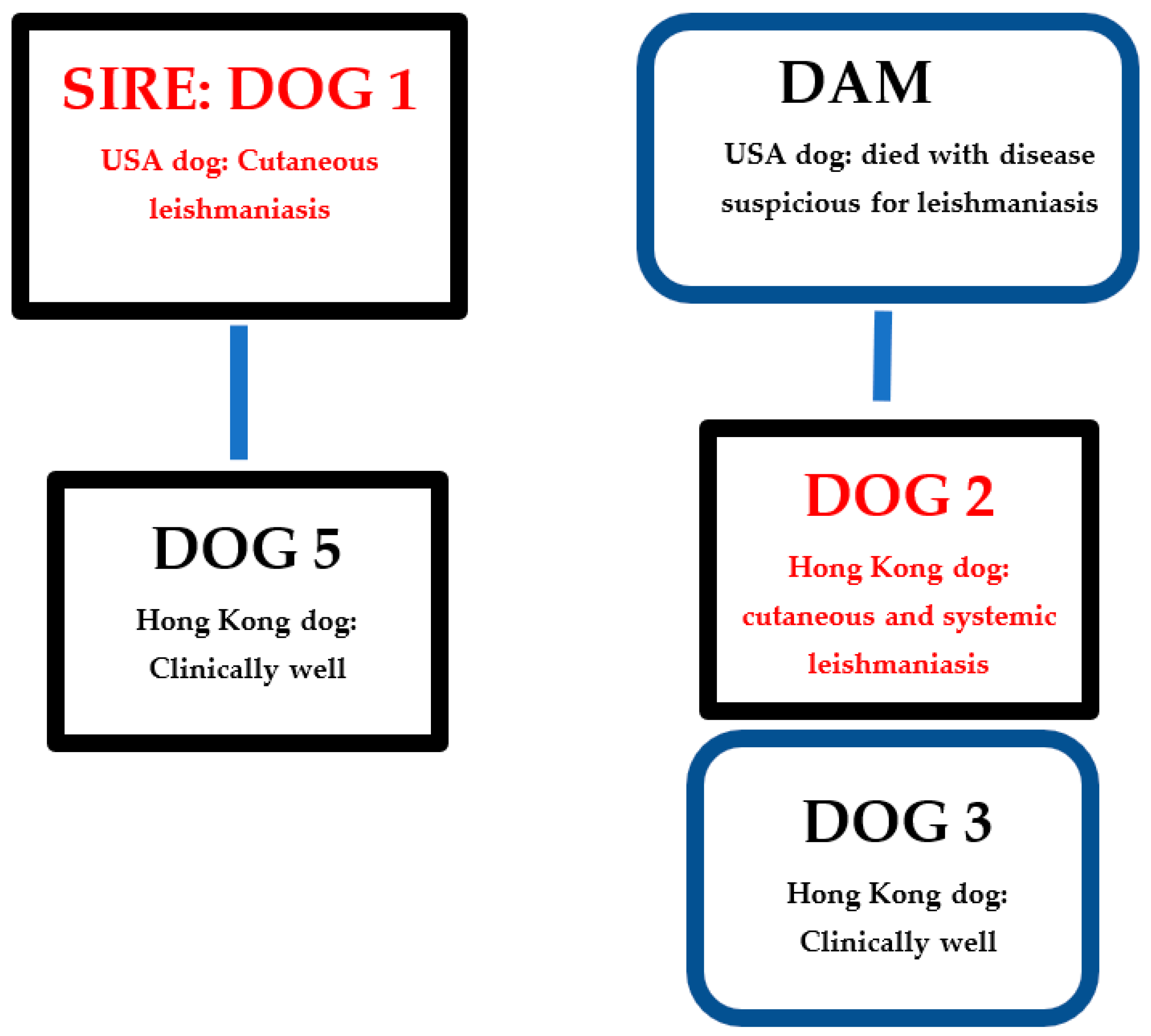
| Dog | Dog Country of Origin | Relationship with Other Dogs in Kennel | Year of Birth | Year of Test | Detection and Anatomic Location of Leishmania Amastigotes by Microscopy | ELISA Serology | PCR Result and Tissue | Leishmania Treatment/Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | USA | Sire of dog 5 | 2011 | 2015 | Yes: Skin | ND | − (blood) * | Skin disease resolved but relapsed |
| 2020 | ND | - | − (blood) + (skin) ** | Skin disease resolved | ||||
| 2 | Hong Kong | Full brother of dog 3 | 2012 | 2018 | Suspected: Skin and nasal mucosa | ND | ND | Skin disease resolved but relapsed and became systemic |
| 2019 | Yes: Spleen and liver | + *** | + (blood, spleen) | Disease resolved | ||||
| 3 | Hong Kong | Full sister of dog 2 | 2012 | 2019 | No | - | − (blood and skin) | NA |
| 4 | Netherlands | None | 2009 | 2019 | No | - | − (blood and skin) | NA |
| 5 | Hong Kong | Son of dog 1 | 2013 | 2020 | ND | - | − (blood) | NA |
| 6 | Hong Kong | None | 2011 | 2020 | ND | - | − (blood) | NA |
| 7 | Sweden | None | 2011 | 2020 | ND | - | − (blood) | NA |
| 8 | Netherlands | None | 2012 | 2020 | ND | - | − (blood) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandy, J.; Matthews, A.; Nachum-Biala, Y.; Baneth, G. First Report of Autochthonous Canine Leishmaniasis in Hong Kong. Microorganisms 2022, 10, 1873. https://doi.org/10.3390/microorganisms10091873
Sandy J, Matthews A, Nachum-Biala Y, Baneth G. First Report of Autochthonous Canine Leishmaniasis in Hong Kong. Microorganisms. 2022; 10(9):1873. https://doi.org/10.3390/microorganisms10091873
Chicago/Turabian StyleSandy, Jeanine, Anthony Matthews, Yaarit Nachum-Biala, and Gad Baneth. 2022. "First Report of Autochthonous Canine Leishmaniasis in Hong Kong" Microorganisms 10, no. 9: 1873. https://doi.org/10.3390/microorganisms10091873
APA StyleSandy, J., Matthews, A., Nachum-Biala, Y., & Baneth, G. (2022). First Report of Autochthonous Canine Leishmaniasis in Hong Kong. Microorganisms, 10(9), 1873. https://doi.org/10.3390/microorganisms10091873







