Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
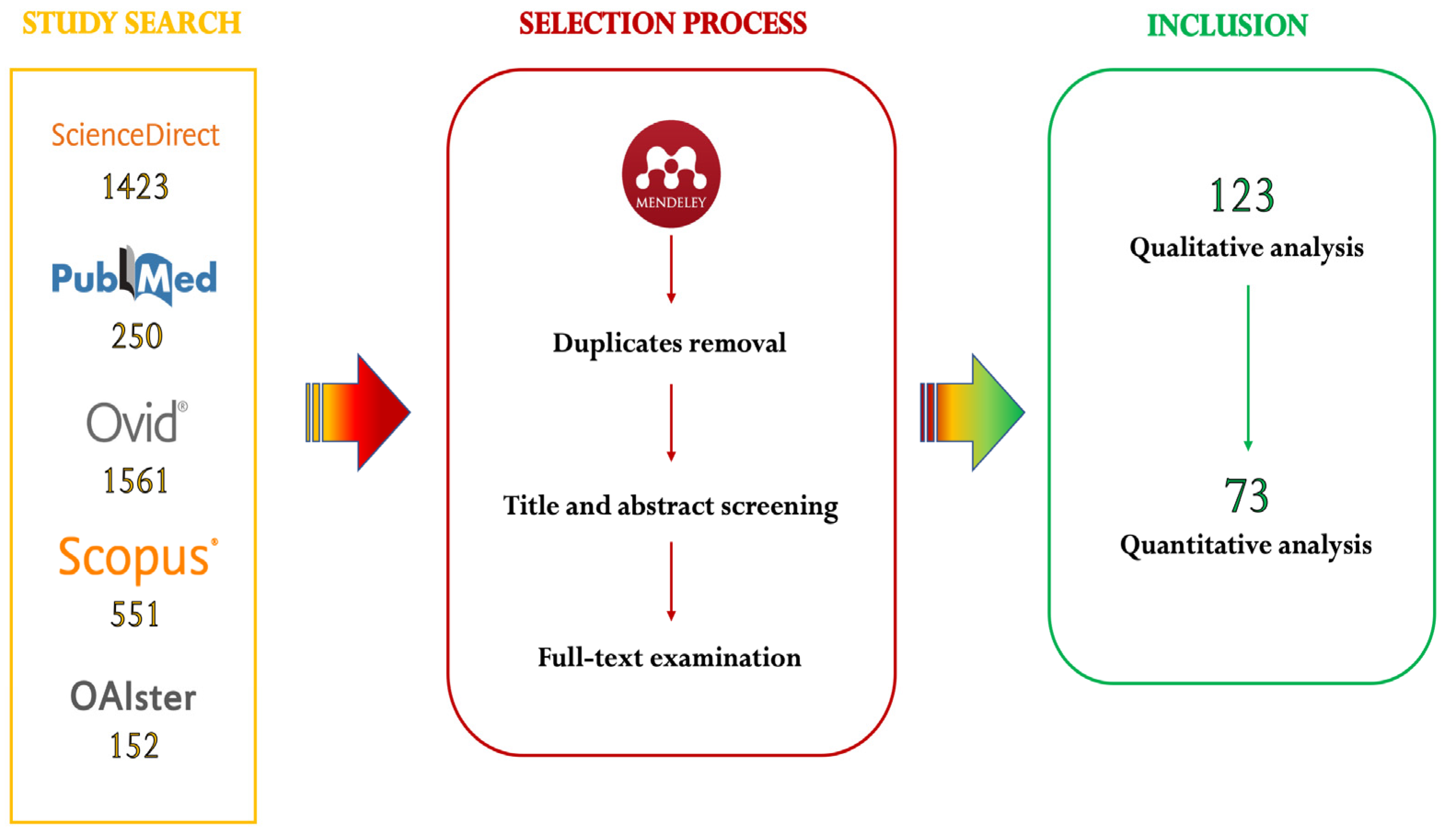
2.1. Search Strategy
2.2. Selection Process
2.3. Data Extraction and Critical Assessment of Included Studies
2.4. Qualitative and Quantitative Analyses
- ○
- Random effects model: the objective of our meta-analysis was to estimate the mean of the distribution of the true prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African tick populations, discarding the assumption that there is one true effect size which is shared between all the included studies (belonging to the fixed effects model). This choice was made on the assumption that microbial prevalence may differ greatly among tick populations based on several variables.
- ○
- Sidik–Jonkman variance estimator, with Hartung–Knapp adjustment: to retrieve more conservative results than the common DerSimonian–Laird method, indicated by wider confidence intervals (CI) [40].
- ○
- Clopper–Pearson confidence interval for individual studies: as above, to obtain wider confidence intervals especially when sample size is small [41], hesnce to retrieve more conservative results.
- ○
- Freeman–Tukey double-arcsine transformation: to avoid overestimation of the weight of studies reporting prevalence close to 0% or 100%. The final pooled estimate and 95% CIs were back-transformed to a proportion.
- ○
- Higgins and Thompson’s I2 statistic and prediction interval (PI): to assess between study heterogeneity. The I2 statistic is defined as the percentage of variability in the effect measure that is not caused by the sampling error. Low heterogeneity is represented by I2 = 25%, values of 50% indicate moderate heterogeneity, while substantial heterogeneity is represented by I2 ≥ 75%. Finally, the PI provides a range between which to expect the effects of future studies to fall based on present evidence [42].
- ○
- Subgroup analyses and multiple meta-regression: to investigate the heterogeneity between studies. In subgroup analyses, we hypothesized that studies in our meta-analysis did not originate from one overall population. We instead assumed that they fell into different subgroups and that each subgroup had its own true overall effect. Our aim was to reject the null hypothesis that there is no difference in effect measured between the subgroups. For each of the results having a moderate to high heterogeneity (i.e., I2 > 70%), we conducted a subgroup analysis where moderators/subgroups were chosen in advance: tick genus, tick species, sampling country, sampling period (categorized in “Before 2002”, “2002–2011” or “2012–2022”), tick origin (domestic animals vs. wild animals vs. environment), tick identification method, sampling strategy, molecular method and risk of bias. Unlike subgroup analyses, in multiple meta-regression, we used more than one predictor to explain variation in effects. A step-wise regression method was adopted to select predictors based on a statistical criterion, i.e., all the moderators that tested significant with the subgroup analysis were first included in the multiple meta-regression model and then removed one by one based on the model fit indexes (residual I2 and R2).
- ○
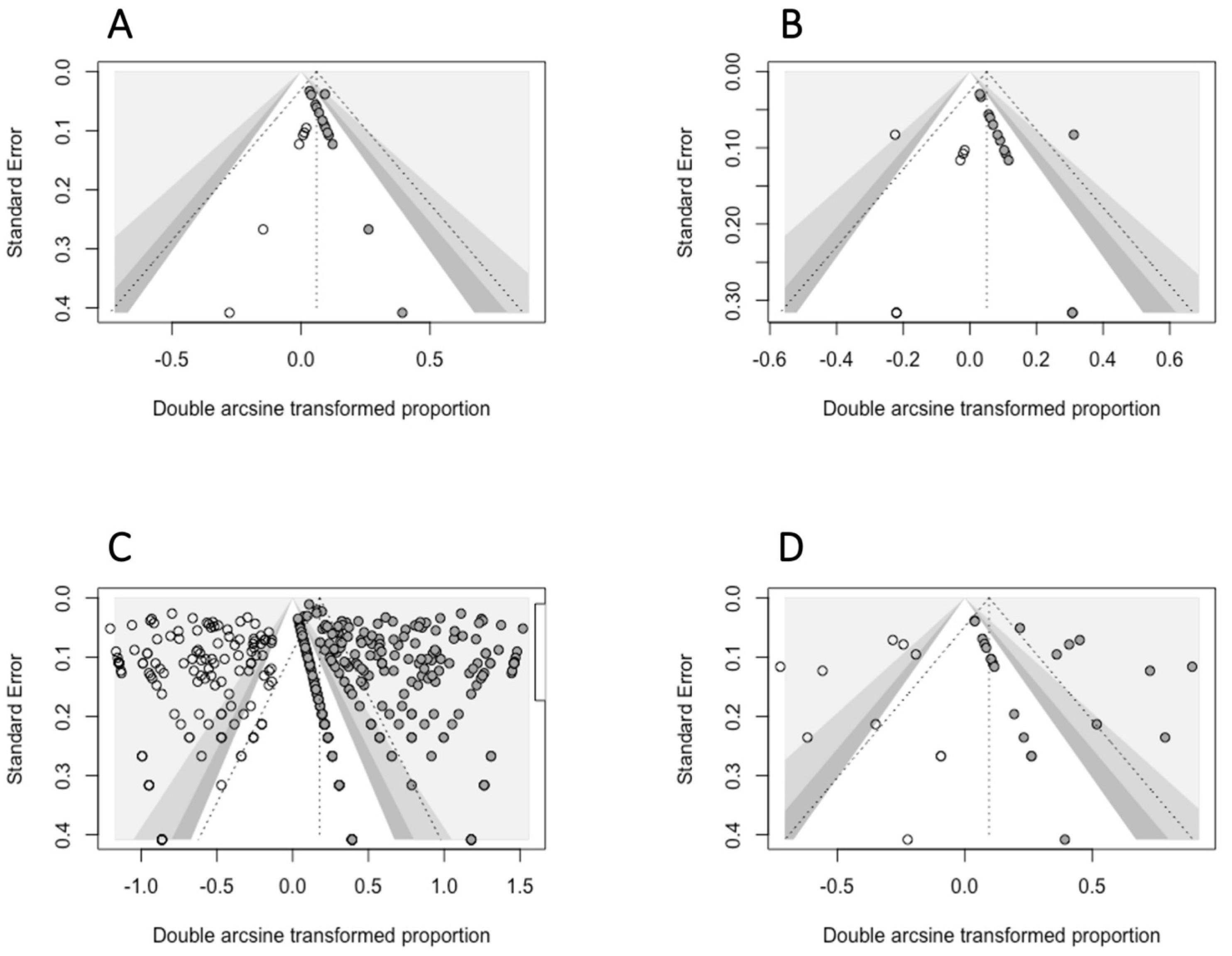
- The small-study-effects method was used to evaluate the presence of publication bias: according to Egger et al., 1997 [43], we assumed that only small studies with a high prevalence are published. This method relies on the evaluation of funnel plot asymmetry, assessed either qualitatively (visual inspection of the funnel plot) or quantitatively, using the Egger’s regression test. For this test, a p < 0.05 was interpreted as the presence of significant asymmetry in the funnel plot. When this condition was satisfied, we used the Duval and Tweedie Trim and Fill Method to adjust for funnel plot asymmetry, selecting the estimator L0 for imputing missing studies [44].
2.5. Quality Assessment of the Body of Evidence
- Initial QoE was based on the study design. In our case, the effect of interest was the molecular prevalence of pathogens in tick populations, which could only be reported by observational studies (prevalence-reporting surveys or cross-sectional studies) [46]. Consequently, the study design did not impact the QoE of our prevalence estimates and the initial QoE was, therefore, set to the same score (3.33) for all the studies.
- Five domains could downgrade the initial QoE to up to 0.67 points each. They were interpreted in the following way:
- Risk of bias: individual studies were classified as high, moderate or low risk of bias, using the AXIS tool. The risk of bias of each prevalence estimate was calculated as a weighted average of the papers included in the respective meta-analysis. Finally, if the average risk of bias was determined to be high, we decreased the QoE by 0.67 points, 0.33 points for moderate risk, while for low bias risk, no points were reduced.
- Publication bias: the QoE was downgraded for publication bias if the Egger’s test indicated significant asymmetry in the funnel plot (p ≤ 0.05).
- Imprecision: downgraded (−0.67 points) if the 95% confidence intervals are wider than 20% (i.e., error level > 20%).
- Inconsistency: our interpretation of inconsistency relied on the heterogeneity that was not explained by the determinants investigated. Therefore, the QoE was downgraded for inconsistency (−0.67 points) if initial (before meta-regression) and residual (after meta-regression) heterogeneity indices (i.e., I2) were higher than 75%.
- Indirectness: among the different interpretations of indirectness provided by the GRADE guidelines, we only considered the indirectness for intervention. More specifically, if the variable “Molecular test” significantly affected the estimated pooled prevalence during subgroup analysis (i.e., p-value of the test for subgroup differences < 0.05), we downgraded the QoE because of indirectness (−0.67 points). Indeed, significantly different results obtained with different molecular tests were due to moderate to high differences in test sensitivity and specificity that may create a biased estimate.
- Three domains could upgrade the QoE: large-effect, dose-response gradient and if residual confounding would only decrease the magnitude of the effect [47]. We considered the large-effect domain applicable to our study. In particular, we upgraded the QoE when a large magnitude of effect was present on either side, i.e., if the lower bound of the CIs was higher than 10% (considering that at least 1 out of 10 ticks was infected) or if the upper bound was less than 1% (considering that less than 1 out of 100 ticks was infected).
2.6. Reliability
2.7. Literate Programming and Search Update
2.8. Abbreviations
3. Results
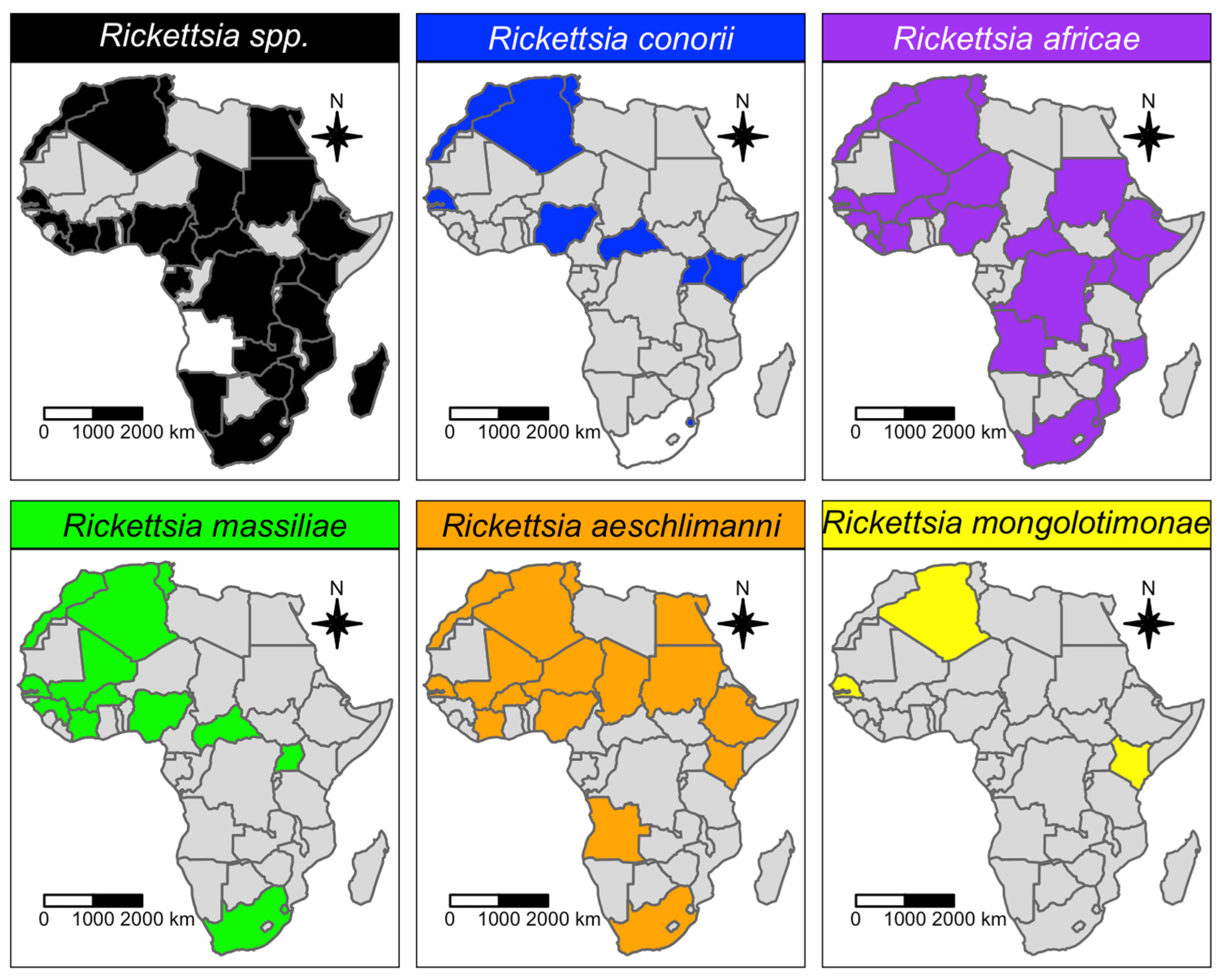
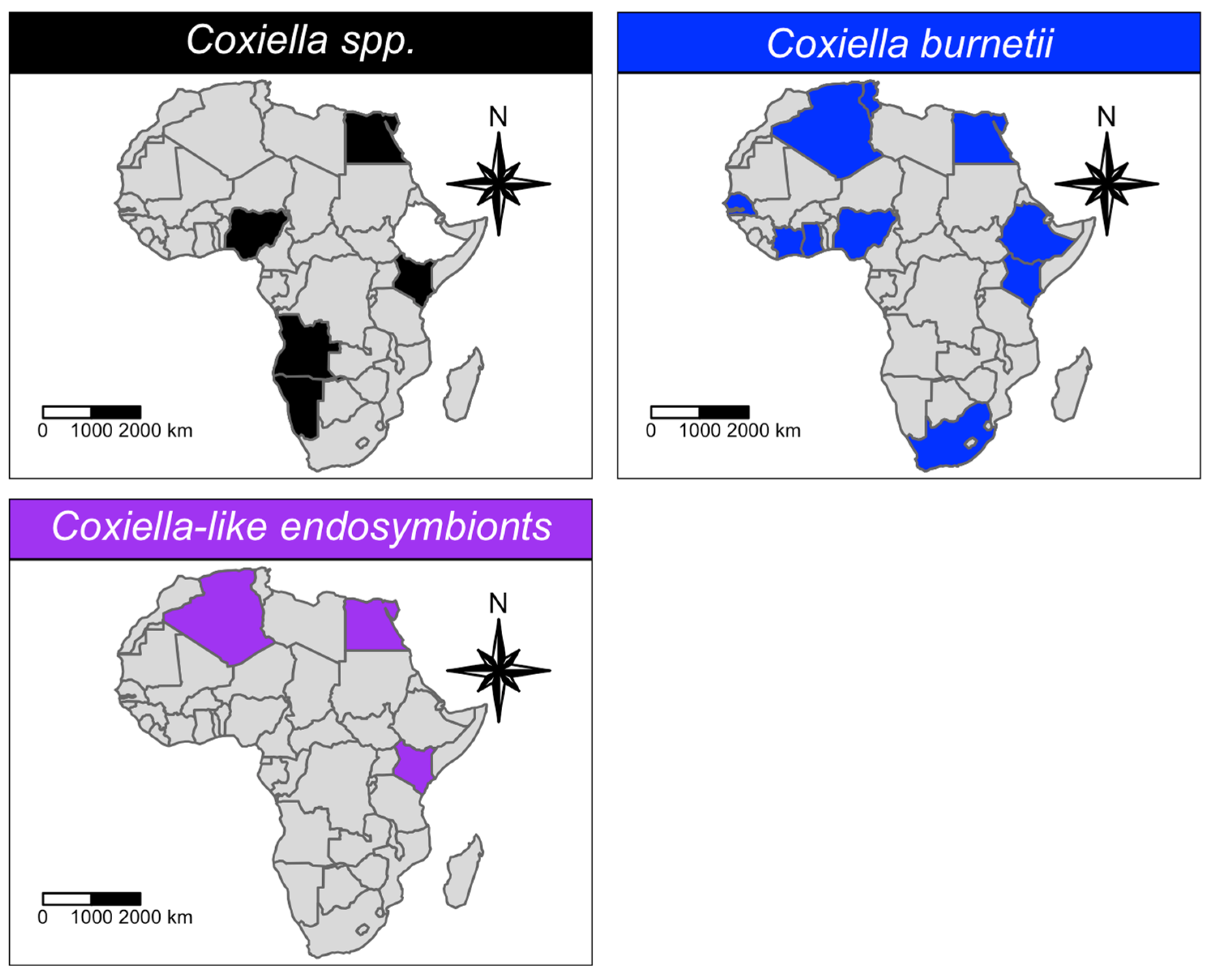
3.1. Qualitative Analysis
3.2. Quantitative Analysis
3.3. Quality of the Body of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peňa, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef]
- Ackermann, R.; Gall, C.; Brayton, K.; Collins, N.; Van Wyk, I.; Wentzel, J.; Kolo, A.; Oosthuizen, M.C. The bacterial microbiome of Rhipicephalus sanguineus ticks in the Mnisi community, South Africa. In Proceedings of the 27th Conference of the World Association for the Advancement of Veterinary Parasitology (WAAVP2019), Madison, WI, USA, 7–11 July 2019. [Google Scholar]
- Duron, O.; Binetruy, F.; Noël, V.; Cremaschi, J.; McCoy, K.D.; Arnathau, C.; Chevillon, C. Evolutionary changes in symbiont community structure in ticks. Int. J. Lab. Hematol. 2017, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Fikrig, E. Tick microbiome: The force within. Trends Parasitol. 2015, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Huynh, T.P.; Davoust, B.; Gomez, J.; Raoult, D.; Parola, P. Transovarial and trans-stadial transmission of Rickettsiae africae in Amblyomma variegatum ticks. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 317–318. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sánchez, S.; Estrada-Peña, A.; Cabezas-Cruz, A.; Fuente, J. de la. Evolutionary Insights into the Tick Hologenome. Trends Parasitol. 2019, 35, 725–737. [Google Scholar] [CrossRef]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef]
- Vautrin, E.; Vavre, F. Interactions between vertically transmitted symbionts: Cooperation or conflict? Trends Microbiol. 2009, 17, 95–99. [Google Scholar] [CrossRef]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-peña, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, Scotland, 2003. [Google Scholar]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combi. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Ehrlichiosis and anaplasmosis. Clin. Infect. Dis. 2010, 30, 1173–1176. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef]
- Ismail, L.; Leulmi, H.; Baziz-Neffah, F.; Lalout, R.; Mohamed, C.; Mohamed, K.; Parola, P.; Bitam, I. Detection of a novel Rickettsia sp. in soft ticks (Acari: Argasidae) in Algeria. Microbes Infect. 2015, 17, 859–861. [Google Scholar] [CrossRef]
- Allsopp, B.A. Heartwater-Ehrlichia ruminantium infection. OIE Rev. Sci. Tech. 2015, 34, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.P.J.; De Vos, S.; Taoufik, A.; Sparagano, O.A.E.; Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002, 89, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, F.; Fokin, S.I.; Modeo, L.; Andreoli, I.; Dini, F.; GÖrtz, H.D.; Verni, F.; Petroni, G. “Candidatus Cryptoprodotis polytropus,” A novel Rickettsia-like organism in the ciliated protist pseudomicrothorax dubius (ciliophora, nassophorea). J. Eukaryot. Microbiol. 2009, 56, 119–129. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Jensenius, M.; Davis, X.; Von Sonnenburg, F.; Schwartz, E.; Keystone, J.S.; Leder, K.; Lopéz-Véléz, R.; Caumes, E.; Cramer, J.P.; Chen, L.; et al. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg. Infect. Dis. 2009, 15, 1791–1798. [Google Scholar] [CrossRef]
- Freedman, D.O.; Weld, L.H.; Kozarsky, P.E.; Fisk, T.; Robins, R.; von Sonnenburg, F.; Keystone, J.S.; Pandey, P.; Cetron, M.S. Spectrum of Disease and Relation to Place of Exposure among Ill Returned Travelers. N. Engl. J. Med. 2006, 354, 119–130. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Saini, N.; Gupta, R.S. A robust phylogenetic framework for members of the order Legionellales and its main genera (Legionella, Aquicella, Coxiella and Rickettsiella) based on phylogenomic analyses and identification of molecular markers demarcating different clades. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2021, 114, 957–982. [Google Scholar] [CrossRef]
- González-Barrio, D.; Ruiz-Fons, F. Coxiella burnetii in wild mammals: A systematic review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef]
- Tozer, S.J.; Lambert, S.B.; Strong, C.L.; Field, H.E.; Sloots, T.P.; Nissen, M.D. Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses Public Health 2014, 61, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Körner, S.; Makert, G.R.; Mertens-Scholz, K.; Henning, K.; Pfeffer, M.; Starke, A.; Nijhof, A.M.; Ulbert, S. Uptake and fecal excretion of Coxiella burnetii by Ixodes ricinus and Dermacentor marginatus ticks. Parasites Vectors 2020, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. Terrestrial Manual—Chapter 3.1.9; “Heartwater”; World Organisation for Animal Health: Paris, France, 2018. [Google Scholar]
- Rahal, M.; Medkour, H.; Diarra, A.Z.; Bitam, I.; Parola, P.; Mediannikov, O. Molecular identification and evaluation of Coxiella-like endosymbionts genetic diversity carried by cattle ticks in Algeria. Ticks Tick-Borne Dis. 2020, 11, 101493. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Noël, V.; McCoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P.; et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef]
- Zhong, J. Coxiella-like endosymbionts; Toman, R., Heinzen, R.A., Samuel, J.E., Mege, J.-L., Eds.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2012; Volume 984, pp. 39–63. [Google Scholar] [CrossRef]
- Seo, M.G.; Lee, S.H.; VanBik, D.; Ouh, I.O.; Yun, S.H.; Choi, E.; Park, Y.S.; Lee, S.E.; Kim, J.W.; Cho, G.J.; et al. Detection and genotyping of Coxiella burnetii and Coxiella-like bacteria in horses in South Korea. PLoS ONE 2016, 11, e0156710. [Google Scholar] [CrossRef]
- Guimard, T.; Amrane, S.; Prudent, E.; El Karkouri, K.; Raoult, D.; Angelakis, E. Case report: Scalp eschar and neck lymphadenopathy associated with bacteremia due to Coxiella-like bacteria. Am. J. Trop. Med. Hyg. 2017, 97, 1319–1322. [Google Scholar] [CrossRef]
- Brenner, A.E.; Muñoz-Leal, S.; Sachan, M.; Labruna, M.B.; Raghavan, R. Coxiella burnetii and Related Tick Endosymbionts Evolved from Pathogenic Ancestors. Genome Biol. Evol. 2021, 13, evab108. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Lalzar, I.; Klasson, L. Distinctive genome reduction rates revealed by genomic analyses of two Coxiella-like endosymbionts in ticks. Genome Biol. Evol. 2015, 7, 1779–1796. [Google Scholar] [CrossRef]
- Guizzo, M.G.; Parizi, L.F.; Nunes, R.D.; Schama, R.; Albano, R.M.; Tirloni, L.; Oldiges, D.P.; Vieira, R.P.; Oliveira, W.H.C.; Leite, M.D.S.; et al. A Coxiella mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep. 2017, 7, 17554. [Google Scholar] [CrossRef]
- Smith, T.A.; Driscoll, T.; Gillespie, J.J.; Raghavan, R. A Coxiella-like endosymbionts a potential vitamin source for the lone star tick. Genome Biol. Evol. 2015, 7, 831–838. [Google Scholar] [CrossRef]
- Ruth Elliman, J.; Owens, L. Confirmation that candidatus Coxiella cheraxi from redclaw crayfish (Cherax quadricarinatus) is a close relative of Coxiella burnetii, the agent of Q-fever. Lett. Appl. Microbiol. 2020, 71, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, A.H.L.; Cadenas, M.B.; Diab, S.S.; Nordhausen, R.; Bradway, D.; Crespo, R.; Breitschwerdt, B. Coxiella -Like Infection in Psittacines and a Toucan. Case Rep. 2008, 52, 426–432. [Google Scholar]
- Angelakis, E.; Mediannikov, O.; Jos, S.L.; Berenger, J.M.; Parola, P.; Raoult, D. Candidatus coxiella massiliensis infection. Emerg. Infect. Dis. 2016, 22, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Vapniarsky, N.; Barr, B.C.; Murphy, B. Systemic Coxiella-like Infection With Myocarditis and Hepatitis in an Eclectus Parrot (Eclectus roratus). Vet. Pathol. 2012, 49, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Munn, Z.; Falavigna, M. How are systematic reviews of prevalence conducted? A methodological study. BMC Med. Res. Methodol. 2020, 20, 96. [Google Scholar] [CrossRef]
- Inthout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamentals of Biostatistics. Am. J. Trop. Med. Hyg. 2016, 18, 479–480. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; Chapman and Hall/CRC: New York, NY, USA, 2021. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Papers Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S. Grading quality of evidence and strength of recommendations. The GRADE Working Group. Br. Med. J. Clin. Res. Ed. 2004, 328, 1490. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Allaire, J.J.; Grolemund, G. R Markdown: The Definitive Guide; Chapman and Hall/CRC: New York, NY, USA, 2018. [Google Scholar]
- Knuth, D.E. Literate programming. Comput. J. 1984, 27, 97–111. [Google Scholar] [CrossRef]
- Biguezoton, A.; Noel, V.; Adehan, S.; Adakal, H.; Dayo, G.-K.; Zoungrana, S.; Farougou, S.; Chevillon, C. Ehrlichia ruminantium infects Rhipicephalus microplus in West Africa. Parasites Vectors 2016, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Esemu, S.N.; Besong, W.O.; Ndip, R.N.; Ndip, L.M. Prevalence of Ehrlichia ruminantium in adult Amblyomma variegatum collected from cattle in Cameroon. Exp. Appl. Acarol. 2013, 59, 377–387. [Google Scholar] [CrossRef]
- Koney, E.B.M.; Dogbey, O.; Walker, A.R.; Bell-Sakyi, L. Ehrlichia ruminantium seroprevalence in domestic ruminants in Ghana. II. Point prevalence survey. Vet. Microbiol. 2004, 103, 183–193. [Google Scholar] [CrossRef]
- Berggoetz, M.; Schmid, M.; Ston, D.; Wyss, V.; Chevillon, C.; Pretorius, A.M.; Gern, L. Protozoan and bacterial pathogens in tick salivary glands in wild and domestic animal environments in South Africa. Ticks Tick-Borne Dis. 2014, 5, 176–185. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Akure, P.C.; Lubembe, D.M.; Sibeko-Matjila, K.; Troskie, M.; Oosthuizen, M.C.; Stoltsz, H. Molecular detection and characterisation of protozoan and rickettsial pathogens in ticks from cattle in the pastoral area of Karamoja, Uganda. Ticks Tick-Borne Dis. 2021, 12, 101709. [Google Scholar] [CrossRef]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; Kacou N’Douba, A.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O.; et al. Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Zannou, O.M.; Biguezoton, A.S.; Kouassi, P.Y.; Belem, A.; Farougou, S.; Oosthuizen, M.; Saegerman, C.; Lempereur, L. Cattle ticks and associated tick-borne pathogens in Burkina Faso and Benin: Apparent northern spread of Rhipicephalus microplus in Benin and first evidence of Theileria velifera and Theileria annulata. Ticks Tick-Borne Dis. 2021, 12, 101733. [Google Scholar] [CrossRef]
- De Waal, D.T.D.T. Anaplasmosis control and diagnosis in South Africa. Ann. N. Y. Acad. Sci. 2000, 916, 474–483. [Google Scholar] [CrossRef]
- Fournier, P.E.; El Karkouri, K.; Leroy, Q.; Robert, C.; Giumelli, B.; Renesto, P.; Socolovschi, C.; Parola, P.; Audic, S.; Raoult, D. Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genom. 2009, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Chiuya, T.; Masiga, D.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef]
- Dupont, H.T.; Cornet, J.P.; Raoult, D. Identification of rickettsiae from ticks collected in the Central African Republic using the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1994, 50, 373–380. [Google Scholar] [CrossRef]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Luus-Powell, W.J.; Oteo, J.A.J.A. Investigation of Rickettsia, Coxiella burnetii and Bartonella in ticks from animals in South Africa. Ticks Tick-Borne Dis. 2016, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Abichu, G.; Meli, M.L.; Tánczos, B.; Sulyok, K.M.; Gyuranecz, M.; Gönczi, E.; Farkas, R.; Hofmann-Lehmann, R. Influence of the biotope on the tick infestation of cattle and on the tick-borne pathogen repertoire of cattle ticks in Ethiopia. PLoS ONE 2014, 9, e106452. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Berger, L.; Busser, S.; Deetman, I.; Jochems, M.; Leenders, T.; De Sitter, B.; Van Der Steen, F.; Wentzel, J.; Stoltsz, H. Amblyomma hebraeum is the predominant tick species on goats in the Mnisi Community Area of Mpumalanga Province South Africa and is co-infected with Ehrlichia ruminantium and Rickettsia africae. Parasites Vectors 2020, 13, 172. [Google Scholar] [CrossRef]
- Koka, H.; Sang, R.; Kutima, H.L.; Musila, L.; Macaluso, K. The detection of spotted fever group rickettsia DNA in tick samples from pastoral communities in Kenya. J. Med. Entomol. 2017, 54, 774–780. [Google Scholar] [CrossRef]
- Omondi, D.; Masiga, D.K.; Fielding, B.C.; Kariuki, E.; Ajamma, Y.U.; Mwamuye, M.M.; Ouso, D.O.; Villinger, J. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent Islands of Lake Victoria and Lake Baringo, Kenya. Front. Vet. Sci. 2017, 4, 73. [Google Scholar] [CrossRef]
- Yssouf, A.; Socolovschi, C.; Kernif, T.; Temmam, S.; Lagadec, E.; Tortosa, P.; Parola, P. First molecular detection of Rickettsia africae in ticks from the Union of the Comoros. Parasites Vectors 2014, 7, 444. [Google Scholar] [CrossRef]
- Abdelkadir, K.; Palomar, A.M.; Portillo, A.; Oteo, J.A.; Ait-Oudhia, K.; Khelef, D. Presence of Rickettsia aeschlimannii, ‘Candidatus Rickettsia barbariae’ and Coxiella burnetii in ticks from livestock in Northwestern Algeria. Ticks Tick-Borne Dis. 2019, 10, 924–928. [Google Scholar] [CrossRef]
- Bitam, I. Vectors of rickettsiae in Africa. Ticks Tick-Borne Dis. 2012, 3, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Demoncheaux, J.P.; Socolovschi, C.; Davoust, B.; Haddad, S.; Raoult, D.; Parola, P. First detection of Rickettsia aeschlimannii in Hyalomma dromedarii ticks from Tunisia. Ticks Tick-Borne Dis. 2012, 3, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Djerbouh, A.; Kernif, T.; Beneldjouzi, A.; Socolovschi, C.; Kechemir, N.; Parola, P.; Raoult, D.; Bitam, I. The first molecular detection of Rickettsia aeschlimannii in the ticks of camels from southern Algeria. Ticks Tick-Borne Dis. 2012, 3, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Romero, L.; Oteo, J.A. Detection of zoonotic agents and a new Rickettsia strain in ticks from donkeys from South Africa: Implications for travel medicine. Travel Med. Infect. Dis. 2018, 26, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Baneth, G.; Apanaskevich, D.A.; Mumcuoglu, K.Y.; Harrus, S. Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Med. Vet. Entomol. 2015, 29, 205–209. [Google Scholar] [CrossRef]
- Leulmi, H.; Aouadi, A.; Bitam, I.; Bessas, A.; Benakhla, A.; Raoult, D.; Parola, P. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in northeastern Algeria. Parasites Vectors 2016, 9, 27. [Google Scholar] [CrossRef]
- Loftis, A.D.; Reeves, W.K.; Szumlas, D.E.; Abbassy, M.M.; Helmy, I.M.; Moriarity, J.R.J.R.; Dasch, G.A. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 2006, 40, 67–81. [Google Scholar] [CrossRef]
- Mura, A.; Socolovschi, C.; Ginesta, J.; Lafrance, B.; Magnan, S.; Rolain, J.M.; Davoust, B.; Raoult, D.; Parola, P. Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 945–949. [Google Scholar] [CrossRef]
- Olivieri, E.; Kariuki, E.; Floriano, A.M.; Castelli, M.; Tafesse, Y.M.; Magoga, G.; Kumsa, B.; Montagna, M.; Sassera, D.A. Multi-country investigation of the diversity and associated microorganisms isolated from tick species from domestic animals, wildlife and vegetation in selected african countries. Exp. Appl. Acarol. 2021, 83, 427–448. [Google Scholar] [CrossRef]
- Parola, P.; Inokuma, H.; Camicas, J.L.; Brouqui, P.; Raoult, D. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 2001, 7, 1014–1017. [Google Scholar] [CrossRef]
- Sambou, M.; Faye, N.; Bassène, H.; Diatta, G.; Raoult, D.; Mediannikov, O. Identification of rickettsial pathogens in ixodid ticks in northern Senegal. Ticks Tick-Borne Dis. 2014, 5, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Messadi, L. Molecular epidemiology and phylogeny of spotted fever group Rickettsia in camels (Camelus dromedarius) and their infesting ticks from Tunisia. Transbound. Emerg. Dis. 2020, 67, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, Y.A.; Elhag, A.M.A.W.; Brima, Y.A.; Abdalla, M.A.; Bakiet, A.O.; Mohmed-Noor, S.E.T.; Lemhöfer, G.; Bestehorn, M.; Poppert, S.; Schaper, S.; et al. Ixodid tick species and two tick-borne pathogens in three areas in the Sudan. Parasitol. Res. 2020, 119, 385–394. [Google Scholar] [CrossRef]
- Tomassone, L.; De Meneghi, D.; Adakal, H.; Rodighiero, P.; Pressi, G.; Grego, E. Detection of Rickettsia aeschlimannii and Rickettsia africae in ixodid ticks from Burkina Faso and Somali Region of Ethiopia by new real-time PCR assays. Ticks Tick-Borne Dis. 2016, 7, 1082–1088. [Google Scholar] [CrossRef]
- Bessas, A.; Leulmi, H.; Bitam, I.; Zaidi, S.; Ait-Oudhia, K.; Raoult, D.; Parola, P. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Boudebouch, N.; Sarih, M.; Socolovschi, C.; Amarouch, H.; Hassar, M.; Raoult, D.; Parola, P. Molecular survey for spotted fever group rickettsiae in ticks from Morocco. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 259–260. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Baneth, G.; Gutiérrez, R.; Nachum-Biala, Y.; Mumcuoglu, K.Y.; Harrus, S. Coxiella burnetii and Rickettsia conorii: Two zoonotic pathogens in peridomestic rodents and their ectoparasites in Nigeria. Ticks Tick-Borne Dis. 2018, 9, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, M.; Socolovschi, C.; Benyettou, M.; Barech, G.; Biche, M.; Kernif, T.; Raoult, D.; Parola, P. Rickettsiae in arthropods collected from the North African Hedgehog (Atelerix algirus) and the desert hedgehog (Paraechinus aethiopicus) in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 117–122. [Google Scholar] [CrossRef]
- Khrouf, F.; M’Ghirbi, Y.; Znazen, A.; Jemaa, M.B.; Hammami, A.; Bouattour, A. Detection of rickettsia in Rhipicephalus sanguineus ticks and Ctenocephalides felis fleas from southeastern tunisia by reverse line blot assay. J. Clin. Microbiol. 2014, 52, 268–274. [Google Scholar] [CrossRef]
- Znazen, A.; Khrouf, F.; Elleuch, N.; Lahiani, D.; Marrekchi, C.; M’Ghirbi, Y.; Ben Jemaa, M.; Bouattour, A.; Hammami, A. Multispacer typing of Rickettsia isolates from humans and ticks in Tunisia revealing new genotypes. Parasites Vectors 2013, 6, 367. [Google Scholar] [CrossRef]
- Beati, L.; Kelly, P.J.; Matthewman, L.A.; Mason, P.R.; Raoult, D. Prevalence of Rickettsia-like organisms and spotted fever group rickettsiae in ticks (Acari: Ixodidae) from Zimbabwe. J. Med. Entomol. 1995, 32, 787–792. [Google Scholar] [CrossRef]
- Boucheikhchoukh, M.; Laroche, M.; Aouadi, A.; Dib, L.; Benakhla, A.; Raoult, D.; Parola, P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 39–49. [Google Scholar] [CrossRef]
- Kernif, T.; Djerbouh, A.; Mediannikov, O.; Ayach, B.; Rolain, J.M.; Raoult, D.; Parola, P.; Bitam, I. Rickettsia africae in Hyalomma dromedarii ticks from sub-Saharan Algeria. Ticks Tick-Borne Dis. 2012, 3, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular Detection of Zoonotic Rickettsiae and Anaplasma spp. in Domestic Dogs and Their Ectoparasites in Bushbuckridge, South Africa. Vector-Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef]
- Maina, A.N.; Jiang, J.; Omulo, S.A.; Cutler, S.J.; Ade, F.; Ogola, E.; Feikin, D.R.; Njenga, M.K.; Cleaveland, S.; Mpoke, S.; et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: Implications for human health. Vector-Borne Zoonotic Dis. 2014, 14, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Proboste, T.; Kalema-Zikusoka, G.; Altet, L.; Solano-Gallego, L.; Fernández De Mera, I.G.I.G.I.G.I.G.; Chirife, A.D.; Muro, J.; Bach, E.; Piazza, A.; Cevidanes, A.; et al. Infection and exposure to vector-borne pathogens in rural dogs and their ticks, Uganda. Parasites Vectors 2015, 8, 306. [Google Scholar] [CrossRef]
- Sarih, M.; Socolovschi, C.; Boudebouch, N.; Hassar, M.; Raoult, D.; Parola, P. Spotted fever group rickettsiae in ticks, Morocco. Emerg. Infect. Dis. 2008, 14, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Sfar, N.; M’Ghirbi, Y.; Letaïef, A.; Parola, P.; Bouattour, A.; Raoult, D. First report of Rickettsia monacensis and Rickettsia helvetica from Tunisia. Ann. Trop. Med. Parasitol. 2008, 102, 561–564. [Google Scholar] [CrossRef]
- Socolovschi, C.; Matsumoto, K.; Marie, J.L.; Davoust, B.; Raoult, D.; Parola, P. Identification of Rickettsiae, Uganda and Djibouti [2]. Emerg. Infect. Dis. 2007, 13, 1508–1509. [Google Scholar] [CrossRef]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817–820. [Google Scholar] [CrossRef]
- Dib, L.; Bitam, I.; Bensouilah, M.; Parola, P.; Raoult, D. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 261–262. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Seo, H.; Choi, Y.; Choi, M.; Kim, H.; Terry, A.K.; Chong, S.; Richards, A.L.; Park, K.-H.; Jang, W.-J. Detection of Rickettsia monacensis from Ixodes nipponensis collected from rodents in Gyeonggi and Gangwon Provinces, Republic of Korea. Exp. Appl. Acarol. 2013, 61, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Collins, N.E.; Brayton, K.A.; Chaisi, M.; Blumberg, L.; Frean, J.; Gall, C.A.; Wentzel, J.M.; Wills-Berriman, S.; De Boni, L.; et al. Anaplasma phagocytophilum and Other Anaplasma spp. in Various Hosts in the Mnisi Community, Mpumalanga Province, South Africa. Microorganisms 2020, 8, 1812. [Google Scholar] [CrossRef] [PubMed]
- Adenyo, C.; Ohya, K.; Qiu, Y.; Takashima, Y.; Ogawa, H.; Matsumoto, T.; Thu, M.J.; Sato, K.; Kawabata, H.; Katayama, Y.; et al. Bacterial and protozoan pathogens/symbionts in ticks infecting wild grasscutters (Thryonomys swinderianus) in Ghana. Acta Tropica 2020, 205, 105388. [Google Scholar] [CrossRef]
- Matsimbe, A.M.; Magaia, V.; Sanches, G.S.; Neves, L.; Noormahomed, E.; Antunes, S.; Domingos, A. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91–102. [Google Scholar] [CrossRef]
- Sarin, M.; M’Ghirbi, Y.; Bouattour, A.; Gern, L.; Baranton, G.; Postic, D. Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J. Clin. Microbiol. 2005, 43, 1127–1132. [Google Scholar] [CrossRef]
- Teshale, S.; Kumsa, B.; Menandro, M.L.; Cassini, R.; Martini, M. Anaplasma, Ehrlichia and rickettsial pathogens in ixodid ticks infesting cattle and sheep in western Oromia, Ethiopia. Exp. Appl. Acarol. 2016, 70, 231–237. [Google Scholar] [CrossRef]
- Adakal, H.; Gavotte, L.; Stachurski, F.; Konkobo, M.; Henri, H.; Zoungrana, S.; Huber, K.; Vachiery, N.; Martinez, D.; Morand, S.; et al. Clonal origin of emerging populations of Ehrlichia ruminantium in Burkina Faso. Infect. Genet. Evol. 2010, 10, 903–912. [Google Scholar] [CrossRef]
- Adelabu, O.A.; Iweriebor, B.C.; Okoh, A.I.; Obi, L.C. Phylogenetic profiling for zoonotic Ehrlichia spp. from ixodid ticks in the Eastern Cape, South Africa. Transbound. Emerg. Dis. 2020, 67, 1247–1256. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Terkawi, M.A.; Jirapattharasate, C.; Cao, S.; Liu, M.; Nakao, R.; Umemiya-Shirafuji, R.; Yokoyama, N.; Sugimoto, C.; Fujisaki, K.; et al. Molecular detection of spotted fever group rickettsiae in Amblyomma variegatum ticks from Benin. Ticks Tick-Borne Dis. 2016, 7, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, B.A.A.; Theron, J.; Coetzee, M.L.L.; Dunsterville, M.T.T. The occurrence of Theileria and Cowdria parasites in African buffalo (Syncerus caffer) and their associated Amblyomma hebraeum ticks. Onderstepoort J. Vet. Res. 1999, 66, 245–249. [Google Scholar] [PubMed]
- Aouadi, A.; Leulmi, H.; Boucheikhchoukh, M.; Benakhla, A.; Raoult, D.; Parola, P. Molecular evidence of tick-borne hemoprotozoan-parasites (Theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 34–39. [Google Scholar] [CrossRef]
- Belkahia, H.; Ben Said, M.; Ghribi, R.; Selmi, R.; Ben Asker, A.; Yahiaoui, M.; Bousrih, M.; Daaloul-Jedidi, M.; Messadi, L. Molecular detection, genotyping and phylogeny of Anaplasma spp. in Rhipicephalus ticks from Tunisia. Acta Tropica 2019, 191, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Bryson, N.R.R.; Horak, I.G.G.; Venter, E.H.H.; Mahan, S.M.M.; Simbi, B.; Peter, T.F.F. The prevalence of Cowdria ruminantium in free-living adult Amblyomma hebraeum collected at a communal grazing area and in 2 wildlife reserves in South Africa. J. S. Afr. Vet. Assoc. 2002, 73, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Geysen, D.; Munstermann, S.; Taoufik, A.; Postigo, M.; Jongejan, F. Molecular detection of Ehrlichia ruminantium infection in Amblyomma variegatum ticks in the Gambia. Exp. Appl. Acarol. 2007, 42, 61–74. [Google Scholar] [CrossRef]
- Fyumagwa, R.D.; Simmler, P.; Meli, M.L.; Hoare, R.; Hofmann-Lehmann, R.; Lutz, H. Prevalence of Anaplasma marginale in different tick species from Ngorongoro Crater, Tanzania. Vet. Parasitol. 2009, 161, 154–157. [Google Scholar] [CrossRef]
- Guo, H.; Adjou Moumouni, P.F.; Thekisoe, O.; Gao, Y.; Liu, M.; Li, J.; Galon, E.M.; Efstratiou, A.; Wang, G.; Jirapattharasate, C.; et al. Genetic characterization of tick-borne pathogens in ticks infesting cattle and sheep from three South African provinces. Ticks Tick-Borne Dis. 2019, 10, 875–882. [Google Scholar] [CrossRef]
- Hornok, S.; Abichu, G.; Takács, N.; Gyuranecz, M.; Farkas, R.; Fernández De Mera, I.G.I.G.; De La Fuente, J. Molecular screening for anaplasmataceae in ticks and tsetse flies from Ethiopia. Acta Vet. Hung. 2016, 64, 65–70. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kwak, Y.S.; Kim, J.Y.; Nam, S.H.; Lee, I.Y.; Mduma, S.; Keyyu, J.; Fyumagwa, R.; Yong, T.S. Prevalence of tick-borne pathogens from ticks collected from cattle and wild animals in Tanzania in 2012. Korean J. Parasitol. 2018, 56, 305–308. [Google Scholar] [CrossRef]
- Ledger, K.J.; Beati, L.; Wisely, S.M. Survey of ticks and tick-borne rickettsial and protozoan pathogens in Eswatini. Pathogens 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Loftis, A.D.; Kelly, P.J.; Paddock, C.D.; Blount, K.; Johnson, J.W.; Gleim, E.R.; Yabsley, M.J.; Levin, M.L.; Beati, L. Panola mountain Ehrlichia in Amblyomma maculatum from the United States and Amblyomma variegatum (acari: Ixodidae) from the Caribbean and Africa. J. Med. Entomol. 2016, 53, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Mahan, S.M.; Peter, T.F.; Simbi, B.H.; Burridge, M.J. PCR detection of Cowdria ruminantium infection in ticks and animals from heartwater-endemic regions of Zimbabwe. Ann. N. Y. Acad. Sci. 1998, 849, 85–87. [Google Scholar] [CrossRef]
- Makenov, M.T.; Toure, A.H.; Korneev, M.G.; Sacko, N.; Porshakov, A.M.; Yakovlev, S.A.; Radyuk, E.V.; Zakharov, K.S.; Shipovalov, A.V.; Boumbaly, S.; et al. Rhipicephalus microplus and its vector-borne haemoparasites in Guinea: Further species expansion in West Africa. Parasitol. Res. 2021, 120, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; D’Amico, G.; Yao, P.K.; Ionica, A.M.; Kanyari, P.W.N.; Daskalaki, A.A.; Dumitrache, M.O.; Sandor, A.D.; Gherman, C.M.; Qablan, M.; et al. Molecular detection of Anaplasma platys infection in free-roaming dogs and ticks from Kenya and Ivory Coast. Parasites Vectors 2016, 9, 157. [Google Scholar] [CrossRef]
- M’ghirbi, Y.; Yach, H.; Ghorbel, A.; Bouattour, A. Anaplasma phagocytophilum in horses and ticks in Tunisia. Parasites Vectors 2012, 5, 180. [Google Scholar] [CrossRef]
- Mtshali, K.; Khumalo, Z.T.H.; Nakao, R.; Grab, D.J.; Sugimoto, C.; Thekisoe, O.M.M. Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J. Vet. Med. Sci. 2016, 77, 1573–1579. [Google Scholar] [CrossRef]
- Mtshali, K.; Nakao, R.; Sugimoto, C.; Thekisoe, O. Occurrence of Coxiella burnetii, Ehrlichia canis, Rickettsia species and Anaplasma phagocytophilum-like bacterium in ticks collected from dogs and cats in South Africa. J. South Afr. Vet. Assoc. 2017, 88, a1390. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Ukegawa, S.Y.; El Hussein, A.R.M.; Rahman, M.B.A.; Gabbar, K.M.A.A.; Chitambo, A.M.; Komiya, T.; Mwase, E.T.; Morita, C.; Tamura, Y. Ehrlichia ruminantium, Sudan. Emerg. Infect. Dis. 2005, 11, 1792–1793. [Google Scholar] [CrossRef]
- Mwamuye, M.M.; Kariuki, E.; Omondi, D.; Kabii, J.; Odongo, D.; Masiga, D.; Villinger, J. Novel Rickettsia and emergent tick-borne pathogens: A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya. Ticks Tick-Borne Dis. 2017, 8, 208–218. [Google Scholar] [CrossRef]
- Nakao, R.; Stromdahl, E.Y.; Magona, J.W.; Faburay, B.; Namangala, B.; Malele, I.; Inoue, N.; Geysen, D.; Kajino, K.; Jongejan, F.; et al. Development of loop-mediated isothermal amplification (LAMP) assays for rapid detection of Ehrlichia ruminantium. BMC Microbiol. 2010, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Ndip, L.M.; Ndip, R.N.; Esemu, S.N.; Walker, D.H.; McBride, J.W. Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel-confined dogs in Limbe, Cameroon. Exp. Appl. Acarol. 2010, 50, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ndip, L.M.; Ndip, R.N.; Ndive, V.E.; Awuh, J.A.; Walker, D.H.; McBride, J.W. Ehrlichia species in Rhipicephalus sanguineus ticks in Cameroon. Vector-Borne Zoonotic Dis. 2007, 7, 221–227. [Google Scholar] [CrossRef]
- Peter, T.F.; Perry, B.D.; O’Callaghan, C.J.; Medley, G.F.; Mlambo, G.; Barbet, A.F.; Mahan, S.M. Prevalence of Cowdria ruminantium infection in Amblyomma hebraeum ticks from heartwater-endemic areas of Zimbabwe. Epidemiol. Infect. 1999, 123, 309–316. [Google Scholar] [CrossRef]
- Pothmann, D.; Poppert, S.; Rakotozandrindrainy, R.; Hogan, B.; Mastropaolo, M.; Thiel, C.; Silaghi, C. Prevalence and genetic characterization of Anaplasma marginale in zebu cattle (Bos indicus) and their ticks (Amblyomma variegatum, Rhipicephalus microplus) from Madagascar. Ticks Tick-Borne Dis. 2016, 7, 1116–1123. [Google Scholar] [CrossRef]
- Sanogo, Y.O.O.; Davoust, B.; Inokuma, H.; Camicas, J.-L.J.-L.L.; Parola, P.; Brouqui, P.; Parola, B.; Brouqui, P. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J. Vet. Res. 2003, 70, 205–212. [Google Scholar] [PubMed]
- Selmi, R.; Ben Said, M.; Dhibi, M.; Ben Yahia, H.; Messadi, L. Improving specific detection and updating phylogenetic data related to Anaplasma platys-like strains infecting camels (Camelus dromedarius) and their ticks. Ticks Tick-Borne Dis. 2019, 10, 101260. [Google Scholar] [CrossRef]
- Socolovschi, C.; Gomez, J.; Marié, J.L.; Davoust, B.; Guigal, P.-M.; Raoult, D.; Parola, P. Ehrlichia canis in Rhipicephalus sanguineus ticks in the Ivory Coast. Ticks Tick-Borne Dis. 2012, 3, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Teshale, S.; Geysen, D.; Ameni, G.; Asfaw, Y.; Berkvens, D. Improved molecular detection of Ehrlichia and Anaplasma species applied to Amblyomma ticks collected from cattle and sheep in Ethiopia. Ticks Tick-Borne Dis. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Tucker, N.S.G.; Weeks, E.N.I.; Beati, L.; Kaufman, P.E. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med. Vet. Entomol. 2021, 35, 147–157. [Google Scholar] [CrossRef]
- Tufa, T.B.; Wölfel, S.; Zubriková, D.; Víchová, B.; Andersson, M.; Rieß, R.; Rutaihwa, L.; Fuchs, A.; Orth, H.M.; Häussinger, D.; et al. Tick species from cattle in the Adama Region of Ethiopia and pathogens detected. Exp. Appl. Acarol. 2021, 84, 459–471. [Google Scholar] [CrossRef]
- Wang’ang’a Oundo, J.; Villinger, J.; Jeneby, M.; Ong’amo, G.; Otiende, M.Y.; Makhulu, E.E.; Musa, A.A.; Ouso, D.O.; Wambua, L. Pathogens, endosymbionts, and blood-meal sources of host-seeking ticks in the fast-changing Maasai Mara wildlife ecosystem. PLoS ONE 2020, 15, e0228366. [Google Scholar] [CrossRef]
- Iweriebor, B.C.; Mmbaga, E.J.; Adegborioye, A.; Igwaran, A.; Obi, L.C.; Okoh, A.I. Genetic profiling for Anaplasma and Ehrlichia species in ticks collected in the Eastern Cape Province of South Africa. BMC Microbiol. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.H.A.M.; Aboelsoued, D.; Farag, T.K.; Abdel-Shafy, S.; Abdel Megeed, K.N.; Parola, P.; Raoult, D.; Mediannikov, O. Molecular characterization of some equine vector-borne diseases and associated arthropods in Egypt. Acta Trop. 2022, 227, 106274. [Google Scholar] [CrossRef] [PubMed]
- AL-Hosary, A.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, A.M.; Silaghi, C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick-Borne Dis. 2021, 12, 101676. [Google Scholar] [CrossRef]
- Aouadi, N.; Benkacimi, L.; Zan Diarra, A.; Laroche, M.; Bérenger, J.-M.; Bitam, I.; Parola, P. Microorganisms associated with the North African hedgehog Atelerix algirus and its parasitizing arthropods in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101726. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, H.; Diarra, A.Z.; Gherissi, D.E.; Bérenger, J.-M.; Benakhla, A.; Parola, P. Molecular and MALDI-TOF MS characterisation of Hyalomma aegyptium ticks collected from turtles and their associated microorganisms in Algeria. Ticks Tick-Borne Dis. 2022, 13, 101858. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.A.; Omar, H.M.; Abuowarda, M.; Ghattas, S.G.; Mahmoud, N.E.; Fahmy, M.M. Screening and phylogenetic characterization of tick-borne pathogens in a population of dogs and associated ticks in Egypt. Parasites Vectors 2022, 15, 222. [Google Scholar] [CrossRef]
- Palomar, A.M.; Molina, I.; Bocanegra, C.; Portillo, A.; Salvador, F.; Moreno, M.; Oteo, J.A. Old zoonotic agents and novel variants of tick-borne microorganisms from Benguela (Angola), July 2017. Parasites Vectors 2022, 15, 140. [Google Scholar] [CrossRef]
- Qiu, Y.; Simuunza, M.; Kajihara, M.; Chambaro, H.; Harima, H.; Eto, Y.; Simulundu, E.; Squarre, D.; Torii, S.; Takada, A.; et al. Screening of tick-borne pathogens in argasid ticks in Zambia: Expansion of the geographic distribution of Rickettsia lusitaniae and Rickettsia hoogstraalii and detection of putative novel Anaplasma species. Ticks Tick-Borne Dis. 2021, 12, 101720. [Google Scholar] [CrossRef]
- Said, Y.; Lahmar, S.; Dhibi, M.; Rjeibi, M.R.; Jdidi, M.; Gharbi, M. First survey of ticks, tick-borne pathogens (Theileria, Babesia, Anaplasma and Ehrlichia) and Trypanosoma evansi in protected areas for threatened wild ruminants in Tunisia. Parasitol. Int. 2021, 81, 102275. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, S.; Allam, N.A.T.; Mediannikov, O.; Parola, P.; Raoult, D. Molecular Detection of Spotted Fever Group Rickettsiae Associated with Ixodid Ticks in Egypt. Vector-Borne Zoonotic Dis. 2012, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Barradas, P.F.; Mesquita, J.R.; Ferreira, P.; Gärtner, F.; Carvalho, M.; Inácio, E.; Chivinda, E.; Katimba, A.; Amorim, I. Molecular identification and characterization of Rickettsia spp. and other tick-borne pathogens in cattle and their ticks from Huambo, Angola. Ticks Tick-Borne Dis. 2021, 12, 101583. [Google Scholar] [CrossRef] [PubMed]
- Beati, L.; Meskini, M.; Thiers, B.; Raoult, D. Rickettsia aeschlimannii sp. nov., a new spotted fever group Rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997, 47, 548–554. [Google Scholar] [CrossRef]
- Benredjem, W.; Leulmi, H.; Bitam, I.; Raoult, D.; Parola, P. Borrelia garinii and Rickettsia monacensis in Ixodes ricinus ticks, Algeria. Emerg. Infect. Dis. 2014, 20, 1776–1777. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I.; Kernif, T.; Harrat, Z.; Parola, P.; Raoult, D. First detection of Rickettsia aeschlimannii in Hyalomma aegyptium from Algeria. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 253–254. [Google Scholar] [CrossRef]
- Bitam, I.; Parola, P.; Matsumoto, K.; Rolain, J.M.; Baziz, B.; Boubidi, S.C.; Harrat, Z.; Belkaid, M.; Raoult, D. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann. N. Y. Acad. Sci. 2006, 1078, 368–372. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Dobler, G.; Schaper, S.; Küpper, T.; Kattner, S.; Wölfel, S. First detection of Rickettsia conorii ssp. caspia in Rhipicephalus sanguineus in Zambia. Parasitol. Res. 2017, 116, 3249–3251. [Google Scholar] [CrossRef]
- Cutler, S.J.; Browning, P.; Scott, J.C. Ornithodoros moubata, a soft tick vector for Rickettsia in East Africa? Ann. N. Y. Acad. Sci. 2006, 1078, 373–377. [Google Scholar] [CrossRef]
- Hsi, T.E.; Hsiao, S.W.; Minahan, N.T.; Yen, T.Y.; de Assunção Carvalho, A.V.; Raoult, D.; Fournier, P.E.; Tsai, K.H. Seroepidemiological and molecular investigation of spotted fever group rickettsiae and Coxiella burnetii in Sao Tome Island: A One Health approach. Transbound. Emerg. Dis. 2020, 67, 36–43. [Google Scholar] [CrossRef]
- Keller, C.; Krüger, A.; Schwarz, N.G.; Rakotozandrindrainy, R.; Rakotondrainiarivelo, J.P.; Razafindrabe, T.; Derschum, H.; Silaghi, C.; Pothmann, D.; Veit, A.; et al. High detection rate of Rickettsia africae in Amblyomma variegatum but low prevalence of anti-rickettsial antibodies in healthy pregnant women in Madagascar. Ticks Tick-Borne Dis. 2016, 7, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Gruszka, K.A.; Majekodunmi, A.; Igweh, A.; Welburn, S.C.; Picozzi, K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg. Infect. Dis. 2013, 19, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Davis, J.; Alam, U.; Korman, A.; Rutherford, J.S.; Rosenberg, R.; Azad, A.F. Spotted fever group Rickettsiae in ticks from the masai mara region of Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Magaia, V.; Taviani, E.; Cangi, N.; Neves, L. Molecular detection of Rickettsia africae in Amblyomma ticks collected in cattle from Southern and Central Mozambique. J. Infect. Dev. Ctries. 2020, 14, 614–622. [Google Scholar] [CrossRef]
- Matsumoto, K.; Parola, P.; Rolain, J.-M.J.M.; Jeffery, K.; Raoult, D. Detection of “Rickettsia sp. strain Uilenbergi” and “Rickettsia sp. strain Davousti” in Amblyomma tholloni ticks from elephants in Africa. BMC Microbiol. 2007, 7, 74. [Google Scholar] [CrossRef]
- Mediannikov, O.; Davoust, B.; Socolovschi, C.; Tshilolo, L.; Raoult, D.; Parola, P. Spotted fever group rickettsiae in ticks and fleas from the Democratic Republic of the Congo. Ticks Tick-Borne Dis. 2012, 3, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Mwamuye, M.M.; Kariuki, E.; Omondi, D.; Kabii, J.; Odongo, D.; Masiga, D.; Villinger, J. Novel tick-borne Rickettsia sp. from wild ticks of Kenya: Implications for emerging vector-borne disease outbreaks. Int. J. Infect. Dis. 2016, 45, 60. [Google Scholar] [CrossRef]
- Nakao, R.; Qiu, Y.; Igarashi, M.; Magona, J.W.; Zhou, L.; Ito, K.; Sugimoto, C. High prevalence of spotted fever group rickettsiae in Amblyomma variegatum from Uganda and their identification using sizes of intergenic spacers. Ticks Tick-Borne Dis. 2013, 4, 506–512. [Google Scholar] [CrossRef]
- Nakao, R.; Qiu, Y.; Salim, B.; Hassan, S.M.; Sugimoto, C. Molecular Detection of Rickettsia africae in Amblyomma variegatum Collected from Sudan. Vector-Borne Zoonotic Dis. 2015, 15, 323–325. [Google Scholar] [CrossRef]
- Norte, A.C.; Harris, D.J.; Silveira, D.; Nunes, C.S.; Núncio, M.S.; Martínez, E.G.; Giménez, A.; Sousa, R.; Lopes de Carvalho, I.; Perera, A. Diversity of microorganisms in Hyalomma aegyptium collected from spur-thighed tortoise (Testudo graeca) in North Africa and Anatolia. Transbound. Emerg. Dis. 2021, 69, 1951–1962. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Rǎileanu, C.; Tauchmann, O.; Fischer, S.; Vasić, A.; Schäfer, M.; Biu, A.A.; Ogo, N.I.; Thekisoe, O.; Silaghi, C. Prevalence and molecular characterization of ticks and tick-borne pathogens of one-humped camels (Camelus dromedarius) in Nigeria. Parasites Vectors 2020, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.K.; Mans, B.J.; Durden, L.A.; Miller, M.M.; Gratton, E.M.; Laverty, T.M. Rickettsia hoogstraalii and a Rickettsiella from the Bat Tick Argas transgariepinus, in Namibia. J. Parasitol. 2020, 106, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, A.; Keller, C.; Krüger, A.; Manchang, T.K.; Hagen, R.M.; Frickmann, H.; Veit, A.; Achukwi, M.D.; Krücken, J.; Poppert, S. Molecular detection of spotted fever group rickettsiae in ticks from Cameroon. Ticks Tick-Borne Dis. 2018, 9, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Chitanga, S.; Chibesa, K.; Sichibalo, K.; Mubemba, B.; Nalubamba, K.S.; Muleya, W.; Changula, K.; Simulundu, E. Molecular Detection and Characterization of Rickettsia Species in Ixodid Ticks Collected From Cattle in Southern Zambia. Front. Vet. Sci. 2021, 8, 684487. [Google Scholar] [CrossRef]
- Elelu, N.; Ola-Fadunsin, S.D.; Bankole, A.A.; Raji, M.A.; Ogo, N.I.; Cutler, S.J. Prevalence of tick infestation and molecular characterization of spotted fever Rickettsia massiliae in Rhipicephalus species parasitizing domestic small ruminants in north-central Nigeria. PLoS ONE 2022, 17, e0263843. [Google Scholar] [CrossRef]
- Hornok, S.; Kontschán, J.; Takács, N.; Chaber, A.-L.; Halajian, A.; Szekeres, S.; Sándor, A.D.; Plantard, O. Rickettsiaceae in two reptile-associated tick species, Amblyomma exornatum and Africaniella transversale: First evidence of Occidentia massiliensis in hard ticks (Acari: Ixodidae). Ticks Tick-Borne Dis. 2022, 13, 101830. [Google Scholar] [CrossRef]
- Mediannikov, O.; Bassene, H.; Aubadie, M.; Raoult, D. Rickettsia felis and related bacteria: An epidemiological enigma. Int. J. Infect. Dis. 2014, 21, 222. [Google Scholar] [CrossRef]
- Nimo-Paintsil, S.C.; Mosore, M.; Addo, S.O.; Lura, T.; Tagoe, J.; Ladzekpo, D.; Addae, C.; Bentil, R.E.; Behene, E.; Dafeamekpor, C.; et al. Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Parasites Vectors 2022, 15, 86. [Google Scholar] [CrossRef]
- Knobel, D.L.; Maina, A.N.; Cutler, S.J.; Ogola, E.; Feikin, D.R.; Junghae, M.; Halliday, J.E.B.; Richards, A.L.; Breiman, R.F.; Cleaveland, S.; et al. Coxiella burnetii in humans, domestic ruminants, and ticks in rural Western Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 513–518. [Google Scholar] [CrossRef]
- Koka, H.; Sang, R.; Kutima, H.L.; Musila, L. Coxiella burnetii Detected in Tick Samples from Pastoral Communities in Kenya. BioMed Res. Int. 2018, 2018, 8158102. [Google Scholar] [CrossRef]
- Kumsa, B.; Socolovschi, C.; Almeras, L.; Raoult, D.; Parola, P. Occurrence and genotyping of Coxiella burnetii in ixodid ticks in oromia, Ethiopia. Am. J. Trop. Med. Hyg. 2015, 93, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Machado-Ferreira, E.; Vizzoni, V.F.; Balsemão-Pires, E.; Moerbeck, L.; Gazeta, G.S.; Piesman, J.; Voloch, C.M.; Soares, C.A.G. Coxiella symbionts are widespread into hard ticks. Parasitol. Res. 2016, 115, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.F.; Sokhna, C.; Trape, J.F.; Raoult, D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef] [PubMed]
- Ndeereh, D.; Muchemi, G.; Thaiyah, A.; Otiende, M.; Angelone-Alasaad, S.; Jowers, M.J. Molecular survey of Coxiella burnetii in wildlife and ticks at wildlife–livestock interfaces in Kenya. Exp. Appl. Acarol. 2017, 72, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, K.M.; Hornok, S.; Abichu, G.; Erdélyi, K.; Gyuranecz, M. Identification of novel Coxiella burnetii genotypes from Ethiopian ticks. PLoS ONE 2014, 9, e113213. [Google Scholar] [CrossRef]
- Moumouni, P.F.A.; Guo, H.; Gao, Y.; Liu, M.; Ringo, A.E.; Galon, E.M.; Vudriko, P.; Umemiya-Shirafuji, R.; Inoue, N.; Suzuki, H.; et al. Identification and genetic characterization of Piroplasmida and Anaplasmataceae agents in feeding Amblyomma variegatum ticks from Benin. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 137–143. [Google Scholar]





| Anaplasmataceae Species | Studies | Rickettsiaceae Species | Studies | Coxiellaceae Species | Studies |
|---|---|---|---|---|---|
| Ehrlichia ruminantium | 27 | Rickettsia spp. | 55 | Coxiella burnetii | 20 |
| Anaplasma marginale | 17 | Rickettsia africae | 38 | Coxiella spp. | 5 |
| Ehrlichia/Anaplasma spp. | 14 | Rickettsia aeschlimanni | 24 | Coxiella-like endosymbionts | 4 |
| Anaplasma platys | 12 | Rickettsia massiliae | 19 | Rickettsiella spp. | 1 |
| Ehrlichia canis | 11 | Rickettsia conorii | 12 | ||
| Anaplasma phagocytophilum | 10 | Rickettsia monacensis | 8 | ||
| Anaplasma ovis | 9 | Rickettsia helvetica | 4 | ||
| Ehrlichia spp. | 7 | Rickettsia rhipicephali | 3 | ||
| Anaplasma bovis | 5 | Rickettsia slovaca | 3 | ||
| Anaplasma centrale | 4 | Rickettsia mongolotimonae | 3 | ||
| Anaplasma spp. | 3 | Rickettsia raoultii | 3 | ||
| Ehrlichia chaffeensis | 3 | Candidatus Rickettsia barbariae | 2 | ||
| Ehrlichia muris | 2 | Rickettsia hoogstraalii | 2 | ||
| Candidatus Ehrlichia rustica | 2 | Rickettsia lusitaniae | 2 | ||
| Ehrlichia minasensis | 2 | Rickettsia conorii ssp. caspia | 1 | ||
| Ehrlichia spp. (EU191229.1) | 1 | Rickettsia japonica | 1 | ||
| Ehrlichia ovina | 1 | Rickettsia africae São Tomé | 1 | ||
| Candidatus Anaplasma ivorensis | 1 | Rickettsia parkeri | 1 | ||
| Candidatus Ehrlichia urmitei | 1 | Rickettsia montanensis | 1 | ||
| Neoehrlichia spp. | 1 | Rickettsia sp. (Uilenbergi) | 1 | ||
| Panola Mountain Ehrlichia (PME) | 1 | Rickettsia sp. (Davousti) | 1 | ||
| Ehrlichia ewingii | 1 | Candidatus Rickettsia kastelanii | 1 | ||
| Anaplasma sp. (Omatjenne) | 1 | Rickettsia israelensis | 1 | ||
| Neoehrlichia mikurensis | 1 | Rickettsia akari | 1 | ||
| Ehrlichia chaffeensis-like | 1 | Occidentia massiliensis | 1 |
| Pathogen Species | N° of Datasets | N° of Ticks Tested | N° of Ticks Positive | Pooled Prevalence | 95% CI (%) | 95% PI (%) | I2 (%) |
|---|---|---|---|---|---|---|---|
| Ehrlichia/Anaplasma spp. | 61 | 2295 | 184 | 2.3% | 0.81–4.34 | 0–19.14 | 62.61 |
| Ehrlichia ruminantium | 44 | 7039 | 552 | 6.4% | 3.97–9.16 | 0–27.85 | 89.82 |
| Ehrlichia canis | 9 | 508 | 47 | 4.3% | 0.04–12.66 | 0–37.44 | 87.36 |
| Anaplasma marginale | 31 | 2322 | 455 | 12.8% | 4.06–24.35 | 0–84.73 | 97.22 |
| Anaplasma centrale | 14 | 913 | 1 | 0.0% | 0–0 | 0–0 | 0 |
| Anaplasma bovis | 14 | 879 | 3 | 0.0% | 0–0 | 0–1.33 | 4.47 |
| Anaplasma ovis | 7 | 657 | 20 | 0.6% | 0–3.73 | 0–10.99 | 81.34 |
| Anaplasma platys | 10 | 1271 | 22 | 0.3% | 0–1.46 | 0–3.61 | 61.02 |
| Anaplasma phagocytophilum | 11 | 689 | 3 | 0.0% | 0–0.15 | 0–0.74 | 0 |
| Rickettsia spp. | 326 | 14,188 | 3252 | 18.4% | 14.23–22.85 | 0–95.75 | 96.63 |
| Rickettsia africae | 24 | 1391 | 285 | 13.5% | 2.76–28.69 | 0–91.91 | 97.67 |
| Rickettsia aeschlimanni | 22 | 815 | 43 | 2.6% | 0–9.48 | 0–45.03 | 83.55 |
| Rickettsia massiliae | 15 | 811 | 75 | 6.9% | 0.21–18.43 | 0–59.89 | 92.06 |
| Rickettsia conorii | 16 | 679 | 77 | 11.3% | 1.77–25.89 | 0–78.99 | 91.63 |
| Coxiella spp. | 32 | 341 | 97 | 27.0% | 10.83–46.03 | 0–100 | 86.86 |
| Coxiella burnetii | 139 | 6442 | 493 | 0.0% | 0–0.25 | 0–15.8 | 80.4 |
| Coxiella-like endosymbionts | 8 | 163 | 119 | 70.5% | 27–99.82 | 0–100 | 94.81 |
| N° of Datasets | Tick Genus | Tick Species | Sampling Country | Sampling Period | Tick Origin | Tick Identification Method | Molecular Test | Risk of Bias | |
|---|---|---|---|---|---|---|---|---|---|
| Ehrlichia ruminantium | 44 | <0.001 | 0.042 | 0.001 | 0.217 | 0.468 | N/A | <0.001 | 0.012 |
| Ehrlichia canis | 9 | <0.001 | <0.001 | <0.001 | N/A | N/A | N/A | <0.001 | <0.001 |
| Anaplasma marginale | 31 | 0.001 | <0.001 | <0.001 | N/A | N/A | N/A | <0.001 | <0.001 |
| Anaplasma ovis | 7 | 0.003 | <0.001 | N/A | N/A | N/A | N/A | N/A | 0.003 |
| Rickettsia spp. | 326 | <0.001 | <0.001 | <0.001 | 0.16 | 0.02 | <0.001 | <0.001 | 0.038 |
| Rickettsia africae | 24 | 0.025 | <0.001 | 0.204 | 0.812 | N/A | N/A | 0.104 | 0.908 |
| Rickettsia aeschlimanni | 22 | <0.001 | <0.001 | 0.042 | 0.176 | N/A | N/A | <0.001 | 0.014 |
| Rickettsia massiliae | 15 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | N/A | 0.241 | 0.156 |
| Rickettsia conorii | 16 | <0.001 | <0.001 | <0.001 | <0.001 | 0.127 | N/A | 0.001 | 0.051 |
| Coxiella spp. | 32 | <0.001 | <0.001 | <0.001 | 1 | 0.122 | N/A | N/A | 1 |
| Coxiella burnetii | 139 | <0.001 | <0.001 | <0.001 | <0.001 | 0.796 | 0.22 | 0.494 | <0.001 |
| Coxiella-like endosymbionts | 8 | <0.001 | <0.001 | <0.001 | N/A | N/A | <0.001 | <0.001 | N/A |
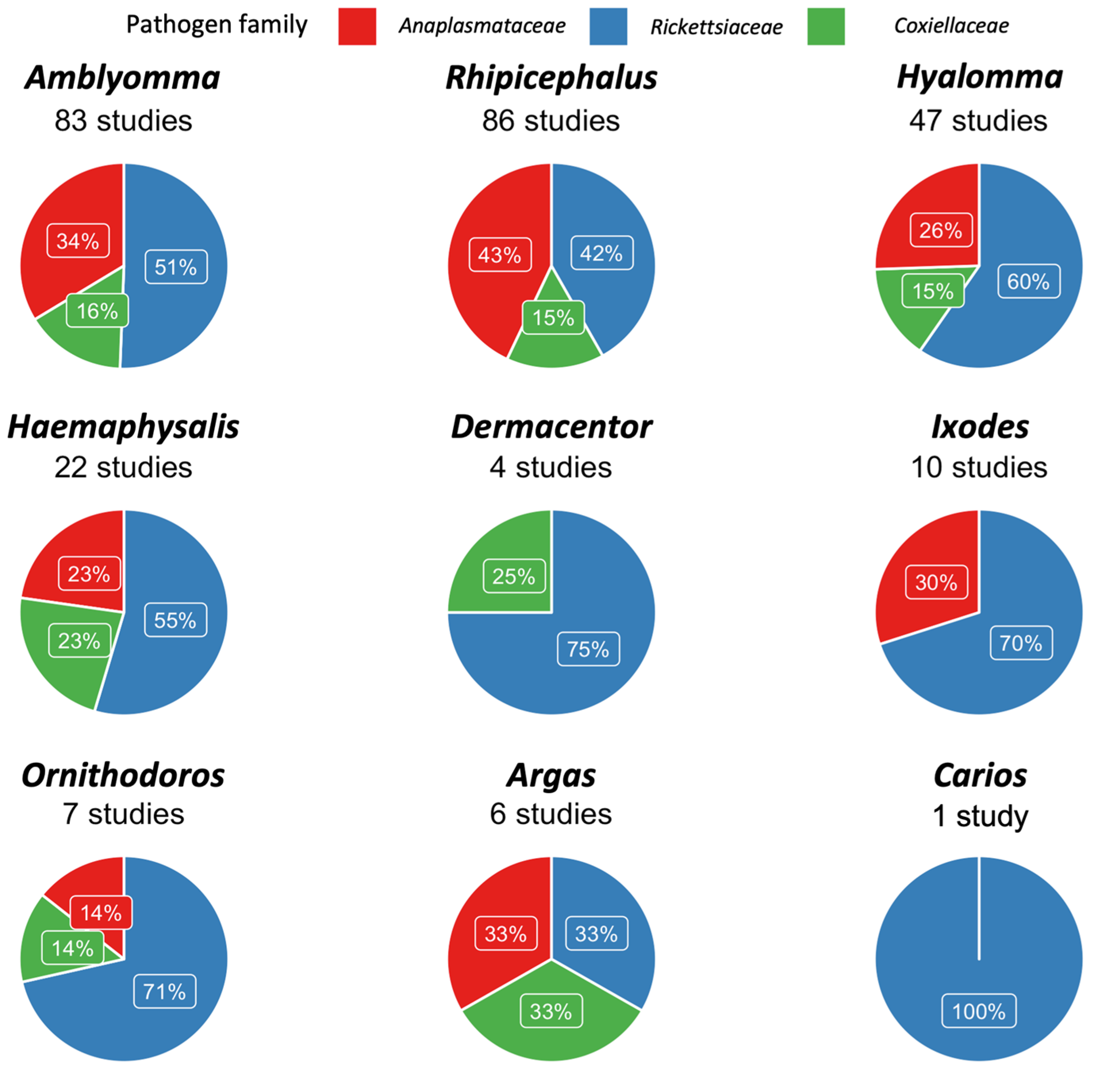
| Tick Genus | E. ruminantium | E. canis | A. marginale | A. ovis | Rickettsia spp. | R. africae | R. aeschlimanni | R. massiliae | R. conorii | Coxiella spp. | C. burnetii | CLEs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amblyomma | 8% [5.6–10.7%] | 0% [0–0.1%] | 12.6% [4.1–24.3%] | 56.6% [45.7–67.2%] | 24.3% [4.3–52.5%] | 0% [0–1%] | 0% [0–1%] | 0% [0–0.1%] | 45.1% [4.4–89.5%] | 0% [0–2.1%] | 99.4% [40.1–100%] | |
| Dermacentor | 38.8% [0.4–88.3%] | 0% [0–38.9%] | 0% [0–50%] | 100% [30.3–100%] | ||||||||
| Haemaphysalis | 12.2% [0.3–32.3%] | 4.2% [0–17%] | 27.3% [4.4–57.9%] | 8.7% [0–50.2%] | 100% [30.3–100%] | |||||||
| Hyalomma | 0% [0–0.4%] | 0% [0–0.4%] | 0% [0–0.1%] | 6.1% [2.5–10.7%] | 13.9% [0–100%] | 13.2% [2.1–28.9%] | 0% [0–0.4%] | 0% [0–2.6%] | 0% [0–1.2%] | 22.5% [4.5–46.4%] | ||
| Ixodes | 5.9% [0–27.4%] | 0% [0–100%] | 3.3% [1–6.6%] | |||||||||
| Rhipicephalus | 10.5% [0–47.8%] | 11.6% [1.7–27.2%] | 21.1% [0–57.9%] | 2.7% [0–10.4%] | 6.1% [2.8–10.2%] | 1% [0–5%] | 0% [0–0%] | 14.9% [2.8–32.4%] | 18.8% [4.3–39%] | 37.4% [12.4–65.6%] | 0% [0–0.4%] |
| Tick Species | E. ruminantium | E. canis | A. marginale | A. ovis | R. africae | R. aeschlimanni | R. massiliae | R. conorii | C. burnetii | CLEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Amblyomma astrion | 0% [0–4.1%] | 97.6% [90.1–100%] | ||||||||
| Amblyomma cohaerens | 5.1% [0–31.4%] | |||||||||
| Amblyomma gemma | 24.4% [13.1–37.1%] | |||||||||
| Amblyomma hebraeum | 9.4% [5.3–14.3%] | 0% [0–0.1%] | 0% [0–0.1%] | 4.2% [0–13.1%] | 0% [0–0.1%] | 0% [0–25%] | ||||
| Amblyomma lepidum | 1.9% [0–5.6%] | 0% [0–6.5%] | ||||||||
| Amblyomma spp. | 0% [0–0%] | |||||||||
| Amblyomma sylvaticum | 0% [0–4.2%] | |||||||||
| Amblyomma variegatum | 7.5% [4.4–11.3%] | 19% [7.7–33.5%] | 72.1% [23–100%] | 0% [0–1%] | 0% [0–1%] | 0.3% [0–5.9%] | 100% [97.2–100%] | |||
| Dermacentor marginatus | 0% [0–50%] | 100% [30.3–100%] | ||||||||
| Haemaphysalis erinacei | 46.9% [29.7–64.4%] | |||||||||
| Haemaphysalis leachi | 4.2% [0–17%] | 0% [0–26.8%] | ||||||||
| Haemaphysalis punctata | 0% [0–100%] | |||||||||
| Haemaphysalis spinulosa | 0% [0–53.9%] | |||||||||
| Haemaphysalis sulcata | 100% [30.3–100%] | |||||||||
| Hyalomma aegyptium | 0% [0–0.7%] | |||||||||
| Hyalomma detritum | 20% [0–67.5%] | 25% [0–79.3%] | ||||||||
| Hyalomma dromedarii | 0% [0–1.1%] | 14.2% [0–79.3%] | 0% [0–6.4%] | |||||||
| Hyalomma excavatum | 0% [0–18.3%] | 0% [0–14.7%] | 36.4% [17.3–57.8%] | |||||||
| Hyalomma impeltatum | 0% [0–1.3%] | 0% [0–44.4%] | 2.4% [0–13.1%] | |||||||
| Hyalomma impressum | 0% [0–100%] | 0% [0–100%] | 100% [0–100%] | 0% [0–88.8%] | 0% [0–100%] | 0% [0–100%] | ||||
| Hyalomma lusitanicum | 0% [0–88.8%] | 33.3% [0–94.1%] | ||||||||
| Hyalomma marginatum | 0% [0–10.5%] | 0% [0–10.5%] | 12.5% [0.3–34.1%] | 37.4% [0–93.8%] | 0% [0–10.5%] | 0% [0–42.5%] | 14.3% [3.3–30.1%] | |||
| Hyalomma rufipes | 3.1% [0–15.2%] | |||||||||
| Hyalomma scupense | 0% [0–99.3%] | |||||||||
| Hyalomma truncatum | 0% [0–6.3%] | 0% [0–6.3%] | 3.7% [0–15.2%] | 11.1% [1.5–26.3%] | 0% [0–6.3%] | 2.1% [0–14.3%] | ||||
| Ixodes ricinus | 0% [0–0%] | |||||||||
| Ixodes vespertilionis | 15.8% [2.3–36.2%] | |||||||||
| Rhipicephalus annulatus | 0% [0–8%] | 0% [0–8%] | 2% [0–100%] | |||||||
| Rhipicephalus appendiculatus | 1.1% [0–4.6%] | 0% [0–2.8%] | ||||||||
| Rhipicephalus bursa | 0% [0–5.7%] | 0% [0–5.7%] | 0.5% [0–4.4%] | |||||||
| Rhipicephalus compositus | 7.1% [0–28.2%] | |||||||||
| Rhipicephalus decoloratus | 0% [0–3.6%] | |||||||||
| Rhipicephalus evertsi | 0% [0–0.4%] | |||||||||
| Rhipicephalus guilhoni | 0.5% [0–8.3%] | |||||||||
| Rhipicephalus lunulatus | 4.3% [0.1–12.7%] | |||||||||
| Rhipicephalus microplus | 14.2% [0–66.4%] | 59.7% [9.1–99.4%] | 3.2% [0–16%] | 0% [0–1.1%] | 0% [0–1.1%] | 0% [0–1.1%] | ||||
| Rhipicephalus muhsamae | 0.7% [0–3%] | 6.9% [3.3–11.7%] | 4.2% [1.4–8.2%] | 0% [0–6.1%] | ||||||
| Rhipicephalus praetextatus | 0.8% [0–4.1%] | |||||||||
| Rhipicephalus pulchellus | 21.3% [7.5–38.7%] | |||||||||
| Rhipicephalus sanguineus | 11.6% [1.7–27.2%] | 0% [0–2.2%] | 2.5% [0–7.5%] | 0% [0–0%] | 25.1% [11.2–41.9%] | 20.8% [4.6–43.3%] | 0% [0–1.5%] | |||
| Rhipicephalus senegalensis | 0% [0–50%] | 0% [0–50%] | 0% [0–50%] | 0% [0–50%] | 60.1% [0–100%] | 0% [0–50%] | ||||
| Rhipicephalus simus | 0% [0–100%] | |||||||||
| Rhipicephalus spp. | 0% [0–5.6%] | |||||||||
| Rhipicephalus sulcatus | 3.1% [0–12.9%] | |||||||||
| Rhipicephalus turanicus | 0% [0–0.8%] | 7.9% [4.7–11.8%] | 0% [0–2.8%] |
| Pathogen Species | Formula | Residual Heterogeneity (I2),% | Amount of Heterogeneity Accounted for (R2),% | Test of Moderators (p-Value) |
|---|---|---|---|---|
| Ehrlichia ruminantium | Sampling_country * Test * Tick_species | 78.4 | 67.03 | <0.001 |
| Ehrlichia canis | Sampling_country | 50.58 | 80.32 | 0.01 |
| Anaplasma marginale | Sampling_country + Tick_species | 82.36 | 87.63 | <0.001 |
| Rickettsia spp. | Sampling_country * Tick_genus * Test * Risk_of_bias | 87.86 | 57.85 | <0.001 |
| Rickettsia africae | Tick_species + Sampling_strategy | 93.24 | 58.87 | 0.007 |
| Rickettsia aeschlimanni | Tick_species + Test | 55.16 | 74.28 | 0.001 |
| Rickettsia massiliae | Tick_species + Sampling_country | 59.78 | 77.68 | 0.07 |
| Rickettsia conorii | Tick_species + Test | 83.29 | 63.18 | 0.003 |
| Coxiella spp. | Sampling_country * Tick_genus | 83.63 | 18.58 | 0.158 |
| Coxiella burnetii | Sampling country * Tick_species | 55.04 | 35.8 | <0.001 |
| Coxiella-like endosymbionts | Sampling_country | 83.32 | 50.22 | 0.018 |
| Pathogen Species | Pooled Estimate (%) [95% CI] | Reasons to Downgrade | Reasons to Upgrade | Score | Resulting QoE |
|---|---|---|---|---|---|
| Ehrlichia/Anaplasma spp. | 2.32 [0.81–4.34] | Risk of bias ~ Moderate Indirectness | 2.33/4 | Moderate +++ | |
| Ehrlichia ruminantium | 6.37 [3.97–9.16] | Risk of bias ~ Low Inconsistency Indirectness | 1.99/4 | Low ++ | |
| Ehrlichia canis | 4.3 [0.04–12.66] | Risk of bias ~ Moderate Indirectness | 2.33/4 | Moderate +++ | |
| Anaplasma marginale | 12.75 [4.06–24.35] | Risk of bias ~ Low Imprecision Inconsistency | 1.99/4 | Low ++ | |
| Anaplasma centrale | 0 [0–0] | Risk of bias ~ Low, Publication bias Indirectness | Large effect | 2.66/4 | Moderate +++ |
| Anaplasma bovis | 0 [0–0] | Risk of bias ~ Low, Publication bias | Large effect | 3.33/4 | High ++++ |
| Anaplasma ovis | 0.55 [0–3.73] | Risk of bias ~ Low Inconsistency Indirectness | 1.99/4 | Low ++ | |
| Anaplasma platys | 0.34 [0–1.46] | Risk of bias ~ Moderate | 3/4 | Moderate +++ | |
| Anaplasma phagocytophilum | 0 [0–0.15] | Risk of bias ~ Low Indirectness | Large effect | 3.33/4 | High ++++ |
| Rickettsia spp. | 18.39 [14.23–22.85] | Risk of bias ~ Moderate Publication bias Inconsistency | Large effect | 2.33/4 | Moderate +++ |
| Rickettsia africae | 13.47 [2.76–28.69] | Risk of bias ~ Low Imprecision Inconsistency | 1.99/4 | Low ++ | |
| Rickettsia aeschlimanni | 2.55 [0–9.48] | Risk of bias ~ Moderate Publication bias Indirectness | 1.66/4 | Low ++ | |
| Rickettsia massiliae | 6.87 [0.21–18.43] | Risk of bias ~ Moderate | 3/4 | Moderate +++ | |
| Rickettsia conorii | 11.28 [1.77–25.89] | Risk of bias ~ Moderate Imprecision Inconsistency | 1.66/4 | Low ++ | |
| Coxiella spp. | 27.02 [10.83–46.03] | Risk of bias ~ Moderate Imprecision Inconsistency | Large effect | 2.33/4 | Moderate +++ |
| Coxiella burnetii | 0 [0–0.25] | Risk of bias ~ Low | Large effect | 4/4 | High ++++ |
| Coxiella-like endosymbionts | 70.47 [27–99.82] | Risk of bias ~ Low Imprecision Inconsistency | Large effect | 2.66/4 | Moderate +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, C.A.; Collins, N.E.; Oosthuizen, M.C.; Menandro, M.L.; Bhoora, R.V.; Vorster, I.; Cassini, R.; Stoltsz, H.; Quan, M.; van Heerden, H. Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 714. https://doi.org/10.3390/microorganisms11030714
Cossu CA, Collins NE, Oosthuizen MC, Menandro ML, Bhoora RV, Vorster I, Cassini R, Stoltsz H, Quan M, van Heerden H. Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis. Microorganisms. 2023; 11(3):714. https://doi.org/10.3390/microorganisms11030714
Chicago/Turabian StyleCossu, Carlo Andrea, Nicola E. Collins, Marinda C. Oosthuizen, Maria Luisa Menandro, Raksha Vasantrai Bhoora, Ilse Vorster, Rudi Cassini, Hein Stoltsz, Melvyn Quan, and Henriette van Heerden. 2023. "Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis" Microorganisms 11, no. 3: 714. https://doi.org/10.3390/microorganisms11030714
APA StyleCossu, C. A., Collins, N. E., Oosthuizen, M. C., Menandro, M. L., Bhoora, R. V., Vorster, I., Cassini, R., Stoltsz, H., Quan, M., & van Heerden, H. (2023). Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis. Microorganisms, 11(3), 714. https://doi.org/10.3390/microorganisms11030714










