Abstract
Coxiella burnetii is a Gram-negative, intracellular bacterium that causes the zoonosis Q fever. Among the many natural isolates of C. burnetii recovered from various sources, the Dugway group exhibits unique genetic characteristics, including the largest C. burnetii genomes. These strains were isolated during 1954–1958 from wild rodents from the Utah, USA desert. Despite retaining phase I lipopolysaccharide and the type 4B secretion system, two critical virulence factors, avirulence has been reported in a guinea pig infection model. Using guinea pig models, we evaluated the virulence, whole-cell vaccine (WCV) efficacy, and post-vaccination hypersensitivity (PVH) potential of a representative Dugway strain. Consistent with prior reports, Dugway appeared to be highly attenuated compared to a virulent strain. Indeed, Dugway-infected animals showed similarly low levels of fever, body weight loss, and splenomegaly like Nine Mile II-infected animals. When compared to a human Q fever vaccine, QVax®, Dugway WCV exhibited analogous protection against a heterologous Nine Mile I challenge. PVH was investigated in a skin-testing model which revealed significantly decreased maximum erythema in Dugway Δdot/icm WCV-skin-tested animals compared to that of QVax®. These data provide insight into this unique bacterial strain and implicate its potential use as a mutated WCV candidate.
1. Introduction
Coxiella burnetii is a Gram-negative, intracellular bacterium with a near worldwide distribution [1,2]. The zoonosis Q fever is caused by this bacterium, typically following inhalation of infectious particles generated by infected animals. Q fever encompasses a wide spectrum of clinical disease; however, the most common manifestation is a flulike illness known as acute Q fever [3]. Due to the potentially debilitating nature of Q fever, C. burnetii’s pronounced environmental stability, and aerosol infection potential, this pathogen is considered a biodefense threat and has been classified as a select agent by the U.S. Centers for Disease Control and Prevention (CDC)-Division of Select Agents and Toxins (DSAT) [4]. C. burnetii Nine Mile II (NMII) Clone 4 (RSA439), is exempt from DSAT regulation and may be manipulated at BSL-2 conditions [5]. This clonal strain expresses truncated lipopolysaccharide (LPS) due to a large chromosomal deletion and has been reported to exhibit avirulence in a guinea pig infection model [6]. In contrast, C. burnetii NMI is a virulent strain expressing full-length LPS and is commonly used as a positive control for virulence in animal studies. Beyond laboratory-generated strains such as NMII, clinically and environmentally derived C. burnetii strains have been isolated from a wide array of organisms including cats, chiggers, cows, dogs, goats, humans, rodents, sheep, and ticks [7,8,9]. These strains exhibit genetic and phenotypic diversity [7,10,11] despite retaining full-length LPS and the type IVB secretion system (T4BSS), two core C. burnetii virulence factors.
In 1959, the isolation of several novel “Dugway” C. burnetii strains was reported [12]. These strains were isolated from three rodent species (Peromyscus maniculatus, Dipodomys ordii, and D. microps) from the Great Salt Lake Desert, Utah USA during a three-year collection period (1954–1957) [12]. This marked the first report of C. burnetii recovery from wild mammals. The unique biologic characteristics of the Dugway strains were reported soon after these strains were isolated. The authors encountered difficulty passaging yolk sac isolates in infected guinea pigs due to apparent avirulence, a hypothesis further bolstered by guinea pig infection studies [13]. Additionally, hamsters appeared to be more susceptible to the Dugway strain infection and pathology than guinea pigs, also developing higher antibody titers following infection. These findings stood in contrast to results obtained for non-rodent derived isolates (e.g., dairy and clinical human isolates). Recent studies confirmed Dugway strain attenuation in guinea pig intraperitoneal [11] and aerosol [10] infection models. Genetic analysis of the Dugway 5J108-111 strain indicated that this strain has the largest genome with the fewest pseudogenes and insertion sequence elements compared to all other C. burnetii strains [14]. These data suggest that the Dugway strains supersede other isolates (e.g., NMI, K Q154, and G Q212) in terms of temporal lineage establishment. Dugway strains possess the unique QpDG plasmid [15] and Dugway-specific plasmid-encoded effector proteins have been identified [16]. It remains to be seen whether chromosomal and/or plasmid genomic sequences lie at the root of Dugway strains’ unique behavior. Further, phylogenetic analysis of various C. burnetii strains based on adaA gene variation suggested that Dugway strains may be the ancestor of all C. burnetii strains [17].
Likely related to host adaptation and tropism, these unique genetic and phenotypic features pose Dugway strains as valuable experimental tools and potential vaccine candidates. Avirulence and/or severe attenuation despite retention of primary virulence factors paired with Dugway strains’ unique genomic content all contribute to its intrigue and utility. Dugway strains possess desired qualities for whole-cell vaccines (WCV); however, protective efficacy has not yet been determined. Further, Q fever WCVs are known to cause potentially severe post-vaccination hypersensitivity responses in pre-immune individuals [18], representing a major roadblock for widespread licensing of existing Q fever vaccines (e.g., Q-VAX®). Although mechanisms of post-Q fever vaccination delayed-type hypersensitivity (DTH) are being uncovered using novel animal models [19,20], causative antigens have not yet been determined. Accordingly, despite Dugway strain uniqueness, the reactogenicity of Dugway-based WCVs has not been reported. Here, we sought to characterize Dugway-host interactions in vivo using guinea pig models of infection, vaccine challenge, and post-vaccination DTH. Further, these studies were designed to evaluate the feasibility of Dugway-based WCVs as an improved Q fever vaccine. Thus, we created a mutant Dugway ∆dot/icm strain, lacking 23 or 26 genes within the dot/icm apparatus encoding the TB4SS apparatus. This strategy has been used for C. burnetii NMI and conferred attenuation, retained immunogenicity, and potentially reduced post-vaccination DTH magnitude [21].
2. Materials and Methods
2.1. Coxiella burnetii Strains, Infection Stocks, and Whole-Cell Vaccines (WCV)
C. burnetii strains (NMI RSA 439, Dugway 7D 77-80, and Dugway Δdot/icm clone 7) were propagated in acidified citrate cysteine medium-2 or -D (ACCM-2 or ACCM-D) [22] at 37 °C, 2.5% O2, and 5% CO2 and were stored at −80 °C in a cell-freezing medium (DMEM with 10% fetal bovine serum and 10% dimethyl sulfoxide) until use. C. burnetii Dugway Δdot/icm was constructed as previously described for the NMI Δdot/icm strain [21]. Whole-cell vaccine (WCV) stocks, used for vaccination and skin testing, were cultured as infection stocks and were fixed in 4% paraformaldehyde for at least 12 h, washed in sterile PBS, and ultimately resuspended in USP-grade saline prior to being stored at −80 °C. C. burnetii infection stock concentrations were quantified using qPCR to enumerate genomic equivalents (GE) [11] while WCV concentrations were determined via direct bacterial count, as previously described [21,23]. Lipopolysaccharide (LPS) from infection stocks was extracted via a modified hot phenol method and visualized by silver stain, as previously described [24] (Supplementary Figure S1). In accordance with standard operating procedures approved by the Rocky Mountain Laboratories Institutional Biosafety Committee, any manipulations of C. burnetii stocks and infected animal tissue were performed in a BSL-3 laboratory.
2.2. Guinea Pigs
Four- to six-week-old Hartley guinea pigs were obtained from Charles River, Wilmington, MA, USA (strain code 051) and were acclimated for at least a week prior to experimental manipulation. Female guinea pigs were utilized in these studies to minimize potentially confounding sex-associated factors (e.g., behavior, body weight, hormonal effects) and are used in accordance with historical Q fever virulence studies [10,25]. Animals were housed in individually ventilated plastic cages (Allentown, Allentown, NJ, USA; two animals per cage) with hardwood Sani-chip bedding (PJ Murphy, Montville, NJ, USA). A high-fiber guinea pig diet (Teklad global high-fiber guinea pig diet; Envigo, Indianapolis, IN, USA, cat n. 2041) and chlorinated, reverse osmosis filtered tap water was administered ad libitum. A 12 h light–dark cycle was maintained in animal housing facilities which were kept at 68–72 °F and 40–60% relative humidity with a 50% set point. Six animals per group were utilized for the experiment evaluating virulence, while four animals per group were utilized in vaccine challenge and post-vaccination hypersensitivity experiments. Animals were housed in approved animal biosafety level 3 (ABSL-3) facilities and manipulated under ABSL-3 standard operating procedures approved by the Rocky Mountain Laboratories Institutional Biosafety Committee and an Institutional Animal Care and Use Committee-approved protocol. Animal experiments and procedures were performed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited NIH/NIAID animal facility.
2.3. Infection Model
On the day of infection, animals were placed under isoflurane-induced sedation using an anesthetic vaporizer with activated charcoal absorption filters (VetEquip Inc., cat. N. 901801 and 931401, Livermore, CA, USA) and subcutaneously implanted with an IPTT-300 transponder (BioMedic Data Systems, Seaford, DE, USA) above the shoulder using a large bore needle. Guinea pigs were then infected with 1 mL of 106–107 GE of C. burnetii in USP-grade saline via intraperitoneal injection. Negative control animals were mock infected with USP-grade saline. Body weights, body temperatures, and any behavioral/clinical changes were recorded daily at a consistent time for 14 days following infection. Body temperatures were collected using a DAS-8007-P reader (BioMedic Data Systems) and a temperature of ≥39.5 °C was defined as fever [10,26,27]. Fourteen days post-infection, animals were euthanized. Blood and spleens were collected at euthanasia and processed as previously described and bacterial outgrowth from spleen tissues was quantified by TaqMan qPCR (groel gene) [21].
2.4. WCV Challenge Model
On the day of vaccination, animals were sedated by isoflurane inhalation and implanted with IPTT-300 transponders as described above. Four guinea pigs per group were vaccinated subcutaneously in the upper back with 0.5 mL of USP-grade saline containing 25–2.5 μg of QVax® or paraformaldehyde-fixed C. burnetii. Negative control animals were mock vaccinated with USP-grade saline. Body weights, body temperatures, and behavioral/clinical changes were recorded daily following vaccination for a total duration of 28 days. At 28 days post-vaccination, animals were infected with 1 mL of 106 GE C. burnetii (NMI) as described above. Upon euthanasia, blood, mesenteric lymph nodes, and spleens were collected and processed as previously described [21].
For mLN and splenic flow cytometric analysis, single-cell suspensions were aliquoted into 96-well U-bottom plates at a density of 1 × 106 cells per well. Cells were washed in a staining buffer (PBS + 1% bovine serum albumin) and stained using a cocktail of antibodies specific for guinea pig cell surface antigens, including B Cells (clone: MsGp10, fluorophore: S/N unconjugated, BioRad, Hercules, CA, USA, cat. N. MCA567) with secondary antibody (anti-mouse IgG1, clone: RMG1-1, fluorophore: AF700, BioLegend, San Diego, CA, USA, cat. N. 406632), CD4 (clone: CT7, fluorophore: RPE, BioRad, cat. n. MCA749PE), and CD8 (clone: CT6, fluorophore: FITC, BioRad, cat. n. MCA752F). Following surface staining, cells were washed in staining buffer and fixed overnight at 4 °C using Cytofix (BD, San Jose, CA, USA, cat. n. 554655). Following fixation, cells were washed in staining buffer and analyzed on a BD FACSymphony flow cytometer using FacsDiva software (BD Biosciences). Data analysis was performed with FlowJo 10.0 software (TreeStar Inc., Ashland, OR, USA). A minimum of 20,000 events were captured for each sample. Single-stained compensation controls and fluorescence minus one staining controls were included to help set gating boundaries.
2.5. Post-Vaccination Hypersensitivity Modeling
The guinea pig post-vaccination hypersensitivity model was performed as previously described [21]. Briefly, four guinea pigs per group were infected with 106 GE of NMI or mock infected with saline and monitored for 42 days. Next, animals were sedated by isoflurane inhalation and skin tested with 0.1 mL of 25, 2.5, and 0.25 μg of C. burnetii WCV in USP-grade saline via intradermal injection at three separate sites on the shaved back. Negative control animals were mock skin tested with USP-grade saline. Body weights, body temperatures, behavioral/clinical changes, and skin metrics were recorded daily for 21 days post-skin tests. Skin-testing sites were shaved one day prior to intradermal inoculation (“skin testing”) and one day prior to subsequent skin metric measurement. Erythema diameter and induration severity were measured as previously described [21]. Animals were euthanized 21 days following skin testing. Blood, mesenteric lymph nodes, spleens, and skin biopsies were collected for subsequent analysis, as previously described [21].
2.6. Histology
Histology was performed as previously described [20]. Briefly, skin biopsies were fixed in 10% Neutral Buffered Formalin for 48 h, placed in tissue cassettes, and processed with a Sakura VIP-6 Tissue Tek (Torrance, CA, USA) on a 12 h automated schedule using a graded series of ethanol, xylene, and PureAffin. Embedded tissues were sectioned at 5 μm, mounted and dried overnight at 42 °C prior to staining with hematoxylin and eosin using established methods. Biopsy specimens were evaluated using an Olympus BX53 microscope (Tokyo, Japan).
2.7. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA, USA). Statistical evidence for differences in group means was assessed using two-sample Welch t tests, allowing for unequal variances between groups. For each comparison, we computed Wald-type 95% confidence intervals and describe statistical significance with two-sided p-values. We represent p-values in equal to or below 0.05 with a single asterisk (*), p-values equal to or below 0.01 with a double asterisk (**), and p-values equal to or below 0.001 with a triple asterisk (***) unless otherwise indicated. Error bars represent the standard deviation of a group mean.
3. Results
3.1. C. burnetii Dugway Is Attenuated in an Intraperitoneal Guinea Pig Model of Q Fever
We first evaluated the virulence of Dugway strain 7D 77-80 using a guinea pig model of intraperitoneal infection (Figure 1A). Guinea pigs were injected with 106−7 genome equivalents (GE) of C. burnetii or saline. Next, body temperatures and weights were recorded for 14 days, followed by euthanasia. Body temperatures for saline mock-infected animals remained stable along with NMII (106), Dugway (106), and Dugway ∆dot/icm infected animals (Figure 1B). Animals infected with 107 NMII and Dugway displayed low-grade, transient fever profiles, in contrast to NMI-infected animals (106) which experienced sustained, robust fever responses. When body temperatures were normalized to the initial readings at day 0, a clear divergence between NMI-infected animals and all other groups emerged (Figure 1C). Body weight change corresponded with body temperature findings, with NMI-infected animals experiencing higher body weight loss than all other groups (Figure 1D). Following the same trend, NMI-infected animals displayed significant splenomegaly compared to saline, NMII, Dugway, and Dugway ∆dot/icm-infected animals (Figure 1E). NMII (107), Dugway (106/7), and Dugway ∆dot/icm (107)-infected animals exhibited significant splenomegaly compared to saline mock-infected animals. Day 14 splenic bacterial burdens were highest in NMI-infected animals but also present in NMII and Dugway-infected animals (Supplementary Figure S2). No C. burnetii DNA was detected in saline or Dugway ∆dot/icm-infected animals.
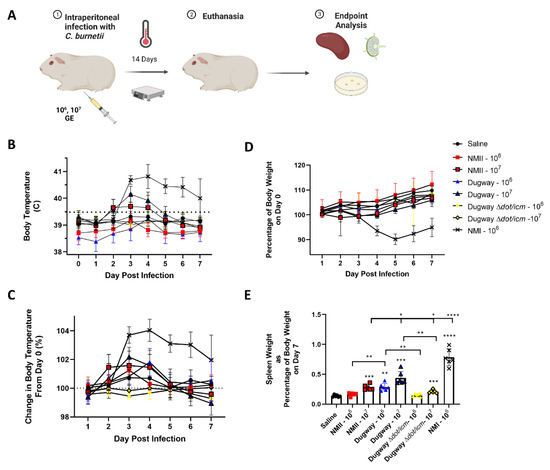
Figure 1.
Coxiella burnetii Dugway strain displays reduced virulence in a robust guinea pig infection model compared to the Nine Mile strain. The guinea pig model of intraperitoneal infection is outlined in (A). Body temperatures (B), body temperature change (C), and body weight change (D) kinetics are displayed for 7 days following infection. Spleens were weighed at euthanasia (day 7) and splenomegaly was determined by normalizing spleen weight to total body weight (E). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001, **** p ≤ 0.0001.
3.2. C. burnetii Dugway Exhibits Heterologous Protection as a Whole-Cell Vaccine (WCV)
Next, we evaluated the efficacy of C. burnetii Dugway as a whole-cell vaccine against a challenge with the virulent Nine Mile I (NMI) strain. Guinea pigs were subcutaneously vaccinated with saline, C. burnetii WCV, or QVax® in the upper back (Figure 2A).
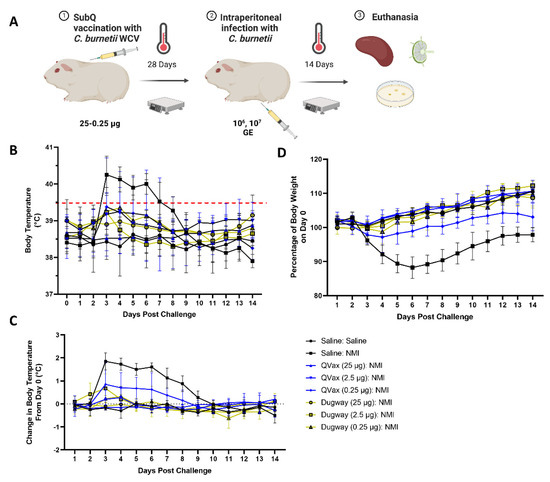
Figure 2.
C. burnetii Dugway whole-cell vaccine exhibits heterologous protection against fever and body weight loss. The guinea pig vaccine-challenge model is outlined in (A). Body temperatures (B), body temperature change (C), and body weight change (D) kinetics are displayed for 14 days following infection.
Body temperature and body weight were taken for 28 days and vaccinations did not induce alterations in body temperature or weight (Supplementary Figure S3A,B). Guinea pigs were then intraperitoneally challenged with C. burnetii NMI (106 GE) and monitored for 14 days before euthanasia. Mock vaccinated and NMI-challenged guinea pigs (Saline:NMI) experienced sustained fever following NMI infection (Figure 2B), serving as a positive infection control. In contrast, Dugway- and QVax®-vaccinated animals appeared to be protected from the development of fever apart from a few transient breakthrough animals at the lowest vaccination dose (0.25 µg). When normalized to day 0 starting body temperatures, these trends persisted (Figure 2C). Body weight change was stable in all groups with the exception of mock vaccinated, NMI-infected animals and QVax® (0.25 µg) vaccinated, NMI-infected animals with both groups losing a higher percentage of body weight than others (Figure 2D). Significant splenomegaly was observed in mock vaccinated, NMI-infected animals compared to uninfected animals (Figure 3A). Significant splenomegaly was not observed in any vaccinated and NMI-infected animals compared to uninfected animals. No significant differences in splenic bacterial burden were detected in vaccinated groups compared to unvaccinated, NMI-infected animals (Figure 3B). Although mesenteric lymph node (mLN) cellularity was not altered in Saline:Saline animals compared to vaccinated groups (Supplementary Figure S4A), spleen cellularity was significantly increased in QVax® (25 and 2.5 µg)- and Dugway (2.5 and 0.25 µg)-vaccinated animals compared to Saline:Saline negative controls (Supplementary Figure S4B). Flow cytometry was performed on secondary lymphoid tissues and the gating strategy for CD4+/CD8+ T cells and B cells is depicted in Supplementary Figure S4C,D, respectively. dLN B cell frequency was significantly decreased in Dugway (25 µg):NMI animals and appeared to be generally reduced in additional vaccinated groups (Supplementary Figure S4E). CD4+ T cell frequency appeared unaltered regardless of treatment (Supplementary Figure S4F). CD8+ T cell frequency was significantly increased in unvaccinated, NMI-infected animals compared to unvaccinated, uninfected animals (Supplementary Figure S4G). Splenic flow cytometry revealed significantly decreased B cell frequency in Saline:NMI, QVax® (2.5 and 0.25 µg):NMI and Dugway:NMI groups compared to that of untreated Saline:Saline animals (Supplementary Figure S4H). CD4+ T cell frequency was significantly decreased in Saline:NMI, QVax® (0.25 µg):NMI and Dugway:NMI groups compared to that of untreated Saline:Saline animals (Supplementary Figure S4I). No significant alterations in splenic CD8+ T cell frequency were observed in treated groups (Supplementary Figure S4J).
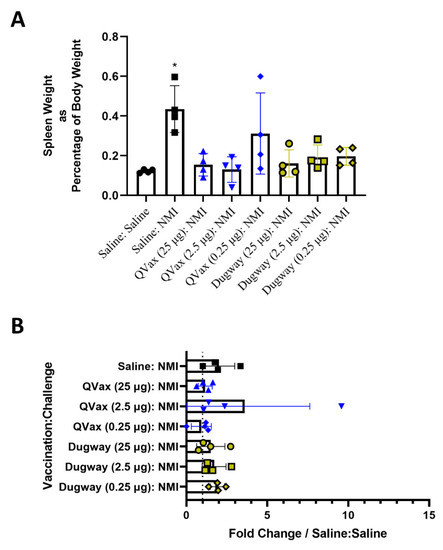
Figure 3.
C. burnetii Dugway whole-cell vaccine prevents post-infectious splenomegaly but does not alter bacterial burden. Spleens were weighed at euthanasia (day 14) and splenomegaly was determined by normalizing spleen weight to total body weight (A). Splenic bacterial burden at euthanasia is represented by fold change in genome equivalents compared to uninfected (Saline:Saline) animals in (B). * p ≤ 0.05.
3.3. Dugway Δdot/icm Strains Exhibit Reduced Post-Vaccination Erythema
A guinea pig post-vaccination hypersensitivity (PVH) model was employed to evaluate the reactogenic potential of various C. burnetii WCVs. Guinea pigs were sensitized by intraperitoneal infection using the NMI or Dugway strains (Figure 4A). Body weights and body temperature were measured for 14 days following inoculation and these data indicated a successful infection (Supplementary Figure S5A,B). Following sensitization, guinea pigs were intradermally injected with saline or C. burnetii WCVs on the back at three different doses (25, 2.5, and 0.25 µg) and skin responses were monitored for 21 days prior to euthanasia. Maximum erythema area was significantly increased for all C. burnetii sensitized (infected) and skin-tested guinea pigs compared to Saline:Saline mock-treated animals (Figure 4B). Further, compared to unsensitized, skin-tested animals (Saline:NMI) maximum erythema for NMI:QVax®, Dugway:Dugway, and NMI:Dugway groups was significantly increased. Compared to highly reactive NMI:QVax® animals, C. burnetii ∆dot/icm WCV skin-tested animals demonstrated significantly reduced maximal erythematous responses. Erythema kinetics reflect maximal values (Supplementary Figure S6A). Induration was assessed at various time points post-skin testing; early (5 days; Supplementary Figure S6B) and late (20 days; Figure 4C) intragroup responses were similar. Generally, induration severity was aligned among all sensitized and skin-tested groups at the 25 µg dose. At the 2.5 and 0.25 µg doses, the NMI:Dugway ∆dot/icm group appeared to experience reduced early and late induration severity. Despite the occurrence of localized PVH responses, body temperature (Supplementary Figure S7A) and body weight (Supplementary Figure S7B) changes due to skin testing were not detected. Significant splenomegaly was detected in NMI:QVax® and NMI:Dugway groups compared to Saline:Saline control animals (Supplementary Figure S7C). Despite this, spleen cellularity (Supplementary Figure S7D) and mesenteric lymph node cellularity (Supplementary Figure S7E) remained unchanged among groups at day 21 post-skin testing.
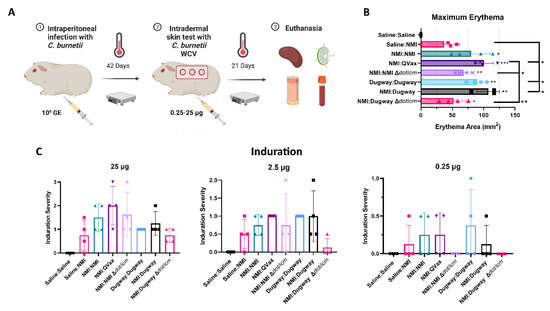
Figure 4.
C. burnetii Dugway ∆dot/icm whole-cell vaccine reduces post-skin testing erythema and late-phase induration magnitude. The guinea pig model of post-vaccination hypersensitivity is outlined in (A). Maximum erythema at any given timepoint post-skin testing is depicted in (B). Induration scoring values at indicated skin testing site at day 20 post-skin testing (C). * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
A standardized histological scoring scheme was applied to guinea pig skin biopsies collected from skin testing sites (Figure 5A). General histological findings associated with sensitization and skin testing occurred in the dermis, hypodermis, and panniculus muscle. Lesions ranged in severity from minimal to severe with abscess formation. Minimal inflammation was characterized by small foci of macrophages, lymphocytes, and sometimes, heterophils within either the dermis, hypodermis, or panniculus muscle. Mildly inflamed foci were increased in size, may occur in more than one tissue layer and contain more macrophages, lymphocytes, and heterophils. Granulomatous to pyogranulomatous inflammation consisted of epithelioid macrophages, multinucleated giant cells, lymphocytes with heterophils which were often degenerative and extended into multiple tissue layers. Moderate to marked pyogranulomatous inflammation consisted of epithelioid macrophages, multinucleated giant cells, lymphocytes, and clusters of degenerate heterophils sometimes associated with necrotic cores. The most severe lesions had a fully developed foci of liquefactive necrosis mixed with degenerative heterophils forming an abscess. There was some blending or overlap of the categories; for instance, a focus of marked pyogranulomatous inflammation was reported just a short distance away from a developing abscess which did not make it into the tissue section. Histological scoring revealed a generally robust inflammatory response in unsensitized Saline:NMI control samples (Figure 5B). Histology score severity was difficult to parse out among groups due to this finding. Notably, NMI:NMI ∆dot/icm animals displayed the least robust histological scores at the lowest skin testing dose site (0.25 µg) and histology scores from NMI:Dugway ∆dot/icm animals were analogous to Saline:NMI unsensitized control guinea pigs (Figure 5C).
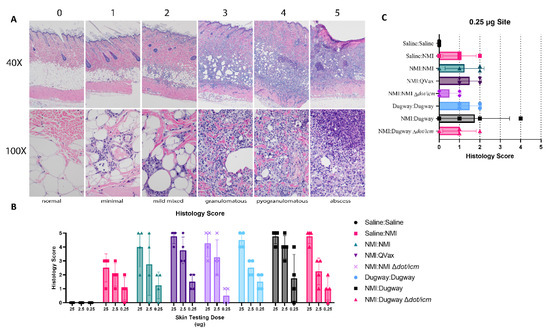
Figure 5.
Low-dose skin testing inflammatory responses are reduced following ∆dot/icm whole-cell vaccination. Skin-testing site biopsy histological scoring is outlined in (A) with inflammatory score (0–5), magnification (40×, 100\×), and brief description denoted. Guinea pigs are identified as follows: 0—Saline:Saline, 25 µg, 1—Saline:NMI, 25 µg, 2—Saline:NMI, 2.5 µg, 3—Saline:NMI, 25 µg, 4—QVax:NMI, 25 µg, 5—QVax:NMI, 25 µg. Inflammatory scoring at euthanasia (day 21 following skin testing) is reported in (B). The legend is formatted as infection Strain:Skin testing WCV. Histological scoring at the 0.25 µg site is highlighted in (C).
4. Discussion
Initial reports regarding Dugway strain characteristics included attenuation or avirulence in a guinea pig model of infection [12] and high infectivity and antibody responsiveness in a hamster model of infection [13]. Compared to high antibody titers induced by virulent strains in hamsters, guinea pigs, and mice, Dugway isolates only induced comparable titers in hamsters. More recently, Dugway strain avirulence in guinea pig infection models has been replicated [10,11]. Building on these studies, we address the potential of Dugway strain virulence, heterologous WCV protective capacity, and post-vaccination reactogenicity. Our guinea pig infection study data indicate that Dugway isolate 7D 77-80 displays similar virulence potential to the exempted NMII strain (RSA 439, clone 4), widely considered to be avirulent or severely attenuated [6,25]. This assessment is based on the effect of infection on body temperature, weight, and changes to the size and histological composition of the spleen. Animals inoculated with Dugway Δdot/icm yielded a similar clinical profile compared to the saline mock-infected control group, apart from slight splenomegaly in animals inoculated with 107 GE. This data complements former reports of Dugway strain attenuation in the guinea pig model [10,11,13] and avirulence in Δdot/icm strains [21]. Considering the physiologic relevance of the guinea pig model with humans in the context of Q fever [28,29] and the lack of any reported human infections involving Dugway strains, they are likely to exhibit attenuation in humans. Other phase I C. burnetii isolates exhibit attenuation in guinea pig infection models, including G Q212, Priscilla/MSU Goat Q177, and P Q238 [10,11]. These isolates are derived from human heart valve samples (G Q212 and P Q238) and a goat cotyledon following abortion (Priscilla/MSU goat Q177). Together, this information indicates factors beyond plasmid type and LPS influence C. burnetii virulence potential.
Despite an incomplete understanding of protective antigens involved in WCV immunity, the role of full-length LPS/O-antigen has been well-established [25]. Given the presence of full-length LPS in Dugway strains, heterologous protection displayed by Dugway WCV was expected. In a direct comparison to QVax®, Dugway WCV demonstrated similar efficacy with neither breakthrough fever nor splenomegaly as evidenced at the lowest dose of QVax®. Despite clear protective efficacy after challenge infection, demonstrated by a lack of fever, body weight change, and splenomegaly, C. burnetii was detectable via qPCR in spleens of all vaccinated animals. Notably, at 14 days post-infection, C. burnetii splenic burden appears to be low and difficult to detect, likely due to host clearance. Regardless, sterilizing immunity was not achieved for QVax® or Dugway WCV, although the absence of clinical disease is notable. Further, a significant increase in mLN CD8+ T cell frequency in the Saline:NMI group following infection was not reflected in vaccinated, challenged animals. This observation recalls data reported in earlier studies [21] and may indicate a role for cytotoxic CD8+ T cells in primary immunity. Here, we present a comprehensive assessment of fever in the guinea pig model in response to QVax® dose escalation in the intraperitoneal guinea pig infection model. This data will likely prove useful for future comparative vaccine studies, as QVax® is considered the gold standard for protection against Q fever.
In a guinea pig PVH model, the Dugway strain appeared to be as reactive as NMI and QVax®. Further, regardless of sensitization strain (Dugway or NMI), Dugway skin-tested animals appeared to experience reactogencitiy comparable to NMI skin-tested animals. Histological characterization of skin-testing sites also appeared comparable between strains. This indicates common PVH antigens shared among Dugway and Nine Mile strains. As previously reported [21], Δdot/icm strains appeared to be less reactive based on several experimental endpoints, including erythema and induration. Indeed, Dugway Δdot/icm demonstrated the most promising reduction in reactogenicity. Beyond the potential contribution of the T4BSS, additional antigens remain to be identified. Newly developed murine PVH models may provide utility in further studies [19,20].
The host species from which Dugway strains were isolated from may contribute to their unique characteristics in guinea pig models. Dugway strains were isolated from deer mice (Peromyscus maniculatus) and kangaroo rats (Dipodomys ordii and D. microps). Despite the recent identification of C. burnetii DNA in deer mice (Canada) and wild rodents (Spain), further strain characterization was not performed [30,31]. In laboratory settings, deer mice, kangaroo rats, and other wild rodent species were shown to be susceptible to intraperitoneal C. burnetii infection, albeit to a lesser degree than guinea pigs [32,33]. Due to the environmental range of C. burnetii, a hypothesis exists that describes wild rodents as disease reservoirs with the potential involvement of ticks in the natural lifecycle of the bacterium separate from or associated with the genesis of the livestock lifecycle of infection [9,32,33]. It is tempting to suggest that wild rodent host adaptation may influence C. burnetii characteristics such as virulence and behavior in a distinct host, such as a guinea pig or human. Further study is needed to address this hypothesis and the Dugway strain group will likely prove a valuable resource in this effort.
The presented data build upon historic findings relating to unique C. burnetii Dugway strains. Our characterization of Dugway host-pathogen interactions in vivo reveals an attenuated strain with vaccine potential. Specifically, the Dugway Δdot/icm strain appears to be a viable WCV candidate, exhibiting significantly reduced reactogenicity. The unique behavior of Dugway isolates paired with the large amount of unique genomic material contained in these isolates raises many important questions. For example, why are Dugway strains attenuated, what do novel genomic regions encode and are they functionally relevant, and does host adaptation play a role in Dugway strain behavior? This manuscript highlights Dugway strain behavior in vivo and provides a framework for future studies to address these inquiries. Indeed, the unique background and phenotype of the Dugway strain group provide a valuable experimental platform for the study C. burnetii pathogenesis and mechanisms of virulence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112261/s1, Figure S1: Infection strain LPS profiles-LPS profiles of C. burnetii strains used in infection modeling are depicted via silver stain (A); Figure S2: Splenic bacterial burdens post-infection-Guinea pig splenic bacterial burden at euthanasia (day 7 post-infection) is represented by C. burnetii GE per spleen; Figure S3: Systemic vaccination tolerance-Body temperature (A) and body weight change (B) kinetics are displayed for 25 days following vaccination. The legend is formatted as-WCV:infection strain; Figure S4: Post-vaccination and challenge flow cytometric analysis of secondary lymphoid organs-Mesenteric lymph node and spleen cellularity at euthanasia (day 14 following challenge) are depicted in A and B, respectively. Flow cytometric gating strategies for T cells (C) and B cells (D) are displayed. Lymph node B cell (E), CD4+ T cell (F), and CD8+ T cell (G) frequency are displayed. Splenic B cell (H), CD4+ T cell (I), and CD8+ T cell (J) frequency are displayed. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; Figure S5: PVH sensitization response-Body temperature (A) and body weight change (B) kinetics are displayed for 9 days following infection. The legend is formatted as- infection strain:skin testing WCV; Figure S6: PVH erythema kinetics and early phase induration response-Erythema measurements for days 0, 7, 14, and 21 following skin testing (A). Early phase induration severity scores (day 7 post-skin test) are displayed for individual animals (B). Figure S7: Systemic PVH responses-Body temperature change (A) and body weight change (B) kinetics are displayed for 21 days following skin testing. The legend is formatted as infection strain:skin testing WCV. Spleen weight (C), spleen cellularity (D), and mesenteric lymph node cellularity (E) are displayed from tissues harvested at euthanasia (day 21 following skin testing) * p ≤ 0.05; *** p ≤ 0.001.
Author Contributions
Conceptualization, M.T., P.A.B., R.A.H. and C.M.L.; investigation, M.T., P.B., D.C., P.A.B., C.S. and C.M.L.; formal analysis, M.T., P.B., C.S. and C.M.L.; writing—original draft preparation, M.T., P.B. and C.M.L.; writing—review and editing, all authors.; supervision and project administration, C.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Disease (ZIAAI001331).
Institutional Review Board Statement
The study was conducted in accordance with ABSL-3 standard operating procedures and approved by the Rocky Mountain Laboratories Institutional Biosafety Committee and an Institutional Animal Care and Use Committee-approved protocol (2021-030-E, approved on 5 May 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Rocky Mountain Veterinary Branch for animal caretaking, Crystal Richards for critical review of the manuscript, and John Stenos of the Australian Rickettsial Reference Laboratory for generously providing QVax®. Figure 1A, Figure 2A and Figure 4A were created in Biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaplan, M.M.; Bertagna, P. The geographical distribution of Q fever. Bull World Health Organ 1955, 13, 829–860. [Google Scholar] [PubMed]
- Wardrop, N.A.; Thomas, L.F.; Cook, E.A.J.; De Glanville, W.A.; Atkinson, P.M.; Wamae, C.N.; Fèvre, E.M. The Sero-epidemiology of Coxiella burnetii in Humans and Cattle, Western Kenya: Evidence from a Cross-Sectional Study. PLoS Negl. Trop. Dis. 2016, 10, e0005032. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.E.; Hendrix, L.R. Laboratory maintenance of Coxiella burnetii. Curr. Protoc. Microbiol. 2009, 15, 6C.1.1–6C.1.16. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Lockhart, M.; Stenos, J.; Graves, S. The attenuated nine mile phase II clone 4/RSA439 strain of Coxiella burnetii is highly virulent for severe combined immunodeficient (SCID) mice. Am. J. Trop. Med. Hyg. 2013, 89, 800–803. [Google Scholar] [CrossRef][Green Version]
- Millar, J.A.; Beare, P.A.; Moses, A.S.; Martens, C.A.; Heinzen, R.A.; Raghavan, R. Whole-Genome Sequence of Coxiella burnetii Nine Mile RSA439 (Phase II, Clone 4), a Laboratory Workhorse Strain. Genome Announc. 2017, 5, e00471. [Google Scholar] [CrossRef]
- Hendrix, L.R.; Samuel, J.E.; Mallavia, L.P. Differentiation of Coxiella burnetii isolates by analysis of restriction-endonuclease-digested DNA separated by SDS-PAGE. J. Gen. Microbiol. 1991, 137, 269–276. [Google Scholar] [CrossRef]
- Hemsley, C.M.; Essex-Lopresti, A.; Norville, I.H.; Titball, R.W. Correlating Genotyping Data of Coxiella burnetii with Genomic Groups. Pathogens 2021, 10, 604. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Pickens, E.G.; Newhouse, V.F.; Lackman, D.B. Isolation of Coxiella Burnetii from Rodents in Western Montana. J. Infect. Dis. 1963, 112, 181–186. [Google Scholar] [CrossRef]
- Russell-Lodrigue, K.E.; Andoh, M.; Poels, M.W.; Shive, H.R.; Weeks, B.R.; Zhang, G.Q.; Tersteeg, C.; Masegi, T.; Hotta, A.; Yamaguchi, T.; et al. Coxiella burnetii isolates cause genogroup-specific virulence in mouse and guinea pig models of acute Q fever. Infect. Immun. 2009, 77, 5640–5650. [Google Scholar] [CrossRef]
- Long, C.M.; Beare, P.A.; Cockrell, D.C.; Larson, C.L.; Heinzen, R.A. Comparative virulence of diverse Coxiella burnetii strains. Virulence 2019, 10, 133–150. [Google Scholar] [CrossRef]
- Stoenner, H.G.; Holdenried, R.; Lackman, D.; Orsborn, J.S., Jr. The occurrence of Coxiella burnetil, Brucella, and other pathogens among fauna of the Great Salt Lake Desert in Utah. Am. J. Trop. Med. Hyg. 1959, 8, 590–596. [Google Scholar] [CrossRef]
- Stoenner, H.G.; Lackman, D.B. The biologic properties of Coxiella burnetii isolated from rodents collected in Utah. Am. J. Hyg. 1960, 71, 45–51. [Google Scholar] [CrossRef]
- Beare, P.A.; Unsworth, N.; Andoh, M.; Voth, D.E.; Omsland, A.; Gilk, S.D.; Williams, K.P.; Sobral, B.W.; Kupko, J.J., 3rd; Porcella, S.F.; et al. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect. Immun. 2009, 77, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Jäger, C.; Lautenschläger, S.; Willems, H.; Baljer, G. Coxiella burnetii plasmid types QpDG and QpH1 are closely related and likely identical. Vet. Microbiol. 2002, 89, 161–166. [Google Scholar] [CrossRef]
- Maturana, P.; Graham, J.G.; Sharma, U.M.; Voth, D.E. Refining the plasmid-encoded type IV secretion system substrate repertoire of Coxiella burnetii. J. Bacteriol. 2013, 195, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Frangoulidis, D.; Splettstoesser, W.D.; Landt, O.; Dehnhardt, J.; Henning, K.; Hilbert, A.; Bauer, T.; Antwerpen, M.; Meyer, H.; Walter, M.C.; et al. Microevolution of the Chromosomal Region of Acute Disease Antigen A (adaA) in the Query (Q) Fever Agent Coxiella burnetii. PLoS ONE 2013, 8, e53440. [Google Scholar] [CrossRef][Green Version]
- Kermode, M.; Yong, K.; Hurley, S.; Marmion, B. An economic evaluation of increased uptake in Q fever vaccination among meat and agricultural industry workers following implementation of the National Q Fever Management Program. Aust. N. Z. J. Public Health 2003, 27, 390–398. [Google Scholar] [CrossRef]
- Fratzke, A.P.; Gregory, A.E.; van Schaik, E.J.; Samuel, J.E. Coxiella burnetii Whole Cell Vaccine Produces a Th1 Delayed-Type Hypersensitivity Response in a Novel Sensitized Mouse Model. Front. Immunol. 2021, 12, 754712. [Google Scholar] [CrossRef]
- Binette, P.; Tesfamariam, M.; Cockrell, D.; Heinzen, R.A.; Richards, C.; Shaia, C.; Long, C.M. Murine Q Fever Vaccination Model Reveals Sex Dimorphism in Early Phase Delayed-Type Hypersensitivity Responses. Front. Immunol. 2022, 13, 894536. [Google Scholar] [CrossRef]
- Long, C.M.; Beare, P.A.; Cockrell, D.C.; Fintzi, J.; Tesfamariam, M.; Shaia, C.I.; Heinzen, R.A. Contributions of lipopolysaccharide and the type IVB secretion system to Coxiella burnetii vaccine efficacy and reactogenicity. NPJ Vaccines 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, K.M.; Beare, P.A.; Cockrell, D.C.; Heinzen, R.A. Complementation of Arginine Auxotrophy for Genetic Transformation of Coxiella burnetii by Use of a Defined Axenic Medium. Appl. Environ. Microbiol. 2016, 82, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, R.; Peacock, M.; Gerloff, R.; Tallent, G.; Wike, D. Limits of rickettsial infectivity. Infect. Immun. 1978, 19, 239–245. [Google Scholar] [CrossRef]
- Beare, P.A.; Jeffrey, B.M.; Long, C.M.; Martens, C.M.; Heinzen, R.A. Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation. PLoS Pathog. 2018, 14, e1006922. [Google Scholar] [CrossRef] [PubMed]
- Moos, A.; Hackstadt, T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 1987, 55, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Kocianová, E.; Kováčová, E.; Literák, I. Comparison of virulence of Coxiella burnetii isolates from bovine milk and from ticks. Folia Parasitol. 2001, 48, 235–239. [Google Scholar] [CrossRef]
- Yun, N.E.; Linde, N.S.; Dziuba, N.; Zacks, M.A.; Smith, J.N.; Smith, J.K.; Aronson, J.F.; Chumakova, O.V.; Lander, H.M.; Peters, C.J.; et al. Pathogenesis of XJ and Romero strains of Junin virus in two strains of guinea pigs. Am. J. Trop. Med. Hyg. 2008, 79, 275–282. [Google Scholar] [CrossRef]
- Benenson, A.S.; Tigertt, W.D. Studies on Q fever in man. Trans. Assoc. Am. Physicians 1956, 69, 98–104. [Google Scholar]
- Tesfamariam, M.; Binette, P.; Long, C.M. Preclinical Animal Models for Q Fever Vaccine Development. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Thompson, M.; Mykytczuk, N.; Gooderham, K.; Schulte-Hostedde, A. Prevalence of the bacterium Coxiella burnetii in wild rodents from a Canadian natural environment park. Zoonoses Public Health 2012, 59, 553–560. [Google Scholar] [CrossRef]
- González-Barrio, D.; Jado, I.; Viñuela, J.; García, J.T.; Olea, P.P.; Arce, F.; Ruiz-Fons, F. Investigating the Role of Micromammals in the Ecology of Coxiella burnetii in Spain. Animals 2021, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Gebhardt, L.P. Susceptibility of Wild Rodents to Experimental Infection with Coxiella Burnetii. Am. J. Trop. Med. Hyg. 1963, 12, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.G.; Marmion, B.P. The spread of Q fever from animals to man; the natural history of a rickettsial disease. Bull World Health Organ 1955, 13, 781–806. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).