Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products
Abstract
1. Introduction
2. Lactic Acid Bacteria as Starter and Not Starter Culture
3. Bacteriocin
3.1. Bacteriocins and Their Classification
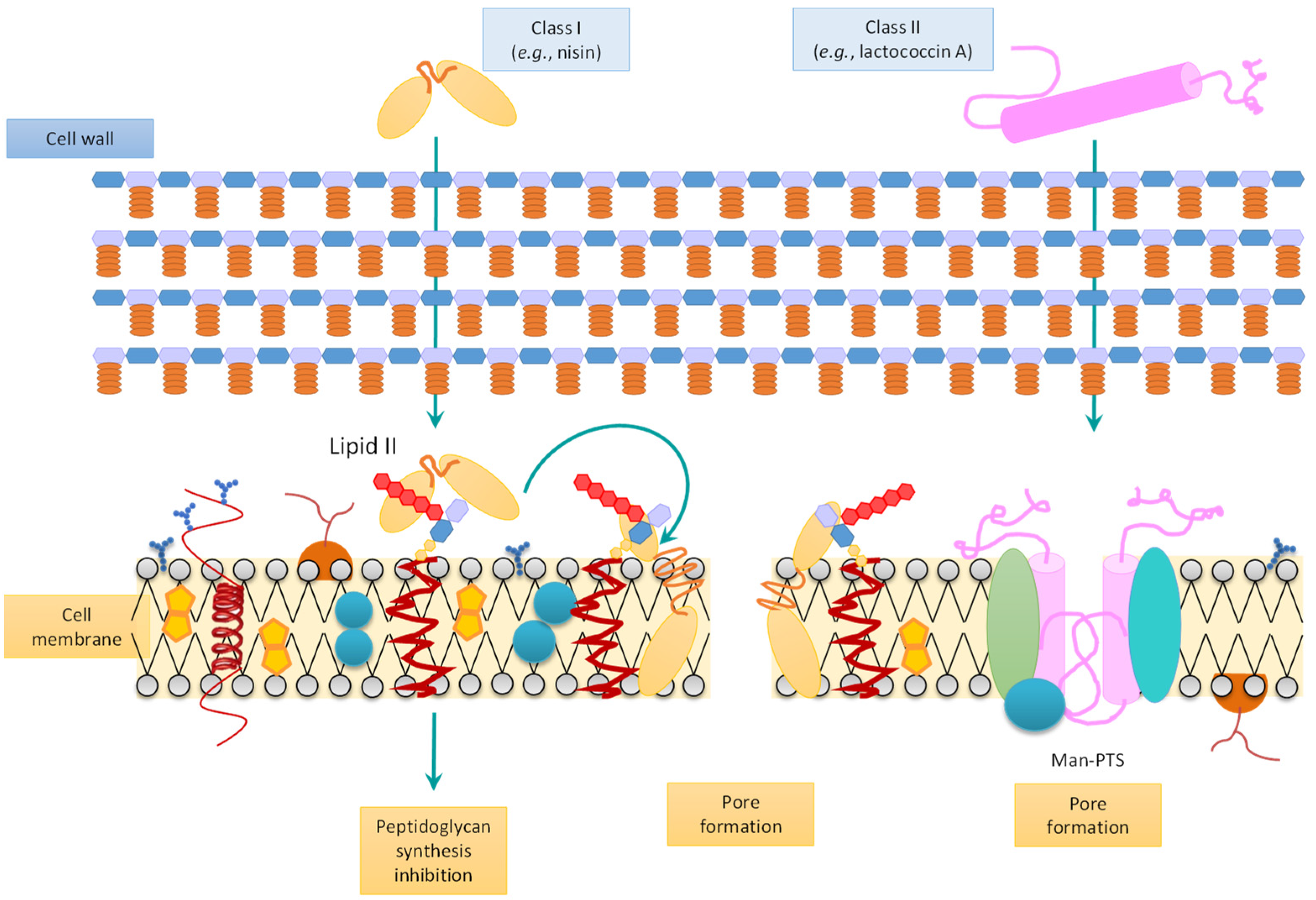
3.2. Synthesis and Mode of Action
3.3. Microbial Resistance
3.4. Purified or Semi-Purified Bacteriocins
4. Bacteriocins Isolated from Meat and Meat Products
4.1. Bacteriocins Used in Meat and Meat Products
4.2. Bacteriocins and Hurdle Technology
5. Active Packaging and Bacteriocins
6. Commercialization and Toxicity
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining Reformulation, Active Packaging and Non-Thermal Post-Packaging Decontamination Technologies to Increase the Microbiological Quality and Safety of Cooked Ready-to-Eat Meat Products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Antioxidant Dietary Fibre Enriched Meat-Based Functional Foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Luong, N.D.M.; Coroller, L.; Zagorec, M.; Membré, J.M.; Guillou, S. Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Škalič, M.; Martínez-Rosell, G.; De Fabritiis, G. KDEEP: Protein-Ligand Absolute Binding Affinity Prediction via 3D-Convolutional Neural Networks. J. Chem. Inf. Model. 2018, 58, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in Proteolysis, Protein Oxidation, Flavor, Color and Texture of Dry-Cured Mutton Ham during Storage. LWT—Food Sci. Technol. 2021, 149, 111860. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Das, A.; Biswas, S. Hazards and Safety Issues of Meat and Meat Products. Food Saf. Hum. Health 2019, 145–168. [Google Scholar] [CrossRef]
- Biswas, O.; Kandasamy, P.; Patnaik, S.; Lorenzo, J.M.; Das, A.K. Effect of Phytochemicals on Quality and Safety Aspects of Meat and Meat Products. Indian J. Anim. Health 2021, 60, 97–108. [Google Scholar] [CrossRef]
- Van Cauteren, D.; Le Strat, Y.; Sommen, C.; Bruyand, M.; Tourdjman, M.; Jourdan-Da Silva, N.; Couturier, E.; Fournet, N.; de Valk, H.; Desenclos, J.C. Estimated Annual Numbers of Foodborne Pathogen–Associated Illnesses, Hospitalizations, and Deaths, France, 2008–2013. Emerg. Infect. Dis. 2017, 23, 1486–1492. [Google Scholar] [CrossRef]
- Ercoli, L.; Gallina, S.; Nia, Y.; Auvray, F.; Primavilla, S.; Guidi, F.; Pierucci, B.; Graziotti, C.; Decastelli, L.; Scuota, S. Investigation of a Staphylococcal Food Poisoning Outbreak from a Chantilly Cream Dessert, in Umbria (Italy). Foodborne Pathog. Dis. 2017, 14, 407–413. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Pradhan, S.R.; Patra, G.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Das, A.K. Comparative Microbial Load Assessment of Meat, Contact Surfaces and Water Samples in Retail Chevon Meat Shops and Abattoirs of Kolkata, W.B., India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 158–164. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, Safety, and Regulatory Considerations in the Use of Nitrite and Nitrate from Natural Origin in Meat Products. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- Zhaxybayeva, E.Z.; Dikhanbayeva, F.; Dimitriev, Z.P.; Imangalieva, Z.; Asenov, R. Development of a Recipe and Technology for the Production of Drinking Yogurt from Camel Milk for Gerodietetic Nutrition Based on the Enzyme, Probiotics and Nutrient Additive. EurAsian J. Biosci. 2020, 14, 355–363. [Google Scholar]
- Buncic, S.; Nychas, G.J.; Lee, M.R.F.; Koutsoumanis, K.; Hébraud, M.; Desvaux, M.; Chorianopoulos, N.; Bolton, D.; Blagojevic, B.; Antic, D. Microbial Pathogen Control in the Beef Chain: Recent Research Advances. Meat Sci. 2014, 97, 288–297. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic Acid Bacteria as Starter Cultures: An Update in Their Metabolism and Genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Imade, E.E.; Omonigho, S.E.; Babalola, O.O.; Enagbonma, B.J. Lactic Acid Bacterial Bacteriocins and Their Bioactive Properties against Food-Associated Antibiotic-Resistant Bacteria. Ann. Microbiol. 2021, 71, 44. [Google Scholar] [CrossRef]
- Balciunas, E.M.; Castillo Martinez, F.A.; Todorov, S.D.; Franco, B.D.G.d.M.; Converti, A.; Oliveira, R.P.d.S. Novel Biotechnological Applications of Bacteriocins: A Review. Food Control 2013, 32, 134–142. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, D.Y.G.; Jin, D.Y.Y.; Jayabalan, D.R.; Yang, D.S.H.; Suh, J.W. Bacteriocins as Food Preservatives: Challenges and Emerging Horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef]
- Trejo-González, L.; Gutiérrez-Carrillo, A.E.; Rodríguez-Hernández, A.I.; del Rocío López-Cuellar, M.; Chavarría-Hernández, N. Bacteriocins Produced by LAB Isolated from Cheeses within the Period 2009–2021: A Review. Probiot. Antimicrob. Proteins 2022, 14, 238–251. [Google Scholar] [CrossRef]
- Da Costa, R.J.; Voloski, F.L.S.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of Meat Products with Bacteriocins Produced by Lactic Acid Bacteria Isolated from Meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Das, A.; Chauhan, G.; Agrawal, R.K.; Das, A.K.; Tomar, S.; Uddin, S.; Satyaprakash, K.; Pateiro, M.; Lorenzo, J.M. Characterization of Crude Extract Prepared from Indian Curd and Its Potential as a Biopreservative. Food Sci. Technol. Int. 2021, 27, 313–325. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Rivas, F.P.; Castro, M.P.; Vallejo, M.; Marguet, E.; Campos, C.A. Sakacin Q Produced by Lactobacillus curvatus ACU-1: Functionality Characterization and Antilisterial Activity on Cooked Meat Surface. Meat Sci. 2014, 97, 475–479. [Google Scholar] [CrossRef]
- Dal Bello, B.; Rantsiou, K.; Bellio, A.; Zeppa, G.; Ambrosoli, R.; Civera, T.; Cocolin, L. Microbial Ecology of Artisanal Products from North West of Italy and Antimicrobial Activity of the Autochthonous Populations. LWT—Food Sci. Technol. 2010, 43, 1151–1159. [Google Scholar] [CrossRef]
- Sakaridis, I.; Soultos, N.; Batzios, C.; Ambrosiadis, I.; Koidis, P. Lactic Acid Bacteria Isolated from Chicken Carcasses with Inhibitory Activity against Salmonella spp. and Listeria monocytogenes. Czech J. Food Sci. 2014, 32, 61–68. [Google Scholar] [CrossRef]
- Kim, J.-D. Antifungal Activity of Lactic Acid Bacteria Isolated from Kimchi against Aspergillus fumigatus. Mycobiology 2005, 33, 210. [Google Scholar] [CrossRef]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The Lactic Acid Bacteria Metabolite Phenyllactic Acid Inhibits Both Radial Growth and Sporulation of Filamentous Fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef]
- Gelinski, J.M.L.N.; Baratto, C.M.; Casagrande, M.; de Oliveira, T.P.; Megiolaro, F.; de Martini Soares, F.A.S.; de Souza, E.M.B.; Vicente, V.A.; Fonseca, G.G. Control of Pathogens in Fresh Pork Sausage by Inclusion of Lactobacillus sakei BAS0117. Can. J. Microbiol. 2019, 65, 831–841. [Google Scholar] [CrossRef]
- Kamiloğlu, A.; Kaban, G.; Kaya, M. Effects of Autochthonous Lactobacillus plantarum Strains on Listeria monocytogenes in Sucuk during Ripening. J. Food Saf. 2019, 39, e12618. [Google Scholar] [CrossRef]
- Nikodinoska, I.; Baffoni, L.; Di Gioia, D.; Manso, B.; García-Sánchez, L.; Melero, B.; Rovira, J. Protective Cultures against Foodborne Pathogens in a Nitrite Reduced Fermented Meat Product. LWT—Food Sci. Technol. 2019, 101, 293–299. [Google Scholar] [CrossRef]
- Zanette, C.M.; Dalla Santa, O.R.; Bersot, L.S. Effect of Lactobacillus plantarum Starter Cultures on the Behavior of Listeria monocytogenes during Sausage Maturation. Int. Food Res. J. 2015, 22, 844–848. [Google Scholar]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat Spoilage: A Critical Review of a Neglected Alteration Due to Ropy Slime Producing Bacteria. Ital. J. Anim. Sci. 2015, 14, 316–326. [Google Scholar] [CrossRef]
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of Leucocin A for Use on Wieners to Inhibit Listeria monocytogenes in the Presence of Spoilage Organisms. Int. J. Food Microbiol. 2017, 255, 25–31. [Google Scholar] [CrossRef]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef]
- Zou, J.; Jiang, H.; Cheng, H.; Fang, J.; Huang, G. Strategies for Screening, Purification and Characterization of Bacteriocins. Int. J. Biol. Macromol. 2018, 117, 781–789. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of Lactic Acid Bacteria: Extending the Family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Hernández-Aquino, S.; Miranda-Romero, L.A.; Fujikawa, H.; de Jesús Maldonado-Simán, E.; Alarcón-Zuñiga, B. Antibacterial Activity of Lactic Acid Bacteria to Improve Shelf Life of Raw Meat. Biocontrol Sci. 2019, 24, 185–192. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.Y. Lantibiotics, Class I Bacteriocins from the Genus Bacillus. J. Microbiol. Biotechnol. 2011, 21, 229–235. [Google Scholar] [CrossRef]
- Ovchinnikov, K.V.; Kristiansen, P.E.; Straume, D.; Jensen, M.S.; Aleksandrzak-Piekarczyk, T.; Nes, I.F.; Diep, D.B. The Leaderless Bacteriocin Enterocin K1 Is Highly Potent against Enterococcus faecium: A Study on Structure, Target Spectrum and Receptor. Front. Microbiol. 2017, 8, 774. [Google Scholar] [CrossRef]
- Ovchinnikov, K.V.; Chi, H.; Mehmeti, I.; Holo, H.; Nes, I.F.; Diep, D.B. Novel Group of Leaderless Multipeptide Bacteriocins from Gram-Positive Bacteria. Appl. Environ. Microbiol. 2016, 82, 5216–5224. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Bacteriocins of Lactic Acid Bacteria as Biotechnological Tools in Food and Pharmaceuticals: Current Applications and Future Prospects. Biocatal. Agric. Biotechnol. 2020, 28, 101750. [Google Scholar] [CrossRef]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological Tools for Bio-Preservation and Shelf-Life Extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Singh, V.P. Recent Approaches in Food Bio-Preservation—A Review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Montalban-Lopez, M.; Sanchez-Hidalgo, M.; Valdivia, E.; Martinez-Bueno, M.; Maqueda, M. Are Bacteriocins Underexploited? NOVEL Applications for OLD Antimicrobials. Curr. Pharm. Biotechnol. 2011, 12, 1205–1220. [Google Scholar] [CrossRef]
- Rea, M.C.; Ross, R.P.; Cotter, P.D.; Hill, C. Classification of Bacteriocins from Gram-Positive Bacteria. In Prokaryotic Antimicrobial Peptides; Springer: New York, NY, USA, 2011; pp. 29–53. [Google Scholar]
- Nissen-Meyer, J.; Rogne, P.; Oppegard, C.; Haugen, H.; Kristiansen, P. Structure-Function Relationships of the Non-Lanthionine-Containing Peptide (Class II) Bacteriocins Produced by Gram-Positive Bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III Bacteriocin Helveticin-M Causes Sublethal Damage on Target Cells through Impairment of Cell Wall and Membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Morton, J.T.; Freed, S.D.; Lee, S.W.; Friedberg, I. A Large Scale Prediction of Bacteriocin Gene Blocks Suggests a Wide Functional Spectrum for Bacteriocins. BMC Bioinform. 2015, 16, 381. [Google Scholar] [CrossRef]
- Mignolet, J.; Fontaine, L.; Sass, A.; Nannan, C.; Mahillon, J.; Coenye, T.; Hols, P. Circuitry Rewiring Directly Couples Competence to Predation in the Gut Dweller Streptococcus Salivarius. Cell Rep. 2018, 22, 1627–1638. [Google Scholar] [CrossRef]
- Field, D.; Cotter, P.; Hill, C.; Ross, R.P. Bacteriocin Biosynthesis, Structure and Function. In Research and Applications in Bacteriocins; Riley, M.A., Gillor, O., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2007; pp. 5–37. [Google Scholar]
- Rashid, R.; Veleba, M.; Kline, K.A. Focal Targeting of the Bacterial Envelope by Antimicrobial Peptides. Front. Cell Dev. Biol. 2016, 4, 55. [Google Scholar] [CrossRef]
- López-Lara, I.M.; Geiger, O. Bacterial Lipid Diversity. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2017, 1862, 1287–1299. [Google Scholar] [CrossRef]
- Gonzales, J.C.H.; Tapia, A.M.; Hernandez, G.L.; Perez, B.E.G.; Jimenez, N.S.C. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar]
- De Freire Bastos, M.D.C.; Varella Coelho, M.L.; da Silva Santos, O.C. Resistance to Bacteriocins Produced by Gram-Positive Bacteria. Microbiology 2015, 161, 683–700. [Google Scholar] [CrossRef]
- Gabrielsen, C.; Brede, D.A.; Hernández, P.E.; Nes, I.F.; Diep, D.B. The Maltose ABC Transporter in Lactococcus lactis Facilitates High-Level Sensitivity to the Circular Bacteriocin Garvicin ML. Antimicrob. Agents Chemother. 2012, 56, 2908–2915. [Google Scholar] [CrossRef]
- Shiraishi, T.; Yokota, S.I.; Fukiya, S.; Yokota, A. Structural Diversity and Biological Significance of Lipoteichoic Acid in Gram-Positive Bacteria: Focusing on Beneficial Probiotic Lactic Acid Bacteria. Biosci. Microbiota Food Health 2016, 35, 147–161. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Popa, L.I.; Marutescu, L.; Gheorghe, I.; Popa, M.; Czobor Barbu, I.; Cristescu, R.; Chifiriuc, M.C. Bacteriocins in the Era of Antibiotic Resistance: Rising to the Challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Kjos, M.; Nes, I.F.; Diep, D.B. Mechanisms of Resistance to Bacteriocins Targeting the Mannose Phosphotransferase System. Appl. Environ. Microbiol. 2011, 77, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wilkinson, B.J.; Standiford, T.J.; Akinbi, H.T.; O’Riordan, M.X.D. Fatty Acids Regulate Stress Resistance and Virulence Factor Production for Listeria monocytogenes. J. Bacteriol. 2012, 194, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, L.M.; Kelleher, P.; Van Rijswijck, I.M.H.; De Waal, P.; Van Peij, N.N.M.E.; Mahony, J.; Van Sinderen, D. Natural Transformation in Gram-Positive Bacteria and Its Biotechnological Relevance to Lactic Acid Bacteria. Annu. Rev. Food Sci. Technol. 2022, 13, 409–431. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and Emerging Applications of Bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Jamuna, M.; Jeevaratnam, K. Isolation and Characterization of Lactobacilli from Some Traditional Fermented Foods and Evaluation of the Bacteriocins. J. Gen. Appl. Microbiol. 2004, 50, 79–90. [Google Scholar] [CrossRef]
- Arief, I.I.; Jenie, B.S.L.; Suryati, T.; Ayuningtyas, G.; Fuziawan, A. Antimicrobial Activity of Bacteriocin from Indigenous Lactobacillus plantarum 2C12 and Its Application on Beef Meatball as Biopreservative. J. Indones. Trop. Anim. Agric. 2012, 37, 90–96. [Google Scholar] [CrossRef]
- Chakchouk-Mtibaa, A.; Smaoui, S.; Ktari, N.; Sellem, I.; Najah, S.; Karray-Rebai, I.; Mellouli, L. Biopreservative Efficacy of Bacteriocin BacFL31 in Raw Ground Turkey Meat in Terms of Microbiological, Physicochemical, and Sensory Qualities. Biocontrol Sci. 2017, 22, 6777. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Natural Antimicrobials as Additives and Ingredients for the Preservation of Foods and Beverages. In Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing: Sawston, UK, 2012; pp. 127–161. [Google Scholar]
- Bédard, F.; Biron, E. Recent Progress in the Chemical Synthesis of Class II and S-Glycosylated Bacteriocins. Front. Microbiol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Gomes, B.C.; Esteves, C.T.; Palazzo, I.C.V.; Darini, A.L.C.; Felis, G.E.; Sechi, L.A.; Franco, B.D.G.M.; De Martinis, E.C.P. Prevalence and Characterization of Enterococcus spp. Isolated from Brazilian Foods. Food Microbiol. 2008, 25, 668–675. [Google Scholar] [CrossRef]
- Castro, M.P.; Palavecino, N.Z.; Herman, C.; Garro, O.A.; Campos, C.A. Lactic Acid Bacteria Isolated from Artisanal Dry Sausages: Characterization of Antibacterial Compounds and Study of the Factors Affecting Bacteriocin Production. Meat Sci. 2011, 87, 321–329. [Google Scholar] [CrossRef]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G.; Saavedra, L. Occurrence of Antilisterial Structural Bacteriocins Genes in Meat Borne Lactic Acid Bacteria. Food Control 2015, 47, 53–59. [Google Scholar] [CrossRef]
- Casquete, R.; Fonseca, S.C.; Pinto, R.; Castro, S.M.; Todorov, S.; Teixeira, P.; Vaz-Velho, M. Evaluation of the Microbiological Safety and Sensory Quality of a Sliced Cured-Smoked Pork Product with Protective Cultures Addition and Modified Atmosphere Packaging. Food Sci. Technol. Int. 2019, 25, 327–336. [Google Scholar] [CrossRef]
- De Azevedo, P.O.S.; Mendonça, C.M.N.; Seibert, L.; Domínguez, J.M.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Bacteriocin-like Inhibitory Substance of Pediococcus pentosaceus as a Biopreservative for Listeria sp. Control in Ready-to-Eat Pork Ham. Braz. J. Microbiol. 2020, 51, 949–956. [Google Scholar] [CrossRef]
- Chopra, L.; Singh, G.; Kumar Jena, K.; Sahoo, D.K. Sonorensin: A New Bacteriocin with Potential of an Anti-Biofilm Agent and a Food Biopreservative. Sci. Rep. 2015, 5, 13412. [Google Scholar] [CrossRef]
- Castellano, P.; Peña, N.; Ibarreche, M.P.; Carduza, F.; Soteras, T.; Vignolo, G. Antilisterial Efficacy of Lactobacillus Bacteriocins and Organic Acids on Frankfurters. Impact on Sensory Characteristics. J. Food Sci. Technol. 2018, 55, 689–697. [Google Scholar] [CrossRef]
- Khalili Sadaghiani, S.; Aliakbarlu, J.; Tajik, H.; Mahmoudian, A. Anti-Listeria Activity and Shelf Life Extension Effects of Lactobacillus along with Garlic Extract in Ground Beef. J. Food Saf. 2019, 39, e12709. [Google Scholar] [CrossRef]
- Isa, J.K.; Razavi, S.H. The Use of Lactobacillus acidophilus and Bifidobacterium animalis ssp. Lactis BB12, as Probiotics to Reduce the Risk of Food Poisoning in Minced Meat. Appl. Food Biotechnol. 2018, 5, 173–183. [Google Scholar] [CrossRef]
- Ünlü, G.; Nielsen, B.; Ionita, C. Inhibition of Listeria monocytogenes in Hot Dogs by Surface Application of Freeze-Dried Bacteriocin-Containing Powders from Lactic Acid Bacteria. Probiot. Antimicrob. Proteins 2016, 8, 102–110. [Google Scholar] [CrossRef]
- Yildirim, Z.; Yerlikaya, S.; Öncül, N.; Sakin, T. Inhibitory Effect of Lactococcin BZ against Listeria innocua and Indigenous Microbiota of Fresh Beef. Food Technol. Biotechnol. 2016, 54, 317–323. [Google Scholar] [CrossRef]
- Casaburi, A.; Di Martino, V.; Ferranti, P.; Picariello, L.; Villani, F. Technological Properties and Bacteriocins Production by Lactobacillus curvatus 54M16 and Its Use as Starter Culture for Fermented Sausage Manufacture. Food Control 2016, 59, 31–45. [Google Scholar] [CrossRef]
- Yan, H.; Lu, Y.; Li, X.; Yi, Y.; Wang, X.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Action Mode of Bacteriocin BM1829 against Escherichia coli and Staphylococcus aureus. Food Biosci. 2021, 39, 100794. [Google Scholar] [CrossRef]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of Novel Synergistic Antimicrobial Activity of Nisin, Lysozyme, EDTA Nanoparticles, and/or ZnO Nanoparticles to Control Foodborne Pathogens on Minced Beef. Food Control 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Li, R.; Yassami, S.; Kiviniemi, E.A.; Qiao, W.; Takala, T.M.; Saris, P.E.J. Listeria Decontamination of Chicken Meat with Beer Brewed with Bacteriocin Producing Saccharomyces boulardii. LWT—Food Sci. Technol. 2021, 152, 112323. [Google Scholar] [CrossRef]
- Li, H.W.; Xiang, Y.Z.; Zhang, M.; Jiang, Y.H.; Zhang, Y.; Liu, Y.Y.; Lin, L.B.; Zhang, Q.L. A Novel Bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: Isolation, Purification, Identification, Antibacterial and Antibiofilm Activity. LWT 2021, 140, 110826. [Google Scholar] [CrossRef]
- Lu, Y.; Aizhan, R.; Yan, H.; Li, X.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Characterization, Modes of Action, and Application of a Novel Broad-Spectrum Bacteriocin BM1300 Produced by Lactobacillus crustorum MN047. Braz. J. Microbiol. 2020, 51, 2033–2048. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, H.; Li, X.; Gu, Y.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Xin, L. Physicochemical Properties and Mode of Action of a Novel Bacteriocin BM1122 with Broad Antibacterial Spectrum Produced by Lactobacillus crustorum MN047. J. Food Sci. 2020, 85, 1523–1535. [Google Scholar] [CrossRef]
- Calumba, K.F.A.; Obsioma, V.P.; Jawa, C.A.E.; Ong, D.G.S. Assessment of Lactobacillus paracasei F2I2 as a Possible Biopreservative for Raw Pork. Mindanao J. Sci. Technol. 2019, 17, 1–17. [Google Scholar]
- De Castilho, N.P.A.; Todorov, S.D.; Oliveira, L.L.; Bersot, L.d.S.; Nero, L.A. Inhibition of Listeria monocytogenes in Fresh Sausage by Bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 and Its Semi-Purified Bacteriocin. LWT—Food Sci. Technol. 2020, 118, 108757. [Google Scholar] [CrossRef]
- Kiran, F.; Osmanagaoglu, O. Inhibition of Listeria monocytogenes in Chicken Meat by Pediocin AcH/PA-1 Produced by Pediococcus pentosaceus OZF. Agro Food Ind. Hi-Tech 2014, 25, 66–69. [Google Scholar]
- Maciel, C.; Komora, N.; Ferreira, V.; Saraiva, J.; Castro, S.M.; Teixeira, P. High Hydrostatic Pressure and Pediocin PA-1 as a Synergistic System to Listeria monocytogenes Inactivation in Fermented Meat Sausage. In Proceedings of the MICROBIOTEC ’17, Book of Abstracts—Congress of Microbiology and Biotechnology, Porto, Portugal, 7–9 December 2017. [Google Scholar]
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined Effect of Pediocin BacHA-6111-2 and High Hydrostatic Pressure to Control Listeria innocua in Fermented Meat Sausage. Int. Food Res. J. 2018, 25, 553–560. [Google Scholar]
- Wu, S.; Zhang, H.; Zhou, H.; Jin, J.; Xie, Y. Synergistic Effect of Plantaricin BM-1 Combined with Physicochemical Treatments on the Control of Listeria monocytogenes in Cooked Ham. J. Food Prot. 2017, 80, 976–981. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of Nisin Z and Lauric Arginate into Pullulan Films to Inhibit Foodborne Pathogens Associated with Fresh and Ready-to-Eat Muscle Foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef]
- Abitayeva, G.K.; Urazova, M.S.; Abilkhadirov, A.S.; Sarmurzina, Z.S.; Shaikhin, S.M. Characterization of a New Bacteriocin-like Inhibitory Peptide Produced by Lactobacillus sakei B-RKM 0559. Biotechnol. Lett. 2021, 43, 2243–2257. [Google Scholar] [CrossRef]
- Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on Ready-to-Eat Meats Using Bacteriocin Mixtures Based on Mode-of-Action. Foods 2017, 6, 22. [Google Scholar] [CrossRef]
- Le, N.T.T.; Bach, L.G.; Nguyen, D.C.; Le, T.H.X.; Pham, K.H.; Nguyen, D.H.; Thi, T.T.H. Evaluation of Factors Affecting Antimicrobial Activity of Bacteriocin from Lactobacillus plantarum Microencapsulated in Alginate-Gelatin Capsules and Its Application on Pork Meat as a Bio-Preservative. Int. J. Environ. Res. Public Health 2019, 16, 1017. [Google Scholar] [CrossRef]
- Kumar, Y.; Kaur, K.; Shahi, A.K.; Kairam, N.; Tyagi, S.K. Antilisterial, Antimicrobial and Antioxidant Effects of Pediocin and Murraya koenigii Berry Extract in Refrigerated Goat Meat Emulsion. LWT—Food Sci. Technol. 2017, 79, 135–144. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, M.; Gao, X.; Shao, Y.; Liu, H.; Jin, J.; Yang, W.; Zhang, H. Development and Antimicrobial Application of Plantaricin BM-1 Incorporating a PVDC Film on Fresh Pork Meat during Cold Storage. J. Appl. Microbiol. 2018, 125, 1108–1116. [Google Scholar] [CrossRef]
- Hongthong, N.; Chumngoen, W.; Tan, F.J. Influence of Sucrose Level and Inoculation of Lactobacillus plantarum on the Physicochemical, Textural, Microbiological, and Sensory Characteristics of Isan Sausage (Thai Fermented Pork Sausage). Anim. Sci. J. 2020, 91, e13312. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined Antimicrobial Effect of Bacteriocins with Other Hurdles of Physicochemic and Microbiome to Prolong Shelf Life of Food: A Review. Sci. Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- De Martinez, Y.B.; Ferrer, K.; Salas, E.M. Combined Effects of Lactic Acid and Nisin Solution in Reducing Levels of Microbiological Contamination in Red Meat Carcasses. J. Food Prot. 2002, 65, 1780–1783. [Google Scholar] [CrossRef]
- Pilevar, Z.; Hosseini, H.; Beikzadeh, S.; Khanniri, E.; Alizadeh, A.M. Application of Bacteriocins in Meat and Meat Products: An Update. Curr. Nutr. Food Sci. 2020, 16, 120–133. [Google Scholar] [CrossRef]
- Kingcha, Y.; Tosukhowong, A.; Zendo, T.; Roytrakul, S.; Luxananil, P.; Chareonpornsook, K.; Valyasevi, R.; Sonomoto, K.; Visessanguan, W. Anti-Listeria Activity of Pediococcus pentosaceus BCC 3772 and Application as Starter Culture for Nham, a Traditional Fermented Pork Sausage. Food Control 2012, 25, 190–196. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Li, P.; Qu, Y. Pentocin 31-1, a Novel Meat-Borne Bacteriocin and Its Application as Biopreservative in Chill-Stored Tray-Packaged Pork Meat. Food Control 2010, 21, 198–202. [Google Scholar] [CrossRef]
- Swetwiwathana, A.; Lotong, N.; Nakayama, J.; Sonomoto, K. Maturation of Nham—A Thai Fermented Meat Product, Effect of Pediocin PA-1 Producer (Pediococcus pentosaceus TISTR 536) as Starter Culture, Nitrite and Garlic on Salmonella anatum during Nham Fermentation. Fleishwirtsch. Int. 2007, 22, 46–49. [Google Scholar]
- Dortu, C.; Huch, M.; Holzapfel, W.H.; Franz, C.M.A.P.; Thonart, P. Anti-Listerial Activity of Bacteriocin-Producing Lactobacillus curvatus CWBI-B28 and Lactobacillus sakei CWBI-B1365 on Raw Beef and Poultry Meat. Lett. Appl. Microbiol. 2008, 47, 581–586. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial Anti-Microbial Peptides and Nano-Sized Drug Delivery Systems: The State of the Art toward Improved Bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Ansari, A.; Ibrahim, F.; Haider, M.S.; Aman, A. In Vitro Application of Bacteriocin Produced by Lactiplantibacillus plantarum for the Biopreservation of Meat at Refrigeration Temperature. J. Food Process. Preserv. 2022, 46, e16159. [Google Scholar] [CrossRef]
- Pato, U.; Yusuf, Y.; Fitriani, S.; Fauzi, D.A.; Ismadiah, G.; Hidayah, M.; Sabiliani, W. Evaluation of Bacteriocin Produced by Pediococcus pentosaceus Strain 2397 as Natural Preservative for Fish Meatballs Stored at Room Temperature. In Proceedings of the 6th International Conference of Food, Agriculture, and Natural Resource (IC-FANRES 2021), Tangerang, Indonesia, 4–5 August 2021; Volume 16, pp. 342–347. [Google Scholar]
- Castellano, P.; Belfiore, C.; Vignolo, G. Combination of Bioprotective Cultures with EDTA to Reduce Escherichia coli O157:H7 in Frozen Ground-Beef Patties. Food Control 2011, 22, 1461–1465. [Google Scholar] [CrossRef]
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha Piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef Meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Melero, B.; Diez, A.M.; Rajkovic, A.; Jaime, I.; Rovira, J. Behaviour of Non-Stressed and Stressed Listeria monocytogenes and Campylobacter jejuni Cells on Fresh Chicken Burger Meat Packaged under Modified Atmosphere and Inoculated with Protective Culture. Int. J. Food Microbiol. 2012, 158, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.R.; Cutter, C.N.; Willett, J.L. Incorporation of Bacteriocin in Plastic Retains Activity and Inhibits Surface Growth of Bacteria on Meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Budde, B.B.; Hornbæk, T.; Jacobsen, T.; Barkholt, V.; Koch, A.G. Leuconostoc Carnosum 4010 Has the Potential for Use as a Protective Culture for Vacuum-Packed Meats: Culture Isolation, Bacteriocin Identification, and Meat Application Experiments. Int. J. Food Microbiol. 2003, 83, 171–184. [Google Scholar] [CrossRef]
- Segli, F.; Melian, C.; Muñoz, V.; Vignolo, G.; Castellano, P. Bioprotective Extracts from Lactobacillus acidophilus CRL641 and Latilactobacillus curvatus CRL705 Inhibit a Spoilage Exopolysaccharide Producer in a Refrigerated Meat System. Food Microbiol. 2021, 97, 103739. [Google Scholar] [CrossRef]
- Orihuel, A.; Bonacina, J.; Vildoza, M.J.; Bru, E.; Vignolo, G.; Saavedra, L.; Fadda, S. Biocontrol of Listeria monocytogenes in a Meat Model Using a Combination of a Bacteriocinogenic Strain with Curing Additives. Food Res. Int. 2018, 107, 289–296. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella Bacteriophage, Nisin and Potassium Sorbate and Their Combination on Safety and Shelf Life of Fresh Chilled Pork. Food Control 2017, 73, 869–877. [Google Scholar] [CrossRef]
- Hammou, B.F.; Skali, N.; Idaomar, M.; Abrini, J. The Antimicrobial Effect of Origanum compactum Essential Oil, Nisin and Their Combination against Escherichia coli in Tryptic Soy Broth (TSB) and in Sheep Natural Sausage Casings during Storage at 25 and 7 °C. Afr. J. Biotechnol. 2011, 10, 15998–16005. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Paula, O.A.L.; Camargo, A.C.; Lopes, D.A.; Nero, L.A. Combined Effect of Bacteriocin Produced by Lactobacillus plantarum ST8SH and Vancomycin, Propolis or EDTA for Controlling Biofilm Development by Listeria monocytogenes. Rev. Argent. Microbiol. 2018, 50, 48–55. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Woraprayote, W.; Kingcha, Y.; Amonphanpokin, P.; Kruenate, J.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Anti-Listeria Activity of Poly(Lactic Acid)/Sawdust Particle Biocomposite Film Impregnated with Pediocin PA-1/AcH and Its Use in Raw Sliced Pork. Int. J. Food Microbiol. 2013, 167, 229–235. [Google Scholar] [CrossRef]
- Ye, M.; Neetoo, H.; Chen, H. Control of Listeria monocytogenes on Ham Steaks by Antimicrobials Incorporated into Chitosan-Coated Plastic Films. Food Microbiol. 2008, 25, 260–268. [Google Scholar] [CrossRef]
- La Storia, A.; Mauriello, G.; Villani, F.; Ercolini, D. Coating-Activation and Antimicrobial Efficacy of Different Polyethylene Films with a Nisin-Based Solution. Food Bioprocess Technol. 2013, 6, 2770–2779. [Google Scholar] [CrossRef]
- Alves, V.F.; Martinez, R.C.R.; Lavrador, M.A.S.; De Martinis, E.C.P. Antilisterial Activity of Lactic Acid Bacteria Inoculated on Cooked Ham. Meat Sci. 2006, 74, 623–627. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.; Zhang, H.; Liu, H.; Jin, J.; Yang, W.; Xie, Y. Development and Antilisterial Activity of PE-Based Biological Preservative Films Incorporating Plantaricin BM-1. FEMS Microbiol. Lett. 2017, 364, fnw283. [Google Scholar] [CrossRef]
- Yang, W.; Xie, Y.; Jin, J.; Liu, H.; Zhang, H. Development and Application of an Active Plastic Multilayer Film by Coating a Plantaricin BM-1 for Chilled Meat Preservation. J. Food Sci. 2019, 84, 1864–1870. [Google Scholar] [CrossRef]
- Trinetta, V.; Floros, J.D.; Cutter, C.N. Sakacin A-Containing Pullulan Film: An Active Packaging System to Control Epidemic Clones of Listeria monocytogenes in Ready-to-Eat Foods. J. Food Saf. 2010, 30, 366–381. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Huq, T.; Khan, A.; Lacroix, M. Antilisterial Effects of Antibacterial Formulations Containing Essential Oils, Nisin, Nitrite and Organic Acid Salts in a Sausage Model. J. Food Sci. Technol. 2016, 53, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, L.; Smith, C.; Deane, S.M.; Dicks, L.M.T.; van Staden, A.D. Migration of Bacteriocins Across Gastrointestinal Epithelial and Vascular Endothelial Cells, as Determined Using In Vitro Simulations. Sci. Rep. 2019, 9, 11481. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.I.; Martinent, C.; Hammami, R.; Fliss, I.; Subirade, M. Dual Coating of Liposomes as Encapsulating Matrix of Antimicrobial Peptides: Development and Characterization. Front. Chem. 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.; Rubio, R.C.; O’Connor, P.M.; Crispie, F.; Brodkorb, A.; Miao, S.; Hill, C.; Ross, R.P.; Cotter, P.D.; Nilaweera, K.N.; et al. Oral Delivery of Nisin in Resistant Starch Based Matrices Alters the Gut Microbiota in Mice. Front. Microbiol. 2018, 9, 1186. [Google Scholar] [CrossRef]

| Class Type | Producing Strain | Subclasses | Characteristic Features | Major Bacteriocins | Ref. |
|---|---|---|---|---|---|
| Class I (Lantibiotics) | Lactococcus lactis subsp. lactis, Staphylococcus epidermidis, Streptococcus mutans Streptococcus salivarius Lactococus uberis Staphylococcus gallinarum | Sub-class AI, AII (Linear and combined molecules) |
| Nisin, Lacticin 481, Enterocin W, Lactocin, Epidermin, Mutacin B-Ny266, Gallidermin, Mersacidin, Salivaricin A, Lacticin 3147 | [38,43,44] |
| Sub-class B (Globular molecules) |
| ||||
| Class II (Non-lantibiotics) | Lactiplantibacillus plantarum, Lactobacillus acidophilus, L sakei, Streptococcus uberis Leuconostocmesenteroides Pediococcusacidilactici, Enterococcus faecalis, Carnobacteriumpiscicoda, C. Divergens | Class II A (Anti-listerialpediocine bacteriocins type) |
| Sakacin A, Pediocin PA-1/Ach, Carnobacteriocin X | [43,45] |
| Class II B |
| Lactococcin G, Lactacin F Plantaracin EF and JK | |||
| Class II C (Other bacteriocins) |
| Acidocin B, Carnobacteriocin A, Divergicin A, Enterocin A, P | |||
| Class III (Large peptides) | Lactobacillus helveticus, L. bulgaricus Lacticaseibacillus casei | Class IIIA (Lytic enzymes) |
| Helvetic in Lactobacillus helveticus, HelveticinV-1829, Enterolysin A | [43,46,47] |
| Class IIIB (Non-lytic proteins) |
| Caseicin 80 Helveticin J | |||
| Class IV Cyclic bacteriocins | E. faecalis L. helveticus 481 |
| Enterocin AS-48 Glycocin F | [48,49] |
| Biopreservative Agent | Product | Major Findings | Ref. |
|---|---|---|---|
| LAB cultures (Lactobacillus sakei ST153) in combination with MAP (either 20% CO2/80% N2 or 40% CO2/60% N2) in RTE sliced ‘lombo’, a traditional cured-smoked pork loin | Microbial growth and sensory attributes of cures-smoked pork loin stored at 5 °C for 124 days |
| [78] |
| Bacteriocin-like inhibitory substances (BLIS) from Pediococcus pentosaceus ATCC 43200 in artificially contaminated RTE pork ham | Physico-chemical and antimicrobial activity of BLIS against Listeria seeligeri NCTC11289 |
| [79] |
| LDPE film coated with sonorensin, a bacteriocin from B. sonorensis MT93 | Chicken meat pieces spiked with 2 mL suspension of Listeria monocytogenes and Staphylococcus aureus at 1.5 × 106 CFU/mL and stored at refrigerated temperature up to 15 days |
| [80] |
| Semi purified bacteriocin BacFL31 (secreted by Enterococcus faecium sp. FL31) at 200 and 400 AU/g | Physicochemical microbial and sensory attributes of turkey meat stored under refrigerated conditions for 14 days |
| [71] |
| Frankfurters in dip solution containing semi-purified bacteriocins (Lactobacillus curvatus CRL705 or L. sakei) in combination with acetic acid or lactic acid at 2.5% | Microbial and sensory studies of vacuum packaged beef frankfurters stored at 10 °C for 36 days |
| [81] |
| Garlic extract (1%) in combination with Limosilactobacillus reuteri (G-LR) and Lactiplantibacillus plantarum (G-LP) | Effect of combination treatment on physico-chemical microbial and sensory characteristics in ground beef samples stored at refrigerated temperature up to 12 days |
| [82] |
| Bacteriocinogenic activity of Lactobacillus acidophilus PTCC 1643 and Bifidobacterium animalis ssp. Lactis BB-12 PTCC 1736 | Anti-microbial activity of bacteriocins on fresh red beef minced meat stored at refrigerated temperature up to 14 days |
| [83] |
| Surface application L. curvatus L442 and L. lactis subsp. cremoris ATCC 14365 bacteriocin (0.6 g/bag) to hotdogs inoculated with L. monocytogenes (4 log CFU/hot dog) | Anti-listerial activity of bacteriocin on vacuum-sealed hot dogs stored under refrigerated conditions for 28 days |
| [84] |
| Lactococcin BZ bacteriocin (produced by L. lactis spp. lactis BZ) | Microbiological quality of fresh beef treated with lactococcin BZ (200–2500 AU/mL) and kept at 4–5 °C for 12 days |
| [85] |
| L. curvatus 54M16 (Sakacins X, T and P) | Fermented sausage |
| [86] |
| Immersion of beef meat in BM1829 bacteriocin derived from Lactobacillus crustorum MN047 | Anti-bacterial effect of bacteriocin and its potential use as a preservative of beef meat stored at refrigerated temperature |
| [87] |
| Nisin derived from L. lactis | Microbial quality of minced beef stored under refrigerated temperature for 15 days |
| [88] |
| Bacteriocin leucocin C (strain of Saccharomyces boulardii CNCM I-745) | Anti-listeria activity of bacteriocin on chicken breast strips marinated overnight in beer spiked with L. monocytogenes |
| [89] |
| Novel bacteriocin (XJS01) from Ligilactobacillus salivarius strain CGMCC2070 | Raw chicken breast piece marinated with beer brewed with bacteriocin |
| [90] |
| Novel bacteriocin BM1300 produced by L. crustorum MN047 | Effect of bacteriocin on beef meat sprayed with S. aureus and E. coli (5 mL, 106 CFU/mL) and stored at refrigerated temperature for 10 days |
| [91] |
| Novel bacteriocin BM1122 derived from L. crustorum MN047 | Anti-bacterial activity of BM1122 in fresh raw beef meat stored under chilled conditions for 10 days |
| [92] |
| Lacticaseibacillus paracasei (LP) bacteriocin | Effect of bacteriocin on microbial and cooking qualities, physico-chemical parameters of raw and roasted pork |
| [93] |
| L. curvatus UFV-NPAC1 or its partially purified bacteriocin at 12.5 mg/g and 6.25 mg/g | Inhibitory activity of bacteriocinogenic strain on L. monocytogenes in fresh pork sausage stored at 7 °C for 10 days |
| [94] |
| Purified pediocin AcH/PA-1, produced by P. pentosaceus OZF | Anti-listeria activity of bacteriocin in chicken meat products radiated and inoculated with L. monocytogenes (105 CFU/g) and stored under refrigerated conditions for 14 days |
| [95] |
| P. acidilactici HA-6111- 2 or its bacteriocin, pediocin PA-1 (1280 AU/g) alone or in combination with mild HHP (300 MPa, 10 °C, 5 min) | Anti-listerial effect of bacteriocin or combination treatment in traditional fermented meat sausages |
| [96] |
| Synergistic effect of pediocin bacHA-6111-2 (in situ and ex situ) in combination with HHP | Control of L. innocua in fermented meat products |
| [97] |
| Cooked ham treated with plantaricin BM-1 5120 AU/g, sodium nitrite 0.075 mg/g, and ultra-high-pressure technology (400 MPa for 5 min) | Control of L. monocytogenes in cooked ham vacuum packaged and stored under refrigerated conditions for 56 days |
| [98] |
| Pullulan films containing lauric arginate (LAE) alone or in combination with nisin Z (produced by L. lactis subsp. Lactis I8-7-3) | Effect of pullulan film on cooked deliham slices vacuum-packaged and stored at refrigerated temperature up to 28 days |
| [99] |
| Sakacin-59 (Sak-59) of Latilactobacillussakei strain | Inhibitory activity against meat spoilage bacteria |
| [100] |
| Bac + strains viz. Latilactobacillus curvatu, L. lactis, Pediococcusacidilactici, Enterococcus faecium (Bac + LAB) and Bac + supernatants cell-free (CFS) mixtures | Effect of surface application of Bac + LAB and Bac + CFS mixtures to prevent the growth of L. monocytogenes in vacuum-packaged RTE meats (hot dogs- beef and pork trimmings) stored at 5 °C up to 12 weeks |
| [101] |
| L. plantarum SC01 bacteriocin microencapsulated in 2.5% alginate -6.0% gelatin, w/v (ALG-GEL) capsules | Antimicrobial activity of bacteriocin and physical quality of pork meat stored in room temperature for 48 h |
| [102] |
| Pediocin bacteriocin from P. pentosaceus in combination with Murraya koenigii berries (MKB) | Anti-listerial and antimicrobial effects of pediocin on raw goat meat emulsion inoculated with L. innocua stored under refrigerated conditions for 9 days |
| [103] |
| Bacteriocin and non-bacteriocin producer strains of Lactiplantibacillus plantarum | Enumeration of L. monocytogenes of pork colonial sausages |
| [33] |
| Plantaricin BM-1 bacteriocin from L. plantarum BM-1 | Antimicrobial effect of plantaricin BM-1 on fresh pork chill stored for 7 days |
| [104] |
| Sucrose 0.3% and 1.2% and L. plantarum | Effect of combination treatment on chemical, textural and sensory characteristics of Isan sausage stored for 28 days |
| [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 2022, 10, 2058. https://doi.org/10.3390/microorganisms10102058
Bhattacharya D, Nanda PK, Pateiro M, Lorenzo JM, Dhar P, Das AK. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms. 2022; 10(10):2058. https://doi.org/10.3390/microorganisms10102058
Chicago/Turabian StyleBhattacharya, Dipanwita, Pramod Kumar Nanda, Mirian Pateiro, José M. Lorenzo, Pubali Dhar, and Arun K. Das. 2022. "Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products" Microorganisms 10, no. 10: 2058. https://doi.org/10.3390/microorganisms10102058
APA StyleBhattacharya, D., Nanda, P. K., Pateiro, M., Lorenzo, J. M., Dhar, P., & Das, A. K. (2022). Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms, 10(10), 2058. https://doi.org/10.3390/microorganisms10102058










