Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus
Abstract
:1. Introduction
2. Materials and Methods
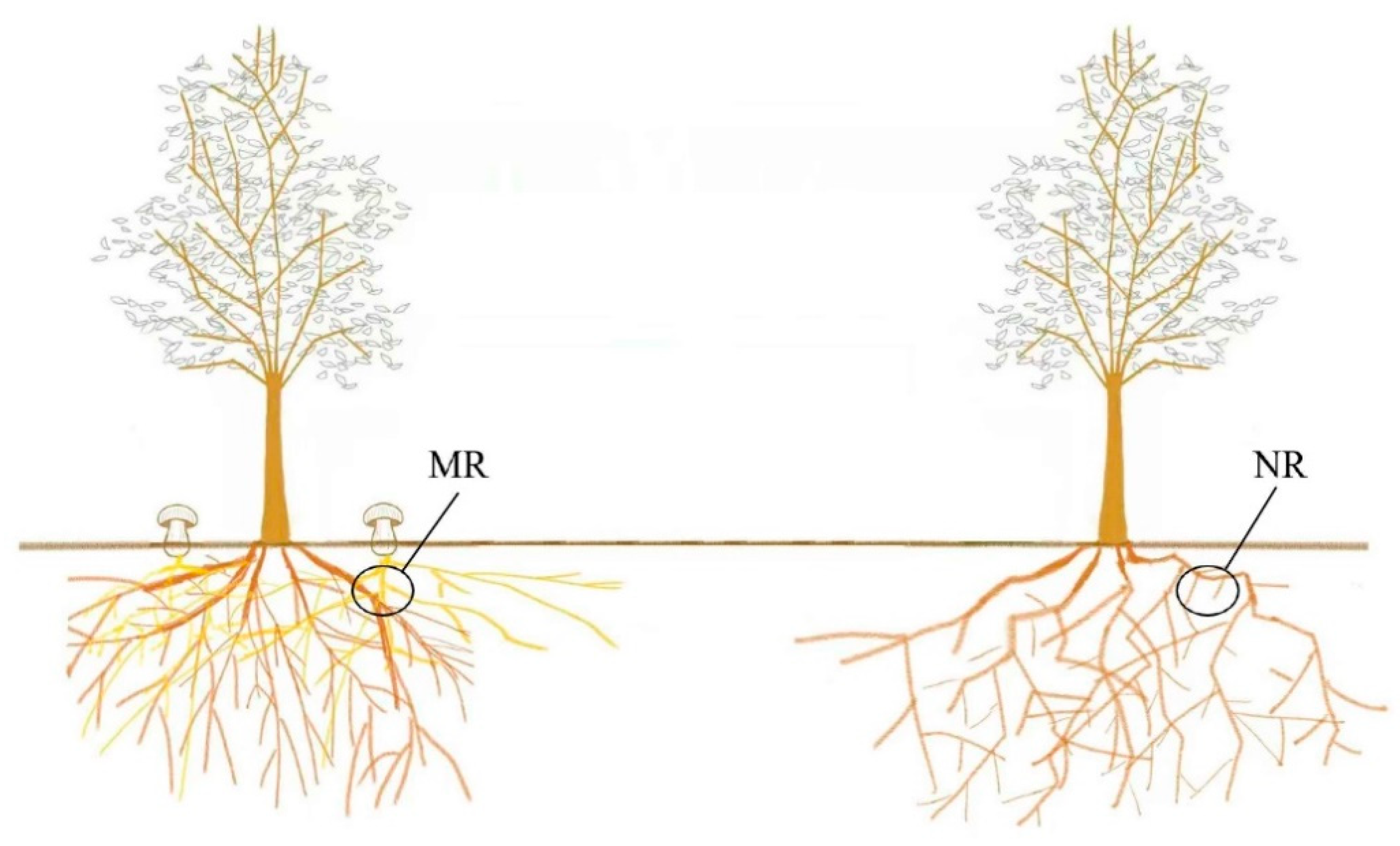
2.1. Sampling and Processing
2.2. DNA Extraction, PCR, and Sequencing
2.3. Bioinformatic Processing
2.4. Chemical Properties
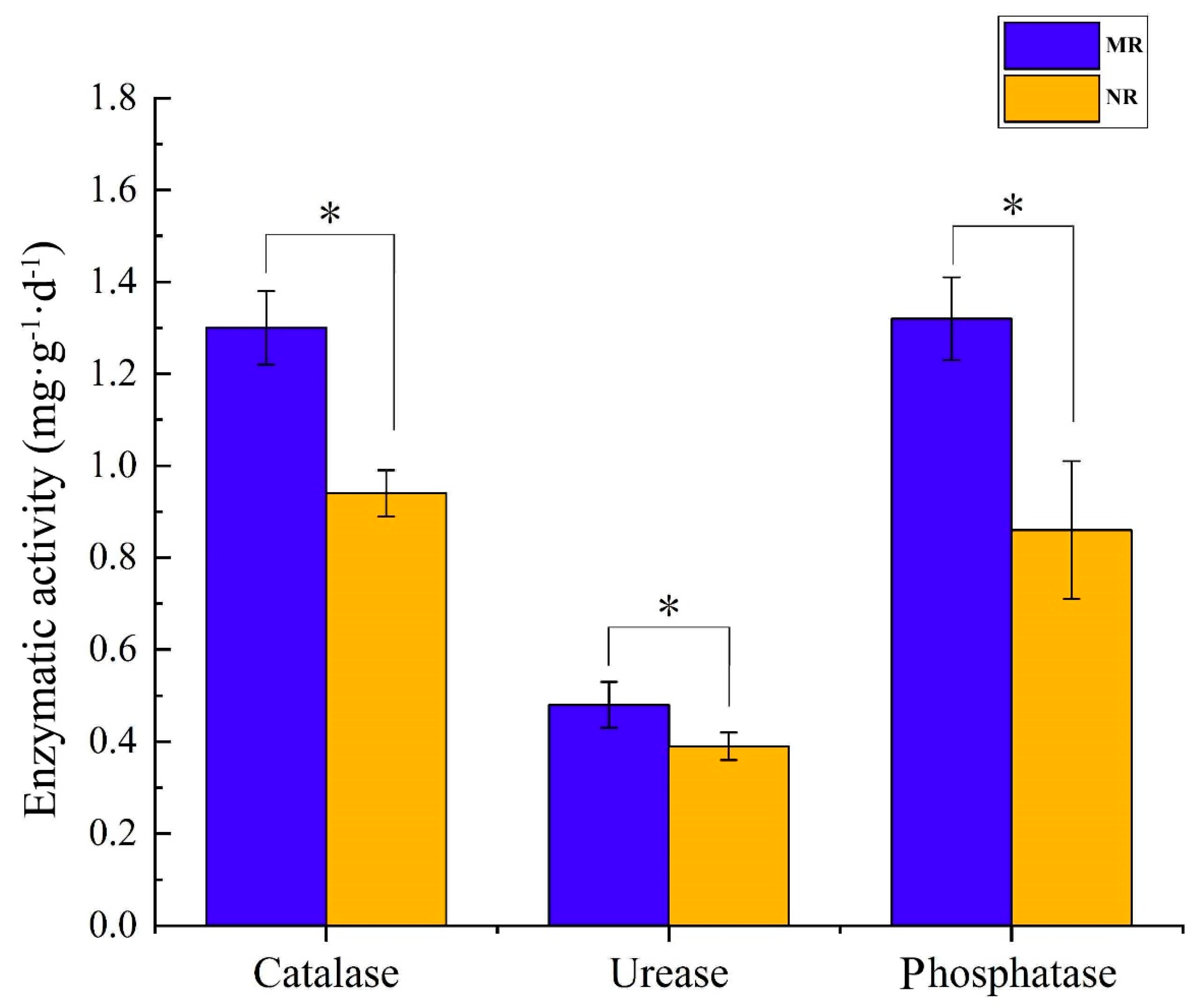
2.5. Enzymic Activity
2.6. Statistical Analysis
3. Results
3.1. Fruiting Body and Mycorrhizal Morphology
3.2. Sequence Data Processing
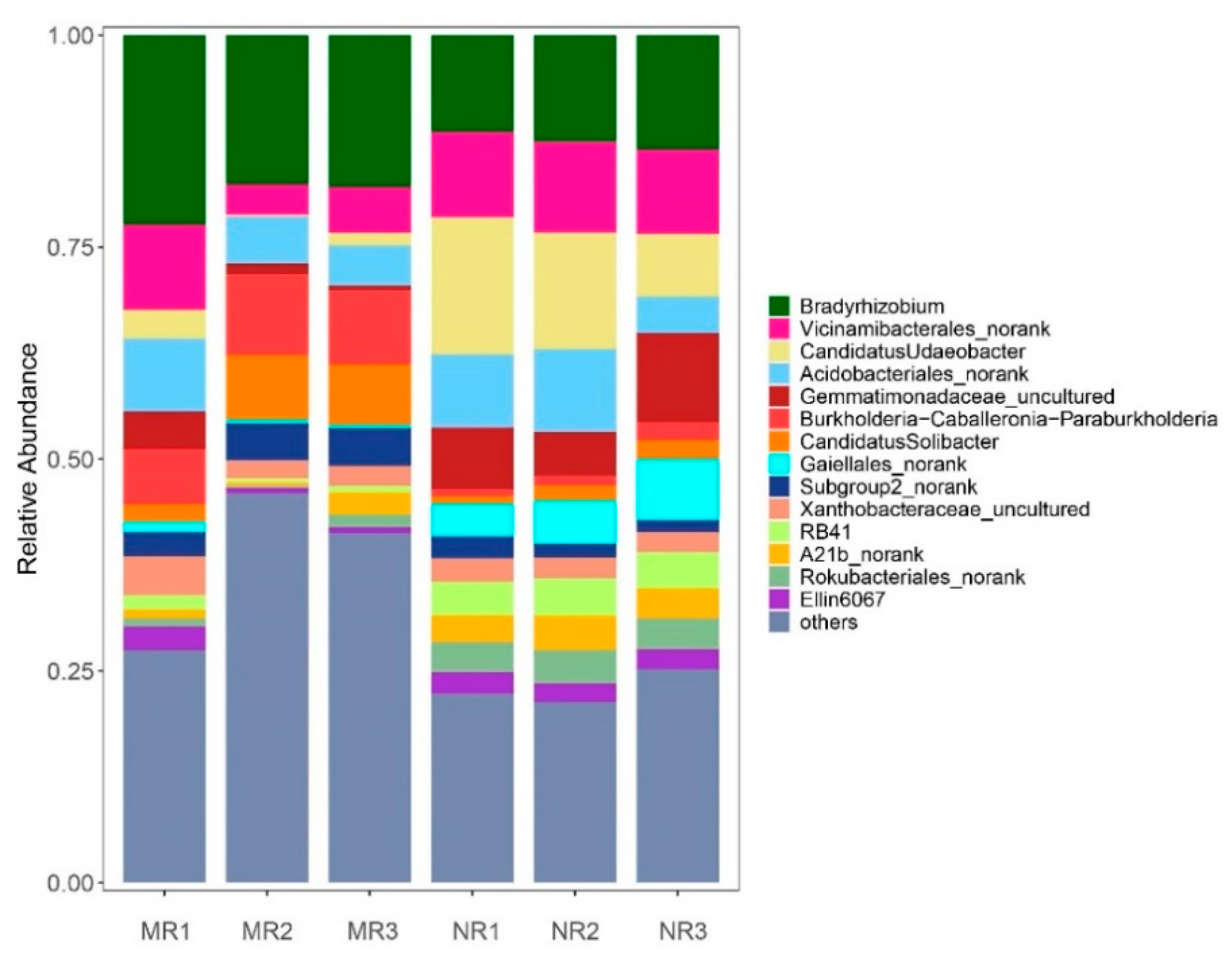
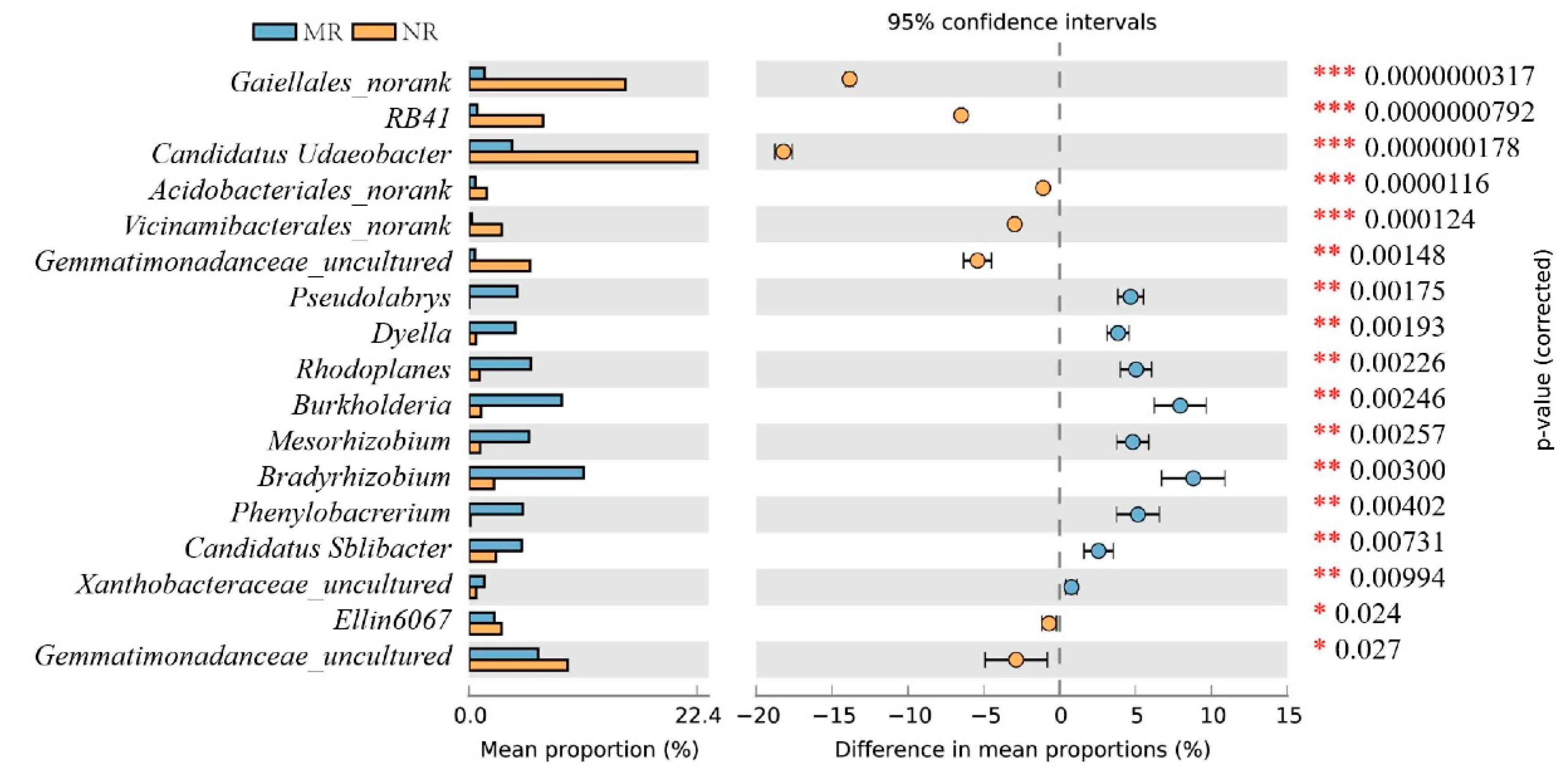
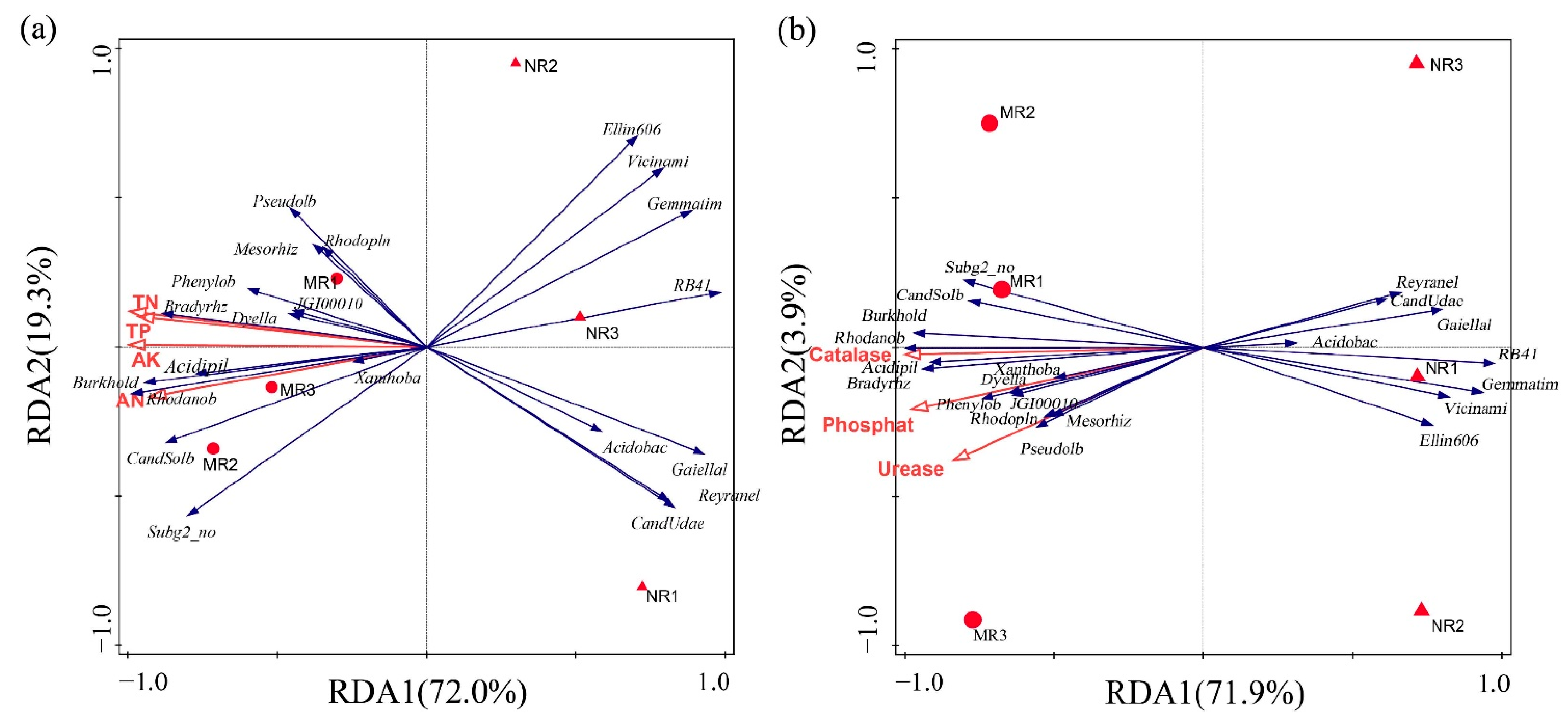
3.3. Clustering and Variation Analysis of Bacterial Communities
3.4. Physicochemical Characteristics of Soil
4. Discussion
4.1. The Relative Abundance of Bacteria in the Mycorrhizal Rhizosphere Was Significantly Higher Than in the Non-Mycorrhizal Rhizosphere
4.2. MHB in Mycorrhizal Rhizosphere Soils Promotes Nutrient Transformation and Indirectly Provides Nutrient Reserves for Sporocarp Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, A.E.; Groten, K. The Costs and Benefits of Plant–Arbuscular Mycorrhizal Fungal Interactions. Annu. Rev. Plant Biol. 2022, 73, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and Common Traits in Mycorrhizal Symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M. Mycorrhizal Types Differ in Ecophysiology and Alter Plant Nutrition and Soil Processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Garbaye, J. Mycorrhiza Helper Bacteria: A Promising Model for the Genomic Analysis of Fungal–Bacterial Interactions. New Phytol. 2005, 168, 4–8. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Fang, S.; Tian, Y.; Guo, J. Variation of Soil Enzyme Activity and Microbial Biomass in Poplar Plantations of Different Genotypes and Stem Spacings. J. For. Res. 2018, 29, 963–972. [Google Scholar] [CrossRef]
- Bengtson, P.; Bengtsson, G. Rapid Turnover of DOC in Temperate Forests Accounts for Increased CO2 Production at Elevated Temperatures. Ecol. Lett. 2007, 10, 783–790. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Q.; Gao, J.; Zheng, Y.; Zhou, J.; Chen, Y.; Yang, Y. Seasonal Response of Extracellular Enzyme Activity to Precipitation Exclusion in a Subtropical Cunninghamia Lanceolata Plantation. Acta Ecol. Sin. 2018, 38, 2119–2127. [Google Scholar] [CrossRef]
- Xiao, S.; You, H.; You, W.; Liu, J.; Cai, C.; Wu, J.; Ji, Z.; Zhan, S.; Hu, Z.; Zhang, Z.; et al. Rhizosphere and Bulk Soil Enzyme Activities in a Nothotsuga Longibracteata Forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J. For. Res. 2017, 28, 521–528. [Google Scholar] [CrossRef]
- Garbaye, J. Tansley Review No. 76 Helper Bacteria: A New Dimension to the Mycorrhizal Symbiosis. New Phytol. 1994, 128, 197–210. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The Mycorrhiza Helper Bacteria Revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Frey, P.; Frey-Klett, P.; Garbaye, J.; Berge, O.; Heulin, T. Metabolic and Genotypic Fingerprinting of Fluorescent Pseudomonads Associated with the Douglas Fir-Laccaria Bicolor Mycorrhizosphere. Appl. Environ. Microbiol. 1997, 63, 1852–1860. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Castro, J.I.; Levenfors, J.J.; Danell, E. Physiological and Genetic Characterization of Fluorescent Pseudomonas Associated with Cantharellus Cibarius. Can. J. Microbiol. 2002, 48, 739–748. [Google Scholar] [CrossRef]
- Brulé, C.; Frey-Klett, P.; Pierrat, J.C.; Courrier, S.; Gérard, F.; Lemoine, M.C.; Rousselet, J.L.; Sommer, G.; Garbaye, J. Survival in the Soil of the Ectomycorrhizal Fungus Laccaria Bicolor and the Effects of a Mycorrhiza Helper Pseudomonas Fluorescens. Soil Biol. Biochem. 2001, 33, 1683–1694. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial Interactions in the Mycorrhizosphere and Their Significance for Sustainable Agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Schrey, S.D.; Schellhammer, M.; Ecke, M.; Hampp, R.; Tarkka, M.T. Mycorrhiza Helper Bacterium Streptomyces AcH 505 Induces Differential Gene Expression in the Ectomycorrhizal Fungus Amanita Muscaria. New Phytol. 2005, 168, 205–216. [Google Scholar] [CrossRef]
- Korhonen, A.; Lehto, T.; Heinonen, J.; Repo, T. Corrigendum: Whole-Plant Frost Hardiness of Mycorrhizal (Hebeloma sp. or Suillus luteus) and Non-Mycorrhizal Scots Pine Seedlings. Tree Physiol. 2019, 39, 951–960. [Google Scholar] [CrossRef]
- Chen, H.; Quan, W.; Liu, H.; Ding, G. Effects of Suillus luteus and S. bovinus on the Physiological Response and Nutrient Absorption of Pinus massoniana Seedlings under Phosphorus Deficiency. Plant Soil 2022, 471, 577–590. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.-Q.; Zhang, C.; Liu, C.; Yang, M.; Li, S. Edible Ectomycorrhizal Fungi and Their Cultivation in China. In Mushrooms, Humans and Nature in a Changing World; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F.-Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 31–60. ISBN 978-3-030-37377-1. [Google Scholar]
- Li, S.; Deng, Y.; Du, X.; Feng, K.; Wu, Y.; He, Q.; Wang, Z.; Liu, Y.; Wang, D.; Peng, X.; et al. Sampling Cores and Sequencing Depths Affected the Measurement of Microbial Diversity in Soil Quadrats. Sci. Total Environ. 2021, 767, 144966. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, M.; Chen, X.; Yin, L.; Gui, H.; Zhu, B. Measuring Rhizosphere Effects of Two Tree Species in a Temperate Forest: A Comprehensive Method Comparison. Rhizosphere 2019, 10, 100153. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Yao, Q.; Hu, X.; Zhang, W.; Mi, G.; Chen, X.; Wang, G. Distinct Soil Bacterial Communities in Response to the Cropping System in a Mollisol of Northeast China. Appl. Soil Ecol. 2017, 119, 407–416. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. (Eds.) Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-866-7. [Google Scholar]
- Weaver, R.W.; Angle, S.; Bottomley, P.; Bezdicek, D.; Smith, S.; Tabatabai, A.; Wollum, A. (Eds.) Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1994; ISBN 978-0-89118-865-0. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, G.; Ma, P.; Lin, Y.; Yang, X.; Cao, C. Isolation and Characterization of a Phosphorus-Solubilizing Bacterium from Rhizosphere Soils and Its Colonization of Chinese Cabbage (Brassica campestris ssp. Chinensis). Front. Microbiol. 2017, 8, 1270. [Google Scholar] [CrossRef]
- Herrero de Aza, C.; Armenteros, S.; McDermott, J.; Mauceri, S.; Olaizola, J.; Hernández-Rodríguez, M.; Mediavilla, O. Fungal and Bacterial Communities in Tuber Melanosporum Plantations from Northern Spain. Forests 2022, 13, 385. [Google Scholar] [CrossRef]
- Reis, F.; Magalhães, A.P.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Bacteria Could Help Ectomycorrhizae Establishment under Climate Variations. Mycorrhiza 2021, 31, 395–401. [Google Scholar] [CrossRef]
- Shinde, S.; Zerbs, S.; Collart, F.R.; Cumming, J.R.; Noirot, P.; Larsen, P.E. Pseudomonas Fluorescens Increases Mycorrhization and Modulates Expression of Antifungal Defense Response Genes in Roots of Aspen Seedlings. BMC Plant Biol. 2019, 19, 4. [Google Scholar] [CrossRef]
- Amini, S.; Hassanpour Asil, M.; Olfati, J.-A.; Mousanejad, S. Effect of Ectomycorrhizal Fungi Symbiosis and Mycorrhiza Helper Bacteria (Bacillus Cereus) on Nutrient Uptake and Growth of Black Pine (Pinus nigra). J. Plant Res. 2020, 27, 249–264. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Lim, Y.W. Root-Associated Bacteria Influencing Mycelial Growth of Tricholoma Matsutake (Pine mushroom). J. Microbiol. 2018, 56, 399–407. [Google Scholar] [CrossRef]
- Leyva-Rojas, J.A.; Coy-Barrera, E.; Hampp, R. Interaction with Soil Bacteria Affects the Growth and Amino Acid Content of Piriformospora indica. Molecules 2020, 25, 572. [Google Scholar] [CrossRef]
- Sangwan, S.; Prasanna, R. Mycorrhizae Helper Bacteria: Unlocking Their Potential as Bioenhancers of Plant–Arbuscular Mycorrhizal Fungal Associations. Microb. Ecol. 2022, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Ceccaroli, P.; Saltarelli, R.; Guidi, C.; Potenza, L.; Basaglia, M.; Fontana, F.; Baldan, E.; Casella, S.; Ryahi, O.; et al. New Evidence for Nitrogen Fixation within the Italian White Truffle Tuber Magnatum. Fungal. Biol.-UK 2010, 114, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Ramezani, A.; Pelletier, D.; Alber, M.; Cannon, W.R. A Multiscale Model of Fungal Impact on Chemotactic Behavior of Mycorrhizal Helper Bacteria. Biophys. J. 2021, 120, 68a–69a. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Bonfante, P. A Burkholderia Strain Living Inside the Arbuscular Mycorrhizal Fungus Gigaspora margarita Possesses the VacB Gene, Which Is Involved in Host Cell Colonization by Bacteria. Microb. Ecol. 2000, 39, 137–144. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Liu, H. Biosynthetic Studies of Aziridine Formation in Azicemicins. J. Am. Chem. Soc. 2009, 131, 18066–18068. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Miao, Q.; Feng, W.-W.; Wang, Y.; Zhu, X.; Xing, K.; Jiang, J.-H. Biodiversity and Plant Growth Promoting Traits of Culturable Endophytic Actinobacteria Associated with Jatropha curcas L. Growing in Panxi Dry-Hot Valley Soil. Appl. Soil Ecol. 2015, 93, 47–55. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant Growth Promotion Under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Hou, L.-L.; Sheng, J.-M.; Ren, J.-H.; Zheng, L.; Chen, D.; Ye, J.-R. Effects of Ectomycorrhizal Fungus Boletus Edulis and Mycorrhiza Helper Bacillus cereus on the Growth and Nutrient Uptake by Pinus thunbergii. Biol. Fertil. Soils 2012, 48, 385–391. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen Transfer in the Arbuscular Mycorrhizal Symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The Uptake, Metabolism, Transport and Transfer of Nitrogen in an Arbuscular Mycorrhizal Symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in Microbial Biomass and Phosphorus Availability in the Rhizosphere of Intercropped Cereal and Legumes under Field Conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Shen, R.F. Stronger Effects of Maize Rhizosphere than Phosphorus Fertilization on Phosphatase Activity and Phosphorus-Mineralizing-Related Bacteria in Acidic Soils. Rhizosphere 2022, 23, 100555. [Google Scholar] [CrossRef]
- Ho, I.; Zak, B. Acid phosphatase activity of six ectomycorrhizal fungi. Can. J. Bot. 1979, 57, 1203–1205. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic Colonization of Plant Roots by Nitrogen-Fixing Bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of Phosphate-Solubilizing Bacteria and Vesicular-Arbuscular Mycorrhizae on Tomato Growth and Soil Microbial Activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Wang, F.; Kertesz, M.A.; Feng, G. Phosphorus Forms Affect the Hyphosphere Bacterial Community Involved in Soil Organic Phosphorus Turnover. Mycorrhiza 2019, 29, 351–362. [Google Scholar] [CrossRef]
- Sharma, S.; Compant, S.; Ballhausen, M.-B.; Ruppel, S.; Franken, P. The Interaction between Rhizoglomus irregulare and Hyphae Attached Phosphate Solubilizing Bacteria Increases Plant Biomass of Solanum lycopersicum. Microbiol. Res. 2020, 240, 126556. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond Nutrient, Fructose, Exuded by an Arbuscular Mycorrhizal Fungus Triggers Phytate Mineralization by a Phosphate Solubilizing Bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef] [Green Version]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of Two Trichoderma Strains on Plant Growth, Rhizosphere Soil Nutrients, and Fungal Community of Pinus sylvestris Var. Mongolica Annual Seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular Enzyme Activity and Stoichiometry: The Effect of Soil Microbial Element Limitation during Leaf Litter Decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Hai, X.; Shangguan, Z.; Deng, L. Dynamics of Soil Microbial C:N:P Stoichiometry and Its Driving Mechanisms Following Natural Vegetation Restoration after Farmland Abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef]
- Orlofsky, E.; Zabari, L.; Bonito, G.; Masaphy, S. Changes in Soil Bacteria Functional Ecology Associated with Morchella rufobrunnea Fruiting in a Natural Habitat. Environ. Microbiol. 2021, 23, 6651–6662. [Google Scholar] [CrossRef]


| Sample | Chao1 Index | Shannon Index | Simpson Index | ACE Index | Coverage |
|---|---|---|---|---|---|
| MR | 208.77 ± 6.41 a | 4.61 ± 0.07 a | 0.0165 ± 0.0006 b | 205.81 ± 6.38 a | 0.9947 ± 0.0022 a |
| NR | 200.25 ± 7.67 a | 4.50 ± 0.04 b | 0.0235 ± 0.0035 a | 193.97 ± 2.37 b | 0.9958 ± 0.0009 a |
| Sample | pH | SOM (g/kg) | TN (g/kg) | TP (g/kg) |
|---|---|---|---|---|
| MR | 5.77 ± 0.026 a | 51.93 ± 2.26 a | 3.96 ± 0.35 a | 0.65 ± 0.04 a |
| NR | 5.83 ± 0.012 b | 45.27 ± 1.96 b | 2.80 ± 0.62 b | 0.52 ± 0.06 b |
| TK (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | |
| MR | 3.72 ± 0.17 a | 57.92 ± 2.27 a | 16.47 ± 1.25 a | 244.67 ± 22.37 a |
| NR | 3.35 ± 0.10 b | 47.77 ± 1.71 b | 13.87 ± 1.19 b | 185.33 ± 20.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shi, Z.; Pang, Q.; Liang, X.; Li, H.; Sui, X.; Li, C.; Song, F. Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus. Microorganisms 2022, 10, 2059. https://doi.org/10.3390/microorganisms10102059
Zhou Y, Shi Z, Pang Q, Liang X, Li H, Sui X, Li C, Song F. Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus. Microorganisms. 2022; 10(10):2059. https://doi.org/10.3390/microorganisms10102059
Chicago/Turabian StyleZhou, Yixin, Zhichao Shi, Qiliang Pang, Xiufeng Liang, Hongtao Li, Xin Sui, Chongwei Li, and Fuqiang Song. 2022. "Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus" Microorganisms 10, no. 10: 2059. https://doi.org/10.3390/microorganisms10102059
APA StyleZhou, Y., Shi, Z., Pang, Q., Liang, X., Li, H., Sui, X., Li, C., & Song, F. (2022). Responses of Bacterial Community Structure, Diversity, and Chemical Properties in the Rhizosphere Soil on Fruiting-Body Formation of Suillus luteus. Microorganisms, 10(10), 2059. https://doi.org/10.3390/microorganisms10102059







