Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes
Abstract
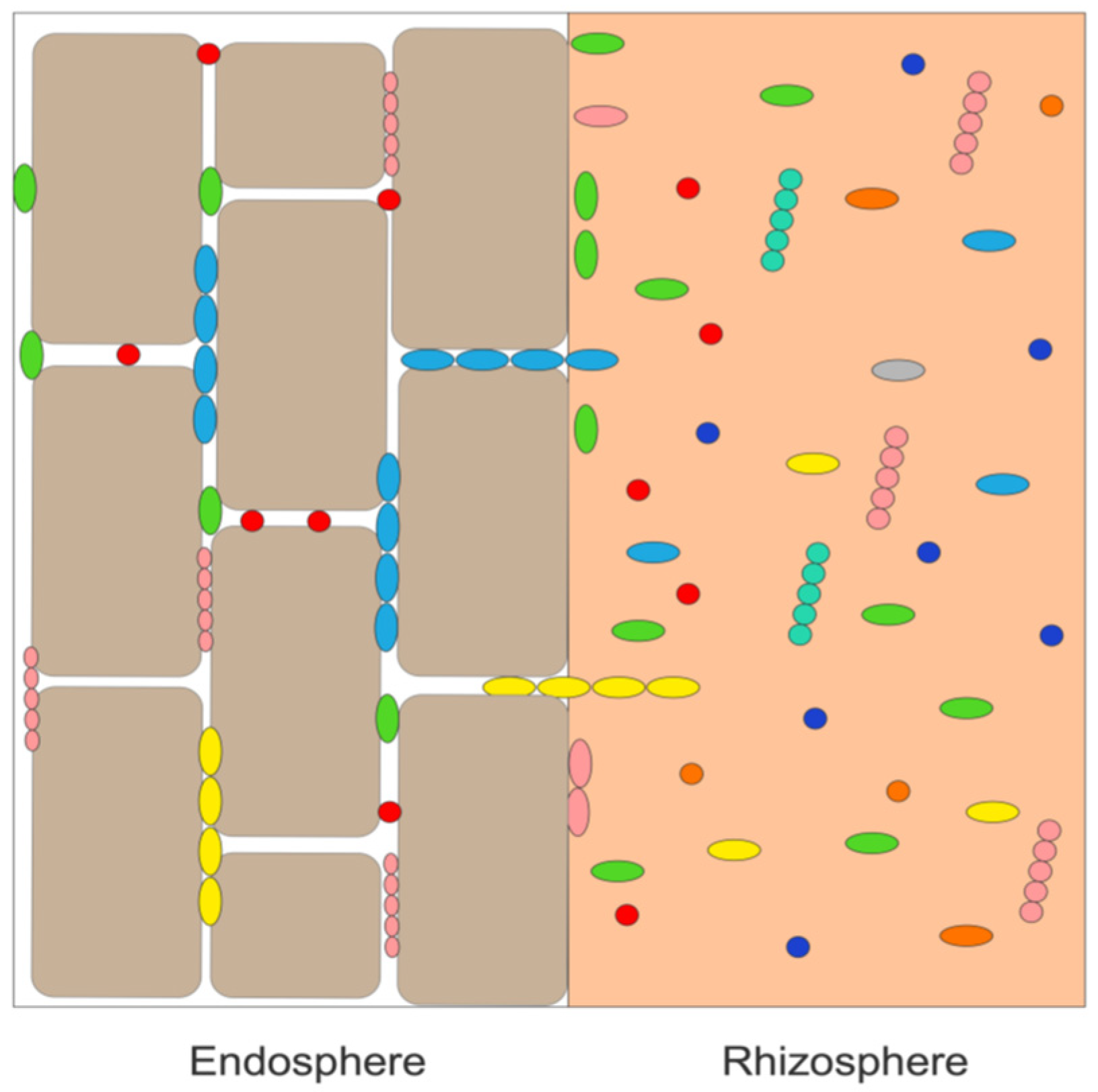
:1. Introduction
2. Plant Bacterial Endophytes
2.1. An Overview of Plant Growth-Promoting Bacterial Endophytes
2.2. Isolation of Bacterial Endophytes
2.3. Endophytic PGPB Mechanisms That Directly Promote Plant Growth
2.4. Endophytic PGPB Mechanisms That Indirectly Promote Plant Growth
2.5. Endophytic PGPB Protect Plants against Abiotic Stresses
3. Production of Secondary Metabolites
3.1. Antibiotics
3.1.1. Lipopeptides
3.1.2. Amino Acid-Rich Peptides
3.1.3. Cyclic Cationic Lipopeptides
3.1.4. Pigments as Antibiotics
3.2. Anti-Cancer Compounds
3.2.1. Cyclic Analogs
3.2.2. Maytansinoids
3.2.3. Extracellular Metabolites
3.3. Anti-Viral Compounds
3.3.1. Flavonoids
3.3.2. Saponins
3.3.3. Nanoparticles
3.4. Other Compounds
Terpenoids and Alkaloids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vargiu, A.V.; Pos, K.M.; Poole, K.; Nikaido, H. Editorial: Bad Bugs in the XXIst Century: Resistance Mediated by Multi-Drug Efflux Pumps in Gram-Negative Bacteria. Front. Microbiol. 2016, 7, 833. [Google Scholar] [CrossRef] [Green Version]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.X.; Ng, S.B. Fungal Endophytes: A. Promising Frontier for Discovery of Novel Bioactive Compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- Petrini, O.; Sieber, T.N.; Toti, L.; Viret, O. Ecology, Metabolite Production, and Substrate Utilization in Endophytic Fungi. Nat. Toxins 1992, 1, 185–196. [Google Scholar] [CrossRef]
- Khan, Z.; Guelich, G.; Phan, H.; Redman, R.; Doty, S. Bacterial and Yeast Endophytes FromPoplar and Willow Promote Growth in Crop Plants and Grasses. ISRN Agron. 2012, 2012, 890280. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Roman, D.; Kintz, T.; delas Alas, M.; Yap, R.; Doty, S. Degradation, Phytoprotection and Phytoremediation of Phenanthrene by Endophyte Pseudomonas Putida, PD1. Environ. Sci. Technol. 2014, 48, 12221–12228. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Kandel, S.L.; Ramos, D.N.; Ettl, G.J.; Kim, S.-H.; Doty, S.L. Increased Biomass of Nursery-Grown Douglas-Fir Seedlings upon Inoculation with Diazotrophic Endophytic Consortia. Forests 2015, 6, 3582–3593. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.-H.; Doty, S.L. Growth Enhancement and Drought Tolerance of Hybrid Poplar upon Inoculation with Endophyte Consortia. Curr. Plant Biol. 2016, 6, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In Vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef] [Green Version]
- Aghai, M.M.; Khan, Z.; Joseph, M.R.; Stoda, A.M.; Sher, A.W.; Ettl, G.J.; Doty, S.L. The Effect of Microbial Endophyte Consortia on Pseudotsuga Menziesii and Thuja Plicata Survival, Growth, and Physiology Across Edaphic Gradients. Front. Microbiol. 2019, 10, 1353. [Google Scholar] [CrossRef]
- Doty, S.L.; Freeman, J.L.; Cohu, C.M.; Burken, J.G.; Firrincieli, A.; Simon, A.; Khan, Z.; Isebrands, J.G.; Lukas, J.; Blaylock, M.J. Enhanced Degradation of TCE on a Superfund Site Using Endophyte-Assisted Poplar Tree Phytoremediation. Environ. Sci. Technol. 2017, 51, 10050–10058. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A Critical Review on Exploiting the Pharmaceutical Potential of Plant Endophytic Fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef]
- Haque, M.A.; Lee, J.H.; Cho, K.M. Endophytic Bacterial Diversity in Korean Kimchi Made of Chinese Cabbage Leaves and Their Antimicrobial Activity against Pathogens. Food Control 2015, 56, 24–33. [Google Scholar] [CrossRef]
- Mishra, S.; Bhardwaj, P.; Sharma, S. Metabolomic Insights into Endophyte-Derived Bioactive Compounds. Front. Microbiol. 2022, 13, 835931. [Google Scholar] [CrossRef] [PubMed]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical Techniques for Metabolomic Studies: A Review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and Chemistry of Endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 383–398. [Google Scholar]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Dwibedi, V.; Rath, S.K.; Joshi, M.; Kaur, R.; Kaur, G.; Singh, D.; Kaur, G.; Kaur, S. Microbial Endophytes: Application towards Sustainable Agriculture and Food Security. Appl. Microbiol. Biotechnol. 2022, 106, 5359–5384. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Monje, D.; Raizada, M.N. Conservation and Diversity of Seed Associated Endophytes in Zea across Boundaries of Evolution, Ethnography and Ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Bacon, C.W.; White, J. Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; p. 500. [Google Scholar]
- Hallmann, J.; Schulz, B.; Berg, G. Isolation procedures for endophytic microorganisms. In Microbial Root Endophytes, 1st ed.; Springer: New York, NY, USA, 2006; pp. 299–314. [Google Scholar]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G. Associations of Bacterial Endophyte Populations from Red Clover and Potato Crops with Potential for Beneficial Allelopathy. Can. J. Microbiol. 1998, 44, 162–167. [Google Scholar] [CrossRef]
- Surette, M.A.; Sturz, A.V.; Lada, R.R.; Nowak, J. Bacterial Endophytes in Processing Carrots (Daucus Carota L. Var. Sativus): Their Localization, Population Density, Biodiversity and Their Effects on Plant Growth. Plant Soil 2003, 253, 381–390. [Google Scholar] [CrossRef]
- Shimaila Rashid. Isolation and Characterization of New Plant Growth-Promoting Bacterial Endophytes. Appl. Soil Ecol. 2012, 61, 217–224. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Khan, M.A.; Zhu, F. Popular Molecular Markers in Bacteria. Mol. Genet. Microbiol. Virol. 2012, 27, 103–107. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B. Mechanisms Used by Plant Growth-Promoting Bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, A.; Poupin, M.J.; Donoso, R.; Ledger, T.; Guiliani, N.; Gutiérrez, R.A.; González, B. Quorum Sensing and Indole-3-Acetic Acid Degradation Play a Role in Colonization and Plant Growth Promotion of Arabidopsis Thaliana by Burkholderia Phytofirmans PsJN. Mol. Plant-Microbe Interact. 2013, 26, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhore, S.J.; Ravichantar, N.; Loh, C.Y. Screening of Endophytic Bacteria Isolated from Leaves of Sambung Nyawa [Gynura Procumbens (Lour.) Merr.] for Cytokinin-like Compounds. Bioinformation 2010, 5, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-Borne Endophytic Bacillus Amyloliquefaciens RWL-1 Produces Gibberellins and Regulates Endogenous Phytohormones of Oryza Sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- da Silveira, A.P.D.; Iório, R.D.P.F.; Marcos, F.C.C.; Fernandes, A.O.; de Souza, S.A.C.D.; Kuramae, E.E.; Cipriano, M.A.P. Exploitation of New Endophytic Bacteria and Their Ability to Promote Sugarcane Growth and Nitrogen Nutrition. Antonie Leeuwenhoek 2019, 112, 283–295. [Google Scholar] [CrossRef]
- Worsley, S.F.; Newitt, J.; Rassbach, J.; Batey, S.F.D.; Holmes, N.A.; Murrell, J.C.; Wilkinson, B.; Hutchings, M.I. Streptomyces Endophytes Promote Host Health and Enhance Growth across Plant Species. Appl. Environ. Microbiol. 2020, 86, e01053-20. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S.; Sharma, S. Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes from Ocimum Sanctum Linn. Against Root Rot Pathogen Fusarium Oxysporum in Pisum Sativum. Front. Plant Sci. 2022, 13, 813686. [Google Scholar] [CrossRef]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus Velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Uwaremwe, C.; Yue, L.; Wang, Y.; Tian, Y.; Zhao, X.; Liu, Y.; Zhou, Q.; Zhang, Y.; Wang, R. An Endophytic Strain of Bacillus Amyloliquefaciens Suppresses Fusarium Oxysporum Infection of Chinese Wolfberry by Altering Its Rhizosphere Bacterial Community. Front. Microbiol. 2022, 12, 782523. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Preface. In Ethylene in Plant Biology, 2nd ed.; Academic Press: New York, NY, USA, 1992; pp. xi–xii. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Ethylene and Abiotic Stress Tolerance in Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 395–412. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.D.C.; Berta, G.; Lingua, G. Screening of Bacterial Endophytes Able to Promote Plant Growth and Increase Salinity Tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd Allah, E.F. Endophytic Bacteria Improve Plant Growth, Symbiotic Performance of Chickpea (Cicer Arietinum L.) and Induce Suppression of Root Rot Caused by Fusarium Solani under Salt Stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; et al. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita Indica. Int. J. Environ. Res. 2022, 16, 62. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Matjaz, R.; Matic, T.; Damjan, J.; Borut, S.; Samo, K.; Ravnikar, M.; Tercelj, M.; Janes, D.; Strukelj, B.; Kreft, S. Antibacterial activity of endophytic fungi isolated from conifer needles. Afr. J. Biotechnol. 2015, 14, 867–871. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic Bacteria: A New Source of Bioactive Compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Zin, N.M.; Baba, M.S.; Zainal-Abidin, A.H.; Latip, J.; Mazlan, N.W.; Edrada-Ebel, R. Gancidin W, a Potential Low-Toxicity Antimalarial Agent Isolated from an Endophytic Streptomyces SUK10. Drug Des. Dev. Ther. 2017, 11, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Hallmann, J. Control of Plant Pathogenic Fungi with Bacterial Endophytes. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–69. [Google Scholar] [CrossRef]
- Azevedo, J.L.; Maccheroni, W., Jr.; Pereira, J.O.; de Araújo, W.L. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 2000, 3. [Google Scholar] [CrossRef]
- Gamalero, E.-G.; Bernard, R. TI-The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology 2020, 9, 381. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M. Bacterial Endophytes as Elicitors of Induced Systemic Resistance. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–52. [Google Scholar] [CrossRef]
- Pandey, P.K.; Samanta, R.; Yadav, R.N.S. Inside the Plant: Addressing Bacterial Endophytes in Biotic Stress Alleviation. Arch. Microbiol. 2019, 201, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Industrial Microbiology. Science 1981, 214, 987–995. [Google Scholar] [CrossRef]
- Tripathi, V.C.; Satish, S.; Horam, S.; Raj, S.; Lal, A.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural Products from Polar Organisms: Structural Diversity, Bioactivities and Potential Pharmaceutical Applications. Polar Sci. 2018, 18, 147–166. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Paradkar, A.S.; Jensen, S.E.; Mosher, R.H. Comparative genetics and molecular biology of beta-lactam biosynthesis. Drugs Pharm. Sci. 1997, 82, 241–277. [Google Scholar]
- Christina, A.; Christapher, V.; Bhore, S.J. Endophytic Bacteria as a Source of Novel Antibiotics: An Overview. Pharmacogn. Rev. 2013, 7, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L. The genus bacillus as a biological control agent and its implications in the agricultural biosecurity. Mex. J. Phytopathol. 2018, 36, 95–130. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Stein, T. Bacillus Subtilis Antibiotics: Structures, Syntheses and Specific Functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.A.; Martin, J.F. Polyene antibiotic. In Biotechnology of Antibiotics, 2nd ed.; Strohl, W.R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 551–575. [Google Scholar]
- Zhao, K.; Penttinen, P.; Guan, T.; Xiao, J.; Chen, Q.; Xu, J.; Lindström, K.; Zhang, L.; Zhang, X.; Strobel, G.A. The Diversity and Anti-Microbial Activity of Endophytic Actinomycetes Isolated from Medicinal Plants in Panxi Plateau, China. Curr. Microbiol. 2011, 62, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.-K.; Tuo, L.; Huang, D.-L.; Osterman, I.A.; Tyurin, A.P.; Liu, S.-W.; Lukyanov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Korshun, V.A.; et al. Diversity, Novelty, and Antimicrobial Activity of Endophytic Actinobacteria from Mangrove Plants in Beilun Estuary National Nature Reserve of Guangxi, China. Front. Microbiol. 2018, 9, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced from Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial Relationships Between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Sharga, B.M.; Lyon, G.D. Bacillus Subtilis BS 107 as an Antagonist of Potato Blackleg and Soft Rot Bacteria. Can. J. Microbiol. 1998, 44, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Emmert, E.A.; Handelsman, J. Biocontrol of Plant Disease: A (Gram-) Positive Perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Beiranvand, M.; Amin, M.; Hashemi-Shahraki, A.; Romani, B.; Yaghoubi, S.; Sadeghi, P. Antimicrobial Activity of Endophytic Bacterial Populations Isolated from Medical Plants of Iran. Iran. J. Microbiol. 2017, 9, 11–18. [Google Scholar]
- Islam, M.N.; Ali, M.S.; Choi, S.-J.; Hyun, J.-W.; Baek, K.-H. Biocontrol of Citrus Canker Disease Caused by Xanthomonas Citri Subsp. Citri Using an Endophytic Bacillus Thuringiensis. Plant Pathol. J. 2019, 35, 486–497. [Google Scholar] [CrossRef]
- Fikri, A.S.I.; Rahman, I.A.; Nor, N.S.M.; Hamzah, A. Isolation and Identification of Local Bacteria Endophyte and Screening of Its Antimicrobial Property against Pathogenic Bacteria and Fungi. AIP Conf. Proc. 2018, 1940, 020072. [Google Scholar] [CrossRef]
- Mamonokane, O.D.; Eunice, U.-J.; Mahloro, H.S.-D. The antibacterial activity of bacterial endophytes isolated from Combretum mole. Afr. J. Biotechnol. 2018, 17, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, Wide-Spectrum Antibiotics Produced by Streptomyces NRRL 30562, Endophytic on Kennedia Nigriscans. Microbiology 2002, 148 Pt 9, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Castillo, U.; Harper, J.K.; Strobel, G.A.; Sears, J.; Alesi, K.; Ford, E.; Lin, J.; Hunter, M.; Maranta, M.; Ge, H.; et al. Kakadumycins, Novel Antibiotics from Streptomyces Sp NRRL 30566, an Endophyte of Grevillea Pteridifolia. FEMS Microbiol. Lett. 2003, 224, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.-H.; Sattler, I.; Lin, W.-H.; Guo, D.-A.; Grabley, S. P-Aminoacetophenonic Acids Produced by a Mangrove Endophyte: Streptomyces Griseus Subsp. J. Nat. Prod. 2005, 68, 1198–1200. [Google Scholar] [CrossRef]
- Ezra, D.; Castillo, U.F.; Strobel, G.A.; Hess, W.M.; Porter, H.; Jensen, J.B.; Condron, M.A.M.; Teplow, D.B.; Sears, J.; Maranta, M.; et al. Coronamycins, Peptide Antibiotics Produced by a Verticillate Streptomyces Sp. (MSU-2110) Endophytic on Monstera Sp. Microbiology 2004, 150 Pt 4, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Ghadin, N.; Zin, N.; Sabaratnam, V.; Badya, N.; Basri, D.F.; Lian, H.H.; Sidik, N.M. Isolation and Characterization of a Novel Endophytic Streptomyces SUK 06 with Antimicrobial Activity from Malaysian Plant. Asian J. Plant Sci. 2008, 7, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Sukari, M.A.; Abdul, A.B.; Taha, M.M.E.; Syam, S.; Ahmad, S.; Lee, K.-H. The Methanolic Extract of Boesenbergia Rotunda (L.) Mansf. and Its Major Compound Pinostrobin Induces Anti-Ulcerogenic Property in Vivo: Possible Involvement of Indirect Antioxidant Action. J. Ethnopharmacol. 2011, 137, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Taechowisan, T.; Chanaphat, S.; Ruensamran, W.; Phutdhawong, W.S. Antibacterial Activity of New Flavonoids from Streptomyces Sp. BT01; an Endophyte in Boesenbergia Rotunda (L.) Mansf. J. Appl. Pharm. Sci. 2014, 4, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Jasim, B.; Sreelakshmi, K.S.; Mathew, J.; Radhakrishnan, E.K. Surfactin, Iturin, and Fengycin Biosynthesis by Endophytic Bacillus Sp. from Bacopa Monnieri. Microb. Ecol. 2016, 72, 106–119. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Zheng, Q.-W.; Wei, T.; Zhang, Z.-Q.; Zhao, C.-F.; Zhong, H.; Xu, Q.-Y.; Lin, J.-F.; Guo, L.-Q. Isolation and Characterization of Fengycins Produced by Bacillus Amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential Against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and Antimicrobial Activity of Biosurfactant Extracts Produced by Bacillus Amyloliquefaciens and Pseudomonas Aeruginosa Isolated from a Wastewater Treatment Plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.M.; Miller, R.V.; Garton-Kenny, D.; Redgrave, B.; Sears, J.; Condron, M.M.; Teplow, D.B.; Strobel, G.A. Ecomycins, Unique Antimycotics from Pseudomonas Viridiflava. J. Appl. Microbiol. 1998, 84, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.; Teplow, D.B.; Rinaldi, M.; Strobel, G. Pseudomycins, a Family of Novel Peptides from Pseudomonas Syringae Possessing Broad-Spectrum Antifungal Activity. J. Gen. Microbiol. 1991, 137, 2857–2865. [Google Scholar] [CrossRef] [Green Version]
- El-Deeb, B.; Fayez, K.; Gherbawy, Y. Isolation and Characterization of Endophytic Bacteria from Plectranthus Tenuiflorus Medicinal Plant in Saudi Arabia Desert and Their Antimicrobial Activities. J. Plant Interact. 2013, 8, 56–64. [Google Scholar] [CrossRef]
- Maggini, V.; De Leo, M.; Mengoni, A.; Gallo, E.R.; Miceli, E.; Reidel, R.V.B.; Biffi, S.; Pistelli, L.; Fani, R.; Firenzuoli, F.; et al. Plant-Endophytes Interaction Influences the Secondary Metabolism in Echinacea Purpurea (L.) Moench: An in Vitro Model. Sci. Rep. 2017, 7, 16924. [Google Scholar] [CrossRef] [Green Version]
- Mengoni, A.; Maida, I.; Chiellini, C.; Emiliani, G.; Mocali, S.; Fabiani, A.; Fondi, M.; Firenzuoli, F.; Fani, R. Antibiotic Resistance Differentiates Echinacea Purpurea Endophytic Bacterial Communities with Respect to Plant Organs. Res. Microbiol. 2014, 165, 686–694. [Google Scholar] [CrossRef]
- Castronovo, L.M.; Calonico, C.; Ascrizzi, R.; Del Duca, S.; Delfino, V.; Chioccioli, S.; Vassallo, A.; Strozza, I.; De Leo, M.; Biffi, S.; et al. The Cultivable Bacterial Microbiota Associated to the Medicinal Plant Origanum Vulgare L.: From Antibiotic Resistance to Growth-Inhibitory Properties. Front. Microbiol. 2020, 11, 862. [Google Scholar] [CrossRef]
- Akter, Y.; Barua, R.; Nasir Uddin, M.; Muhammad Sanaullah, A.F.; Marzan, L.W. Bioactive Potentiality of Secondary Metabolites from Endophytic Bacteria against SARS-CoV-2: An in-Silico Approach. PLoS ONE 2022, 17, e0269962. [Google Scholar] [CrossRef]
- Miller, K.; Qing, C.; Sze, D.; Neilan, B. Investigation of the Biosynthetic Potential of Endophytes in Traditional Chinese Anticancer Herbs. PLoS ONE 2012, 7, e35953. [Google Scholar] [CrossRef] [Green Version]
- Komura, S.; Kurahashi, K. Biosynthesis of Polymyxin E. III. Total Synthesis of Polymyxin E by a Cell-Free Enzyme System. Biochem. Biophys. Res. Commun. 1980, 95, 1145–1151. [Google Scholar] [CrossRef]
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the Potential of Endophytes from Medicinal Plants as Sources of Antimycobacterial Compounds. Microbiol. Res. 2014, 169, 483–495. [Google Scholar] [CrossRef]
- Semenzato, G.; Alonso-Vásquez, T.; Del Duca, S.; Vassallo, A.; Riccardi, C.; Zaccaroni, M.; Mucci, N.; Padula, A.; Emiliani, G.; Palumbo Piccionello, A.; et al. Genomic Analysis of Endophytic Bacillus-Related Strains Isolated from the Medicinal Plant Origanum Vulgare L. Revealed the Presence of Metabolic Pathways Involved in the Biosynthesis of Bioactive Compounds. Microorganisms 2022, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Pelo, S.; Mavumengwana, V.; Green, E. Diversity and Antimicrobial Activity of Culturable Fungal Endophytes in Solanum Mauritianum. Int. J. Environ. Res. Public Health 2020, 17, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashitha, A.; Radhakrishnan, E.K.; Mathew, J. Antibacterial Potential and Apoptosis Induction by Pigments from the Endophyte Burkholderia Sp. WYAT7. Curr. Microbiol. 2020, 77, 2475–2485. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Veronese, F.M. PEG Conjugates in Clinical Development or Use as Anticancer Agents: An Overview. Adv. Drug Deliv. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.R.; Molina, G.; Dionísio, A.P.; Maróstica Junior, M.R.; Pastore, G.M. The Use of Endophytes to Obtain Bioactive Compounds and Their Application in Biotransformation Process. Biotechnol. Res. Int. 2011, 2011, 576286. [Google Scholar] [CrossRef]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive Products from Plant-Endophytic Gram-Positive Bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Conti, R.; Chagas, F.O.; Caraballo-Rodriguez, A.M.; Melo, W.G.D.P.; do Nascimento, A.M.; Cavalcanti, B.C.; de Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V.; Krogh, R.; et al. Endophytic Actinobacteria from the Brazilian Medicinal Plant Lychnophora Ericoides Mart. and the Biological Potential of Their Secondary Metabolites. Chem. Biodivers. 2016, 13, 727–736. [Google Scholar] [CrossRef]
- Kim, N.; Shin, J.C.; Kim, W.; Hwang, B.Y.; Kim, B.S.; Hong, Y.-S.; Lee, D. Cytotoxic 6-Alkylsalicylic Acids from the Endophytic Streptomyces Laceyi. J. Antibiot. 2006, 59, 797–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieber, B.; Nüske, J.; Ritzau, M.; Gräfe, U. Alnumycin a New Naphthoquinone Antibiotic Produced by an Endophytic Streptomyces Sp. J. Antibiot. 1998, 51, 381–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assad, B.M.; Savi, D.C.; Biscaia, S.M.; Mayrhofer, B.F.; Iantas, J.; Mews, M.; de Oliveira, J.C.; Trindade, E.S.; Glienke, C. Endophytic Actinobacteria of Hymenachne Amplexicaulis from the Brazilian Pantanal Wetland Produce Compounds with Antibacterial and Antitumor Activities. Microbiol. Res. 2021, 248, 126768. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Filho, J.A. Endophytic Microbes as a Novel Source for Producing Anticancer Compounds as Multidrug Resistance Modulators, Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2008; pp. 343–381. [Google Scholar]
- Vu, H.-N.T.; Nguyen, D.T.; Nguyen, H.Q.; Chu, H.H.; Chu, S.K.; Chau, M.V.; Phi, Q.-T. Antimicrobial and Cytotoxic Properties of Bioactive Metabolites Produced by Streptomyces Cavourensis YBQ59 Isolated from Cinnamomum Cassia Prels in Yen Bai Province of Vietnam. Curr. Microbiol. 2018, 75, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Miura, S.-S.; Fujita, T.; Furumai, T. Pterocidin, a Cytotoxic Compound from the Endophytic Streptomyces Hygroscopicus. J. Antibiot. 2006, 59, 193–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Shen, Y. A New Macrolide Antibiotic with Antitumor Activity Produced by Streptomyces Sp. CS, a Commensal Microbe of Maytenus Hookeri. J. Antibiot. 2003, 56, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebola, T.E.; Uche-Okereafor, N.C.; Tapfuma, K.I.; Mekuto, L.; Green, E.; Mavumengwana, V. Evaluating Antibacterial and Anticancer Activity of Crude Extracts of Bacterial Endophytes from Crinum Macowanii Baker Bulbs. MicrobiologyOpen 2019, 8, e914. [Google Scholar] [CrossRef] [Green Version]
- Kusari, S.; Lamshöft, M.; Kusari, P.; Gottfried, S.; Zühlke, S.; Louven, K.; Hentschel, U.; Kayser, O.; Spiteller, M. Endophytes Are Hidden Producers of Maytansine in Putterlickia Roots. J. Nat. Prod. 2014, 77, 2577–2584. [Google Scholar] [CrossRef]
- Zhao, P.-J.; Fan, L.-M.; Li, G.-H.; Zhu, N.; Shen, Y.-M. Antibacterial and Antitumor Macrolides from Streptomyces Sp. Is9131. Arch. Pharm. Res. 2005, 28, 1228–1232. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yuan, Q.; Shan, L.-T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor Activity of Bacterial Exopolysaccharides from the Endophyte Bacillus Amyloliquefaciens Sp. Isolated from Ophiopogon Japonicus. Oncol. Lett. 2013, 5, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Taechowisan, T.; Chaisaeng, S.; Phutdhawong, W.S. Antibacterial, Antioxidant and Anticancer Activities of Biphenyls from Streptomyces Sp. BO-07: An Endophyte in Boesenbergia Rotunda (L.) Mansf A. Food Agric. Immunol. 2017, 28, 1330–1346. [Google Scholar] [CrossRef] [Green Version]
- Goris, T.; Pérez-Valero, Á.; Martínez, I.; Yi, D.; Fernández-Calleja, L.; San León, D.; Bornscheuer, U.T.; Magadán-Corpas, P.; Lombó, F.; Nogales, J. Repositioning Microbial Biotechnology against COVID-19: The Case of Microbial Production of Flavonoids. Microb. Biotechnol. 2021, 14, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Arumugaswami, V.; Raychaudhuri, S.; Yeh, G.K.; Maloney, E.M.; Wang, J.; Dasgupta, A.; French, S.W. Divergent Antiviral Effects of Bioflavonoids on the Hepatitis C Virus Life Cycle. Virology 2012, 433, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Raihan, T.; Rabbee, M.F.; Roy, P.; Choudhury, S.; Baek, K.-H.; Azad, A.K. Microbial Metabolites: The Emerging Hotspot of Antiviral Compounds as Potential Candidates to Avert Viral Pandemic Alike COVID-19. Front. Mol. Biosci. 2021, 8, 732256. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Münch, J.; Goerls, H.; Maier, A.; Fiebig, H.-H.; Lin, W.-H.; Hertweck, C. Xiamycin, a Pentacyclic Indolosesquiterpene with Selective Anti-HIV Activity from a Bacterial Mangrove Endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar] [CrossRef]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of Influenza A Virus Infection by Ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Liu, Q.; Zang, P.; Li, X.; Ji, Q.; He, Z.; Zhao, Y.; Yang, H.; Zhao, X.; Zhang, L. An Endophytic Bacterium Isolated from Panax Ginseng C.A. Meyer Enhances Growth, Reduces Morbidity, and Stimulates Ginsenoside Biosynthesis. Phytochem. Lett. 2015, 11, 132–138. [Google Scholar] [CrossRef]
- Chu, L.L.; Bae, H. Bacterial Endophytes from Ginseng and Their Biotechnological Application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.-H.; Yin, C.-Y. Biotransformation of Ginsenoside Rb1 to Ginsenoside Rg3 by Endophytic Bacterium Burkholderia Sp. GE 17-7 Isolated from Panax Ginseng. J. Appl. Microbiol. 2017, 122, 1579–1585. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Bilal Khan Niazi, M.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tariq, F.; Mumtaz, R.; Li, B. Bioprospecting a Native Silver-Resistant Bacillus Safensis Strain for Green Synthesis and Subsequent Antibacterial and Anticancer Activities of Silver Nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of Antibacterial Silver Nanoparticles Using the Endophytic Bacterium Bacillus Cereus Isolated from Garcinia Xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.W.-Y.; Chen, R.; Chung, N.P.-Y.; Ho, C.-M.; Lin, C.-L.S.; Che, C.-M. Silver Nanoparticles Fabricated in Hepes Buffer Exhibit Cytoprotective Activities toward HIV-1 Infected Cells. Chem. Commun. 2005, 5059–5061. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.; Che, C.-M. Silver Nanoparticles Inhibit Hepatitis B Virus Replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of Herpes Simplex Virus Type 1 Infection by Silver Nanoparticles Capped with Mercaptoethane Sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Sidhu, R.S.; Ford, E.J.; Long, D.M.; Hess, W.M.; Strobel, G.A. The Induction of Taxol Production in the Endophytic Fungus—Periconia Sp from Torreya Grandifolia. J. Ind. Microbiol. Biotechnol. 1998, 20, 259–264. [Google Scholar] [CrossRef]
- Tiwari, R.; Awasthi, A.; Mall, M.; Shukla, A.K.; Srinivas, K.V.; Syamasundar, K.V.; Kalra, A. Bacterial Endophyte-Mediated Enhancement of in Planta Content of Key Terpenoid Indole Alkaloids and Growth Parameters of Catharanthus Roseus. Ind. Crops Prod. 2013, 43, 306–310. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, K.; Chen, F.; Yuan, J.; Li, X.; Dai, C. Endophytic Pseudomonas Induces Metabolic Flux Changes That Enhance Medicinal Sesquiterpenoid Accumulation in Atractylodes Lancea. Plant Physiol. Biochem. 2018, 130, 473–481. [Google Scholar] [CrossRef]
- Pu, X.; Chen, F.; Yang, Y.; Qu, X.; Zhang, G.; Luo, Y. Isolation and Characterization of Paenibacillus Polymyxa LY214, a Camptothecin-Producing Endophytic Bacterium from Camptotheca Acuminata. J. Ind. Microbiol. Biotechnol. 2015, 42, 1197–1202. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Liang, Z. Endophytic Bacteria from Pinellia Ternata, a New Source of Purine Alkaloids and Bacterial Manure. Pharm. Biol. 2015, 53, 1545–1548. [Google Scholar] [CrossRef]
- Pandey, S.S.; Singh, S.; Babu, C.S.V.; Shanker, K.; Srivastava, N.K.; Kalra, A. Endophytes of Opium Poppy Differentially Modulate Host Plant Productivity and Genes for the Biosynthetic Pathway of Benzylisoquinoline Alkaloids. Planta 2016, 243, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline Alkaloid Metabolism: A Century of Discovery and a Brave New World. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ptak, A.; Morańska, E.; Warchoł, M.; Gurgul, A.; Skrzypek, E.; Dziurka, M.; Laurain-Mattar, D.; Spina, R.; Jaglarz, A.; Simlat, M. Endophytic Bacteria from in Vitro Culture of Leucojum Aestivum L. a New Source of Galanthamine and Elicitor of Alkaloid Biosynthesis. Sci. Rep. 2022, 12, 13700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.-D.; Liu, C.-X.; Yuan, J.-H.; Wang, X.-J.; Xiang, W.-S. A New Prenylated Indole Derivative from Endophytic Actinobacteria Streptomyces Sp. Neau-D50. Nat. Prod. Res. 2014, 28, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dubey, A.K. Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microbiol. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Ding, L.; Maier, A.; Fiebig, H.-H.; Lin, W.-H.; Hertweck, C. A Family of Multicyclic Indolosesquiterpenes from a Bacterial Endophyte. Org. Biomol. Chem. 2011, 9, 4029–4031. [Google Scholar] [CrossRef]
- Gos, F.M.W.R.; Savi, D.C.; Shaaban, K.A.; Thorson, J.S.; Aluizio, R.; Possiede, Y.M.; Rohr, J.; Glienke, C. Antibacterial Activity of Endophytic Actinomycetes Isolated from the Medicinal Plant Vochysia Divergens (Pantanal, Brazil). Front. Microbiol. 2017, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.T.; Lim, W.J.; Kim, E.J.; Yun, H.D.; Lee, Y.H.; Cho, K.M. Endophytic Bacterial Diversity in the Young Radish and Their Antimicrobial Activity against Pathogens. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 493–503. [Google Scholar] [CrossRef]
- Chandrakar, S.; Gupta, A. Actinomycin-Producing Endophytic Streptomyces Parvulus Associated with Root of Aloe Vera and Optimization of Conditions for Antibiotic Production. Probiot. Antimicrob. Proteins 2019, 11, 1055–1069. [Google Scholar] [CrossRef]
- Xu, W.; Ren, H.; Ou, T.; Lei, T.; Wei, J.; Huang, C.; Li, T.; Strobel, G.; Zhou, Z.; Xie, J. Genomic and Functional Characterization of the Endophytic Bacillus Subtilis 7PJ-16 Strain, a Potential Biocontrol Agent of Mulberry Fruit Sclerotiniose. Microb. Ecol. 2019, 77, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Ghiasvand, M.; Makhdoumi, A.; Matin, M.M.; Vaezi, J. Exploring the Bioactive Compounds from Endophytic Bacteria of a Medicinal Plant: Ephedra Foliata (Ephedrales: Ephedraceae). Adv. Tradit. Med. 2020, 20, 61–70. [Google Scholar] [CrossRef]
- Anjum, N.; Chandra, R. Endophytic Bacteria of Catharanthus Roseus as an Alternative Source of Vindoline and Application of Response Surface Methodology to Enhance Its Production. Arch. Biol. Sci. 2019, 71, 27–38. [Google Scholar] [CrossRef]
- Shweta, S.; Bindu, J.H.; Raghu, J.; Suma, H.K.; Manjunatha, B.L.; Kumara, P.M.; Ravikanth, G.; Nataraja, K.N.; Ganeshaiah, K.N.; Uma Shaanker, R. Isolation of Endophytic Bacteria Producing the Anti-Cancer Alkaloid Camptothecine from Miquelia Dentata Bedd. (Icacinaceae). Phytomedicine 2013, 20, 913–917. [Google Scholar] [CrossRef]
- Xu, D.-B.; Ye, W.-W.; Han, Y.; Deng, Z.-X.; Hong, K. Natural Products from Mangrove Actinomycetes. Mar. Drugs 2014, 12, 2590–2613. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, X.; Yang, Y.; Zhao, L.; Xu, L.; Ding, Z. A New Anthracycline from Endophytic Streptomyces Sp. YIM66403. J. Antibiot. 2015, 68, 216–219. [Google Scholar] [CrossRef]
- Igarashi, Y.; Trujillo, M.E.; Martínez-Molina, E.; Yanase, S.; Miyanaga, S.; Obata, T.; Sakurai, H.; Saiki, I.; Fujita, T.; Furumai, T. Antitumor Anthraquinones from an Endophytic Actinomycete Micromonospora Lupini Sp. Nov. Bioorg. Med. Chem. Lett. 2007, 17, 3702–3705. [Google Scholar] [CrossRef]
| Strain Name | Biological Activity | Plant Host | Chemical Class | References |
|---|---|---|---|---|
| Pseudomonas viridiflava EB273 | Antifungal | Lactuca sativa (lettuce) | Ecomycin | [92] |
| Pseudomonas syringae | Antifungal | Nicotiana benthamiana (tobacco) | Pseudomycin | [93] |
| Streptomyces sp. | Antifungal | Glycine max (Soybean) | 3-acetonylidene-7-prenylindolin-2-one (Alkaloid) | [145] |
| Streptomyces sp. | Antifungal Antitumor | Allium tuberosum (Chinese chives) | 6-Prenylindole (Alkaloid) | [146] |
| Streptomyces sp. strain NRRL 30562 | Antibacterial | Kennedia nigricans (Black kennedia) | Munumbicin | [82] |
| Streptomyces sp. NRRL 30566 | Antibacterial | Grevillea pteridifolia (Darwin silky oak) | Kakadumycin | [83] |
| Streptomyces sp. HK 10595 | Antibacterial | Kandelia candel (mangrove) | Xiamycin B | [147] |
| Aeromicrobium pontii | Antibacterial | Vochysia divergens (Tropical evergreen tree) | 1-acetyl-b-carboline (Alkaloid) | [148] |
| Actinomycetes | Antibacterial | Chinese mangrove plants | Erythromycin and levofloxacin-like antibiotics | [73] |
| Bacillus sp. | Antibacterial | Combretum mole (medicinal plant) | Flavonoids | [81] |
| Streptomyces sp. MSU-2110 | Antibacterial | Monstera sp. (tropical plant) | Coronamycins | [85] |
| Streptomyces sp. | Antibacterial | Alnus glutinosa alder tree) | Alnumycin | [111] |
| Enterobacter sp. YRL01 B.subtilis sp. YRL02 | Antibacterial | Raphanus sativus L. (Raddish) | Antibiotics | [149] |
| Actinomyces | Antibacterial Antifungal | Chinese medicinal plants | NRPS and PKS | [72] |
| Streptomyces parvulus Av-R5 | Antibacterial Antifungal | Aloe barbadensis miller (Aloe vera) | Actinomycins | [150] |
| Bacillus sp. 7PJ-16 | Antimicrobial | Morus alba (Mulberry) | Bacteriocins (Subtilin, subtilosin A) | [151] |
| Streptomyces sp. Is9131 | Anti-tuberculosis | Maytenus hookeri (medicinal plant) | Maytansine (an ansamycine antibiotic) | [119] |
| Kytococcus schroeter | Anti-cancer | Ephedra foliate (Medicinal shrub) | Camptothecin (Alkaloid) | [152] |
| Microbacterium sp. Burkholderia sp. | Anti-cancer (Leukemia) | Coptis teeta (medicinal herb) | Vindoline (Alkaloid) | [153] |
| Bacillus cereus | Anti-cancer | Miquelia dentata Bedd. (Wet forest plant) | Camptothecine | [154] |
| Actinomyces sp. | Anti-cancer | Bruguiera gymnorrhiza (mangrove) | Indolocarbazoles (Alkaloid) | [155] |
| Streptomyces sp. YIM66403 | Anti-cancer | Isodon eriocalyx (medicinal plant) | Anthracyclin | [156] |
| Bacillus amyloliquefaciens | Anti-cancer (gastric) | Ophiopogon japonicus (medicinal plant) | Exopolysaccharide | [120] |
| Micromonospora lupini | Anti-cancer(colon) | Lupinus angustifolius (Lupin) | Anthroquinones | [157] |
| Streptomyces sp. | Anti-cancer(leukemia) | Alnus glutinosa (alder tree) | Alnumycin | [111] |
| Streptomyces sp. | Anti-cancer (lung) | Maytenus hookeri (medicinal plant) | Maytansine | [116] |
| Streptomyces sp. strain Is9131 | Anti-cancer (gastric, liver, leukemia, lung) | Maytenus hookeri (medicinal plant) | Maytansine | [119] |
| Streptomyces sp. BO-07 | Anti-cancer (HeLa, HepG2, Huh7 cancer cell lines) | Boesenbergia rotunda (medicinal herb) | Biphenyls | [121] |
| Streptomyces cavourensis YBQ59 | Anti-cancer (lung) | Cinnamomum cassia (medicinal plant) | Bafilomycin D | [114] |
| Streptomyces hygroscopicus | Anti-cancer (NCI-H522, OVCAR-3, SF539, LoX-IMVI cell lines) | Herbaceus plants | Pterocidin | [115] |
| Streptomyces laceyi MS53 | Anti-cancer(breast) | Ricinus communis (Castor plant) | Salaceyins A, B | [110] |
| Burkholderia sp. | Anti-cancer | Panax ginseng (Asian ginseng) | Ginsenoside Rg3 (Saponin) | [146] |
| Streptomyces sp. GT2002/1503 | Anti-HIV | Bruguiera gymnorrhiza (mangrove) | Xiamycin A | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. https://doi.org/10.3390/microorganisms10102008
Narayanan Z, Glick BR. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms. 2022; 10(10):2008. https://doi.org/10.3390/microorganisms10102008
Chicago/Turabian StyleNarayanan, Zareen, and Bernard R. Glick. 2022. "Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes" Microorganisms 10, no. 10: 2008. https://doi.org/10.3390/microorganisms10102008
APA StyleNarayanan, Z., & Glick, B. R. (2022). Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms, 10(10), 2008. https://doi.org/10.3390/microorganisms10102008








