New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extract Preparation
2.3. Phytochemical Analysis
2.4. Microbial Strains
2.5. Assessment of Antimicrobial Activity
2.5.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal/Fungicidal Concentration (MBC/MFC)
2.5.2. Microbial Growth Analysis
2.5.3. Time-Kill Assay
2.6. Evaluation of Membrane Integrity
2.7. Scanning Electron Microscopy (SEM)
2.8. In Vitro Biofilm Formation
2.9. Checkerboard Assay
2.10. Statistical Analysis
3. Results
3.1. Antimicrobial Activity of P. longifolia Extracts
3.2. The Chemical Composition of PlLm and PlLe Extracts
3.2.1. P. longifolia Extracts Inhibited S. aureus Growth in a Dose and Time Dependent Manner
3.2.2. P. longifolia Leaf Extracts Have a Potent Bactericidal Activity against S. aureus Cells
3.3. Mode of Action
3.3.1. PlLm and PlLe Extracts Impair the Integrity of the Cellular Membrane
3.3.2. Irreversible Cell Morphological Damage Is Induced by PlLm Extract
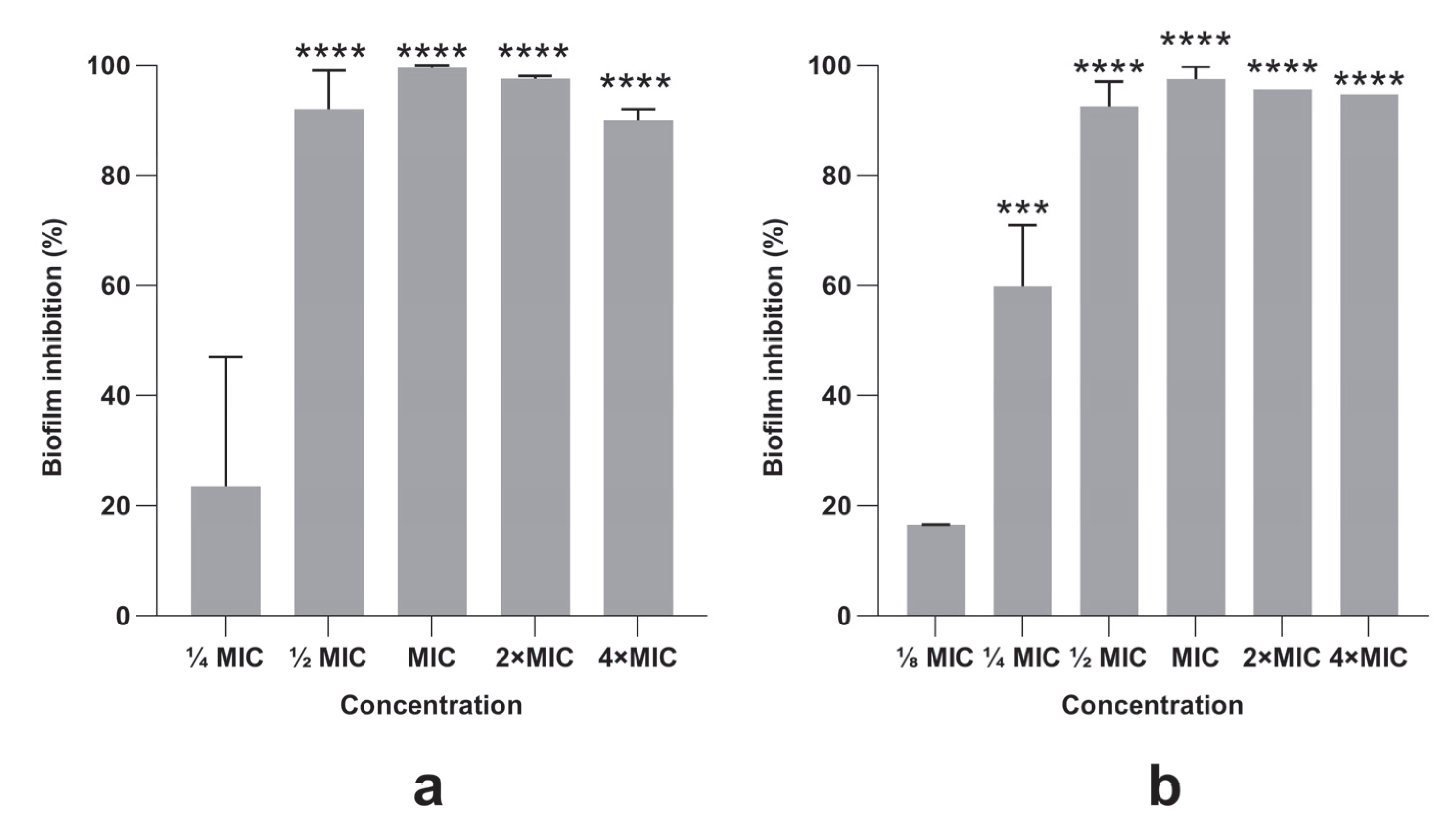
3.4. Bacterial Biofilm Formation Was Inhibited by PlLm and PlLe Extracts
3.5. Effect of P. longifolia Extracts in Combination with Penicillin against a MRSA Strain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention Control, World Health Organization. Antimicrobial Resistance Surveillance in Europe: 2020 Data; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Founou, L.L.; Founou, R.C.; Essack, S. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; p. 232. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tessema, A.; Bitew, A.; Lema, T. Antimicrobial Susceptibility pattern of bacterial isolates from wound infections at all Africa leprosy, tuberculosis and rehabilitation training center, Addis Ababa Ethiopia. EC Microbiol. 2018, 14, 391–399. [Google Scholar]
- Bate, P.N.N.; Orock, A.E.; Nyongbela, K.D.; Babiaka, S.B.; Kukwah, A.; Ngemenya, M.N. In vitro activity against multi-drug resistant bacteria and cytotoxicity of lichens collected from Mount Cameroon. J. King Saud Univ. Sci. 2018, 32, 614–619. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K. Annonaceae: Breaking the Wall of Inflammation. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, X.; Liu, Y.; Luo, Y.; Li, X.; Zhou, Y.; Yang, A.; Yan, Z.; Ye, L.; Chen, S.; et al. Chemical constituents from ethanol extract of Polyalthia rumphii branches and their cytotoxicity evaluation. Rev. Bras. de Farm. 2018, 28, 235–238. [Google Scholar] [CrossRef]
- Chanda, S.; Nair, R. Antimicrobial activity of Polyalthia longifolia (Sonn.) Thw. var. Pendula leaf extracts against 91 clinically important pathogenic microbial strains. Chin. Med. 2010, 1, 31–38. [Google Scholar] [CrossRef]
- Ghosh, G.; Subudhi, B.B.; Ghosh, G.; Mishra, S.K. Antihyperglycemic activity of root bark of Polyalthia longifolia var. pendula and aerial parts of Sida rhombifolia Linn. and its relationship with antioxidant property. Asian J. Chem. 2011, 23, 141–144. [Google Scholar]
- Faizi, S.; Khan, R.A.; Mughal, N.R.; Malik, M.S.; Sajjadi, K.E.; Ahmad, A. Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: Isolation of active principles from the leaves and the berries. Phytother Res. 2008, 22, 907–912. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Shukla, P.K. Antimicrobial evaluation of clerodane diterpenes from Polyalthia longifolia var. pendula. Nat. Prod. Commun. 2009, 4, 1934578X0900400305. [Google Scholar]
- El-Akhal, J.; Humulescu, I.; Ionita, R.; Postu, P.; Ungureanu, E.; Hancianu, M.; Bencheikh, R.; Robu, S.; Cioanca, O.; Hritcu, L. Anxiolytic and antidepressant-like effects of Conyza canadensis aqueous extract in the scopolamine rat model. Plants 2021, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Savu, M.; Motrescu, I.; Birsa, L.M.; Sarbu, L.G.; Stefan, M. The antibacterial synthetic flavonoid BrCl-Flav exhibits important anti-Candida activity by damaging cell membrane integrity. Pharmaceuticals 2021, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.-N.; Gostin, I.; Mihai, C.-T.; Sârbu, L.-G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Pournaras, S.; Vrioni, G.; Neou, E.; Dendrinos, J.; Dimitroulia, E.; Poulou, A.; Tsakris, A. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time–kill assay. Int. J. Antimicrob. Agents 2011, 37, 244–247. [Google Scholar] [CrossRef]
- Caleffi-Ferracioli, K.R.; Maltempe, F.G.; Siqueira, V.L.D.; Cardoso, R.F. Fast detection of drug interaction in Mycobacterium tuberculosis by a checkerboard resazurin method. Tuberculosis 2013, 93, 660–663. [Google Scholar] [CrossRef]
- Lebaron, P.; Catala, P.; Parthuisot, N. Effectiveness of SYTOX green stain for bacterial viability assessment. Appl. Environ. Microbiol. 1998, 64, 2697–2700. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug discovery from plant sources: An integrated approach. AYU (An Int. Q. J. Res. Ayurveda) 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Nagore, P.; Ghotekar, S.; Mane, K.; Ghoti, A.; Bilal, M.; Roy, A. structural properties and antimicrobial activities of Polyalthia longifolia leaf extract-mediated CuO nanoparticles. BioNanoScience 2021, 11, 579–589. [Google Scholar] [CrossRef]
- Mani, T.; Sivaraj, R. Phytochemical analysis and antimicrobial activity of Polyalthia longifolia. Int. J. Pharma. Bio. Sci. 2010, 1, 6288–6299. [Google Scholar]
- Olanrewaju, I.O.; Mordi, R.C.; Echeme, J.O.; Bolade, O.P.; Bashir, M.; Ekwuribe, S.; Ayo-Ajayi, J.I. In-vitro anti-microbial studies and GC/MS analysis of the leaf extract and fractions of Polyalthia longifolia (Engl. & Diels) Verde. J. Physics: Conf. Ser. 2019, 1299, 012087. [Google Scholar] [CrossRef]
- Uzama, D.; Omosimua, R.; Machan, D.B.; Asuquo, T.S. Comparative study on the antimicrobial activities of the ethanolic extracts of lemon grass and Polyalthia longifolia. J. Appl. Pharm. Sci. 2011, 1, 174–176. [Google Scholar]
- Khan, A.K.; Ahmed, A.; Hussain, M.; Khan, I.A.; Ali, S.A.; Farooq, A.D.; Faizi, S. Antibiofilm potential of 16-oxo-cleroda-3, 13(14) E-diene-15 oic acid and its five new γ-amino γ-lactone derivatives against methicillin resistant Staphylococcus aureus and Streptococcus mutans. Eur. J. Med. Chem. 2017, 138, 480–490. [Google Scholar] [CrossRef]
- Shimamura, T.; Zhao, W.-H.; Hu, Z.-Q. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infect. Agents Med. Chem. 2007, 6, 57–62. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, A.; Faizi, S. Antibiofilm potential of isolated diterpenes from Plongifolia and its derivatives against methicillin-resistant Staphylococcus aureus. Int. J. Infect. Dis. 2020, 101, 309. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 1–19. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A. Applications of catechins in the treatment of bacterial infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Escandón, R.A.; del Campo, M.; López-Solis, R.; Obreque-Slier, E.; Toledo, H. Antibacterial effect of kaempferol and (−)-epicatechin on Helicobacter pylori. Eur. Food Res. Technol. 2016, 242, 1495–1502. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- Sitheeque, M.; Panagoda, G.; Yau, J.; Amarakoon, A.; Udagama, U.; Samaranayake, L. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy 2009, 55, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–modes of antimicrobial and antibiofilm activities of a common plant polyphenol. South Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Res. Int. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Jothy, S.L.; Yeng, C.; Sasidharan, S. Chromatographic and spectral fingerprinting of Polyalthia longifolia, a source of phytochemicals. BioRes 2013, 8, 5102–5119. [Google Scholar] [CrossRef]
- Sampath, M. Isolation and identification of gallic acid from Polyalthia longifolia (Sonn.) Thawaites. Int. J. Pharma. Bio. Sci. 2013, 4, 966–972. [Google Scholar]
- Sari, D.P.; Ninomiya, M.; Efdi, M.; Santoni, A.; Ibrahim, S.; Tanaka, K.; Koketsu, M. Clerodane diterpenes isolated from Polyalthia longifolia induce apoptosis in human leukemia HL-60 cells. J. Oleo Sci. 2013, 62, 843–848. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, K. Bioassay-guided isolation and identification of antifungal compounds from Polyalthia longifolia Benth. & Hook. J. Biol. Act. Prod. Nat. 2013, 3, 106–114. [Google Scholar] [CrossRef]
- Gacche, R.; Shaikh, D.R.; Pund, M. In-vitro evaluation of anticancer and antimicrobial activity of selected medicinal plants from Ayurveda. Asian J. Tradit. Med. 2011, 6, 1–7. [Google Scholar]
- Ojewuyi, O.B.; Ajiboye, T.O.; Adebanjo, E.O.; Balogun, A.; Mohammed, A.O. Proximate composition, phytochemical and mineral contents of young and mature Polyalthia longifolia Sonn.leaves. Fountain J. Nat. Appl. Sci. 2014, 3, 10–19. [Google Scholar] [CrossRef]
- Wal, P.; Saxena Pal, R.; Pal, Y.; Rai, A.; Wal, A.; Srivastava, A.; Chandra, S.; Saraswat, N. Physico-chemical and phytochemical evaluation of crude drug powder (leaves) of Polyalthia longifolia. J. Pharmacogn. Phytochem. 2016, 53, 212–213. [Google Scholar]
- Billet, K.; Houillé, B.; De Bernonville, T.D.; Besseau, S.; Oudin, A.; Courdavault, V.; Delanoue, G.; Guérin, L.; Clastre, M.; Giglioli-Guivarc’H, N.; et al. Field-based metabolomics of Vitis vinifera L. stems provides new insights for genotype discrimination and polyphenol metabolism structuring. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Mishra, A.; Kehri, H.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 9. [Google Scholar] [CrossRef]
- Nakahara, K.; Kawabata, S.; Ono, H.; Ogura, K.; Tanaka, T.; Ooshima, T.; Hamada, S. Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans Streptococci. Appl. Environ. Microbiol. 1993, 59, 968–973. [Google Scholar] [CrossRef]
- Ohemeng, K.; Schwender, C.; Fu, K.; Barrett, J. DNA gyrase inhibitory and antibacterial activity of some flavones(1). Bioorganic Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Rodríguez, E.; Seguer, J.; Rocabayera, X.; Manresa, A. Cellular effects of monohydrochloride of l-arginine, Nα-lauroyl ethylester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J. Appl. Microbiol. 2004, 96, 903–912. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin impacts the growth of the gut microbiota and alters the gene expression of Enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Dutta, B.; Mukherjee, I.; Ghosh, S.; Dey, A.; Banerjee, R.; Ray, R.R. Catechin as the most efficient bioactive compound from Azadirachta indica with antibiofilm and anti-quorum sensing activities against dental biofilm: An in vitro and in silico study. Appl. Biochem. Biotechnol. 2021, 193, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2018, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sasidharan, S. Synergistic effect of Polyalthia longifolia leaf and antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) by microscopic technique. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 323–334. [Google Scholar] [CrossRef]
- Gupta, V.K.; Verma, S.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Darokar, M.P. In vivo efficacy and synergistic interaction of 16α-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide, a clerodane diterpene from Polyalthia longifolia against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 9121–9131. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Dziedzic, A.; Wąsik, T.J. Catechin hydrate augments the antibacterial action of selected antibiotics against Staphylococcus aureus clinical strains. Molecules 2016, 21, 244. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. Biomed. Res. Int. 2018, 7413504. [Google Scholar] [CrossRef] [Green Version]
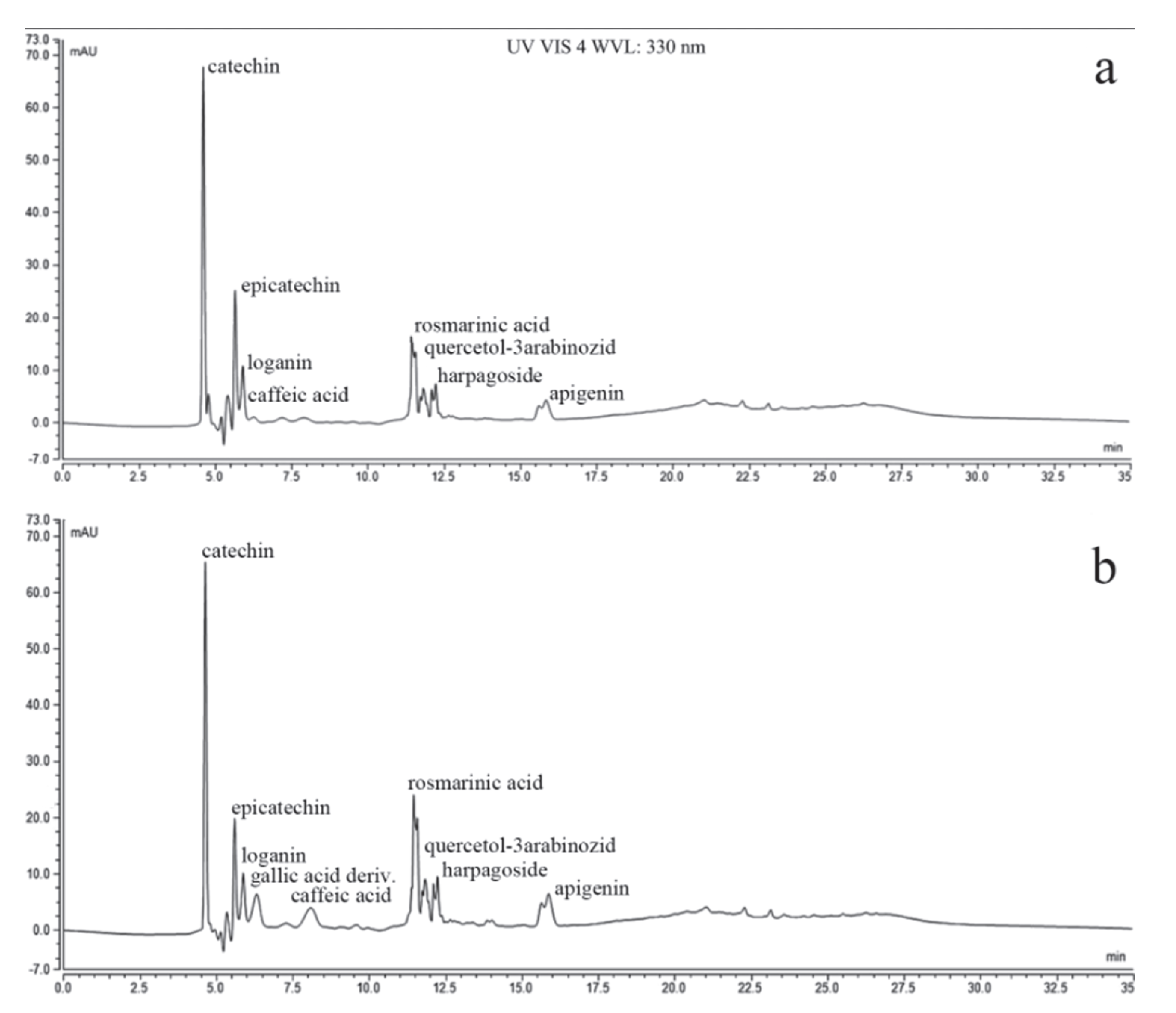
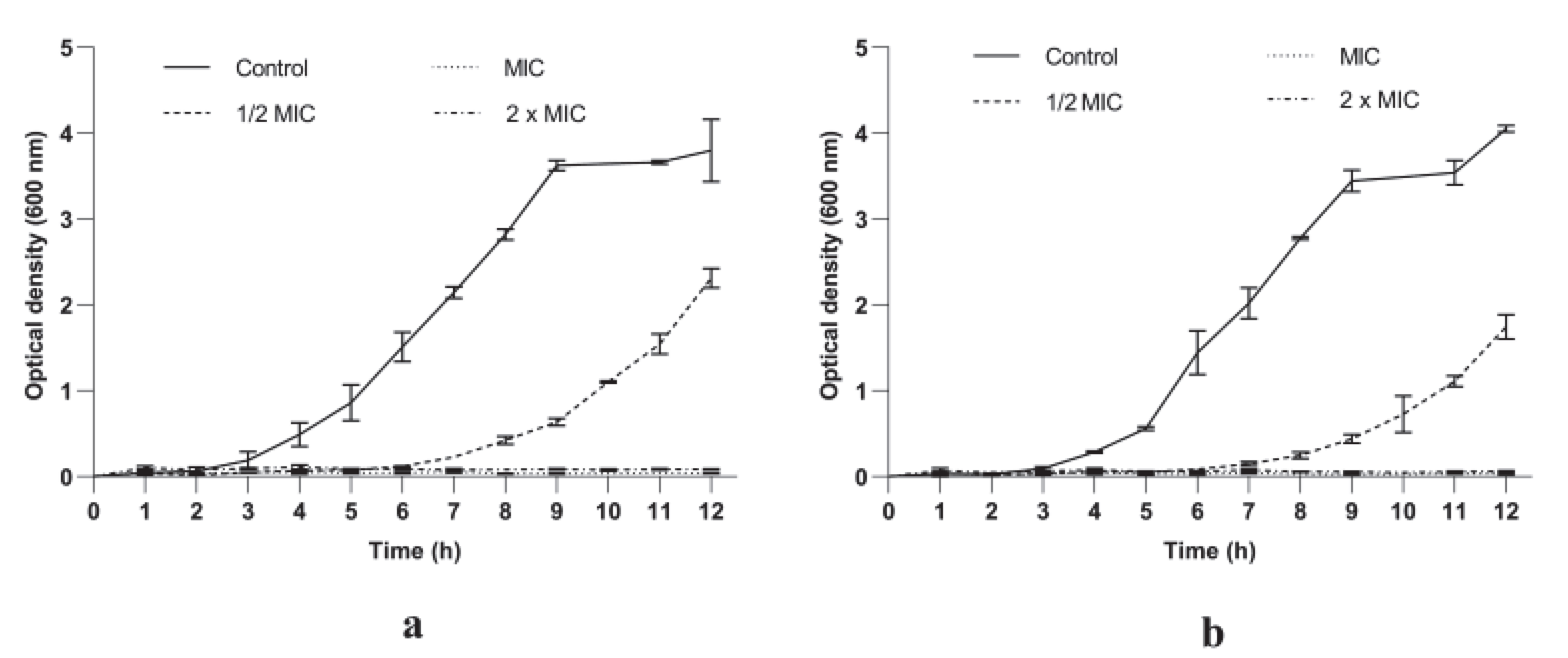
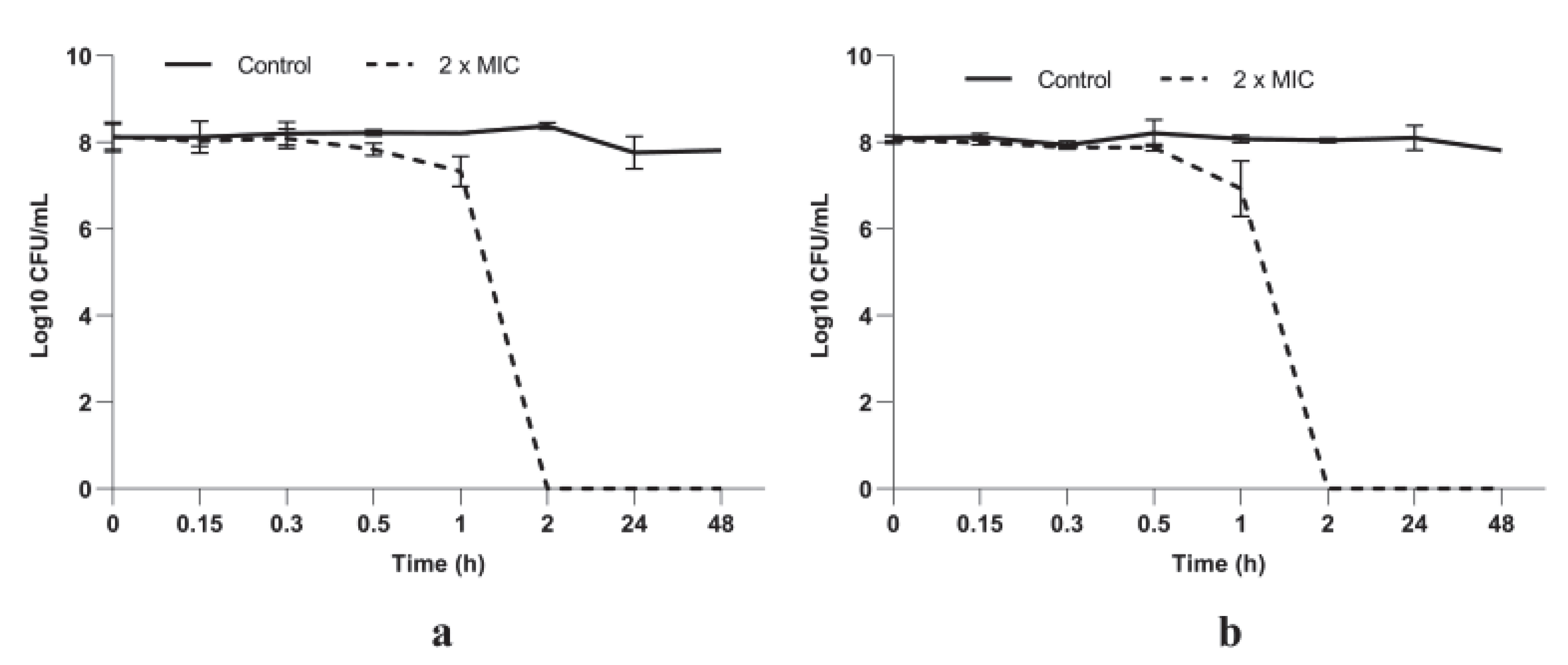
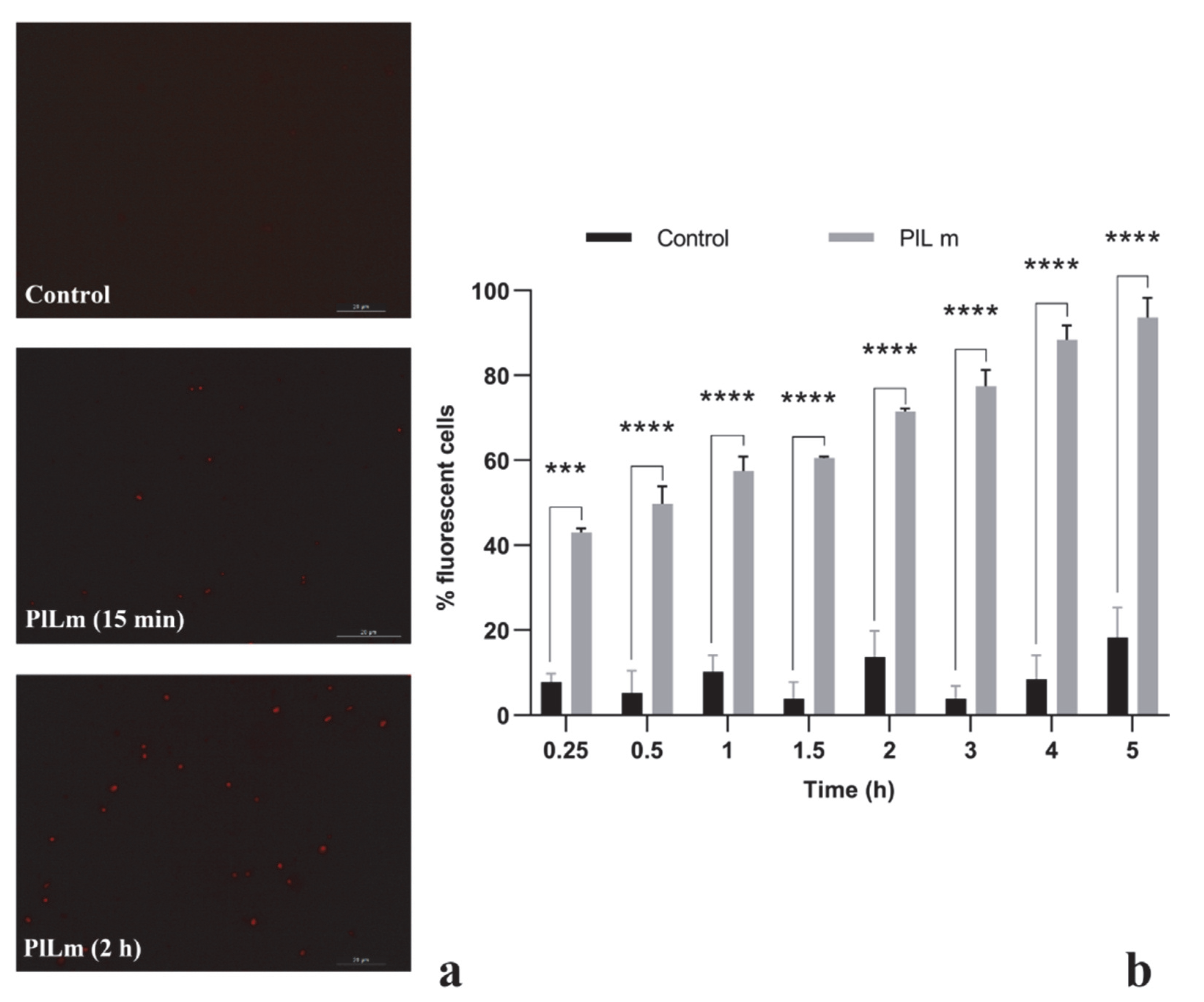

| Extract | S. aureus ATCC 25923 | E. coli ATCC 25922 | C. albicans P37037 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MFC | |
| PlLm | 0.039 | 0.078 | 10 | 20 | 5 | 10 |
| PlLe | 0.078 | 0.156 | 10 | 20 | 10 | 10 |
| PlLw | 5 | 10 | 5 | 10 | 5 | 5 |
| PlSm | 0.310 | 20 | 10 | 20 | 5 | 10 |
| PlSe | 0.152 | 20 | 10 | 20 | 5 | 10 |
| PlSw | 5 | 20 | 5 | 20 | 5 | 5 |
| Ampicillin | 0.00781 | - | 0.00781 | - | - | - |
| Kanamycin | 0.00195 | - | 0.00195 | - | - | - |
| Nystatin | - | - | - | - | 0.03 | - |
| Compound | Extracts | |
|---|---|---|
| PlLm | PlLe | |
| Flavonoids (mg/mL *) | ||
| 1. Catechin | 42.8096 ± 0.0213 | 41.8657 ± 0.0511 |
| 2. Epicatechin | 22.7416 ± 0.0110 | 19.5180 ± 0.0155 |
| 3. Gallic acid derivatives | 0.0093 ± 0.0012 | 9.8197 ± 0.0153 |
| 6. Quercetin-3-arabinoside | 11.2497 ± 0.0312 | 11.2647 ± 0.0222 |
| 7. Apigenin | 22.0347 ± 0.0322 | 13.2238 ± 0.0113 |
| Polyphenolcarboxylic acids (mg/mL) | ||
| 4. Caffeic acid | 5.3001 ± 0.0201 | 0.0028 ± 0.0010 |
| 5. Rosmarinic acid | 25.1654 ± 0.0412 | 19.333 ± 0.0313 |
| Iridoids (mg/mL) | ||
| 8. Harpagoside | 1.0231 ± 0.0152 | 0.0093 ± 0.0021 |
| 9. Loganin | 0.0254 ± 0.0031 | 0.0145 ± 0.0013 |
| MIC (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| Strain | Alone | In Combination | ||||
| PlLm Extract | Penicillin | PlLm Extract | Penicillin | FICI | Interaction | |
| MRSA | 0.0003 | 2 | 2 | Indifference | ||
| 0.0003 | 1 | 1 | Indifference | |||
| 0.001 | 0.5 | 0.5 | Additivity | |||
| 0.009 | 0.25 | 0.27 | Synergy | |||
| 0.019 | 0.125 | 0.18 | Synergy | |||
| 0.312 | 1 | 0.039 | 0.062 | 0.18 | Synergy | |
| 0.078 | 0.031 | 0.28 | Synergy | |||
| 0.156 | 0.015 | 0.51 | Additivity | |||
| PlLe Extract | Penicillin | PlLe Extract | Penicillin | FICI | Interaction | |
| 0.001 | 2 | 2 | Indifference | |||
| 0.002 | 1 | 1 | Indifference | |||
| 0.002 | 0.5 | 0.5 | Additivity | |||
| 0.624 | 1 | 0.019 | 0.25 | 0.28 | Synergy | |
| 0.039 | 0.125 | 0.18 | Synergy | |||
| 0.078 | 0.06 | 0.18 | Synergy | |||
| 0.015 | 0.03 | 0.28 | Synergy | |||
| 0.312 | 0.01 | 0.51 | Additivity | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savu, M.; Simo, M.K.; Fopokam, G.X.; Olaru, S.M.; Cioanca, O.; Boyom, F.F.; Stefan, M. New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus. Microorganisms 2022, 10, 1943. https://doi.org/10.3390/microorganisms10101943
Savu M, Simo MK, Fopokam GX, Olaru SM, Cioanca O, Boyom FF, Stefan M. New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus. Microorganisms. 2022; 10(10):1943. https://doi.org/10.3390/microorganisms10101943
Chicago/Turabian StyleSavu, Mihaela, Marguerite Kamdem Simo, Gabriel Xavier Fopokam, Stefan Mihaita Olaru, Oana Cioanca, Fabrice Fekam Boyom, and Marius Stefan. 2022. "New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus" Microorganisms 10, no. 10: 1943. https://doi.org/10.3390/microorganisms10101943
APA StyleSavu, M., Simo, M. K., Fopokam, G. X., Olaru, S. M., Cioanca, O., Boyom, F. F., & Stefan, M. (2022). New Insights into the Antimicrobial Potential of Polyalthia longifolia—Antibiofilm Activity and Synergistic Effect in Combination with Penicillin against Staphylococcus aureus. Microorganisms, 10(10), 1943. https://doi.org/10.3390/microorganisms10101943







