Biosynthesis of Polyhydroxyalkanoate Terpolymer from Methanol via the Reverse β-Oxidation Pathway in the Presence of Lanthanide
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Condition
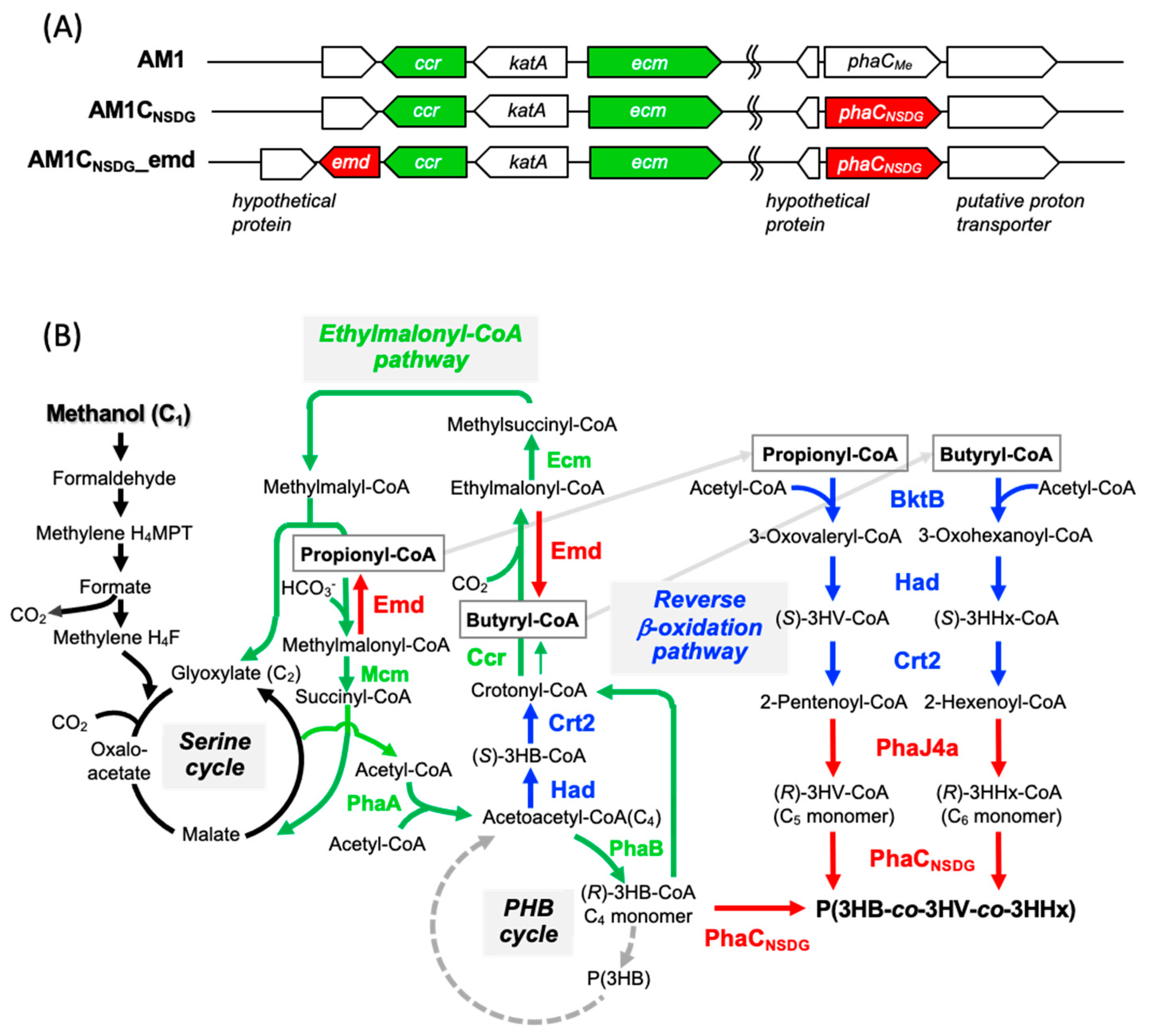
2.2. Construction of Host Strains
2.3. Construction of Expression Plasmids
2.4. PHA Analyses and Measurement of Methanol Concentration
2.5. Methanol Dehydrogenase Assay
3. Results
3.1. Introduction of Ethylmalonyl-CoA Decarboxylase into M. extorquens
3.2. Enhancement of the Reverse β-Oxidation (RBO) Pathway
3.3. PHA Production by the Engineered Strains of M. extorquens
3.4. Both Methylotrophic Growth and PHA Production Were Restored by Addition of La3+
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borrelle, S.; Rochman, C.M.; Liboiron, M.; Bond, A.; Lusher, A.; Bradshaw, H.; Provencher, J.F. Opinion: Why we need an international agreement on marine plastic pollution. Proc. Natl. Acad. Sci. USA 2017, 114, 9994–9997. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Cotton, C.A.; Claassens, N.J.; Vaquerizo, S.B.; Bar-Even, A. Renewable methanol and formate as microbial feedstocks. Curr. Opin. Biotechnol. 2019, 62, 168–180. [Google Scholar] [CrossRef]
- Crowther, G.J.; Kosály, G.; Lidstrom, M.E. Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1. J. Bacteriol. 2008, 190, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, A.; Sonntag, F.; Buchhaupt, M.; Schrader, J.; Vorholt, J.A. Methylobacterium extorquens: Methylotrophy and biotechnological applications. Appl. Microbiol. Biotechnol. 2014, 99, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Orita, I.; Nishikawa, K.; Nakamura, S.; Fukui, T. Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions. Appl. Microbiol. Biotechnol. 2014, 98, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Insomphun, C.; Xie, H.; Mifune, J.; Kawashima, Y.; Orita, I.; Nakamura, S.; Fukui, T. Improved artificial pathway for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with high C6-monomer composition from fructose in Ralstonia eutropha. Metab. Eng. 2015, 27, 38–45. [Google Scholar] [CrossRef]
- Zhang, M.; Kurita, S.; Orita, I.; Nakamura, S.; Fukui, T. Modification of acetoacetyl-CoA reduction step in Ralstonia eutropha for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from structurally unrelated compounds. Microb. Cell Fact. 2019, 18, 147. [Google Scholar] [CrossRef]
- Yang, S.; Sadilek, M.; Synovec, R.E.; Lidstrom, M.E. Liquid chromatography–tandem quadrupole mass spectrometry and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry measurement of targeted metabolites of Methylobacterium extorquens AM1 grown on two different carbon sources. J. Chromatogr. A 2009, 1216, 3280–3289. [Google Scholar] [CrossRef]
- Hu, B.; Lidstrom, M.E. Metabolic engineering of Methylobacterium extorquens AM1 for 1-butanol production. Biotechnol. Biofuels 2014, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Berg, I.A.; Brecht, V.; Muller, M.; Fuchs, G.; Alber, B.E. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: The ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 10631–10636. [Google Scholar] [CrossRef]
- Erb, T.J.; Brecht, V.; Fuchs, G.; Muller, M.; Alber, B.E. Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc. Natl. Acad. Sci. USA 2009, 106, 8871–8876. [Google Scholar] [CrossRef]
- Okubo, Y.; Skovran, E.; Guo, X.; Sivam, D.; Lidstrom, M.E. Implementation of Microarrays for Methylobacterium extorquens AM1. OMICS A J. Integr. Biol. 2007, 11, 325–340. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Marx, C.J.; Lidstrom, M.E. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 2001, 147, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Mifune, J.; Nakamura, S.; Fukui, T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from vegetable oil. Polym. Degrad. Stab. 2010, 95, 1305–1312. [Google Scholar] [CrossRef]
- Woodall, C.A. Electroporation of E. coli. Methods Mol. Biol. 2003, 235, 55–69. [Google Scholar] [PubMed]
- Kawashima, Y.; Cheng, W.; Mifune, J.; Orita, I.; Nakamura, S.; Fukui, T. Characterization and functional analyses of R-specific enoyl coenzyme A hydratases in polyhydroxyalkanoate-producing Ralstonia eutropha. Appl. Environ. Microbiol. 2011, 78, 493–502. [Google Scholar] [CrossRef]
- Kato, M.; Bao, H.J.; Kang, C.K.; Fukui, T.; Doi, Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium chain length 3-hydroxyalkanaic acids by Pseudomonas sp 61–3 from sugars. Appl. Microbiol. Biotechnol. 1996, 45, 363–370. [Google Scholar] [CrossRef]
- Fukui, T.; Doi, Y. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bacteriol. 1997, 179, 4821–4830. [Google Scholar] [CrossRef]
- Day, D.J.; Anthony, C. Methanol dehydrogenase from Methylobacterium extorquens AM1. Methods Enzymol. 1990, 188, 210–216. [Google Scholar] [CrossRef]
- Tsuge, T.; Watanabe, S.; Shimada, D.; Abe, H.; Doi, Y.; Taguchi, S. Combination of N149S and D171G mutations in Aeromonas caviae polyhydroxyalkanoate synthase and impact on polyhydroxyalkanoate biosynthesis. FEMS Microbiol. Lett. 2007, 277, 217–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linster, C.L.; Noël, G.; Stroobant, V.; Vertommen, D.; Vincent, M.-F.; Bommer, G.; Veiga-Da-Cunha, M.; Van Schaftingen, E. Ethylmalonyl-CoA decarboxylase, a new enzyme involved in metabolite proofreading. J. Biol. Chem. 2011, 286, 42992–43003. [Google Scholar] [CrossRef]
- Šmejkalová, H.; Erb, T.J.; Fuchs, G. Methanol assimilation in Methylobacterium extorquens AM1: Demonstration of all enzymes and their regulation. PLoS ONE 2010, 5, e13001. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Muller, J.E.; Kiefer, P.; Vorholt, J.A.; Schrader, J.; Buchhaupt, M. High-level production of ethylmalonyl-CoA pathway-derived dicarboxylic acids by Methylobacterium extorquens under cobalt-deficient conditions and by polyhydroxybutyrate negative strains. Appl. Microbiol. Biotechnol. 2015, 99, 3407–3419. [Google Scholar] [CrossRef]
- Segawa, M.; Wen, C.; Orita, I.; Nakamura, S.; Fukui, T. Two NADH-dependent (S)-3-hydroxyacyl-CoA dehydrogenases from polyhydroxyalkanoate-producing Ralstonia eutropha. J. Biosci. Bioeng. 2019, 127, 294–300. [Google Scholar] [CrossRef]
- Peyraud, R.; Schneider, K.; Kiefer, P.; Massou, S.; Vorholt, J.A.; Portais, J.C. Genome-scale reconstruction and system level investigation of the metabolic network of Methylobacterium extorquens AM1. BMC Syst. Biol. 2011, 5, 189. [Google Scholar] [CrossRef]
- Nakagawa, T.; Mitsui, R.; Tani, A.; Sasa, K.; Tashiro, S.; Iwama, T.; Hayakawa, T.; Kawai, K. A Catalytic Role of XoxF1 as La3+-dependent methanol dehydrogenase in Methylobacterium extorquens strain AM1. PLoS ONE 2012, 7, e50480. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.; Subuyuj, G.A.; Vijayakumar, S.; Good, N.M.; Martinez-Gomez, N.C.; Skovran, E. Lanthanide-dependent regulation of methanol oxidation systems in Methylobacterium extorquens AM1 and their contribution to methanol growth. J. Bacteriol. 2016, 198, 1250–1259. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Polen, T.; Bott, M.; Marienhagen, J. Reversal of β-oxidative pathways for the microbial production of chemicals and polymer building blocks. Metab. Eng. 2017, 42, 33–42. [Google Scholar] [CrossRef]
- Tsuge, T. Metabolic improvements and use of inexpensive carbon sources in microbial production of polyhydroxyalkanoates. J. Biosci. Bioeng. 2002, 94, 579–584. [Google Scholar] [CrossRef]
- Fukui, T.; Abe, A.H.; Doi, Y. Engineering of Ralstonia eutropha for Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Fructose and Solid-State Properties of the Copolymer. Biomacromolecules 2002, 3, 618–624. [Google Scholar] [CrossRef]
- Bhubalan, K.; Lee, W.H.; Loo, J.C.Y.; Yamamoto, T.; Tsuge, T.; Doi, Y.; Sudesh, K. Controlled biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym. Degrad. Stab. 2008, 93, 17–23. [Google Scholar] [CrossRef]
- Pastawan, V.; Suganuma, S.; Mizuno, K.; Wang, L.; Tani, A.; Mitsui, R.; Nakamura, K.; Shimada, M.; Hayakawa, T.; Fitriyanto, N.A.; et al. Regulation of lanthanide-dependent methanol oxidation pathway in the legume symbiotic nitrogen-fixing Bacterium bradyrhizobium sp. strain Ce-3. J. Biosci. Bioeng. 2020, 130, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Suzuki, Y.; Fujitani, Y.; Mitsui, R.; Nakagawa, T.; Shintani, M.; Tani, A. Lanthanide-dependent regulation of methylotrophy in Methylobacterium aquaticum strain 22A. Msphere 2018, 3, e00462-17. [Google Scholar] [CrossRef]
- Good, N.M.; Moore, R.S.; Suriano, C.J.; Martinez-Gomez, N.C. Contrasting in vitro and in vivo methanol oxidation activities of lanthanide-dependent alcohol dehydrogenases XoxF1 and ExaF from Methylobacterium extorquens AM1. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bourque, D.; Pomerleau, Y.; Groleau, D. High cell density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: Production of high-molecular-mass PHB. Appl. Microbiol. Biotechnol. 1995, 44, 367–376. [Google Scholar] [CrossRef]
- Mokhtari-Hosseini, Z.B.; Vasheghani-Farahani, E.; Shojaosadati, S.A.; Karimzadeh, R.; Heidarzadeh-Vazifekhoran, A. Effect of feed composition on PHB production from methanol by HCDC of Methylobacterium extorquens (DSMZ 1340). J. Chem. Technol. Biotechnol. 2009, 84, 1136–1139. [Google Scholar] [CrossRef]



| Strains or Plasmids | Relevant Characteristics | Source or Reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | deoR, endA1, gyrA96, hsdR17 (rK− mK+), recA1, relA1, supE44, thi-l, ∆(lacZYA-argFV169), φ80lacZ∆M15, F−, λ− | Clontech (Palo Alto, CA) |
| Methylorubrum extorquens | ||
| AM1 | Wild type | |
| AM1CNSDG | AM1 derivative; phaCMe::phaCNSDG | This study |
| AM1CNSDG_emd | AM1CNSDG derivative; emd downstream of ccr | This study |
| Plasmids | ||
| pK18mobsacB | pMB1 ori, RP4 mob, modified sacB, lacZa, Kanr | [15] |
| pK18_ phaCNSDG | pK18mobsacB carrying phaCNSDG | This study |
| pTAKN-2-emdMm | pTAKN-2 cloning vector carrying emdMm | [8] |
| pK18_emd | pK18mobsacB carrying emdMm | This study |
| pCM80Km | pCM80 derivative; Tcr::Kanr | [7,16] |
| pCM80Km_emd | pCM80Km carrying emdMm | This study |
| pCM80Km_emdbktB | pCM80Km carrying emdMm-bktBRe | This study |
| pUC118 | Ampr general cloning vector | Takara Bio (Ohtsu, Japan) |
| pUC-hadcrt2 | pUC118 carrying hadRe-crt2Re | This study |
| pCM80Km-ehcjb | pCM80Km carrying emdMm-hadRe-crt2Re-phaJ4aRe-bktBRe | This study |
| pCM80Km-hcjb | pCM80Km carrying hadRe-crt2Re-phaJ4aRe-bktBRe | This study |
| pCM80PphaA-hcjb | pCM80Km-hcjb derivative; PmxaF::PphaA | This study |
| Strain | EDTA | Dry Cell Weight (g/L) | PHA Content (wt%) | PHA (g/L) | Residual Cell Weight (g/L) | Monomer Composition (mol%) | ||
|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3HHx | ||||||
| AM1CNSDG | + | 0.96 ± 0.11 | 28.0 ± 2.6 | 0.27 ± 0.02 | 0.69 ± 0.10 | 99.3 ± 0.1 | 0.45 ± 0.09 | 0.23 ± 0.02 |
| AM1CNSDG_emd | 0.28 ± 0.06 | 42.5 ± 8.1 | 0.11 ± 0.01 | 0.16 ± 0.06 | 99.3 ± 0.2 | 0.39 ± 0.20 | 0.33 ± 0.01 | |
| AM1CNSDG | − | 1.11 ± 0.07 | 33.1 ± 1.9 | 0.37 ± 0.01 | 0.74 ± 0.07 | 99.6 ± 0.0 | 0.18 ± 0.01 | 0.18 ± 0.01 |
| AM1CNSDG_emd | 1.08 ± 0.03 | 23.1 ± 1.0 | 0.25 ± 0.01 | 0.83 ± 0.02 | 96.6 ± 0.0 | 0.20 ± 0.01 | 0.17 ± 0.02 | |
| Strain | Plasmid | La | Dry cell Weight (g/L) | PHA Content (wt%) | PHA (g/L) | Residual Cell Weight (g/L) | Monomer Composition (mol%) | ||
|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3HHx | |||||||
| AM1CNSDG | pCM80Km | − | 0.85 ± 0.02 | 27.1 ± 1.2 | 0.23 ± 0.01 | 0.62 ± 0.02 | 98.6 ± 0.3 | 1.09 ± 0.22 | 0.31 ± 0.04 |
| pCM80Km-emd | 0.07 ± 0.01 | 27.4 ± 1.7 | 0.02 ± 0.00 | 0.05 ± 0.01 | 98.1 ± 0.3 | 0.97 ± 0.21 | 0.89 ± 0.11 | ||
| AM1CNSDG_emd | pCM80Km | 0.93 ± 0.04 | 35.5 ± 1.8 | 0.33 ± 0.03 | 0.59 ± 0.02 | 98.5 ± 0.4 | 1.15 ± 0.40 | 0.32 ± 0.03 | |
| pCM80Km-hcjb | 0.44 ± 0.16 | 42.3 ± 2.8 | 0.18 ± 0.05 | 0.26 ± 0.10 | 95.8 ± 1.7 | 3.00 ± 1.67 | 1.17 ± 0.15 | ||
| pCM80PphaA-hcjb | 0.20 ± 0.02 | 39.7 ± 2.1 | 0.08 ± 0.01 | 0.12 ± 0.01 | 95.0 ± 1.4 | 4.11 ± 1.12 | 0.94 ± 0.40 | ||
| AM1CNSDG | pCM80Km | + | 0.72 ± 0.09 | 22.2 ± 2.5 | 0.16 ± 0.04 | 0.56 ± 0.05 | 98.3 ±0.5 | 1.43 ± 0.48 | 0.32 ± 0.05 |
| pCM80Km-emd | 0.61 ± 0.06 | 27.2 ± 0.8 | 0.17 ± 0.02 | 0.44 ± 0.04 | 97.9 ± 0.1 | 1.55 ± 0.04 | 0.55 ± 0.04 | ||
| AM1CNSDG_emd | pCM80Km | 0.77 ± 0.02 | 33.6 ± 1.6 | 0.26 ± 0.01 | 0.51 ± 0.02 | 97.9 ± 0.3 | 1.75 ± 0.34 | 0.37 ± 0.02 | |
| pCM80Km-hcjb | 0.83 ± 0.02 | 36.2 ± 2.3 | 0.30 ± 0.02 | 0.53 ± 0.02 | 96.4 ± 0.2 | 3.02 ± 0.17 | 0.55 ± 0.04 | ||
| pCM80PphaA-hcjb | 0.83 ± 0.01 | 41.3 ± 2.6 | 0.34 ± 0.02 | 0.49 ± 0.02 | 93.7 ± 1.0 | 5.42 ± 0.89 | 0.90 ± 0.12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orita, I.; Unno, G.; Kato, R.; Fukui, T. Biosynthesis of Polyhydroxyalkanoate Terpolymer from Methanol via the Reverse β-Oxidation Pathway in the Presence of Lanthanide. Microorganisms 2022, 10, 184. https://doi.org/10.3390/microorganisms10010184
Orita I, Unno G, Kato R, Fukui T. Biosynthesis of Polyhydroxyalkanoate Terpolymer from Methanol via the Reverse β-Oxidation Pathway in the Presence of Lanthanide. Microorganisms. 2022; 10(1):184. https://doi.org/10.3390/microorganisms10010184
Chicago/Turabian StyleOrita, Izumi, Gento Unno, Risa Kato, and Toshiaki Fukui. 2022. "Biosynthesis of Polyhydroxyalkanoate Terpolymer from Methanol via the Reverse β-Oxidation Pathway in the Presence of Lanthanide" Microorganisms 10, no. 1: 184. https://doi.org/10.3390/microorganisms10010184
APA StyleOrita, I., Unno, G., Kato, R., & Fukui, T. (2022). Biosynthesis of Polyhydroxyalkanoate Terpolymer from Methanol via the Reverse β-Oxidation Pathway in the Presence of Lanthanide. Microorganisms, 10(1), 184. https://doi.org/10.3390/microorganisms10010184






