Abstract
Citrus stubborn was initially observed in California in 1915 and was later proven as a graft-transmissible disease in 1942. In the field, diseased citrus trees have compressed and stunted appearances, and yield poor-quality fruits with little market value. The disease is caused by Spiroplasma citri, a phloem-restricted pathogenic mollicute, which belongs to the Spiroplasmataceae family (Mollicutes). S. citri has the largest genome of any Mollicutes investigated, with a genome size of roughly 1780 Kbp. It is a helical, motile mollicute that lacks a cell wall and peptidoglycan. Several quick and sensitive molecular-based and immuno-enzymatic pathogen detection technologies are available. Infected weeds are the primary source of transmission to citrus, with only a minor percentage of transmission from infected citrus to citrus. Several phloem-feeding leafhopper species (Cicadellidae, Hemiptera) support the natural spread of S. citri in a persistent, propagative manner. S. citri-free buds are used in new orchard plantings and bud certification, and indexing initiatives have been launched. Further, a quarantine system for newly introduced types has been implemented to limit citrus stubborn disease (CSD). The present state of knowledge about CSD around the world is summarized in this overview, where recent advances in S. citri detection, characterization, control and eradication were highlighted to prevent or limit disease spread through the adoption of best practices.
1. Introduction
Stubborn is a worldwide citrus disease that reduces the productivity and growth of affected trees [1]. Although the disease does not kill citrus trees in most cases [2,3], it has a significant economic impact [2], particularly when it infects them early in their growth cycle (severe plant stunting is observed) [2,3]. Citrus stubborn disease (CSD) was originally discovered in California Navel sweet orange (Citrus sinensis (L.) Osb.) trees in 1915 [4]. It was not until 1942 when stubborn was identified as a virus-like infection. Furthermore, it was only afterward that the exact nature of this bacterial pathogen was revealed [3]. The term “stubborn” refers to the reactions of buds that do not grow as expected after top-dressing sick trees. The disease was also called “acorn disease” for the numerous acorn-shaped fruits produced by infected trees [1]. The disease is present in the majority of nations where citrus grows in dry or semi-arid environments. It can be found in the warmer parts of Arizona and California, as well as in the bulk of North African, Near-Middle Eastern, and Arabian Peninsula countries. However, CSD is a rare phenomenon in cooler climates, since the vector, as well as the causative agent, are favored by warmer temperatures [1,2].
Given the importance of citrus pathology and the significant research progress in recent years, this review focuses on advancements linked to stubborn by highlighting the current knowledge on the characterization of the causal agent and the symptoms it causes, the development of reliable and speedy diagnosis methods, potential vectors, and management options, among other topics. A brief overview of the current state of CSD in the Mediterranean Basin is included, with a focus on its spread in Morocco’s citrus-growing regions.
2. Taxonomy, Genome Structure, and Organization
Spiroplasma citri, a fastidious wall-less bacterium limited to phloem, is the causal agent of CSD. The shape and motility of this pathogen are helical [5]. S. citri belongs to the domain Bacteria, phylum Firmicutes, class Mollicutes, order Entomoplasmatales, and family Spiroplasmataceae [6]. S. citri is a Gram-positive bacterium belonging to a phylogenetic group of microorganisms with low G-C concentration [7]. The earliest Spiroplasma to be isolated in pure culture, and, as a result, the first to be assigned to the genus as S. citri, was the causal agent of CSD [5,8]. Serological traits such as cross-serological growth inhibition and organism deformation can be used to classify members of the Spiroplasma group [9]. S. citri is classified as a member of Serogroup I, Subgroup I-1 [9,10]. With a genome size of roughly 1.8 Mbp and a single 16S-23S-5S ribosomal RNA (rRNA) operon, S. citri possesses one of the largest genomes among Mollicutes [11]. The total genome size of five strains of S. citri described by Yokomy et al. [12] ranged from 1,611,714 to 1,832,173 bp in plants and 1,968,976 to 2,155,613 bp in leafhoppers [12]. In addition, between 1908 and 2556 coding sequences were predicted in this study. In strains from the United States, one set of rRNA genes and 32 transfer RNA (tRNA) genes were predicted. This result is in line with the R8-A2 T strain, a Moroccan strain that was originally isolated from an S. citri-diseased sweet orange tree [13,14].
In addition to the circular chromosome, S. citri has plasmids and virus genomes that contribute to genetic information [15,16]. S. citri’s genome is characterized by a high adenosine-thymidine concentration and the use of UGA to encode tryptophan rather than serving as a stop codon [15,16]. The number of plasmids from plant hosts varies between one and seven [13,14,17], and eight or nine plasmids from beet leafhoppers [17]. Most plasmids were found in beet leafhopper strains, followed by carrot, Chinese cabbage, horseradish, and citrus strains, respectively. One plasmid with high similarity to plasmid pSci6 was found in all S. citri strains [13]. pSciA and pSci1 to pSci6 are plasmids that are replicated 10 to 14 times in each cell. Plasmids pSci1 to pSci5 encode surface proteins of the S. citri adhesion-related protein (ScARP) family, with pSci6 conferring insect transmissibility [18]. SpV1 was the first virus bacteriophage to infect S. citri and introduce DNA through horizontal transference. The biological significance of viral sequences introduced into cells is currently unknown. In contrast, the physical map of the S. citri genome indicates that this bacteriophage might be present in up to 17 copies within the genome, representing up to 8% of the total genome content [19,20]. The S. citri chromosome can be entirely or partially integrated by this single-stranded circular DNA virus [19]. SpVl-like sequences are involved in large-scale genomic rearrangements such as inversions, transpositions, and deletions of vast DNA regions, as well as chromosome size changes [21]. The presence of prophage sequences in the genomes of S. citri could help it broaden its host range [13].
DNA acquisition and loss, DNA replication and repair, homologous recombination, and transposition are all factors in the genetic diversity of S. citri [16]. Due to chromosomal and extrachromosomal inversions and deletions, graft transfer or many passages in medium cultures can lead to genome changes [22,23]. For example, due to chromosomal inversion and genomic deletions in the BR3-3X strain of S. citri, continual graft transmission from periwinkle (Catharanthus roseus (L.) G. Don) to periwinkle resulted in a lack of transmissibility by the natural vector leafhopper Circulifer tenellus Baker (synonym: Neoaliturus tenellus Baker). The high passage in the artificial medium also affects the transmissibility of S. citri [22,24]. Crucially, transcriptional gene regulation in S. citri likely plays a key part in its ability to adapt to its hosts and could be a useful tool for altering the spiroplasma surface in response to changing environmental conditions [25].
The genome of S. citri GII-3 (1820 Kbp) [18], a strain obtained from its Moroccan leafhopper vector Circulifer haematoceps Mulsant and Rey (synonym: Neoaliturus haematoceps Baker) [26], encodes 645 membrane proteins, including 68 putative lipoproteins [27], and 577 transmembrane proteins [28]. Many mycoplasma species’ interactions with their hosts have been discovered to be dependent on membrane proteins, particularly surface proteins [29,30]. Spiralin is an amphiphilic polypeptide with an apparent molecular size of 26 to 28 KDa that is the most abundant protein in the S. citri membrane [31,32]. Spiralin is not required for disease or motility, but it is requested for successful spiroplasma transmission via the insect vector [33,34]. The central coding region of the 9.6 Kbp sequence of S. citri BR3-3X—of which one copy was suppressed in the insect’s non-transmissible lineage, BR3-G—contained several genes, including the putative membrane protein P58 coding gene [35]. This gene contained its proper promoter and terminator signal sequences for transcription and the Shine–Dalgarno sequence for translation. Spiroplasma-insect vector interactions are mediated by the P58 protein. However, the lack of a copy of the P58 gene in the insect-non-transmissible mutant line, BR3-G, might not explain the resulting loss of its transmittance to insects. The non-deleted copy of this gene in BR3-G appears to be functional, as the P58 protein was detected in this line [15]. Extrachromosomal DNA with a high molecular mass carries the P32 gene, which corresponds to a bigger plasmid of 35.5 Kbp. The P32 gene may have a role in the transmission and might be used as a marker for the transmissibility of S. citri by leafhoppers [36]. In spiroplasma, there was a link between the loss of high-molecular-mass plasmids and the non-transmissible phenotype [18]. P89 (Sarp1), a potential adhesion-related protein from S. citri, is found on a plasmid and in the pathogen genome [37,38,39] and is involved in S. citri’s adhesion to vector cells, specifically C. tenellus cells [38]. The surface lipoprotein Sc76, homolog to a solute-binding protein of an ABC transporter, was also identified to be relevant, as disrupting the gene drastically reduced S. citri’s ability to be transmitted by C. haematoceps [40]. There are 466 amino acids in the Sc76 gene product (51.8 KDa). This gene is involved in the transport of glucose [41].
Molecular phylogenetic inference of 39 spiroplasmas was performed utilizing the NCBI database’s 16S rRNA genes. The S. citri strains are closely related, but not identical, according to this gene sequence analysis. S. citri strains formed a monophyletic group with plant pathogenic Spiroplasma kunkelii, Spiroplasma phoeniceum, and a honeybee pathogen, Spiroplasma melliferum, according to the 16S rRNA gene phylogeny [13]. Citrus strains C189 from southern California and R8-A2 from Morocco were clustered together in phylogenetic analyses using core orthologous sequences among S. citri strains. CC-2, a Chinese cabbage isolate, and C5, a carrot isolate, belong to the same group. The strains LB 319 (citrus), BLH-13 (beet leafhopper), BLH-MB (beet leafhopper), and BR12 (horseradish) formed a distinct clade [13].
3. Symptoms and Economical Impact
CSD symptoms are similar to those caused by other biotic and abiotic stresses, thereby making it difficult to distinguish between a diseased and a healthy S. citri tree [42]. In most cases, stubborn-infected trees do not die, but instead, establish a state of equilibrium. The growth of the trees slows and the tops of the trees flatten. Flowering can occur at any time of year, but the fruits are of poor quality, and their number declines over time [43]. The fruits are non-homogeneous in color, have a gland shape, and a green stylar end (Figure 1) [44,45,46]. Numerous papers have described the symptoms reported to be associated with CSD [2,3,14,43,45,47,48,49,50,51,52,53,54]. Multiple axillary buds, a large number of shoots, and erect, bunchy growth with short internodes are all signs of aberrant growth. On severely damaged trees, twigs become stunted and die, and they become more susceptible to both cold and heat. While lower branches of some diseased trees show symptoms, many trees can still provide typical-looking leaves and fruits on their shaded lower limbs. In some cases, trees show symptoms of CSD only on one or a few branches and may remain in this state for years. Infected trees have smaller leaves than healthy trees. The leaves might have a healthy or diseased appearance (mottled, cup-shaped, distorted in different ways, and pinched in near the tip). It is worth noting that diseased trees’ mottled leaves look a lot like leaves with zinc, iron, or manganese deficiencies (Figure 1).
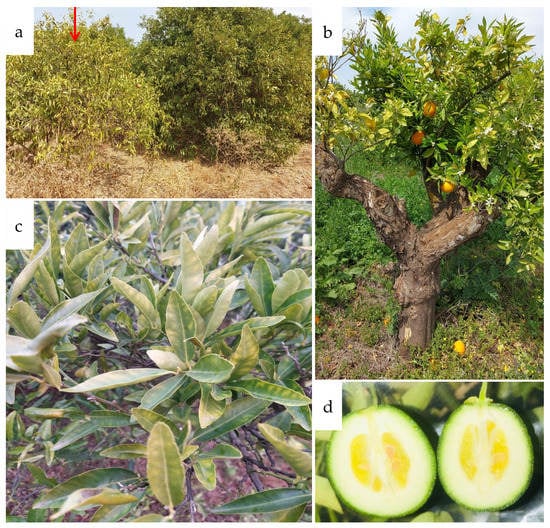
Figure 1.
Field symptoms of citrus stubborn disease observed in Moroccan citrus orchards in the Tadla region (situated in the center of Morocco) in growing season 2021. (a) Compressed and stunted Navel sweet orange tree (red arrow); (b) clementine tree with different phenological stages; (c) Navel sweet orange leaves showing nutritional deficiency-like symptoms; (d) Navel sweet orange fruits with a gland shape.
In severely damaged branches, premature leaf drop can occur. Out of season, diseased trees may have several phases of limited blooming, resulting in the appearance of fruits at various stages of maturity. The produced fruits may be of a smaller size with an asymmetric shape (acorn shape), display greening at the stylar-end, and have a high level of seed abortion, notably in Valencia oranges. Two types of symptoms have been identified, depending on the severity of the condition: (i) “severe”, when the entire tree canopy is damaged, i.e., all branches have mottling and short internodes, and many show off-season blooming; and (ii) “mild”, when the tree is practically asymptomatic or the symptoms, such as short internodes and leaf mottling, are limited to a few branches [42,52].
All these symptoms are likely related to the fact that S. citri needs energy supplies (such as sterols and carbohydrates) from its host plant to grow [55,56]. S. citri competes with its host for these energy sources while living in the plant, leading to the depletion of some hormones and sugars and the accumulation of others [52]. Furthermore, it has been demonstrated that S. citri mutants that are unable to utilize fructose have been shown to exhibit only modest and delayed symptoms. This is because fructose uptake by sieve-tube-restricted wild-type spiroplasmas is believed to deplete companion cells of fructose, thus suppressing sucrose loading in sieve tubes [41]. As a result of this imbalance, the normal citrus plant metabolism is disrupted, resulting in CSD symptoms (stunting, leaf mottling, reduction in fruit size and number, and the occurrence of off-season blooming) [52].
Numerous elements, particularly environmental conditions, may alter the symptom expression of CSD [57]. It is critical to note that elevated temperatures enhance the symptoms of CSD [2,58,59]. In other words, warm temperatures (27 °C at night and 35 °C during the day) cause the mottled-leaf symptom of CSD on sweet orange and grapefruit to appear within 2 months, whereas cool temperatures (23 °C at night and 27 °C during the day) tend to delay the emergence of the same symptom (up to 5 months) [58]. Bové et al. [59] observed similar results on Madam Vinous seedlings. Warm temperatures (27 °C for 8 h nights and 32 °C for 16 h days) have been found to promote the onset of severe CSD symptoms on Madam Vinous seedlings within 5 weeks, whereas cool temperatures (22 °C for 8 h nights and 24 °C for 16 h days) tend to induce only mild symptoms after a long period (26 weeks). It is worth noting that the classic symptoms of CSD, such as tiny, cupped leaves with pale-green tips and mottling, were only seen in warm weather [59]. Calavan and Bové [60] suggested that symptom severity was linked to bacterial titer and/or strain virulence. Further, Mello et al. [61] found that CSD severity is related to S. citri titer but not to bacterial genotype. Indeed, the titer of S. citri in fruits harvested from severely symptomatic trees is about 6000 times higher than in fruits harvested from mildly symptomatic trees. It is worth noting that the genotypes found in this study were found in trees that were both severely and mildly symptomatic. This confirms that genetic differences in S. citri populations have no impact on disease severity [61]. CSD may be influenced by the rootstock chosen. Indeed, a study conducted in Sicily to assess the vulnerability of four rootstocks to CSD (Cleopatra mandarin (Citrus reshni Tanaka), Rangpur lime, sour orange, and Volkamer lemon (Citrus volkameriana V.Ten. and Pasq.)) revealed that C. volkameriana is susceptible to CSD. The inoculated seedlings of the three other rootstocks showed no significant variations in vegetative growth or other signs on leaves and stem after one year. However, the C. volkameriana seedlings showed a temporary decline in growth five months after inoculation [57]. CSD severity may also change depending on the citrus cultivar. This was demonstrated in a three-year study conducted in California to monitor CSD progress in 12 orchards. In two orchards of grapefruit, the severity of CSD increased drastically from 0 (healthy) to 3 (26–50% of a tree showing symptoms), although the Valencia orange showed the smallest increase in disease severity. The CSD severity reaction in Navel orange was middling. It is worth noting that in the second year of testing, Navel and Valencia sweet orange trees showed a pronounced stubborn symptom remission for about four months [47].
Several field trials on various citrus species, cultivars, and rootstocks have been performed in various agroecosystems to assess the impact of CSD on vegetative growth and yield (Table 1). This is the case, for example, with 12-year-old Navel sweet orange trees that were naturally infected with S. citri and whose fruit yield was investigated. The yield was higher in trees with minor CSD symptoms, according to the findings. On the other hand, trees with mild symptoms yielded 20 Kg less, on average, than healthy trees [62]. The fruit yield of CSD-infected Navel and Valencia sweet orange trees was likewise significantly reduced [63]. Diseased trees of both cultivars had a considerable loss in mean fruit weight when compared to healthy trees, with reductions of about 19 and 34% for Navel and Valencia, respectively [63]. Recent research on Navel oranges has revealed the impact of CSD on fruit production [52]. The disease’s impact on Navel sweet orange production was underlined by the findings, which revealed that diseased trees, particularly those with severe symptoms, show a considerable reduction in fruit number. In other words, in 2006 and 2007, the productivity of S. citri-positive trees was 25% and 32% lower than that of S. citri-free plants, respectively. It is worth noting that the disease had a greater influence on Navel yield on severely symptomatic trees (52 and 45% lower in 2006 and 2007, respectively) than on mildly symptomatic trees (no statistical difference). On S. citri-infected trees, yield reduction might be attributed to both earlier fruit drop and the production of lighter and smaller fruit than on S. citri-free trees [52]. Furthermore, CSD has been associated with reductions in fruit size, particularly with Valencia oranges [54]. Further, the CSD has also an impact on tree height and canopy diameter [52].

Table 1.
Results summary of the known field trials carried out in different citrus-growing countries to evaluate the effect of Spiroplasma citri on vegetative growth and yield of different citrus scion and rootstock combinations.
The available studies on the impact of CSD on fruit quality and quantity are inconclusive. Indeed, Mello et al. [52] and Kyriakou et al. [63] reported that both juice quantity and quality are not affected by CSD. The S. citri-positive trees, on the other hand, produced insipid, sour, or bitter-tasting fruits [65]. Furthermore, CSD is regarded as one of the primary causes of citrus fruit quality degradation in Egypt [46]. Indeed, S. citri-positive trees produced fewer, lower-quality fruits (reduced size and asymmetric shape) than S. citri-negative trees [52].
4. Transmission and Epidemiology
The citrus stubborn spiroplasma is vectored by many leafhopper species. The disease is also propagated by grafting or collecting bud material from diseased plants. Several factors, associated with the causal agent, its plant hosts, vectors, management practices, and the environment, influence the disease epidemiology. Indeed, the transmission level of S. citri is correlated with temperature and is higher in warm conditions [59,66]. S. citri is an obligate parasite that lives in the phloem sieve tubes of infected plants [53,67]. Leafhoppers have been proven to transfer the mollicute from and to a wide range of weeds and vegetable hosts [68]. Infected weeds became stunted and yellow, and as they dried up in warm or hot temperatures, S. citri vectors moved from these hosts to young citrus trees, which are more susceptible than older ones. Transmission is primarily from infected weeds to citrus, with less transmission from infected citrus to citrus [69]. Infection with S. citri can also be spread through grafting using contaminated scions [4]. As a result, in areas where the disease is not endemic, the use of S. citri-free buds is required to prevent infection [70].
In Arizona and California, ornamental periwinkle seedlings were the first hosts to be naturally found infected with S. citri [71,72], and they were later used experimentally to learn more about the spiroplasma’s natural transmission in both Morocco and Syria [71,72,73,74,75,76]. It is vital to note that periwinkle is employed as a model host plant for mollicutes research in plant pathology. This is due to the ornamental plant’s high susceptibility to phytoplasma and spiroplasma infection from various crops [77], which can be transmitted by insect vectors feeding on S. citri-infected trees [78], dodder transmission [79], and/or mechanical inoculation [80]. A rapid reduction in the size and number of plant flowers, reduction in leaf size, chlorosis of leaf tips and margins, stunting, and mortality are all indicators of S. citri-infected periwinkles [81].
Leafhoppers belonging to the Cicadellidae family (Deltocephalinae subfamily) are responsible for persistent and propagative insect transmission of S. citri [82,83]. C. haematoceps is the main vector of S. citri in the Mediterranean region. Turkey, Morocco, Syria, and France (Corsica) have all reported it [76]. C. haematoceps is also the most common species in Asia, particularly Iran, however it appears that the beet leafhopper, C. tenellus, is more common there than in the Mediterranean Basin. As a result, both C. haematoceps and C. tenellus can be vectors in the Middle East. The major vector of S. citri in the United States is C. tenellus [69]. Indeed, S. citri was found to be transmitted to citrus and periwinkle by a beet leafhopper collected in California citrus plantations [84]. C. haematoceps host plants have been identified in Syria and France, stating that their presence along the Mediterranean coast explains various epidemic scenarios, including those in citrus fields with nucellar trees [69].
Crossing the insect vector’s intestinal and salivary gland barriers is required for the persistent and propagative transmission of S. citri. These crossings are based on an endocytosis/exocytosis mechanism wherein bacterial protein complexes identify certain patterns on eukaryotic cell surfaces [85]. The S. citri infects the entire insect via crossing a circulative route after being acquired from the phloem vessels of an infected plant by leafhopper vectors. Spiroplasmas enter the insect gut wall, multiply, circulate, and infiltrate most of the insect organs, including the salivary glands, before being discharged into the primary salivary duct leading to the stylet’s salivary canal. During feeding, they are delivered into the plant phloem with salivary secretions [86,87,88]. S. citri multiplies in the phloem sieve components of the host plant, causing severe symptoms [25]. S. citri was found to lose its ability to cross the gut and salivary gland barriers and to be transmitted after numerous plant grafts or several sub-cultures in in vitro culture without insect passage [24]. The absence of several proteins (146, 144, and 92 KDa) thought to be important in transmission explains this study [39]. S. citri transmission by insect vectors has been linked to several proteins. Spiralin [33,89], Sc76 (the solute binding protein of an ABC transporter) [40], and P89, are all encoded on pBJS-O plasmid [38,90]. In vitro, the P89 protein was found to be necessary for insect cell adhesion [91]. Further, the S. citri transmission also involves P58 [15] and P32 encoded on plasmid pSci6 [36,37].
5. Methods to Detect the Disease
5.1. Biological Indexing
Calavan and Christiansen [92] developed biological indexing of CSD for the first time in 1965, where sensitive varieties such as Madam Vinous or Pineapple sweet orange were inoculated with the examined tissue [92]. To ensure the effectiveness of indexing, these citrus cultivars, known as indicator plants, should be kept at warm temperatures [93]. Both side and young leaf piece grafts have been demonstrated to successfully transmit the disease to Madam Vinous sweet orange indicator plant seedlings. These two inoculum sources were shown to be more effective than buds or blind buds in disease transmission [94,95]. Madam Vinous seedlings used as indicator plants produced new growth within 3 weeks and displayed significant symptoms of CSD within 5 weeks when kept in a greenhouse under warm conditions (30 °C to 40 °C maximum days and 26 °C to 27 °C night). It is worth noting that the disease’s classic symptoms, such as small, cupped leaves with pale-green tips and mottling, were only seen in hot climates [59,96]. Mannaa et al. [46] developed an effective biological indexing technique called inverse inoculation. This approach allows the symptoms of CSD to be observed only 4 weeks after inoculation and improves transmissibility (85%) compared to standard inoculation, which takes 3 months and has a low success rate (35%) of transmission [46]. It is worth noting that biological indexing has revealed the irregular distribution of S. citri throughout the plants, making this technique difficult to utilize regularly. However, due to the presence of mild forms of S. citri on the one hand, and the uneven distribution of the causal agent on the other, this observation helps to explain why some experimentally infected plants do not develop symptoms [48,94,97,98,99,100].
The use of biological indexing in CSD diagnosis has two fundamental drawbacks. The first is S. citri’s low rate of greenhouse transmission. The standard approach of biological indexing on indicator host plants was used to calculate this rate. The second is the length of time it takes for the symptoms to develop on the indicator plants. The prior limits could be explained by the low concentration of S. citri in the bud stick used, especially during cold seasons. To address the problem, adjustments to the standard method of biological indexing must be undertaken to boost the rate of successful pathogen greenhouse transfer [46]. In addition, biological indexing, which includes mechanical inoculation, must be supplemented by laboratory indexing, including serological, molecular, and chemical assays [51].
5.2. Isolation and Culturing
Two research groups were the first to describe S. citri in in vitro culture [101,102]. Since then, S. citri isolation and culture have been used to diagnose CSD in field trees as the gold standard [4,103]. For initial isolation and routine cultivation of S. citri from both plant material and leafhopper hosts, several culture mediums have been devised. C-3G [104], LD8 [105], SP4 [106], and R2 [107] are just a few examples (Table 2). The requirement of cholesterol for growth, as well as a total resistance to penicillin, are the two most important cultural characteristics [103]. Most mycoplasma mediums contain complicated elements such as basis compounds (PPLO broth base, animal serum, and yeast extract), as well as other substances (tryptone, peptone, and animal tissue culture medium) that are frequently included. Other, simpler media have been created, such as R2 [107].
S. citri, like other bacteria, has a sigmoid growth pattern. At 29 °C, helix doubling takes 20 h in the exponential phase. When the pH of the culture medium dropped to 5.4 or below, S. citri lost motility and helicity [108]. A second culture is required after 2 days to ensure the ongoing growth of S. citri, with the maximal concentration of inoculum in the second cultivation is required after 2 weeks [104]. In 0.8% agar media, fried egg-shaped colonies (small in size and round in form) can be seen [44,51,104]. During the exponential phase, filament branching was common, and the helical filaments stuck to each other and formed aggregates in the old cultures [108].


Table 2.
Summary of the major medium culture used for Spiroplasma citri isolation and growth.
Table 2.
Summary of the major medium culture used for Spiroplasma citri isolation and growth.
| Ingredients | Medium Name [References] | ||||
|---|---|---|---|---|---|
| C-3G [104] | R2 [107] | M1D [109,110] | LD8 [105] | SP4 [106,111] | |
| Distilled water | 72 mL | 76 mL | Fill to 100 mL | 1.2 mL | 61.5 mL |
| PPLO broth base w/o | 1.5 g | 1.5 g | - | 1.2 g | 0.35 g |
| Mycoplasma broth base (BBL) | - | - | 700 mg | 9 g | 0.35 g |
| Sucrose | 12 g | 8 g | 332 mg | 6 g | - |
| Glucose | - | - | 33.2 mg | 400 mg | 0.5 g |
| Fructose | - | - | 33.2 mg | 0.4 g | |
| Phenol red (0.2%) | 1 mL | 1 mL | 1 mL | - | 2 mL |
| Horse serum | 20 mL | 15 mL | - | - | |
| Fetal bovine serum (heated at 56 °C for 1 h) | - | - | 16.6 mL | 10 mL | 17 mL |
| Penicillin (1 MU/g) | 100 mg | 100 mg | 100 mg | - | 100 mg |
| Sorbitol | - | - | 2.5 g | - | - |
| Peptone | - | - | 266 mg | - | 0.53 g |
| 1.0 n NaOH | - | - | As needed | - | - |
| Schneider’s insect medium | - | - | 53.4 mL | - | - |
| Tryptone 10.0 g | - | - | 332 mg | - | 1 g |
| CMRL 1066 medium (10×) (with glutamine) (Gibco 154) | - | - | - | - | 5 mL |
| Fresh yeast extract (25% solution) | - | - | - | 5 mL | 3.5 mL |
| Yeastolate (2% solution, sterile) | - | - | - | 0.2 mL | 10 mL |
| HEPES buffer | - | - | - | 1.5 mL | - |
| Organic acids | - | - | - | - | |
| α-Ketoglutaric acid | 0.04 g | ||||
| Pyruvic acid | 0.04 g | ||||
| Inorganic salts | - | - | - | ||
| KCl | 0.04 g | ||||
| KH2PO4 | 0.03 g | ||||
| MgSO4 7H2O | 0.02 g | ||||
| NaCl | 0.14 g | ||||
| NaHPO4 | 0.02 g | ||||
| NaSO3 | 0.05 g | ||||
| Amino acids | - | - | - | ||
| L-Arginine | 0.06 g | ||||
| L-Asparagine | 0.06 g | ||||
| L-Cysteine HCl | 0.04 g | ||||
| L-Glutamine | 0.06 g | ||||
| Methionine | 0.04 g | ||||
Although S. citri isolation and culture are a very sensitive and specific method for CSD diagnosis, its time-consuming nature (it takes 2 to 3 weeks) prevents its routine usage. Furthermore, contamination by non-target organisms is regarded as a limitation that could limit the widespread application of this diagnostic approach [112].
5.3. Antibodies-Antigen-Based Methods
S. citri was found to be detectable in infected host plant tissue, arthropod hosts, and pure culture using an enzyme-linked immunosorbent assay (ELISA) [113,114,115]. Indeed, ELISA has been the most often used diagnosis technology in the preliminary sanitary evaluation of propagating material due to its ease of use and ability to evaluate a large number of samples [116]. ELISA’s sensitivity is comparable to that of culture. In citrus, both assays can detect S. citri in 95% of symptomatic nursery or field trees in citrus [117]. The tests are not sensitive enough to accurately detect S. citri in trees that do not show any symptoms. The limited number of spiroplasmas present in the phloem makes the early detection of the pathogen difficult during the plant disease establishment [117,118]. Furthermore, when utilizing citrus leaves as samples, the results were uneven [114].
The immunocapture-based polymerase chain reaction (IC-PCR) is thought to be a promising technology for detecting low levels of S. citri in citrus plants and insect cells. Indeed, the sensitivity of IC-PCR has been compared to that of two other techniques, ELISA and polymerase chain reaction (PCR). The lowest number of spiroplasmas detected per milliliter (mL) of plant extract was roughly 10−6 to 10−7 per mL using ELISA. By contrast, PCR found around 10−4 spiroplasmas per mL, while IC-PCR detected approximately 10−3 per mL [119]. Two primer pairs were used to provide an enhanced IC-PCR test: D/D’ [120], which is specific for the entire spiralin gene, and SC/SC’, which is specific for a portion of the spiralin gene. The two primer pairs amplified 1,035 bp and 330 bp from infected but not healthy citrus trees, respectively [121]. In a short, IC-PCR is a sensitive and specific method for detecting S. citri [121].
Because CSD diagnosis is difficult due to low and variable concentrations of S. citri in diseased trees and the random distribution of S. citri, a new diagnostic approach based on an S. citri-secreted protein as a detection marker has been devised. This approach is based on the fact that microbial pathogens, such as S. citri, release a large number of proteins during infection. In diseased plants, the vascular flow allows systemic dispersion. As a result, the presence of these proteins may be more widespread than just pathogen infection sites, and they could be used as biological detection markers. With mass spectrophotometry analysis, a novel secreted protein for S. citri has been identified, which is strongly expressed in the presence of citrus phloem extract. ScCCPP1, an antibody raised against this protein, was able to differentiate diseased citrus and periwinkle plants from healthy ones. In summary, using the secreted protein as a marker is a successful diagnostic strategy for large-scale CSD field surveys. For this application, however, extra validation and specificity confirmation tests of the approach are required. This is because this technique can create critical data, making it unsuitable for use in the field. This can arise, for example, when field samples are taken from other citrus species, at various tree ages and stages of development, from trees infected with other bacteria and viruses associated with citrus, at various times of the year, and in various geographic locations [80]. Using the antiserum ScCCPP1, a simple direct tissue print assay has been developed. This technique was used to evaluate six adult trees that were known to be naturally infected with CSD. All of the examined plants developed positive signals from the phloem area of the stem print utilizing ScCCPP1. Using direct tissue prints on a nitrocellulose membrane, diseased S. citri trees were effectively diagnosed in a highly specific and reliable manner. It is worth noting that in the field, this direct print tissue assay is more sensitive than real-time PCR in diagnosing CSD. To put it another way, the results of real-time PCR did not match those of the imprint data (eight positive samples with direct tissue print assay instead of only six with real-time PCR). Positive signals have been detected without the presence of S. citri cells in phloem-rich tissues. This finding suggests that the pathogen detection signal was disseminated throughout the phloem via the transportation flow [80].
5.4. Nucleic Acid-Based Methods
CSD field diagnosis is typically challenging since culture on artificial media, biological indexing, or serological techniques to detect the causal agent are laborious, expensive, and/or time-consuming [122]. As a result, molecular assays based on PCR have been designed to address the major limitations of serological testing and culture assays for the identification of CSD (sporadic distribution of the mollicute in infected plants, seasonal concentration changes of S. citri, etc.) [122]. The sensitivity of PCR is 100 to 1000 times higher than with ELISA or culture assays [118,122]. Using primers amplifying the spiralin gene or multicopy genes encoding membrane proteins, several PCR-based detection techniques have been devised. P58 and P89 are among the genes used for this purpose [120,122]. Table 3 lists all of the PCR and associated assays that have been developed to detect S. citri. Yokomi et al. [122] found that primers based on P89 and P58 were at least 1000 times more sensitive in recognizing S. citri in field samples than those based on the spiralin gene [120]. S. citri PCR assays are highly sensitive, need a small amount of sample, and can be reliably conducted at high throughput [122]. Other primers have also been designed to target viruses associated with S. citri [19,112,118,122,123]. Depending on the S. citri isolate and the targeted gene, the detection method’s performance varies [44].

Table 3.
Primer sequences and their annealing temperature (Tm), primer/probe location, and expected size of PCR products for each primer pair when used to amplify Spiroplasma citri by PCR and related tests.
Highly sensitive and reliable real-time PCR assays were developed to address the problem of the non-detection of some S. citri isolates by the traditional PCR test previously developed by Yokomi et al. [122]. A real-time PCR assay based on sequences from the P58 putative adhesin multigene of S. citri enhances sensitivity from 8 × 10−5 to 1.2 × 10−6 ng of S. citri DNA (6.14 × 105 to 9.6 × 103 copies of target gene) per milligram (mg) of field citrus tree tissue. It is worth noting that the titer of S. citri was consistently higher in the fruit columella than in the leaf midribs, making the former the best choice for sampling [125]. Another primer pair (Scif/Scir) has been designed to target the pE gene of the pSci1 plasmid of S. citri. The real-time PCR test developed with this primer pair was more sensitive than those based on the spiralin gene (D/D’ and F1/R1) or 16S ribosomal DNA (rDNA) (ScR16F1/ScR16R1 and ScR16F1A/ScR16R2) [120,123,128]. Additionally, designing primer pairs has resulted in the development of a very sensitive and reliable real-time PCR test. This includes Php-orf1, which targets conserved prophage sequences in the S. citri genome [126]. It is crucial to note that this test improves the sensitivity of detection of S. citri by 4.91 and 3.65 cycle threshold (Cq) units, respectively, as compared to spiralin and P58 putative adhesin gene housekeeping gene primers [126].
The S. citri strain diversity can be studied using restriction fragment length polymorphism (RFLP) analysis of 16S rDNA, amplified by nested PCR with S. citri 16S rDNA-based primers [123]. The restriction enzymes AluI, HhaI, HaeIII, and MseI were used to digest the products of nested PCR tests using the primer pair SCR16F1A/ScR16R2 [123]. RFLP analysis of the PCR-amplified sequences digested with these restriction enzymes revealed that the sequences obtained from the carrot samples tested positive for S. citri have identical restriction profiles to those of the S. citri reference strain with all four enzymes and a different profile from that of the S. citri reference strain (Cir3B isolated from beet leaf) with all four enzymes [123].
To detect S. citri, a droplet digital PCR test (ddPCR) was developed [125]. A comparison of ddPCR and real-time PCR revealed a difference in detection sensitivity [125]. Two sets of spiralin and SpV1 open reading frame 1 (ORF1) primers/probes were also compared [122,125,126]. For the identification of S. citri in both culture media and field samples, ddPCR is more accurate than real-time PCR. In a 20 µL reaction, this approach enables the absolute quantification of one copy of the target [125]. It is worth noting that ORF1 primers, which target the SpV1-ORF1 prophage (X51344), are more robust than SP1 primers (targeting the spiralin gene) in CSD diagnosis, according to both real-time PCR and ddPCR data [125].
A loop-mediated isothermal amplification (LAMP) PCR has recently been developed as a highly specific approach for detecting S. citri. The target DNA may be detected to a concentration of 100 fg/L using this approach, which is unaffected by crude plant extracts. This LAMP assay, on the other hand, is nine times less sensitive than real-time PCR with pure DNA templates [127].
Table 4 provides a comparison of the detection methods described above in terms of both sensitivity and specificity.

Table 4.
Summary of the main findings from the different Spiroplasma citri detection tests.
6. Strategies to Control the Disease
6.1. S. citri Sanitation
Some infections, such as S. citri, are difficult to remove from the citrus mother plant, according to preliminary research. Their eradication does necessitate the use of specific sanitation techniques [132]. The ability of in vitro shoot-tip grafting to assure the eradication of S. citri and the development of healthy citrus stocks has been investigated. It has proven to be a very effective strategy for eradicating citrus graft-transmissible diseases, such as CSD (with a 100% success rate) [133]. The S. citri was eradicated from four citrus cultivars using this approach, including Madam Vinous, Navel orange, Valencia orange, and Redblush grapefruit. The results revealed that all plants derived from S. citri-infected shoots of the four cultivars and kept for a year under warm circumstances were CSD-free [96].
6.2. Cultural Practices
Preventing S. citri from achieving and infecting young sensitive plants [50] is the most effective way to avoid CSD, especially in the early-nursery phase [1]. The most efficient way to avoid infection with S. citri is to follow a variety of cultural practices [50]. CSD management begins at the nursery, where weeds are monitored, stunted nursery trees are removed, and citrus nursery plants are kept under a screen. All of those precautions are critical to avoid primary infection with S. citri. This is because the major source of infection is vectors feeding on weeds and then transferring to young nursery plants when the weeds dry up [1]. Furthermore, because S. citri is disseminated by several insect vectors, trap plants appear to be a viable method of limiting CSD propagation. This is the case, for example, with sugar beet, a plant that attracts insect vectors, particularly the beet leafhopper, but is resistant to CSD. Because older trees are less vulnerable to S. citri infection, preventing infection while the tree is still growing is critical. As a result, young trees (up to 6 years old) should be removed because they will never produce fruits. However, for infected trees older than six years, an individual assessment should be performed to determine whether symptomatic areas should be removed or the diseased trees should be replaced with healthy trees [50]. CSD can be passed down through grafting. As a result, it is crucial to make sure the mother tree is free of S. citri before starting the propagation process. The use of plants obtained from S. citri-free areas is essential for avoiding the disease’s introduction into a new orchard. Furthermore, it is critical to keep an eye on weeds in these groves to ensure that they are not disease-prone hosts and to eradicate any susceptible ones as soon as possible [50].
6.3. Chemotherapy
In the past, insecticides and tetracycline-based antibiotics were employed to remove insect vectors and alleviate symptoms in spiroplasma-infected plants, respectively [134].
The antibiotic sensitivity of S. citri has been widely studied, and antibiotics have been utilized to suppress this wall-less pathogen in the field [135]. Indeed, previous research suggests that erythromycin, tylosin, and numerous other antibiotics in the tetracycline group may be effective in the treatment of CSD [136]. The effects of tetracycline compounds, which have an impact on protein synthesis pathways [137], on the development of CSD symptoms were studied in terms of uptake, translocation, and impact. These compounds, applied to the roots as a dip or in hydroponic culture, fully reduced stubborn symptom development in infected seedlings. However, tetracycline compounds in the form of quartz sand drenches were found to be ineffective in preventing CSD symptom development. It is important to highlight that Achromycin, which looked to be more stable than Aureomycin, was more effective in preventing the onset of symptoms [138]. Although several antibiotics have been proven effective in the treatment of CSD in vitro, it is important to remember that factors such as antibiotic stability and translocation in plants can have a significant impact on antibiotic performance in vivo when compared to their activity in vitro [136]. Field investigations show that after a lengthy period of use on yellows-diseased plants, including CSD, tetracyclines eventually lose their effectiveness. Antibiotic-resistant Spiroplasma strains were suspected to arise in tetracycline-treated diseased plants [135].
The susceptibility of S. citri to a variety of systemic insecticides (cygon, furadan, and lannate) and fungicides (benomyl, thiabendazole, and thiophanate M) has been investigated in vitro. S. citri was resistant to most of the three systemic insecticides tested. Only thiabendazole in 5% dimethylsulfoxide showed an inhibitory effect equivalent to that of some antibiotics [136].
Although, like other phloem-colonizing and insect-transmitted bacterial pathogens, S. citri has shown antibiotic sensitivity in vitro and remission of disease symptoms in planta, there is no practical therapy for CSD once a tree is infected [135,136,138]. Furthermore, the widespread use of such biocides in the field is not only ineffective but also environmentally unsound, as antibiotic and insecticide resistance would surely develop in spiroplasmas and their insect vectors [134]. Therefore, to avoid the use of antibiotics in agriculture and the emergence of resistant microbial strains, it is advised that molecules with various modes of action, such as ribosome-inactivating proteins, plant hormones, and resistance inducers such as plasma-activated water, should reasonably be expected [137]. To summarize, it seems that CSD effective control is mainly reliant on preventative and roughing measures, which are themselves reliant on precise and early detection [80].
6.4. Genetically Engineered Resistance
There are currently no plants that show natural resistance to S. citri. Artificial resistance in plants is now achievable thanks to advances in molecular biology and biotechnology [134]. The in planta expression of genes encoding antimicrobial peptides (AMPs), a novel class of antimicrobial compounds that offers an alternative to standard antibiotics, is one of the ways to engineer cellular pathogen resistance [139]. In other words, employing AMPs to engineer artificial plant resistance has been viewed as a possible approach for controlling agronomically significant spiroplasmal diseases, including CSD. A recent study focused on screening AMPs that have the potential to inhibit the growth of S. citri. For the in vitro growth inhibition test, four AMPs were selected: Novispirin T7, Caerin 1.1, Tricholongin, and Dhvar4. For rapid qualitative and quantitative analyses of the AMPs, a liquid assay method was designed. Novispirin T7 and Caerin 1.1 inhibited the growth of S. citri with efficacy comparable to tetracycline. Spiroplasma cultures treated with these two peptides showed cell deformations, indicating that the AMPs interact with the spiroplasma cell membranes. Because Novispirin T7 and Caerin 1.1 are both short, linear peptides that are water-soluble, they can be synthesized chemically and supplied exogenously. Alternatively, using a gene expression cassette, the peptides can be engineered into spiroplasma-susceptible plants. The expression of engineered AMPs in plants may improve plant resistance against CSD [134].
7. Disease Situation in the Mediterranean Region: Focus on Morocco
Citrus stubborn is a common disease in the Mediterranean. S. citri has been found in nearly every Mediterranean country and is one of the most common citrus infections [140]. Furthermore, in the Mediterranean region, citrus stubborn is considered an endemic vector-borne disease [2]. The development of reliable diagnostic procedures has enabled extensive surveys of S. citri in various areas of the region. S. citri was identified in several countries, including Morocco [5,14,43,69,114,141,142,143,144], Algeria [43,145], Tunisia [43,146,147,148], Egypt [44], Syria [43,49,69], Lebanon [43], Palestine [69], Israel [4,43], Turkey [4,43,45], Spain [4], Italy [4,57], Cyprus [63], and France [149]. In Italy and Spain, CSD appears to be of minor importance [2], while some citrus-growing Mediterranean countries, such as Malta, Croatia, and Portugal, have been reported to be free of S. citri [150]. The natural transmission of S. citri by different leafhopper species and the deployment of buds from infected trees are thought to be the key explanatory causes underlying the prevalence of CSD in the Mediterranean area [69].
In Morocco, stubborn was once thought to be one of the rare citrus diseases [142]. The disease’s causal agent has been found in every citrus-growing region of the country, including Gharb, Haouz, Loukkos, Moulouya, Souss [143], and Tadla [5,141,143]. In Morocco, a genetic investigation of S. citri has never been performed. However, it should be underlined that a first complete nucleotide sequence of the circular chromosome, as well as two plasmids from S. citri of Moroccan origin, have been recently documented [14].
8. Conclusions and Future Prospects
Although CSD has been studied for decades, the accurate detection of the disease remains difficult due to the disease’s uneven distribution and low titers in diseased trees, as well as considerable seasonal fluctuations [103,122,151]. In other words, a variety of factors, such as sampling season and growing circumstances, as well as S. citri strains, can influence the accuracy of disease diagnosis [124]. Even though biological indexing is required in certification programs, the limitations of traditional biological indexing, such as the low concentration of S. citri, the pathogen’s unsatisfactory transmission rates in the greenhouse, and the long delay in symptom expression, have limited its widespread use [46]. It is worth noting that the use of ScCCPP1 is the first documented serological diagnosis of CSD that is not dependent on the presence of S. citri in the analyzed sample [80]. Briefly, it is not easy to choose one CSD diagnosis approach over another, even if the results are consistent. The best option will be determined by the diagnosis goals and the facilities of each laboratory.
The control of CSD is still an enigma. This is owing to the airborne transmission of the disease by leafhopper vectors that primarily feed on weeds. Since the discovery that a mollicute is the cause of CSD, significant control attempts have been made. This was accomplished through the use of a variety of methods, including whitewash sprays and preventative net covering. None of these techniques, however, has been demonstrated to be successful in preventing the spread of CSD. This is related to the difficulties of conducting studies with a disease that has a sporadic natural infection [2]. The key to preventing CSD propagation is to stay on top of the sanitary state of citrus donor plants to produce S. citri-free propagating material. In vitro shoot-tip grafting is successful in removing S. citri from propagating material [96,132,133].
In terms of the current situation of S. citri across Morocco, this paper presents an overview of the disease’s spread in the citrus-growing regions of the country. Virus-free (certified) saplings, vector management, regular monitoring of citrus orchards to enable the early diagnosis of the disease, and grubbing up of affected trees could all help to prevent the introduction and spread of CSD in Moroccan citrus groves. This overview includes references to many disciplines of research on CSD in Morocco and worldwide.
The characterization of S. citri strains, the identification of potential leafhopper vectors, the search for secondary hosts, and the development of sustainable control measures are some of the research topics that could be pursued as prospects. Investigating functional genomics in the citrus–S. citri interaction using transcriptomic and/or proteomic approaches would be an interesting way to learn more about the full mechanisms underlying the complex and varied events associated with such an interactome and thus aid in the development of new diagnostic methods and plant protection strategies. The relationship between S. citri and other citrus pathogens (viruses and viroids) prevailing in Moroccan citrus orchards [152,153,154] should be deeply investigated in the future to learn more about the whole range of interactions that these pathogens have during infection and their impact on CSD severity. This advanced research will contribute to a better knowledge of the epidemiology of S. citri and the mechanisms behind its spread worldwide, including Morocco, therefore helping to develop novel pathogen control measures.
Author Contributions
Conceptualization, T.S. and Z.B.; methodology, Z.B., T.S. and R.L.; software, Z.B. and T.S.; validation, R.L., A.T., E.A.B. and N.R.; data curation, T.S. and Z.B., resources, Z.B. and R.L.; writing—original draft preparation, T.S. and Z.B.; writing—review and editing, R.L., A.T. and E.A.B.; supervision, N.R., R.L. and E.A.B.; project administration, Z.B. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used for the analyses in this study are available within the article.
Acknowledgments
This research was financially supported by the INRA Institute (CRRA de Oujda; Qualipole de Berkane), the Department of Plant Protection, Ecole Nationale d’Agriculture de Meknes, and Faculté des Sciences et Techniques de Mohammedia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roistacher, C.N. Diagnosis and Management of Virus and Virus-like Diseases of Citrus. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2004; Volume 1, pp. 109–189. [Google Scholar]
- Bar-Joseph, M.; Catara, A. Endemic and Emerging Vector-Borne Mediterranean Citrus Diseases and their Epidemiological Consequences. Integr. Control Citrus Pests Mediterr. Reg. 2010, 19, 137–155. [Google Scholar] [CrossRef]
- Brown, L.G. Stubborn disease of citrus. Plant Pathol. Circ. 1992, 673, 1–2. [Google Scholar]
- Roistacher, C.N. Graft-Transmissible Diseases of Citrus, Hand Book for Detection and Diagnosis; FAO: Rome, Italy, 1991; p. 285. [Google Scholar]
- Saglio, P.; Lhospital, M.; Laflèche, D.; Dupont, G.; Bové, J.M.; Tully, J.G.; Freundt, E.A. Spiroplasma citri gen. and sp. n.: A Mycoplasma—Like Organism Associated with “Stubborn” Disease of Citrus. Int. J. Syst. Evol. Microbiol. 1973, 23, 191–204. [Google Scholar] [CrossRef]
- Gasparich, G.E. Spiroplasmas: Evolution, adaptation and diversity. Front. Biosci. 2002, 7, 619–640. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Tully, J.G.; Rose, D.L.; Petzel, J.P.; Oyaizu, H.; Yang, D.; Mandelco, L.; Sechrest, J.; Lawrence, T.G.; Etten, J.V.; et al. A Phylogenetic Analysis of the Mycoplasmas: Basis for Their Classification. J. Bacteriol. 1989, 171, 6455–6467. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.M.; Tully, J.G.; Popkin, T.J.; Bové, J.M. Morphology, Ultrastructure, and Bacteriophage Infection of the Helical Mycoplasma-Like Cultured from “Stubborn” Disease of Citrus. J. Bacteriol. 1973, 115, 367–386. [Google Scholar] [CrossRef]
- Davis, R.E.; Lee, I.M.; Basciano, L.K. Spiroplasmas: Serological grouping of strains associated with plants and insects. Can. J. Microbiol. 1979, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M.; Mouches, C.; Carle-junca, P.; Degorce-Dumas, J.R.; Tully, J.G.; Whitcomb, R.F. Spiroplasmas of Group I: The Spiroplasma citri Cluster. Yale J. Biol. Med. 1983, 56, 573–582. [Google Scholar]
- Carle, P.; Saillard, C.; Carrère, N.; Carrère, S.; Duret, S.; Eveillard, S.; Gaurivaud, P.; Gourgues, G.; Gouzy, J.; Salar, P.; et al. Partial Chromosome Sequence of Spiroplasma citri Reveals Extensive Viral Invasion and Important Gene Decay. Appl. Environ. Microbiol. 2010, 76, 3420–3426. [Google Scholar] [CrossRef]
- Yokomi, R.; Rattner, R.; Osman, F.; Maheshwari, Y.; Selvaraj, V.; Pagliaccia, D.; Chen, J.; Vidalakis, G. Whole-genome sequence of five strains of Spiroplasma citri isolated from different host plants and its leafhopper vector. BMC Res. Notes 2020, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Rattner, R.; Thapa, S.P.; Dang, T.; Osman, F.; Selvaraj, V.; Maheshwari, Y.; Pagliaccia, D.; Espindola, A.S.; Hajeri, S.; Chen, J.; et al. Genome analysis of Spiroplasma citri strains from different host plants and its leafhopper vectors. BMC Genom. 2021, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Shao, J.; Zhao, Y.; Gasparich, G.E.; Gaynor, B.J.; Donofrio, N. Complete Genome Sequence of Spiroplasma citri Strain R8-A2T, Causal Agent of Stubborn Disease in Citrus Species. Genome Announc. 2017, 5, e00206-17. [Google Scholar] [CrossRef]
- Ye, F.; Melcher, U.; Fletcher, J. Molecular characterization of a gene encoding a membrane protein of Spiroplasma citri. Gene 1997, 189, 95–100. [Google Scholar] [CrossRef]
- Melcher, U.; Fletcher, J. Genetic Variation in Spiroplasma citri. Eur. J. Plant Pathol. 1999, 105, 519–533. [Google Scholar] [CrossRef]
- Yokomi, R.; Chen, J.; Rattner, R.; Selvaraj, V.; Maheshwari, Y.; Osman, F.; Pagliaccia, D.; Vidalakis, G. Genome sequence resource for Spiroplasma citri, strain CC-2, associated with citrus stubborn disease in California. Phytopathology 2020, 110, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Saillard, C.; Carle, P.; Duret-Nurbel, S.; Henri, R.; Killiny, N.; Carrère, S.; Gouzy, J.; Bové, J.M.; Renaudin, J.; Foissac, X. The abundant extrachromosomal DNA content of the Spiroplasma citri GII3-3X genome. BMC Genom. 2008, 9, 195. [Google Scholar] [CrossRef]
- Bébéar, C.M.; Aullo, P.; Bové, J.M.; Renaudin, J. Spiroplasma citri Virus SpV1: Characterization of Viral Sequences Present in the Spiroplasmal Host Chromosome. Curr. Microbiol. 1996, 32, 134–140. [Google Scholar] [CrossRef]
- Ye, F.; Laigret, F.; Whitley, J.C.; Citti, C.; Finch, L.R.; Carle, P.; Renaudin, J.; Bové, J.M. A physical and genetic map of the Spiroplasma citri genome. Nucleic Acids Res. 1992, 20, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Laigret, F.; Carle, P.; Bové, J.M. Chromosomal Heterogeneity among Various Strains of Spiroplasma citri. Int. J. Syst. Bacteriol. 1995, 45, 729–734. [Google Scholar] [CrossRef]
- Ye, F.; Melcher, U.; Rascoe, J.E.; Fletcher, J. Extensive chromosome aberrations in Spiroplasma citri strain BR3. Biochem. Genet. 1996, 34, 269–286. [Google Scholar] [CrossRef]
- Gasparich, G.E.; Hackett, K.J.; Clark, E.A.; Renaudin, J.; Whitcomb, R.F. Occurrence of extrachromosomal deoxyribonucleic acids in spiroplasmas associated with plants, insects, and ticks. Plasmid 1993, 29, 81–93. [Google Scholar] [CrossRef]
- Wayadande, A.C.; Fletcher, J. Transmission of Spiroplasma citri lines and their ability to cross gut and salivary gland barriers within the leafhopper vector Circulifer tenellus. Phytopathology 1995, 85, 1256–1259. [Google Scholar] [CrossRef]
- Dubrana, M.P.; Béven, L.; Arricau-bouvery, N.; Duret, S.; Claverol, S.; Renaudin, J.; Saillard, C. Differential expression of Spiroplasma citri surface protein genes in the plant and insect hosts. BMC Microbiol. 2016, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Vignault, J.C.; Bové, J.M.; Saillard, C.; Vogel, R.; Faro, A.; Venegas, L. Culture of Spiroplasma From Plant Material and Insects From Mediterranean Countries and the Near-East. Comptes Rendus Hebd. Seances L‘Acad. Sci. 1980, 290, 775–778. [Google Scholar]
- Fos, A.; Bové, J.M.; Lallemand, J.; Saillard, C.; Vignault, J.C.; Ali, Y.; Brun, P.; Vogel, R. The Leafhopper Neoaliturus haematoceps (Mulsant & Rey) is a vector of Spiroplasma citri in the Mediterranean. Microbiologie 1986, 137, 97–107. [Google Scholar] [CrossRef]
- Brown, D.R.; Whitcomb, R.F.; Bradbury, J.M. Revised minimal standards for description of new species of the class Mollicutes (division Tenericutes). Int. J. Syst. Evol. Microbiol. 2007, 57, 2703–2719. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, A.; Boyer, M.J.; Wroblewski, H. Membrane protein structure. In Mycoplasmas: Molecular Biology and Pathogenesis; Maniloff, J., McElhaney, R., Finch, L., Baseman, J.B., Eds.; Springer: New York, NY, USA, 1992; pp. 93–112. [Google Scholar]
- Wise, K.S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993, 1, 59–63. [Google Scholar] [CrossRef]
- Wroblewski, H.; Johansson, K.E.; Hjerten, S. Purification and characterization of spiralin, the main protein of the Spiroplasma citri membrane. Biochim. Biophys. Acta 1977, 465, 275–289. [Google Scholar] [CrossRef]
- Wroblewski, H. Amphiphilic Nature of Spiralin, the Major Protein of the Spiroplasma citri Cell Membrane. J. Bacteriol. 1979, 140, 738–741. [Google Scholar] [CrossRef]
- Duret, S.; Berho, N.; Danet, J.L.; Garnier, M.; Renaudin, J. Spiralin Is Not Essential for Helicity, Motility, or Pathogenicity but Is Required for Efficient Transmission of Spiroplasma citri by Its Leafhopper Vector Circulifer haematoceps. Appl. Environ. Microbiol. 2003, 69, 6225–6234. [Google Scholar] [CrossRef]
- Breton, M. Les Plasmides pSci de Spiroplasma citri GII3: Caractérisation Fonctionnelle et Rôle dans la Transmission par l’Insecte Ecteur. Ph.D. Thesis, Universié Victor Segalen Bordeaux 2, Bordeaux, France, December 2009. [Google Scholar]
- Comer, J.; Fletcher, J.; Davis, R.E.; Melcher, U. Evolution of the Spiroplasma P58 Multigene Family. Biochem. Genet. 2007, 45, 25–32. [Google Scholar] [CrossRef]
- Killiny, N.; Batailler, B.; Foissac, X.; Saillard, C. Identification of a Spiroplasma citri hydrophilic protein associated with insect transmissibility. Microbiology 2006, 152, 1221–1230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berho, N.; Duret, S.; Renaudin, J. Absence of plasmids encoding adhesion-related proteins in non-insect-transmissible strains of Spiroplasma citri. Microbiology 2006, 152, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wayadande, A.C.; Fletcher, J. Spiroplasma citri Surface Protein P89 Implicated in Adhesion to Cells of the Vector Circulifer tenellus. Phytopathology 2000, 90, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Shaw, M.E.; Baker, G.R.; Dugan, K.J.; Ye, F.; Sha, Y.; Zuck, P.D.; Myers, G.D. Molecular characterization of Spiroplasma citri BR3 lines that differ in transmissibility by the leafhopper Circulifer tenellus. Can. J. Microbiol. 1996, 42, 124–131. [Google Scholar] [CrossRef]
- Boutareaud, A.; Danet, J.L.; Garnier, M.; Saillard, C. Disruption of a Gene Predicted To Encode a Solute Binding Protein of an ABC Transporter Reduces Transmission of Spiroplasma citri by the Leafhopper Circulifer haematoceps. Appl. Environ. Microbiol. 2004, 70, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M.; Renaudin, J.; Saillard, C.; Foissac, X.; Garnier, M. Spiroplasma citri, A Plant Pathogenic Mollicute: Relationships with Its Two Hosts, the Plant, and the Leafhopper Vector. Annu. Rev. Phytopathol. 2003, 41, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.F.S.; Yokomi, R.K.; Melcher, U.; Chen, J.; Civerolo, E.; Wayadande, A.C.; Fletcher, J. New Perspectives on the Epidemiology of Citrus Stubborn Disease in California Orchards. Plant Health Prog. 2010, 11, 37. [Google Scholar] [CrossRef]
- Chapot, H. First Studies on the Stubborn Disease of Citrus in Some Mediterranean Countries; International Organization of Citrus Virologists: Riverside, CA, USA, 1959; pp. 109–117. [Google Scholar]
- Abd El-Fatah, W.; Egiza, A.O.; Youssef, S.A.; Shalaby, A.A. Isolation and Identification of Spiroplasma citri Associated with Citrus Stubborn Disease in Egypt. Int. J. Adv. Res. Biol. Sci. 2016, 3, 223–231. [Google Scholar]
- Çaglar, B.K.; Satar, G.; Balogu, S.; Drais, M.I.; Djelouah, K. Detection of Spiroplasma citri from citrus trees in Turkey by molecular techniques. Mediterr. Agric. Sci. 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Mannaa, M.; D’Onghia, A.M.; Djelouah, K.; Cavallo, G.; Valentini, F. Improvement of Detection Methods and Further Characterization of Spiroplasma citri, the Causal Agent of Citrus Stubborn Disease in Egypt. Am. J. Plant Sci. 2013, 4, 245–249. [Google Scholar] [CrossRef]
- Osorio-Acosta, F. Citrus Stubborn Disease Progression, Detection, and Yield Loss. Ph.D. Thesis, University of California Riverside, Riverside, CA, USA, June 1999. [Google Scholar]
- Hilgeman, R.H. Response of Stubborn-Infected Trees to Iron Chelates. In Proceedings of the Integrated Control of Citrus Virologists, 1961; pp. 84–92. Available online: https://escholarship.org/uc/item/6r35f72p (accessed on 11 December 2021).
- Bové, J.M.; Saillard, C.; Vignault, J.C.; Fos, A. Citrus Stubborn Disease in Iraq and Syria: Correlation Between Symptom Expression and Detection of Spiroplasma citri by Culture and ELISA. In Proceedings of the Ninth International Organization of Citrus Virologists Conference, Foz do Iguaçu, Brazil, 9–13 May 1983; pp. 145–152. [Google Scholar]
- Kiptoo, J.J.; Mubeen, M.; Usman, H.M.; Abbas, A.; Pixley, K.; Chemoiwa, E.; Nderitu, P.W.; Godfrey, R.; Kiptoo, G.J. Stubborn disease of citrus caused by Spiroplasma citri: A short note. Plant Prot. 2021, 5, 117–120. [Google Scholar] [CrossRef]
- Iftikhar, Y.; Bakhtawar, F.; Hussain, I.; Sajid, A.; Mubeen, M.; Zeshan, M.A.; Sohail, M.A.; Fatima, N.; Umer, M.; Iqbal, S. Detection of Spiroplasma citri causing citrus stubborn disease in Sargodha, Pakistan. Int. J. Bot. Stud. 2020, 5, 481–485. [Google Scholar]
- Mello, A.F.S.; Yokomi, R.K.; Payton, M.E.; Fletcher, J. Effect of citrus stubborn disease on navel orange production in a commercial orchard in California. J. Plant Pathol. 2010, 92, 429–438. [Google Scholar]
- Jakeli, E.; Nikolaishvili, A.; Gulua, M.; Gulua, L. Study of Citrus Stubborn Disease Symptoms in Georgia Orange Orchards; Durmishidze Institute of Biochemistry and Biotechnology: Tbilisi, Georgia, 2004. [Google Scholar]
- Chapot, H. Morphological Modifications Induced by Stubborn Disease on Citrus Fruits. In Proceedings of the Second International Organization of Citrus Virologists Conference Proceedings (1957–2010), Gainesville, FL, USA, 7–11 November 1960; University of Florida Press: Gainesville, FL, USA, 1961; pp. 79–83. [Google Scholar]
- André, A.; Maucourt, M.; Moing, A.; Rolin, D.; Renaudin, J. Sugar Import and Phytopathogenicity of Spiroplasma citri: Glucose and Fructose Play Distinct Roles. Mol. Plant-Microbe Interact. 2005, 18, 33–42. [Google Scholar] [CrossRef]
- Chang, C.J. Pathogenicity of Aster Yellows Phytoplasma and Spiroplasma citri on Periwinkle. Phytopathology 1998, 88, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Catara, A.A.; Cartia, G. Observations on Stubborn Disease in Sicily. In Proceedings of the Fourth International Organization of Citrus Virologists Conference Proceedings (1957–2010), Rome, Italy, 2–12 October 1966; pp. 128–130. [Google Scholar]
- Olsen, E.O.; Rogers, B. Effects of temperature on expression and transmission of stubborn disease of citrus. Plant Dis. Rep. 1969, 53, 45. [Google Scholar]
- Bové, J.M.; Calavan, E.C.; Capoor, S.P.; Cortez, R.E.; Schwarz, R.E. Influence of Temperature on Symptoms of California Stubborn, South Africa Greening, India Citrus Decline, and Philippines Leaf Mottling Diseases. In Proceedings of the Sixth International Organization of Citrus Virologists Conference Proceedings (1957–2010), 1972; pp. 12–15. Available online: https://escholarship.org/uc/iocv_proceedings (accessed on 11 December 2021).
- Calavan, E.C.; Bové, J.M. Ecology of Spiroplasma citri. In The Mycoplasmas; Whitcomb, R.F., Tully, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1989; pp. 425–485. [Google Scholar]
- Mello, A.F.S.; Yokomi, R.K.; Melcher, U.; Chen, J.C.; Fletcher, J. Citrus Stubborn Severity Is Associated with Spiroplasma citri Titer But Not with Bacterial Genotype. Plant Dis. 2010, 94, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, J.E.; Gumpf, D.J.; Ohr, H.D. Living with Stubborn Disease in Central California Citrus. In Proceedings of the Tenth International Organization of Citrus Virologists Conference Proceedings (1957–2010), Valencia, Spain, 17–21 November 1986; Volume 10, pp. 304–306. [Google Scholar]
- Kyriakou, A.; Eliades, G.; Ioannou, N.; Kapari-Isaia, T. Effect of stubborn disease on growth, yield, and fruit quality of Frost Washington Navel and Frost Valencia oranges in Cyprus. J. Hortic. Sci. 1996, 71, 461–467. [Google Scholar] [CrossRef]
- Calavan, E.C.; Christiansen, D.W. Effects of stubborn disease on various varieties of citrus trees. Isr. J. Bot. 1966, 15, 121–132. [Google Scholar]
- Calavan, E.C.; Oldfield, G.N. Symptomatology of spiroplasmal plant diseases. In The Mycoplasmas; Whitcomb, R.F., Tully, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1979; Volume 3, pp. 37–65. [Google Scholar]
- Calavan, E.C.; Blue, R.L.; Harjung, M.K.; Cartia, G.; Granett, A.L.; Rana, G.L.; Gumpf, D.J. Seasonal and Geographical Variations in Natural Spread of Citrus Stubborn Disease. In Proceedings of the Seventh International Organization of Citrus Virologists Conference Proceedings (1957–2010), Athens, Greece, 29 September–4 October 1975; pp. 7–9. [Google Scholar]
- Nejat, N.; Vadamalai, G.; Dickinson, M. Spiroplasma citri: A wide host range phytopathogen. Plant Pathol. J. 2011, 10, 46–56. [Google Scholar] [CrossRef][Green Version]
- Oldfield, G.N.; Calavan, E.C. Stubborn disease in non-rutaceous plants. In Description and Illustration of Virus and Virus-Like Diseases of Citrus: A Collection of Color Slides; Bové, J.M., Vogel, R., Eds.; FAO: Rome, Italy, 1980. [Google Scholar]
- Bové, J.M.; Fos, A.; Lallemand, J.; Raie, A.; Ali, Y.; Ahmed, N.; Saillard, C.; Vignault, J.C. Epidemiology of Spiroplasma citri in the Old World. In Proceedings of the Tenth International Organization of Citrus Virologists Conference, Valencia, Spain, 17–21 November 1986; pp. 295–299. [Google Scholar]
- Anonyme the Citrus Clonal Protection Program (CCPP) and the Study of Pathogenic and Host Regulating Graft Transmissible Agents of Citrus. Available online: https://reeis.usda.gov/web/crisprojectpages/0215675-the-citrus-clonal-protection-program-ccpp-and-the-study-of-pathogenic-and-host-regulating-graft-transmissible-agents-of-citrus.html (accessed on 1 August 2021).
- Allen, R.M. Spiroplasma organism found in naturally infected periwinkle. Citograph 1975, 60, 428–446. [Google Scholar]
- Granett, A.L.; Blue, R.L.; Harjung, M.K.; Calavan, E.C.; Gumpf, D.J. Occurrence of Spiroplasma citri in periwinkle in California. Calif. Agric. 1976, 30, 18. [Google Scholar]
- Bové, J.M.; Vignault, J.C.; Garnier, M.; Saillard, C.; Garcia-Jurado, O.; Bové, C.; Nhami, A. Mise en evidence de Spiroplasma citri, l’agent causal de la maladie du stubborn des agrumes, dans des pervenches (Vinca rosea L.) ornementales de la ville de Rabat, Maroc. Comptes Rendus L’académie Sci. 1978, 286, 57–60. [Google Scholar]
- Bové, J.M.; Nhami, A.; Saillard, C.; Vignault, J.C.; Mouches, C.; Garnier, M.; Moutous, G.; Fos, A.; Bonfils, J.; Abassi, M.; et al. Présence au Maroc de Spiroplasma citri, l’agent causaide la maladie du stubborn des agrumes dans des pervenches (Vinca rosea) implantees en bordures d’orangeraies malades et contamination probable du chiendent Cynodon dactylon L. (Pers) par le spiroplasme. Comptes Rendus L’académie Sci. 1979, 288, 399–402. [Google Scholar]
- Bové, J.M. Mycoplasma infections of plants. Isr. J. Med. Sci. 1981, 17, 572–585. [Google Scholar]
- Bové, J.M. Stubborn and its natural transmission in the Mediterranean area and the Near East. FAO Plant Prot. Bull. 1986, 34, 15–23. [Google Scholar]
- Lee, I.M.; Davis, R.E.; Gundersen-Rindal, D.E. Phytoplasma: Phytopathogenic Mollicutes. Annu. Rev. Microbiol. 2000, 54, 221–255. [Google Scholar] [CrossRef]
- Foissac, X.; Danet, J.L.; Saillard, C.; Whitcomb, R.F.; Bové, J.M. Experimental infections of plants by spiroplasmas. In Molecular Diagnostic Procedures in Mycoplasmology; Academic Press: Cambridge, MA, USA, 1996; Volume 2, pp. 385–389. [Google Scholar]
- Přibylová, J.; Spak, J. Dodder transmission of phytoplasmas. Methods Mol. Biol. 2013, 938, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Pagliaccia, D.; Morgan, R.; Qiao, Y.; Pan, S.; Vidalakis, G.; Ma, W. Novel Diagnosis for Citrus Stubborn Disease by Detection of a Spiroplasma citri-Secreted Protein. Phytopathology 2014, 104, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Nejat, N.; Vadamalai, G.; Sijam, K.; Dickinson, M. First report of Spiroplasma citri (-induced ) associated with periwinkle lethal yellows in Southeast Asia. Plant Dis. 2011, 95, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.; Fulton, D.; Bai, X.; Meulia, T.; Hogenhout, S.A. An attachment tip and pili-like structures in insect- and plant-pathogenic spiroplasmas of the class Mollicutes. Arch. Microbiol. 2004, 181, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Melcher, U.; Wayadande, A. The phytopathogenic Spiroplasmas. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 905–947. ISBN 9780387254944. [Google Scholar]
- Oldfield, G.N.; Kaloostian, G.H.; Pierce, H.D.; Calavan, E.C.; Granett, A.L.; Blue, R.L. Beet leafhopper transmits citrus stubborn disease. Calif. Agric. 1976, 30, 15. [Google Scholar] [CrossRef]
- Labroussaa, F.; Dubrana, M.P.; Arricau-Bouvery, N.; Béven, L.; Saillard, C. Involvement of a Minimal Actin-Binding Region of Spiroplasma citri Phosphoglycerate Kinase in Spiroplasma Transmission by Its Leafhopper Vector. PLoS ONE 2011, 6, e17357. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Wayadande, A.; Melcher, U.; Ye, F. The phytopathogenic mollicute-insect vector interface: A closer look. Phytopathology 1998, 88, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Gumpf, D.J.; Oldfield, G.N.; Calavan, E.C. The Relationship of Spiroplasma citri and Circulifer tenellus. Am. Phytopathol. Soc. 1983, 733, 585–590. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gumpf, D.J.; Oldfield, G.N.; Calavan, E.C. Transmission of Spiroplasma citri by Circulifer tenellus. Am. Phytopathol. Soc. 1983, 73, 582–585. [Google Scholar] [CrossRef]
- Killiny, N.; Castroviejo, M.; Saillard, C. Spiroplasma citri Spiralin Acts In Vitro as a Lectin Binding to Glycoproteins from Its Insect Vector Circulifer haematoceps. Phytopathology 2005, 95, 541–548. [Google Scholar] [CrossRef]
- Joshi, B.D.; Berg, M.; Rogers, J.; Fletcher, J.; Melcher, U. Sequence comparisons of plasmids pBJS-O of Spiroplasma citri and pSKU146 of S. kunkelii: Implications for plasmid evolution. BMC Genom. 2005, 6, 117. [Google Scholar] [CrossRef]
- Berg, M.; Melcher, U.; Fletcher, J. Characterization of Spiroplasma citri adhesion-related protein SARP1, which contains a domain of a novel family designated sarpin. Gene 2001, 275, 57–64. [Google Scholar] [CrossRef]
- Calavan, E.C.; Christiansen, D.W. Rapid indexing for stubborn disease of citrus. Phytopathology 1965, 55, 1053. [Google Scholar]
- Rangel, B.; Krueger, R. Stubborn Disease of Citrus in California; Topics in Subtropics. Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=9392 (accessed on 10 October 2021).
- Calavan, E.C.; Roistacher, C.N.; Christiansen, D.W. Distribution of stubborn disease virus in trees of Citrus sinensis and C. paradisi at different seasons. In Proceedings of the Fourth International Organization of Citrus Virologists Conference Proceedings (1957–2010), Rome, Italy, 2–12 October 1966; pp. 145–153. [Google Scholar]
- Calavan, E.C.; Olson, E.O.; Christiansen, D.W. Transmission of the stubborn pathogen in citrus by leaf-piece grafts. In Proceedings of the Fifth International Organization of Citrus Virologists, Japan 1969; pp. 11–14. Available online: https://escholarship.org/uc/item/0858d7hv (accessed on 11 December 2021).
- Roistacher, C.N.; Navarro, L.; Murashige, T. Recovery of Citrus Selections Free of Several Viruses, Exocortis Viroid, and Spiroplasma citri by Shoot-Tip Grafting in vitro. In Proceedings of the Seventh International Organization of Citrus Virologists Conference Proceedings (1957–2010), Athens, Greece, 29 September–4 October 1975; pp. 186–193. [Google Scholar]
- Calavan, E.C.; Christiansen, D.W. Stunting and chlorosis induced in young-line citrus plants by inoculations from navel orange trees having symptoms of stubborn disease. In Proceedings of the Second International Organization of Citrus Virologists Conference Proceedings (1957–2010), Gainesville, FL, USA, 7–11 November 1960; University of Florida Press: Gainesville, FL, USA, 1961; pp. 69–76. [Google Scholar]
- Carpenter, J.B. Present status of some investigations on stubborn disease of citrus in the United States. In Citrus Virus Diseases; Wallace, J.M., Ed.; University of California Division of Agriculture: Berkeley, CA, USA, 1959; pp. 101–107. [Google Scholar]
- Cassin, J. Research on stubborn disease in Morocco. In Proceedings of the Third International Organization of Citrus Virologists, Campinas, Brazil; Sao Paulo, Brazil, 1963; pp. 204–206. Available online: https://escholarship.org/uc/item/9hx1925c (accessed on 11 December 2021).
- Chapot, H.; Cassin, J. Maladies et troubles divers affectant les citrus au Maroc. Al Awamia 1961, 1, 107–142. [Google Scholar]
- Saglio, P.; La Flèche, D.; Bonisol, C.; Bové, J.M. Isolement et culture in vitro des mycoplasmes associés au “stubborn” des agrumes et leur observation au microscope électronique. Comptes Rendus L’académie Sci. 1971, 272, 1387–1390. [Google Scholar]
- Fudl-Allah, A.E.A.; Calavan, E.C.; Igwegbe, E.C.K. Culture of a Mycoplasmalike Organism Associated with Stubborn Disease of Citrus. Phytopathology 1972, 62, 729–731. [Google Scholar] [CrossRef]
- Gumpf, D.J.; Calavan, E.C. Stubborn disease of citrus. In Mycoplasma Diseases of Trees and Shrubs; Maramorosch, K., Raychaudhuri, S.P., Eds.; Academic Press: Cambridge, MA, USA, 1981; pp. 97–134. [Google Scholar]
- Chen, T.A.; Liao, C.H. Corn Stunt Spiroplasma: Isolation, Cultivation, and Proof of Pathogenicity. Science 1975, 188, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Davis, R.E. New media for rapid growth of Spiroplasma citri and corn stunt Spiroplasma. Phytopathology 1984, 74, 84–89. [Google Scholar] [CrossRef]
- Whitcomb, R.F. Culture media for spiroplasmas. In Methods in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 1, pp. 147–158. [Google Scholar]
- Moulder, R.W.; French, F.E.; Chang, C.J. Simplified media for spiroplasmas associated with tabanid flies. Can. J. Microbiol. 2002, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.C.; Chen, T.A. Growth, morphology and ultrastructural studies of a spiroplasma associated with Bermudagrass showing witches’-broom symptoms/Wachstum, Morphologie und Ultrastruktur von Spiroplasma in hexenbesenkrankem Bermudagras. J. Plant Dis. Prot. 1980, 87, 37–45. [Google Scholar]
- Jones, A.L.; Whitcomb, R.F.; Williamson, D.L.; Coan, M.E. Comparative Growth and Primary Isolation of Spiroplasmas in Media Based on Insect Tissue Culture Formulations. Phytopathology 1977, 67, 738–746. [Google Scholar] [CrossRef]
- Williamson, D.L.; Whitcomb, R.F. Plant Mycoplasmas: A Cultivable Spiroplasma Causes Corn Stunt Disease. Science 1975, 188, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.G.; Whitcomb, R.F.; Clark, H.F.; Williamson, D.L. Pathogenic Mycoplasmas: Cultivation and Vertebrate Pathogenicity of a New Spiroplasma. Science 1977, 195, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Rangel, B.; Krueger, R.R.; Lee, R.F. Current research on Spiroplasma citri in California. In Proceedings of the Sixteenth International Organization of Citrus Virologists Conference Proceedings, Monterrey, Mexico, 7–12 November 2004; pp. 439–442. [Google Scholar]
- Clark, M.F.; Flegg, C.L.; Bar-Joseph, M.; Rottem, S. The Detection of Spiroplasma citri by Enzyme-linked Immunosorbent Assay (ELISA). Phytopathology 1978, 92, 332–337. [Google Scholar] [CrossRef]
- Saillard, C.; Garcia-Jurado, O.; Bové, J.M.; Vignault, J.C.; Moutous, G.; Fos, A.; Bonfils, J.; Nhami, A.; Vogel, R.; Viennot-Bourgin, G. Application of ELISA to the Detection of Spiroplasma citri in Plants and Insects. In Proceedings of the Eighth International Organization of Citrus Virologists Conference Proceedings, Mildura, VC, Australia, 23–25 May 1979; pp. 145–152. [Google Scholar]
- Archer, D.B.; Townsend, R.; Markham, P.G. Detection of Spiroplasma citri in plants and insect hosts by ELISA. Plant Pathol. 1982, 31, 299–306. [Google Scholar] [CrossRef]
- Ghezli, C.; Aouane, B. Evaluation des maladies des agrumes transmissibles par greffage sur le matériel végétal de multiplication de l’ITAF. In Mediterranean Research Network on Certification of Citrus (MNCC): 1998–2001; D’Onghia, A.M., Djelouah, K., Roistacher, C.N., Eds.; CIHEAM: Bari, Italy, 2002; pp. 101–104. [Google Scholar]
- Bové, J.M.; Vignault, J.C.; Saillard, C. Spiroplasma citri detection by enzyme-linked immunosorbent assay (ELISA), culture and dot hybridization. J. Med. Sci. 1987, 23, 729–731. [Google Scholar]
- Saillard, C.A.; Nhami, A.; Moreno, P.; Garnier, M.; Bové, J.M. Spiroplasma citri detection by immuno-capture PCR. In Proceedings of the Thirteenth International Organization of Citrus Virologists Conference Proceedings (1957–2010); p. 413.
- Saillard, C.; Barthe, C.; Renaudin, J.; Bové, J.M.; Moreno, P. Detection of Spiroplasma citri by culture, ELISA, dot-blot hybridization PCR and immuno-capture PCR: An evaluation. In Proceedings of the Twelfth International Organization of Citrus Virologists, New Delhi, India, 23–27 November 1992; p. 467. [Google Scholar]
- Foissac, X.; Saillard, C.; Gandar, J.; Zreik, L.; Bove, J. Spiralin Polymorphism in Strains of Spiroplasma citri Is Not Due to Differences in Posttranslational Palmitoylation. J. Bacteriol. 1996, 178, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, O.M.; AbouZeid, A.A.; Fawzya, I.M.; Azza, G.F. Immunocapture Polymerase Chain Reaction (I C- PCR) And Nucleic acid hybridization Techniques for Detection of Spiroplasma citri. Int. J. Virol. 2005, 1, 13. [Google Scholar]
- Yokomi, R.K.; Mello, A.F.S.; Saponari, M.; Fletcher, J. Polymerase chain reaction-based detection of Spiroplasma citri associated with citrus stubborn disease. Plant Dis. 2008, 92, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Bottner, K.D.; Munyaneza, J.E.; Davis, R.E.; Crosslin, J.M.; Toit, L.J.; Crosby, T. New Diseases and Epidemics Carrot Purple Leaf: A New Spiroplasmal Disease Associated with Carrots in Washington State. Plant Dis. 2006, 90, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Khanchezar, A.; Izadpanah, K.; Salehi, M. Partial Characterization of Spiroplasma citri Isolates Associated with Necrotic Yellows Disease of Safflower in Iran. J. Plant Pathol. 2012, 160, 331–336. [Google Scholar] [CrossRef]
- Maheshwari, Y.; Selvaraj, V.; Hajeri, S.; Yokomi, R. Application of droplet digital PCR for quantitative detection of Spiroplasma citri in comparison with real-time PCR. PLoS ONE 2017, 12, e0184751. [Google Scholar] [CrossRef]
- Wang, X.; Doddapaneni, H.; Chen, J.; Yokomi, R.K. Improved Real-Time PCR Diagnosis of Citrus Stubborn Disease by Targeting Prophage Genes of Spiroplasma citri. Plant Dis. 2015, 99, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Drais, M.I.; Maheshwari, Y.; Selvaraj, V.; Varvaro, L.; Yokomi, R.; Djelouah, K. Development and validation of a loop-mediated isothermal amplification technique (LAMP) for the detection of Spiroplasma citri, the causal agent of citrus stubborn disease. Eur. J. Plant Pathol. 2019, 155, 125–134. [Google Scholar] [CrossRef]
- Khanchezar, A.; Izadpanah, K.; Salehi, M.; Taghavi, S.M. Novel isolate of Spiroplasma citri from leafhopper Circulifer haematoceps from Fars province. Iran. J. Plant Pathol. 2010, 46, Pe281. [Google Scholar]
- Grulet, O.; Humphery-Smith, I.; Sunyach, C.; Le Goff, F.; Chastel, C. “Spiromed”: A rapid and inexpensive Spiroplasma isolation technique. J. Microbiol. Methods 1993, 17, 123–128. [Google Scholar] [CrossRef]
- Whitcomb, R.F.; Chen, T.A.; Williamson, D.L.; Lia, C.; Tully, J.G.; Bové, J.M.; Rose, D.L.; Coan, M.E.; Clark, T.B. Spiroplasma kunkelii sp.nov.: Characterization of the Etiological Agent of Corn Stunt Disease. Int. J. Syst. Biol. 1986, 36, 170–178. [Google Scholar]
- Yokomi, R.K.; Mello, A.F.S.; Fletcher, J.; Saponari, M. Estimation of Citrus Stubborn Incidence in Citrus Groves by Real-Time PCR. In Proceedings of the Seventh International Organization of Citrus Virologists, Athens, Greece, 29 September–4 October 1975; pp. 131–141. [Google Scholar]
- Chand, L.; Sharma, S.; Dalal, R.; Poonia, A.K. In vitro shoot tip grafting in citrus species—A review. Agric. Rev. 2013, 34, 279–287. [Google Scholar] [CrossRef]
- Carimi, F.; De Pasquale, F.; Fiore, S.; D’Onghia, A.M. Sanitation of citrus germplasm by somatic embryogenesis and shoot-tip grafting. Options Méditerranéennes 2001, 33, 61–65. [Google Scholar]
- Wei, W.; Davis, R.E.; Mowery, J.D.; Zhao, Y. Growth inhibition of phytopathogenic spiroplasmas by membrane-interactive antimicrobial peptides Novispirin T7 and Caerin 1.1. Ann. Appl. Biol. 2021, 180, 109–117. [Google Scholar] [CrossRef]
- Liao, C.H.; Chen, T.A. In vitro Susceptibility and Resistance of Two Spiroplasmas to Antibiotics. Phytopathology 1981, 71, 442–445. [Google Scholar] [CrossRef]
- Bowyer, J.W.; Calavan, E.C. Antibiotics Sensitivity In Vitro of the Mycoplasmalike Organism Associated with Citrus Stubborn Disease. Phytopathology 1974, 64, 346–349. [Google Scholar] [CrossRef]
- Bertaccini, A. Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics 2021, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, E.C.K.; Calavan, E.C. Effect of Tetracycline Antibiotics on Symptom Development of Stubborn Disease and Infectious Variegation of Citrus Seedlings. Phytopathology 1973, 63, 1044–1048. [Google Scholar] [CrossRef]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Maladies à virus des citrus dans les pays du Bassin méditerranéen. Fruits 1967, 22, 125–140. [Google Scholar]
- Nhami, A.; Bove, J.M.; Bové, C.; Monsion, M.; Garnier, M.; Saillard, C.; Moutous, G.; Fos, A. Natural Transmission of Spiroplasma citri to Periwinkles in Morocco. In Proceedings of the Eighth International Organization of Citrus Virologists Conference Proceedings, Mildura, VC, Australia, 23–25 May 1979; pp. 153–161. [Google Scholar]
- El-Otmani, M.; Coggins, J.C.W.; Duymovic, A. Citrus Cultivars and Production in Morocco. HortScience 1990, 25, 1343–1346. [Google Scholar] [CrossRef]
- Afechtal, M. Present status of virus and virus-like diseases of citrus in Morocco. Integr. Control Citrus Fruit Crop. IOBC-WPRS Bull. 2018, 132, 215–220. [Google Scholar]
- Hafidi, B.; Bencheqroun, N.; Fischer, H.U.; Vanderveken, J. Application of the immuno-enzymatic “ELISA” technique for the detection of Spiroplasma citri, the causal agent of citrus stubborn disease, in Moroccan orange orchards. Parasitica 1979, 35, 141–151. [Google Scholar]
- Drais, M.I.; Abou Kubaa, R.; Ghezli, C.; Varvaro, L.; Djelouah, K. First molecular identification and characterization of Spiroplasma citri, the causal agent of citrus stubborn disease in Algerian citrus groves. J. Plant Pathol. 2019, 101, 783. [Google Scholar] [CrossRef]
- Najar, A.; Duran-Vila, N.; Khlij, A.; Bové, M. Virus and Virus-Like Diseases of Citrus in Tunisia. In Proceedings of the Sixteenth International Organization of Citrus Virologists Conference Proceedings, Monterrey, Mexico, 7–12 November 2004; pp. 484–486. [Google Scholar]
- Najar, A.; Bouachem, S.; Danet, J.L.; Saillard, C.; Garnier, M.; Bové, J.M. Présence en Tunisie de Spiroplasma citri, l’agent causal du stubborn des agrumes et de son vecteur, la cicadelle Circulifer haematoceps. Contamination de C. haematoceps et de C. opacipennis par S. citri. Fruits 1998, 53, 391–396. [Google Scholar]
- Montaigne, E.; El Hadad-gauthier, F.; Khefifi, L. Challenges in Citrus Market Chains in Tunisia and Morocco. In Sustainable Agricultural Development; Springer: New York, NY, USA, 2015; pp. 227–253. ISBN 9783319178134. [Google Scholar]
- Vogel, R.; Bové, J.M. Studies of Stubborn Disease in Corsica. In Proceedings of the Sixth International Organization of Citrus Virologists Conference Proceedings (1957–2010), Swaziland, 1972; pp. 23–25. Available online: https://escholarship.org/uc/item/4sc3m33d (accessed on 11 December 2021).
- Anonym Scientific Opinion on pest the categorisation of Spiroplasma citri. EFSA J. 2014, 12, 1–29. [CrossRef]
- Bové, J.M.; Garnier, M. Stubborn. In Compendium of Citrus Diseases; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; Amer Phytopathological Society: St. Paul, MN, USA, 2000; pp. 48–50. [Google Scholar]
- Belabess, Z.; Afechtal, M.; Benyazid, J.; Sagouti, T.; Rhallabi, N. Prévalence des phytovirus associés aux agrumes dans la région de Berkane. Afr. Mediterr. Agric. J.-Al Awamia 2020, 129, 144–160. [Google Scholar]
- Belabess, Z.; Sagouti, T.; Rhallabi, N.; Tahiri, A.; Massart, S.; Tahzima, R.; Lahlali, R.; Jijakli, M.H. Citrus Psorosis Virus: Current Insights on a Still Poorly Understood Ophiovirus. Microorganisms 2020, 8, 1197. [Google Scholar] [CrossRef] [PubMed]
- Belabess, Z.; Radouane, N.; Sagouti, T.; Tahiri, A.; Lahlali, R. A Current Overview of Two Viroids Prevailing in Citrus Orchards: Citrus Exocortis Viroid and Hop Stunt Viroid. In Citrus; Khan, M.S., Ahmad, I., Eds.; Taschen: Cologne, Germany, 2021; p. 27. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).