Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience?
Abstract
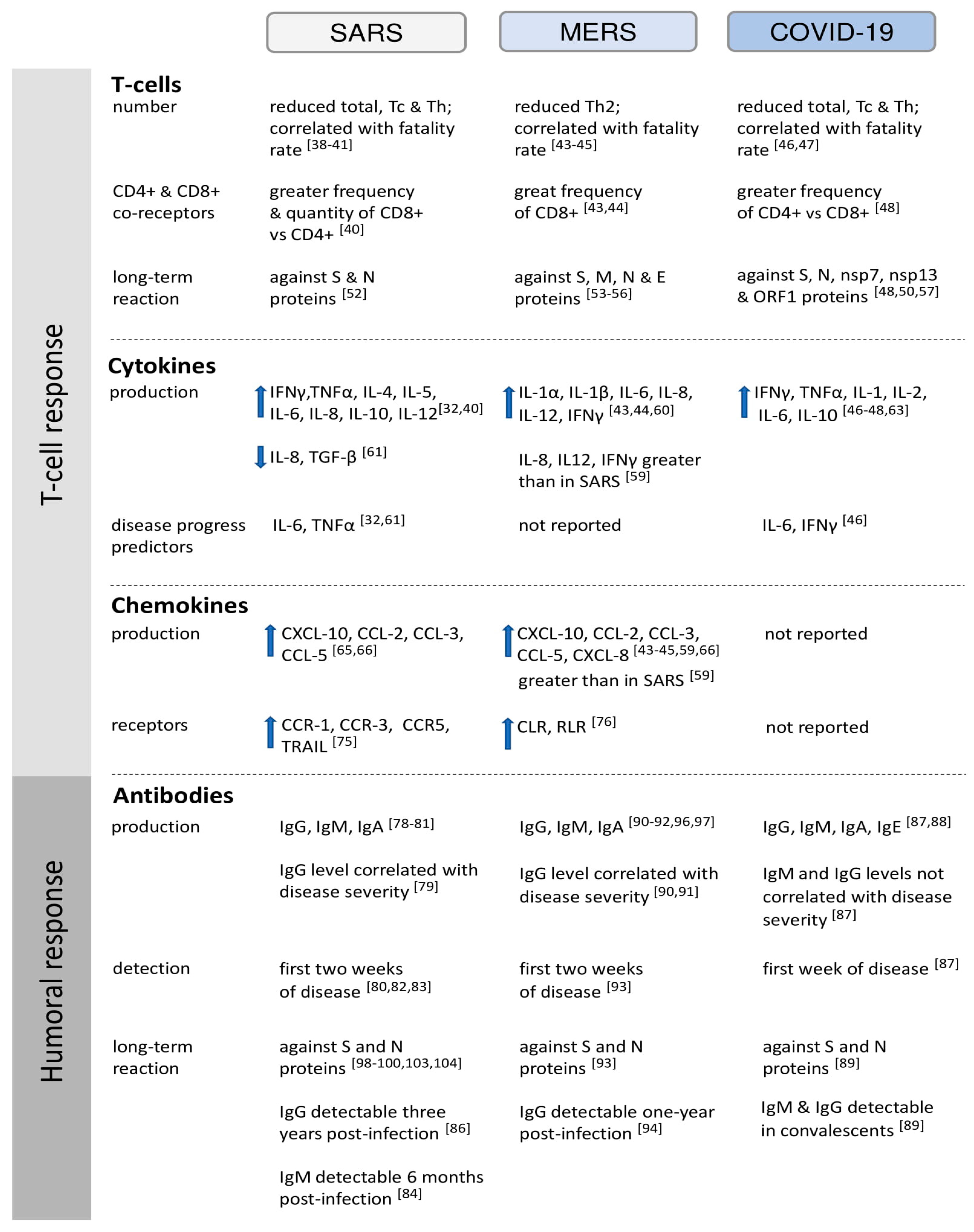
:1. Introduction
2. Cell-Mediated Immunity
2.1. T Cell Response to HCoVs
2.2. Cytokines Secretion in HCoV Infections
2.2.1. Interleukins and Tumor Necrosis Factors
2.2.2. Chemokines
2.2.3. Interferons
2.2.4. Cytokine Receptors and Ligands
3. Humoral Immunity
3.1. Kinetics of Antibody Production in Response to HCoVs
3.2. Differentiation of the Immune Response Depending on HCoV Structural Proteins
3.3. Antibody-Dependent Enhancement in HCoV Infections
4. Complement System
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Lai, M.M.C.; Holmes, K.V. Coronaviridae: The viruses and their replication. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1163–1185. [Google Scholar]
- Kahn, J.S.; McIntosh, K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005, 24, 223–227. [Google Scholar] [CrossRef]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar]
- Neuman, B.W.; Adair, B.D.; Yoshioka, C.; Quispe, J.D.; Orca, G.; Kuhn, P.; Milligan, R.A.; Yeager, M.; Buchmeier, M.J. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006, 80, 7918–7928. [Google Scholar] [CrossRef] [Green Version]
- Barcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.A.; Varkleij, A.; Rttier, P.J.M.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.M.C.; Liao, C.L.; Lin, Y.J.; Zhang, X. Coronavirus: How a large RNA viral genome is replicated and transcribed. Infect. Agents Dis. 1994, 3, 98–105. [Google Scholar]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses 2015, 1282, 1–23. [Google Scholar]
- World-Health-Organization Update 49—SARS Case Fatality Ratio, Incubation Period. Available online: https://www.who.int/csr/sars/archive/2003_05_07a/en/ (accessed on 31 January 2020).
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Yajin, Q.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- World-Health-Organization Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/emergencies/mers-cov/en/ (accessed on 31 January 2020).
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2014, 40, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.; Hu, Y.; Tao, Z.; Tian, J.; Pei, Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Gorbalenya, A.E. The species severe acute eespiratory syndrome related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Qun, L.; Xuhua, G.; Peng, W.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel CoronavirusInfected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar]
- Burke, R.M.; Midgley, C.M.; Dratch, A.; Fenstersheib, M.; Haupt, T.; Holshue, M.; Ghinai, I.; Jarashow, C.M.; Lo, J.; McPherson, T.D.; et al. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 – United States, January-February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 245–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.; Liu, L.; Zhang, Z. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26, 1320–1323. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.E.; Li, Z.; Chiew, C.J.; Yong, S.E.; Toh, M.P.; Lee, V.J. Presymptomatic Transmission of SARS-CoV-2—Singapore, 23 January–16 March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Hasoksuz, M.; Spiro, D.; Halpin, R.; Wang, S.; Vlasova, A.; Janies, D.; Jones, L.R.; Ghedin, E.; Saif, L.J. Quasipecies of bovine enteric and respiratory coronaviruses based on complete genome sequences and genetic changes after tissue culture adaptation. Virology 2007, 363, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020, 92, 433–440. [Google Scholar] [CrossRef]
- Yesilbag, Y.; Aytogu, G. Coronavirus host divergence and novel coronavirus (Sars-CoV-2) outbreak. CEOTI 2020, 2, 1–7. [Google Scholar]
- Rabenau, H.F.; Kampf, G.; Cinatl, J.; Doerr, H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005, 61, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Yang, L.; Li, J.; Lu, Z.; Wang, L.; Koup, R.A.; Bailer, R.T.; Wu, C. Human Memory T Cell Responses to SARS-CoV E Protein. Microbes Infect. 2006, 8, 2424–2431. [Google Scholar] [CrossRef]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS Coronavirus Spike Protein-Induced Innate Immune Response Occurs via Activation of the NF-kappaB Pathway in Human Monocyte Macrophages in Vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef]
- Aboagye, J.O.; Yew, C.W.; Ng, O.; Manteil, V.M.; Mirazimi, A.; Tan, Y. Overexpression of the Nucleocapsid Protein of Middle East Respiratory Syndrome Coronavirus Up-Regulates CXCL10. Biosci. Rep. 2018, 38, BSR20181059. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Samosundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-COV-2. Turk. J. Med. Sci. 2020, 21, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Kruger, N.; Muller, M.; Drosten, C.; Pohlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Taisheng, L.; Zhifeng, Q.; Linqi, Z.; Yang, H.; Wei, H.; Zhengyin, L.; Xiaojun, M.; Hongwei, F.; Wei, L.; Jing, X.; et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004, 189, 648–651. [Google Scholar]
- Li, T.S.; Qiu, Z.F.; Han, Y.; Wang, Z.; Fan, H.; Lu, W.; Xie, J.; Ma, X.; Wang, A. Rapid loss of both CD4+ and CD8+ T lymphocyte subsets during the acute phase of severe acute respiratory syndrome. Chin. Med. J. (Engl.) 2003, 116, 985–987. [Google Scholar]
- Li, C.K.; Wu, H.; Yan, H.; Ma, S.; Wang, L.; Zhang, M.; Tang, X.; Temperton, N.J.; Weiss, R.A.; Brenchley, J.M.; et al. T Cell Responses to Whole SARS Coronavirus in Humans. J. Immunol. 2008, 181, 5490–5500. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Fan, H.W.; Li, T.S.; Qiu, Z.; Han, Y. Dynamic changes of T lymphocyte subsets in the long-term follow-up of severe acute respiratory syndrome patients. Chin. Acad. Med. Sci. 2006, 28, 253–255. [Google Scholar]
- Chu, H.; Zhou, J.; Wong, B.H.; Li, C.; Chan, J.F.; Cheng, Z.; Yang, D.; Wang, D.; Lee, A.C.; Li, C.; et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Kim, Y.; Kim, G.; Lee, J.Y.; Jeong, I.; Joh, J.; Kim, H.; Chang, E.; Sim, S.Y.; Park, J.; et al. Immune Responses to Middle East Respiratory Syndrome Coronavirus During the Acute and Convalescent Phases of Human Infection. Clin. Infect. Dis. 2019, 68, 984–992. [Google Scholar] [CrossRef] [Green Version]
- Alosaimi, B.; Hamed, M.E.; Naeem, A.; Alsharef, A.A.; AlQahtani, S.Y.; AlDosari, K.M.; Alamri, A.A.; Al-Eisa, K.; Khojah, T.; Assiri, A.M.; et al. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine 2020, 126, 154895. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Choi, J.; Hong, S.; Lee, J.; Kwon, J.; Kim, S.; Park, S.Y.; Rhee, J.; Kim, B.; Choi, H.J.; et al. Predictors of Mortality in Middle East Respiratory Syndrome (MERS). Thorax 2018, 73, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; Van der Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; Van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Rydyzynski-Moderbacher, C.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Baruah, V.; Bose, S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 2020, 92, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses 2020, 12, 254. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.G.; Pun, M.C.K.; Chen, Z.L.; Feng, L.Q.; Li, Z.T.; Huang, J.C.; Ke, C.W.; Deng, X.; Ling, Y.; Wu, S.G.; et al. Characteristics of traveler with Middle East respiratory syndrome, China, 2015. Emerg. Infect. Dis. 2015, 21, 2278–2280. [Google Scholar]
- Veit, S.; Jany, S.; Fux, R.; Sutter, G.; Volz, A. CD8+ T Cells Responding to the Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Delivered by Vaccinia Virus MVA in Mice. Viruses 2018, 10, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, P.Y.; Won, L.Y.R.; Fung, C.L.; Siu, K.L.; Yeung, M.L.; Yuen, K.S.; Chan, C.P.; Woo, P.C.Y.; Yuen, K.Y.; Jin, D.Y. Middle East respiratory syndrome coronavirus M protein suppresses type I interferon expression through the inhibition of TBK1-dependent phosphorylation of IRF3. Emerg. Microbes Infect. 2016, 5, e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.H.; Liu, H.M.; Chang, M.F.; Chang, S.C. Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Suppresses Type I and Type III Interferon Induction by Targeting RIG-I Signaling. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.; Cheng, Z.; Poon, V.K.; Sun, T.; Lau, C.C.; Wong, K.K.; Chan, J.Y.; et al. Active Replication of Middle East Respiratory Syndrome Coronavirus and Aberrant Induction of Inflammatory Cytokines and Chemokines in Human Macrophages: Implications for Pathogenesis. J. Infect. Dis. 2014, 209, 1331–1342. [Google Scholar] [CrossRef]
- Kim, E.S.; Choe, P.G.; Park, W.B.; Oh, H.S.; Kim, E.J.; Nam, E.Y.; Na, S.H.; Kim, M.; Song, K.H.; Bang, J.H.; et al. Clinical Progression and Cytokine Profiles of Middle East Respiratory Syndrome Coronavirus Infection. J. Korean Med. Sci. 2016, 31, 1717–1725. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhan, Y.; Wu, L.; Yu, X.; Zhang, W.; Ye, L.; Xu, S.; Sun, R.; Wang, Y.; et al. Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immun. 2004, 72, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.; Zhu, Y.; Li, B.; Huang, C.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Subbarao, K. The Immunobiology of SARS. Annu. Rev. Immunol. 2007, 25, 443–472. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.; Dickey, R.; Carter, W. Sensitivity of SARS/MERS CoV to Interferons and Other Drugs Based on Achievable Serum Concentrations in Humans. Infect. Disord. Drug Targets. 2014, 14, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Trombetti, S.; Cicetti, S.; Antonelli, S.; Selvaggi, C.; Perrone, L.; Visca, M.; Romano, S.; Antonelli, G. Severe Acute Respiratory Syndrome Coronavirus Elicits a Weak Interferon Response Compared to Traditional Interferon-Inducing Viruses. Intervirology 2008, 51, 217–223. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Jou, Y.; Huang, S.; Hsiao, L.; Wan, L.; Lin, Y.; Kung, S.; Lin, C. SARS Coronavirus Papain-Like Protease Inhibits the TLR7 Signaling Pathway Through Removing Lys63-Linked Polyubiquitination of TRAF3 and TRAF6. Int. J. Mol. Sci. 2016, 17, 678. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yen, Y.; Singh, S.; Kao, C.; Wu-Hsieh, B.A. SARS-CoV Regulates Immune Function-Related Gene Expression in Human Monocytic Cells. Viral Immunol. 2012, 25, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Castilletti, C.; Bordi, L.; Lalle, E.; Rozera, G.; Poccia, F.; Agrati, C.; Abbate, I.; Capobianchi, M.R. Coordinate Induction of IFN-alpha and -Gamma by SARS-CoV Also in the Absence of Virus Replication. Virology 2005, 341, 163–169. [Google Scholar] [CrossRef]
- Totura, A.L.; Whitmore, A.; Agnihothram, S.; Schafer, A.; Katze, M.G.; Heise, M.T.; Baric, R.S. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. MBio 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Al-Qahtani, A.A.; Lyroni, K.; Aznaourova, M.; Tseliou, M.; Al-Anazi, M.R.; Al-Ahdal, M.N.; Alkathani, S.; Sourvinos, G.; Tsatsanis, C. Middle East Respiratory Syndrome Corona Virus Spike Glycoprotein Suppresses Macrophage Responses via DPP4-mediated Induction of IRAK-M and PPARγ. Oncotarget 2017, 8, 9053–9066. [Google Scholar] [CrossRef] [Green Version]
- Inn, K.; Kim, Y.; Aigerim, A.; Park, U.; Hwang, E.; Choi, M.; Kim, Y.; Cho, N. Reduction of Soluble Dipeptidyl Peptidase 4 Levels in Plasma of Patients Infected with Middle East Respiratory Syndrome Coronavirus. Virology 2018, 518, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Law, H.K.W.; Cheung, C.Y.; Sia, S.F.; Chan, Y.O.; Peiris, M.; Lau, Y.L. Toll-like Receptors, Chemokine Receptors and Death Receptor Ligands Responses in SARS Coronavirus Infected Human Monocyte Derived Dendritic Cells. BMC Immunol. 2009, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Chu, H.; Wong, B.H.; Chiu, M.C.; Wang, D.; Li, C.; Liu, X.; Yang, D.; Poon, V.K.; Cai, J.; et al. Activation of C-Type Lectin Receptor and (RIG)-I-Like Receptors Contributes to Proinflammatory Response in Middle East Respiratory Syndrome Coronavirus-Infected Macrophages. J. Infect. Dis. 2020, 221, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020, 92, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Chen, X.; Xu, A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003, 349, 508–509. [Google Scholar] [CrossRef]
- Chen, W.J.; Xu, Z.Y.; Mu, J.S.; Yang, L.; Gan, H.; Mu, F.; Fan, B.; He, B.; Huang, S.; You, B.; et al. Antibody response and viraemia during the course of severe acute respiratory syndrome (SARS)-associated coronavirus infection. J. Med. Microbiol. 2004, 53, 435–438. [Google Scholar] [CrossRef]
- He, Z.; Dong, Q.; Zhuang, H.; Song, S.; Peng, G.; Luo, G.; Dwyer, D.E. Kinetics of Severe Acute Respiratory Syndrome (SARS) Coronavirus-Specific Antibodies in 271 Laboratory-Confirmed Cases of SARS. Clin. Diagn. Lab. Immunol. 2004, 11, 792–794. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, P.R.; Huang, L.M.; Chen, P.J.; Kao, C.L.; Yang, P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin. Microbiol. Infect. 2004, 10, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Corner, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Peiris, J.S.; Chu, C.M.; Cheng, V.C.C.; Chan, K.S.; Hung, I.F.N.; Poon, L.L.M.; Law, K.I.; Tang, B.S.F.; Hon, T.Y.W.; Chan, C.S.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Mo, H.; Xu, J.; Ren, X.; Zeng, G.; Tan, Y.; Chen, R.; Chan-Yeung, M.; Zhong, N. Evaluation by Indirect Immunofluorescent Assay and Enzyme Linked Immunosorbent Assay of the Dynamic Changes of Serum Antibody Responses Against Severe Acute Respiratory Syndrome Coronavirus. Chin. Med. J. (Engl.) 2005, 118, 446–450. [Google Scholar] [PubMed]
- Tang, F.; Quan, Y.; Xin, Z.-T.; Wrammert, J.; Ma, M.-J.; Lv, H.; Wang, T.-B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: A six-year follow-up study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Liu, L.; Du, L.; Zhang, C.; Jiang, S.; Li, T.; He, Y. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol. J. 2010, 7, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.; Wang, T.; Zhang, B.; Luo, Y.; Mao, L.; Wang, F.; Wu, S.; Sun, Z. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin. Transl. Immunol. 2020, 9, e1136. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, H.X.; Hu, K.; Wu, X.J.; Zhang, Y.T.; Wang, M.M.; Wang, T.; Zheng, Z.S.; Li, X.C.; Zeng, S.L. Abnormal immunity of non-survivors with COVID-19: Predictors of mortality. Infect. Dis. Poverty 2020, 9, 108. [Google Scholar]
- Ni, L.; Ye, F.; Cheng, M.L.; Feng, Y.; Deng, Y.Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef]
- Park, W.B.; Perera, R.A.P.M.; Choe, P.G.; Lau, E.H.Y.; Choi, S.J.; Chun, J.Y.; Oh, H.S.; Song, K.H.; Bang, J.H.; Kim, E.S.; et al. Kinetics of Serologic Responses to MERS Coronavirus Infection in Humans, Korea. Emerg. Infect. Dis. 2015, 21, 2186–2189. [Google Scholar] [CrossRef]
- Corman, V.M.; Albarrak, A.M.; Omrani, A.S.; Albarrak, M.M.; Farah, M.E.; Almasri, M.; Muth, D.; Sieberg, A.; Meyer, B.; Assiri, A.M.; et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin. Infect. Dis. 2016, 62, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Al-Kahlout, R.A.; Nasrallah, G.H.; Farag, E.A.; Wang, L.; Lattwein, E.; Muller, M.A.; El-Zowalaty, M.E.; Al-Romaihi, H.E.; Graham, B.S.; Al-Thani, A.A.; et al. Comparative Serological Study for the Prevalence of Anti-MERS Coronavirus Antibodies in High- And Low-Risk Groups in Qatar. J. Immunol. Res. 2019, 2019, 1386740. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, H.; Deng, Y.; Song, T.; Lan, J.; Wu, G.; Ke, C.; Tan, W. Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China. Emerg. Microbes Infect. 2016, 5, e113. [Google Scholar] [CrossRef]
- Choe, P.G.; Perera, R.A.P.M.; Park, W.B.; Song, K.; Bang, J.H.; Kim, E.S.; Kim, H.B.; Ko, L.W.R.; Park, S.W.; Kim, N.; et al. MERS-CoV Antibody Responses 1 Year After Symptom Onset, Korea, 2015. Emerg. Infect. Dis. 2017, 23, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Alhetheel, A.; Altalhi, H.; Albarrag, A.; Shakoor, Z.; Mohamed, D.; El-Hazmi, M.; Somily, A.; Barry, M.; Bakhrebah, M.; Nassar, M. Assessing the Detection of Middle East Respiratory Syndrome Coronavirus IgG in Suspected and Proven Cases of Middle East Respiratory Syndrome Coronavirus Infection. Viral Immunol. 2017, 30, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muth, D.; Corman, V.M.; Meyer, B.; Assiri, A.; Al-Masri, M.; Farah, M.; Steinhagen, K.; Lattwein, E.; Al-Tawfig, J.A.; Albarrak, A.; et al. Infectious Middle East Respiratory Syndrome Coronavirus Excretion and Serotype Variability Based on Live Virus Isolates from Patients in Saudi Arabia. J. Clin. Microbiol. 2015, 53, 2951–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Muller, M.A.; Seok, H.; Park, G.E.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E.; Baek, J.Y.; Kim, S.H.; Kang, J.M.; et al. Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: Performance analysis of serologic tests. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kong, W.; Huang, Y.; Roberts, A.; Murphy, B.R.; Subbarao, K.; Nabel, G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004, 428, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Deming, D.; Sheahan, T.; Heise, M.; Yount, B.; Davis, N.; Sims, A.; Suthar, M.; Harkema, J.; Whitmore, A.; Pickles, R.; et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006, 3, e525. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Lu, X.; Tao, L.; Bai, B.; Zhang, Z.; Chen, Y.; Zheng, F.; Chen, J.; Chen, Z.; Wanh, H. Induction of Specific Immune Responses by Severe Acute Respiratory Syndrome Coronavirus Spike DNA Vaccine with or Without interleukin-2 Immunization Using Different Vaccination Routes in Mice. Clin. Vaccine Immunol. 2007, 14, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA Vaccine Induces Neutralizing Antibody and Cellular Immune Responses in Healthy Adults in a Phase I Clinical Trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Mitchison, N.A. T-cell-B-cell cooperation. Nat. Rev. Immunol. 2004, 4, 308–312. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Li, J.; Yin, J.; Zhu, Q.; Wang, H.; Yang, Y.; Qin, E.; You, B.; Li, W.; et al. Assessment of immunoreactive synthetic peptides from the structural proteins of severe acute respiratory syndrome coronavirus. Clin. Chem. 2003, 49, 1989–1996. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Shi, Y.; Li, P.; Li, L.; Yi, Y.; Ma, Q.; Cao, C. Profile of antibodies to the nucleocapsid protein of the severe acute respiratory syndrome (SARS)-associated coronavirus in probable SARS patients. Clin. Vaccine Immunol. 2004, 11, 227–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peron, J.P.S.; Nakaya, H. Susceptibility of the Elderly to SARS-CoV-2 Infection: ACE-2 Overexpression, Shedding, and Antibody-dependent Enhancement (ADE). Clinics 2020, 75, e1912. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Tirado, S.M.; Yoon, K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Vennema, H.; De Groot, R.J.; Harbour, D.A.; Dalderup, M.; Gruffydd-Jones, T.; Horzinek, M.C.; Spaan, W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 1990, 64, 1407–1409. [Google Scholar] [CrossRef] [Green Version]
- Hohdatsu, T.; Yamada, M.; Tominaga, R.; Makino, K.; Kida, K.; Koyama, H. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 1998, 60, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Kam, Y.W.; Kien, F.; Roberts, A.; Cheung, Y.C.; Lamirande, E.W.; Vogel, L.; Chu, S.L.; Tse, J.; Guarner, J.; Zaki, S.R.; et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine 2007, 25, 729–740. [Google Scholar] [CrossRef]
- Jaume, M.; Yip, M.S.; Cheung, C.Y.; Leung, N.H.L.; Li, P.H.; Kien, F.; Dutry, I.; Callendret, B.; Escriou, N.; Altmeyer, R.; et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 2011, 85, 10582–10597. [Google Scholar] [CrossRef] [Green Version]
- Yip, M.S.; Leung, N.H.L.; Cheung, C.Y.; Li, P.H.; Yeung, H.H.; Daeron, M.; Peiris, J.S.M.; Bruzzone, R.; Jaume, M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. J. Virol. 2014, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, L.; Kuwahara, K.; Li, L.; Liu, Z.; Li, T.; Zhu, H.; Liu, J.; Xu, Y.; Xie, J.; et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect. Dis. 2016, 2, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4, e123158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Yu, W.; He, T.; Yu, J.; Yi, C.E.; Ba, L.; Li, W.; Farzan, M.; Chen, Z.; et al. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J. Med. Virol. 2006, 78, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Zhao, G.; Yang, Y.; Qiu, H.; Wang, L.; Kou, Z.; Tao, X.; Yu, H.; Sun, S.; Tseng, C.T.; et al. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 7045–7053. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020, 94, e02015–e02019. [Google Scholar] [CrossRef] [Green Version]
- The Protein Cell Atlas Webpage. Available online: https://www.proteinatlas.org/ENSG00000143226-FCGR2A/tissue (accessed on 30 June 2020).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Ooi, G.C.; Daqing, M. Sars: Radiological features. Respirology 2003, 8, S15–S19. [Google Scholar] [CrossRef]
- Bost, P.; Giladi, A.; Liu, Y.; Bendjelal, Y.; Xu, G.; David, E.; Bletcher-Gonen, R.; Cohen, M.; Medaglia, C.; Li, H.; et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell 2020, 181, 1475–1488. [Google Scholar] [CrossRef]
- Tan, W.; Lu, Y.; Zhang, J.; Wang, J.; Dan, Y.; Tan, Z.; He, X.; Qian, C.; Sun, Q.; Hu, Q.; et al. Viral kinetics and antibody responses in patients with COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Fierz, W.; Waltz, B. Antibody Dependent Enhancement Due to Original Antigenic Sin and the Development of SARS. Front. Immunol. 2020, 11, 1120. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.L.; Li, X.; Meng, Y.; Xiao, B.; Ma, Q.; Ying, S.S.; Wu, P.S.; Zhang, Z.S. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin. Exp. Pharmacol. Physiol. 2010, 37, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Sedokani, A.; Feizollahzadeh, S. Plasmapheresis, Anti-ACE2 and Anti-FcγRII Monoclonal Antibodies: A Possible Treatment for Severe Cases of COVID-19. Drug Des. Dev. Ther. 2020, 14, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zhao, G.; Song, N.; Li, P.; Chen, Y.; Guo, Y.; Li, J.; Du, L.; Jiang, S.; Guo, R.; et al. Blockade of the C5a-C5aR Axis Alleviates Lung Damage in hDPP4-transgenic Mice Infected With MERS-CoV. Emerg. Microbes Infect. 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yang, Y.L.; Jang, S.; Jang, Y. Human β-Defensin 2 Plays a Regulatory Role in Innate Antiviral Immunity and Is Capable of Potentiating the Induction of Antigen-Specific Immunity. Virol. J. 2018, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, Y.L.; Jang, Y. Human β-Defensin 2 Is Involved in CCR2-mediated Nod2 Signal Transduction, Leading to Activation of the Innate Immune Response in Macrophages. Immunobiology 2019, 224, 502–510. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinderewicz, E.; Czelejewska, W.; Jezierska-Wozniak, K.; Staszkiewicz-Chodor, J.; Maksymowicz, W. Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience? Pathogens 2020, 9, 739. https://doi.org/10.3390/pathogens9090739
Sinderewicz E, Czelejewska W, Jezierska-Wozniak K, Staszkiewicz-Chodor J, Maksymowicz W. Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience? Pathogens. 2020; 9(9):739. https://doi.org/10.3390/pathogens9090739
Chicago/Turabian StyleSinderewicz, Emilia, Wioleta Czelejewska, Katarzyna Jezierska-Wozniak, Joanna Staszkiewicz-Chodor, and Wojciech Maksymowicz. 2020. "Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience?" Pathogens 9, no. 9: 739. https://doi.org/10.3390/pathogens9090739
APA StyleSinderewicz, E., Czelejewska, W., Jezierska-Wozniak, K., Staszkiewicz-Chodor, J., & Maksymowicz, W. (2020). Immune Response to COVID-19: Can We Benefit from the SARS-CoV and MERS-CoV Pandemic Experience? Pathogens, 9(9), 739. https://doi.org/10.3390/pathogens9090739




