Mayaro Virus Pathogenesis and Transmission Mechanisms
Abstract
1. Introduction
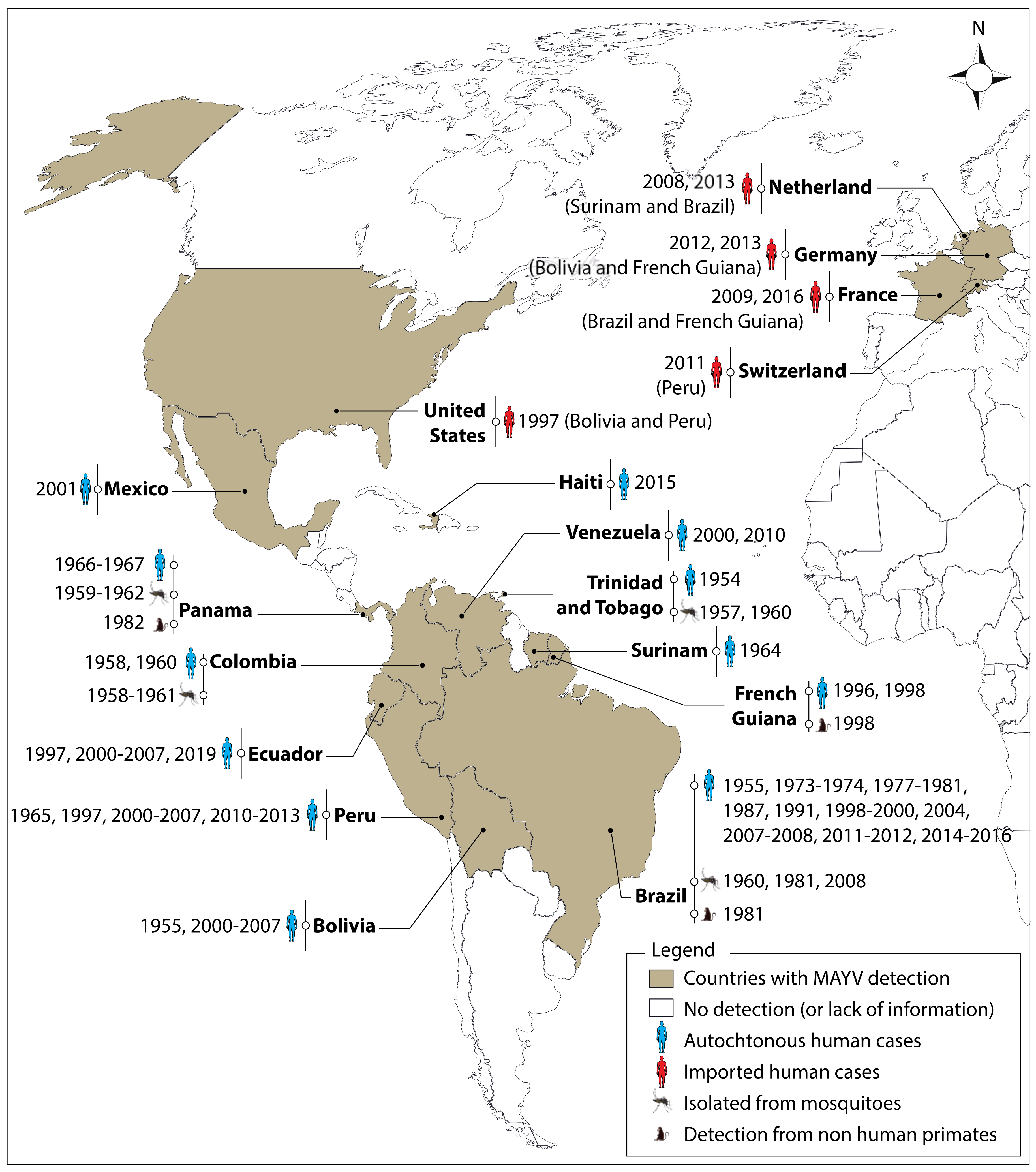
2. Distribution and Significance to Health
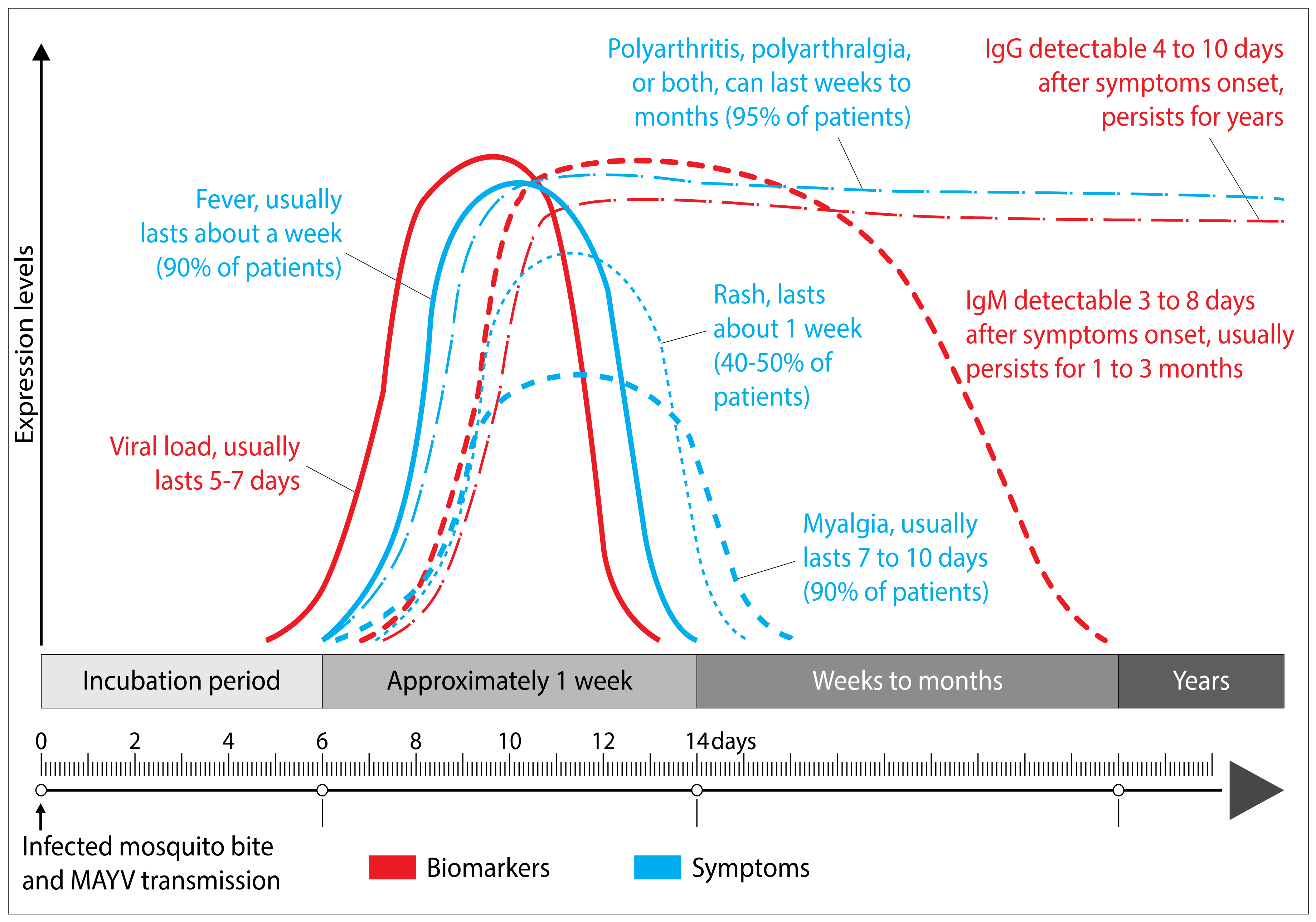
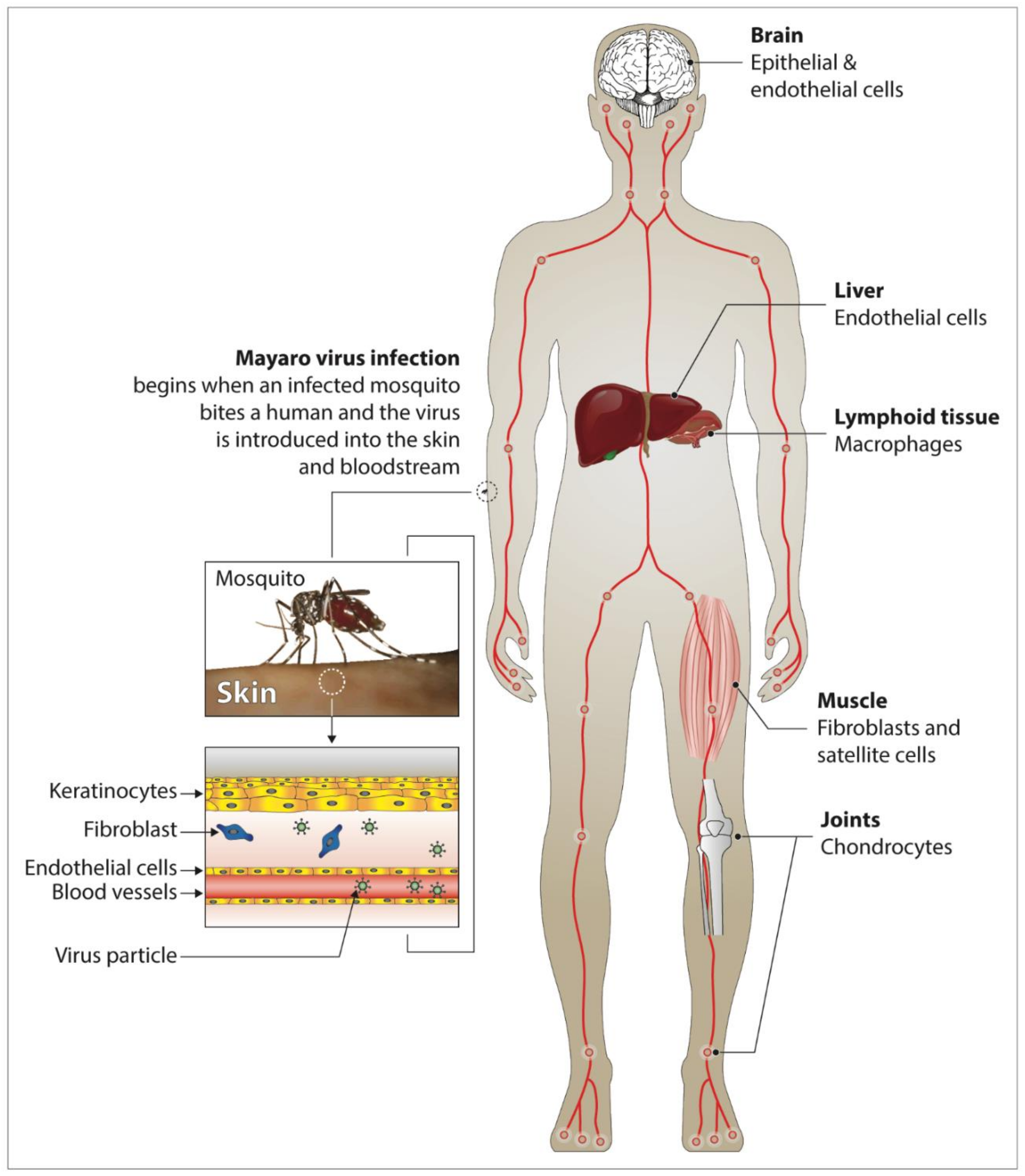
3. Clinical Manifestations and Pathogenesis of Mayaro Fever in Humans
4. Diagnosis
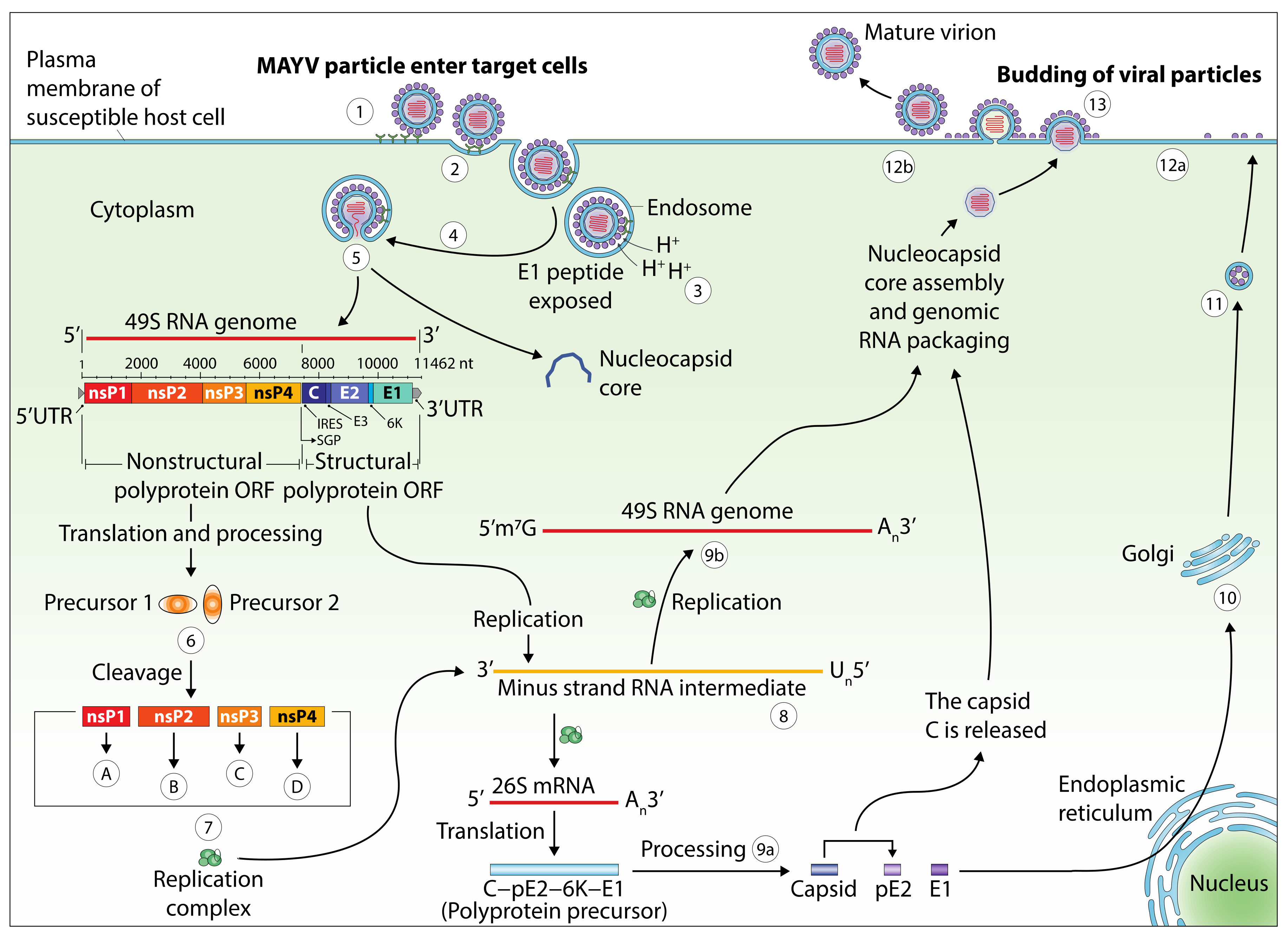
5. Genomic Structure and Viral Life Cycle
6. Phylogeny
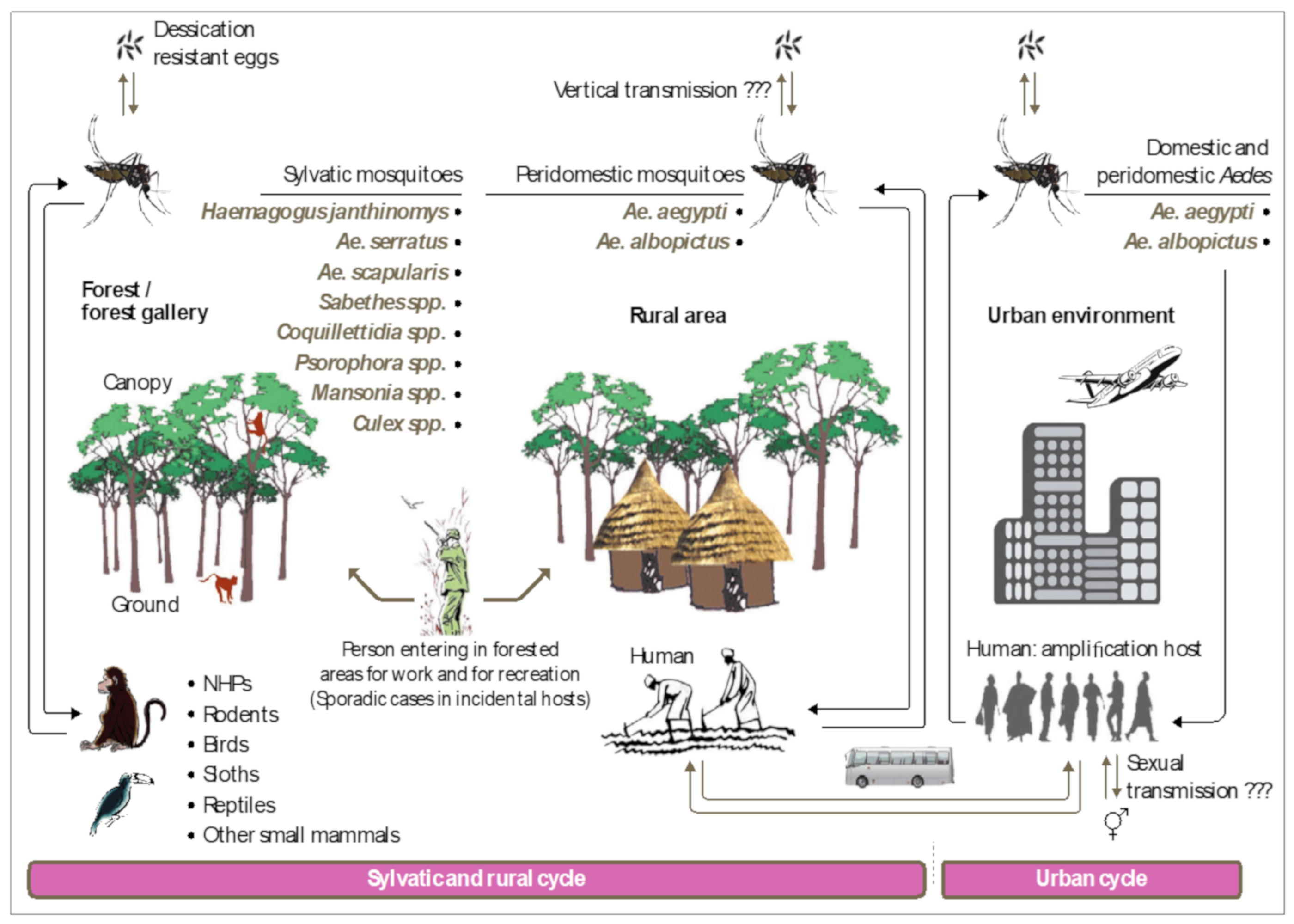
7. Transmission Cycle
8. MAYV Vectors: Field and Laboratory Observations
8.1. Evidence from the Field
8.2. Vector Competence Studies
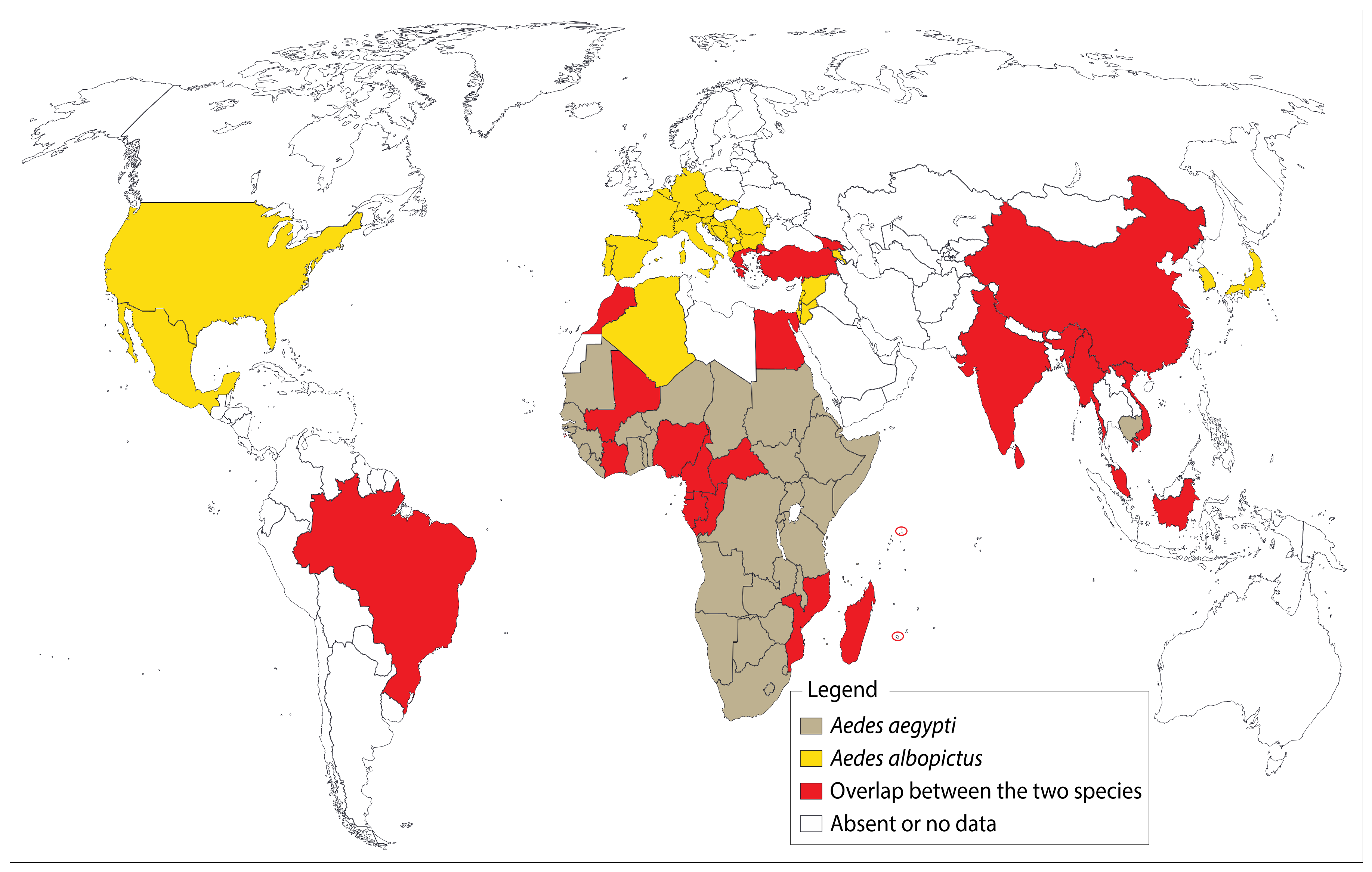
9. Major Urban MAYV Vectors: Extension and Risk to Developed and Developing Countries
9.1. Aedes aegypti
9.2. Aedes albopictus
10. Other Potential Vertebrate Hosts for MAYV
11. MAYV Transmission in Nonhuman Primates
12. Emergence Mechanisms of Urban MAYV Transmission
13. Entomological Surveillance
14. Prevention and Control
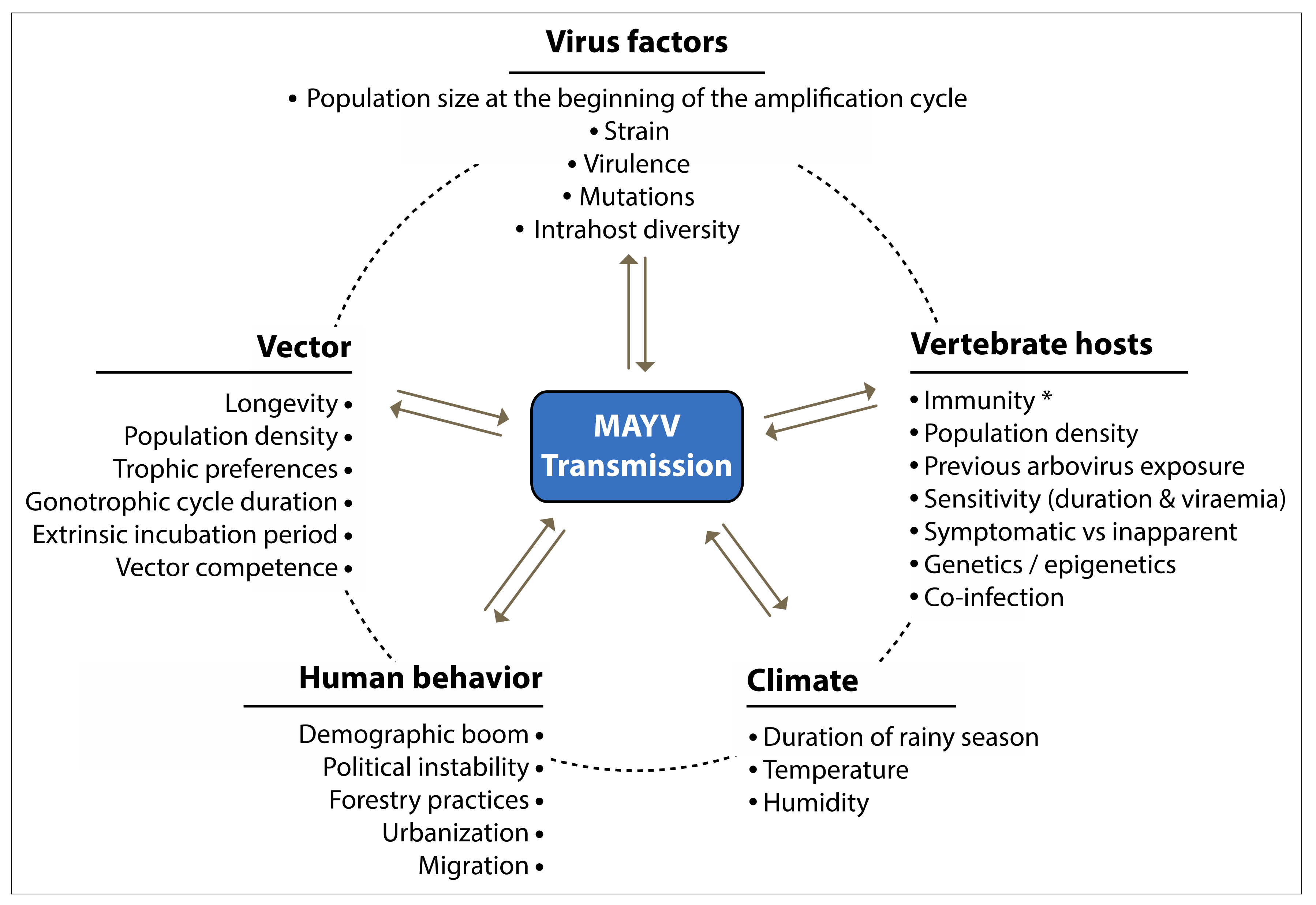
15. Factors Associated with MAYV Emergence and Invasion
16. Future Trends
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro Virus: A New Human Disease Agent. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Pinheiro, F.P.; da Rosa, A.P.A.T. An Outbreak of Mayaro Virus Disease in Belterra, Brazil II. Epidemiology. Am. J. Trop. Med. Hyg. 1981, 30, 682–688. [Google Scholar] [CrossRef]
- Schaeffer, M.; Gajdusek, D.C.; Lema, A.B.; Eichenwald, H. Epidemic Jungle Fevers Among Okinawan Colonists in the Bolivian Rain Forest. Am. J. Trop. Med. Hyg. 1959, 8, 372–396. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.A.M.; Silva, J.L.; Oliveira, A.C.; Gomes, A.M.O. On the entry of an emerging arbovirus into host cells: Mayaro virus takes the highway to the cytoplasm through fusion with early endosomes and caveolae-derived vesicles. PeerJ 2017, 5, e3245. [Google Scholar] [CrossRef] [PubMed]
- Mezencio, J.M.S.; de Souza, W.; Fonseca, M.E.F.; Rebello, M.A. Ultrastructural study of Mayaro virus replication in BHK-21 cells. Arch. Virol. 1990, 114, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mezencio, J.M.S.; de Souza, W.; Fonseca, M.E.F.; Rebello, M.A. Replication of Mayaro virus in Aedes albopictus cells: An electron microscopic study. Arch. Virol. 1989, 104, 299–308. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Travassos da Rosa, A.; Rodrigues, S.G.; Travassos da Rosa, E.S.; Dégallier, N.; Travassos da Rosa, J.F. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad. Saúde Pública 2001, 17, S155–S164. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Freitas, R.B.; da Rosa, J.F.T.; Gabbay, Y.B.; Mello, W.A.; LeDuc, J.W. An Outbreak of Mayaro Virus Disease in Belterra, Brazil I. Clinical and Virological Findings. Am. J. Trop. Med. Hyg. 1981, 30, 674–681. [Google Scholar] [CrossRef]
- Izurieta, R.O.; DeLacure, D.A.; Izurieta, A.; Hoare, I.A.; Reina Ortiz, M. Mayaro virus: The jungle flu. Virus Adapt. Treat. 2018, 10, 9–17. [Google Scholar] [CrossRef][Green Version]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Theilacker, C.; Held, J.; Allering, L.; Emmerich, P.; Schmidt-Chanasit, J.; Kern, W.V.; Panning, M. Prolonged polyarthralgia in a German traveller with Mayaro virus infection without inflammatory correlates. BMC Infect. Dis. 2013, 13, 369. [Google Scholar] [CrossRef]
- Tesh, R.B.; Watts, D.M.; Russell, K.L.; Damodaran, C.; Calampa, C.; Cabezas, C.; Ramirez, G.; Vasquez, B.; Hayes, C.G.; Rossi, C.A.; et al. Mayaro Virus Disease: An Emerging Mosquito-Borne Zoonosis in Tropical South America. Clin. Infect. Dis. 1999, 28, 67–73. [Google Scholar] [CrossRef]
- Phillips, P.A.; Lehmann, D.; Spooner, V.; Barker, J.; Tulloch, S.; Sungu, M.; Canil, K.A.; Pratt, R.D.; Lupiwa, T.; Alpers, M.P. Viruses associated with acute lower respiratory tract infections in children from the Eastern Highlands of Papua New Guinea (1983–1985). Southeast Asian J. Trop. Med. Public Health 1990, 21, 373–382. [Google Scholar]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An emerging viral threat? Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Aguilar-Luis, M.A.; del Valle-Mendoza, J.; Silva-Caso, W.; Gil-Ramirez, T.; Levy-Blitchtein, S.; Bazán-Mayra, J.; Zavaleta-Gavidia, V.; Cornejo-Pacherres, D.; Palomares-Reyes, C.; del Valle, L.J. An emerging public health threat: Mayaro virus increases its distribution in Peru. Int. J. Infect. Dis. 2020, 92, 253–258. [Google Scholar] [CrossRef]
- Pego, P.N.; Gomes, L.P.; Provance, D.W., Jr.; De Simone, S.G. Mayaro Virus Disease. J. Hum. Virol. Retrovirol. 2014. [Google Scholar] [CrossRef]
- Azevedo, R.S.S.; Silva, E.V.P.; Carvalho, V.L.; Rodrigues, S.G.; Neto, J.P.N.; Monteiro, H.A.O.; Peixoto, V.S.; Chiang, J.O.; Nunes, M.R.T.; Vasconcelos, P.F.C. Mayaro Fever Virus, Brazilian Amazon. Emerg. Infect. Dis. 2009, 15, 1830–1832. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Srihongse, S.; Rodaniche, E.D.; Grayson, M.A. An Ecological Survey for Arboviruses in Almirante, Panama, 1959–1962. Am. J. Trop. Med. Hyg. 1966, 15, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.; Travassos da Rosa, A.; Freitas, R.; Travassos da Rosa, J.; Vasconcelos, P. Aspectos clínico-epidemiológicos das arboviroses. Inst. Evandro Chagas 1986, 50, 375–408. [Google Scholar]
- Seymour, C.; Peralta, P.H.; Montgomery, G.G. Serologic Evidence of Natural Togavirus Infections in Panamanian Sloths and Other Vertebrates. Am. J. Trop. Med. Hyg. 1983, 32, 854–861. [Google Scholar] [CrossRef]
- de Oliveira Mota, M.T.; Ribeiro, M.R.; Vedovello, D.; Nogueira, M.L. Mayaro virus: A neglected arbovirus of the Americas. Future Virol. 2015, 10, 1109–1122. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E. Mayaro virus: A forest virus primed for a trip to the city? Microbes Infect. 2016, 18, 724–734. [Google Scholar] [CrossRef]
- Galindo, P.; Srihongse, S. Transmission of Arboviruses to Hamsters by the Bite of Naturally Infected Culex (Melanoconion) Mosquitoes. Am. J. Trop. Med. Hyg. 1967, 16, 525–530. [Google Scholar] [CrossRef]
- Muñoz, M.; Navarro, J.C. Mayaro: A re-emerging Arbovirus in Venezuela and Latin America. Biomédica 2012, 32, 286–302. [Google Scholar] [CrossRef]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; dos Santos, F.A.L.; Slhessarenko, R.D. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef]
- Mourão, M.P.G.; de Souza Bastos, M.; de Figueiredo, R.P.; Gimaque, J.B.L.; dos Santos Galusso, E.; Kramer, V.M.; de Oliveira, C.M.C.; Naveca, F.G.; Figueiredo, L.T.M. Mayaro Fever in the City of Manaus, Brazil, 2007–2008. Vector-Borne Zoonotic Dis. 2011, 12, 42–46. [Google Scholar] [CrossRef]
- Halsey, E.S.; Siles, C.; Guevara, C.; Vilcarromero, S.; Jhonston, E.J.; Ramal, C.; Aguilar, P.V.; Ampuero, J.S. Mayaro Virus Infection, Amazon Basin Region, Peru, 2010–2013. Emerg. Infect. Dis. 2013, 19, 1839–1842. [Google Scholar] [CrossRef]
- Lednicky, J.; De Rochars, V.M.B.; Elbadry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Okech, B.; et al. Mayaro Virus in Child with Acute Febrile Illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Spinsanti, L.I.; Almiron, W.R.; Contigiani, M.S. UNA virus: First report of human infection in Argentina. Rev. Inst. Med. Trop. São Paulo 2003, 45, 109–110. [Google Scholar] [CrossRef]
- Forshey, B.M.; Guevara, C.; Laguna-Torres, V.A.; Cespedes, M.; Vargas, J.; Gianella, A.; Vallejo, E.; Madrid, C.; Aguayo, N.; Gotuzzo, E. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl. Trop. Dis. 2010, 4, e787. [Google Scholar] [CrossRef] [PubMed]
- Blohm, G.; Elbadry, M.A.; Mavian, C.; Stephenson, C.; Loeb, J.; White, S.; Telisma, T.; Chavannes, S.; Beau De Rochar, V.M.; Salemi, M.; et al. Mayaro as a Caribbean traveler: Evidence for multiple introductions and transmission of the virus into Haiti. Int. J. Infect. Dis. 2019, 87, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Hassing, R.-J.; Leparc-Goffart, I.; Blank, S.N.; Thevarayan, S.; Tolou, H.; van Doornum, G.; van Genderen, P.J. Imported Mayaro virus infection in the Netherlands. J. Infect. 2010, 61, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Cramer, J.P.; Field, V.; Caumes, E.; Jensenius, M.; Gkrania-Klotsas, E.; de Vries, P.J.; Grobusch, M.P.; Lopez-Velez, R.; Castelli, F.; et al. Infectious diseases among travellers and migrants in Europe, EuroTravNet 2010. Eurosurveillance 2012, 17, 20205. [Google Scholar] [CrossRef]
- Receveur, M.C.; Grandadam, M.; Pistone, T.; Malvy, D. Infection with Mayaro virus in a French traveller returning from the Amazon region, Brazil, January, 2010. Eurosurveillance 2010, 15, 19563. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, A.; Gabriel, M.; Fritz, J.; Günther, S.; Hatz, C.; Schmidt-Chanasit, J.; Blum, J. Mayaro Virus Infection in Traveler Returning from Amazon Basin, Northern Peru. Emerg. Infect. Dis. 2012, 18, 695–696. [Google Scholar] [CrossRef]
- Llagonne-Barets, M.; Icard, V.; Leparc-Goffart, I.; Prat, C.; Perpoint, T.; André, P.; Ramière, C. A case of Mayaro virus infection imported from French Guiana. J. Clin. Virol. 2016, 77, 66–68. [Google Scholar] [CrossRef]
- Coimbra, T.L.M.; Santos, C.L.S.; Suzuki, A.; Petrella, S.M.C.; Bisordi, I.; Nagamori, A.H.; Marti, A.T.; Santos, R.N.; Fialho, D.M.; Lavigne, S.; et al. Mayaro virus: Imported cases of human infection in São Paulo State, Brazil. Rev. Inst. Med. Trop. São Paulo 2007, 49, 221–224. [Google Scholar] [CrossRef]
- Talarmin, A.; Chandler, L.J.; Kazanji, M.; de Thoisy, B.; Debon, P.; Lelarge, J.; Labeau, B.; Bourreau, E.; Vié, J.C.; Shope, R.E.; et al. Mayaro virus fever in French Guiana: Isolation, identification, and seroprevalence. Am. J. Trop. Med. Hyg. 1998, 59, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Arenívar, C.; Rodríguez, Y.; Rodríguez-Morales, A.J.; Anaya, J.-M. Osteoarticular manifestations of Mayaro virus infection. Curr. Opin. Rheumatol. 2019, 31, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Grand, R.L.; Gras, G.; Roques, P. Chikungunya Disease: Infection-Associated Markers from the Acute to the Chronic Phase of Arbovirus-Induced Arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Her, Z.; Ong, E.K.S.; Chen, J.; Dimatatac, F.; Kwek, D.J.C.; Barkham, T.; Yang, H.; Rénia, L.; Leo, Y.-S.; et al. Persistent Arthralgia Induced by Chikungunya Virus Infection is Associated with Interleukin-6 and Granulocyte Macrophage Colony-Stimulating Factor. J. Infect. Dis. 2011, 203, 149–157. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Noret, M.; Herrero, L.; Rulli, N.; Rolph, M.; Smith, P.N.; Li, R.W.; Roques, P.; Gras, G.; Mahalingam, S. Interleukin 6, RANKL, and Osteoprotegerin Expression by Chikungunya Virus-Infected Human Osteoblasts. J. Infect. Dis. 2012, 206, 455–457. [Google Scholar] [CrossRef]
- Morrison, T.E.; Oko, L.; Montgomery, S.A.; Whitmore, A.C.; Lotstein, A.R.; Gunn, B.M.; Elmore, S.A.; Heise, M.T. A Mouse Model of Chikungunya Virus–Induced Musculoskeletal Inflammatory Disease: Evidence of Arthritis, Tenosynovitis, Myositis, and Persistence. Am. J. Pathol. 2011, 178, 32–40. [Google Scholar] [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Tappe, D.; Pérez-Girón, J.V.; Just-Nübling, G.; Schuster, G.; Gómez-Medina, S.; Günther, S.; Muñoz-Fontela, C.; Schmidt-Chanasit, J. Sustained Elevated Cytokine Levels during Recovery Phase of Mayaro Virus Infection. Emerg. Infect. Dis. 2016, 22, 750–752. [Google Scholar] [CrossRef]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015, 9, e0004104. [Google Scholar] [CrossRef]
- Santos, F.M.; Dias, R.S.; de Oliveira, M.D.; Costa, I.C.T.A.; de Souza Fernandes, L.; Pessoa, C.R.; da Matta, S.L.P.; Costa, V.V.; Souza, D.G.; da Silva, C.C.; et al. Animal model of arthritis and myositis induced by the Mayaro virus. PLoS Negl. Trop. Dis. 2019, 13, e0007375. [Google Scholar] [CrossRef] [PubMed]
- Churchman, S.M.; Ponchel, F. Interleukin-7 in rheumatoid arthritis. Rheumatology 2008, 47, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-A.; Yoon, H.-J.; Kim, H.-S.; Chae, C.-B.; Falco, S.D.; Cho, C.-S.; Kim, W.-U. Role of placenta growth factor and its receptor flt-1 in rheumatoid inflammation: A link between angiogenesis and inflammation. Arthritis Rheum. 2009, 60, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; da Costa Guerra, J.F.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Oxidative stress in Mayaro virus infection. Virus Res. 2017, 236, 1–8. [Google Scholar] [CrossRef] [PubMed]
- da Silva Caetano, C.C.; Camini, F.C.; Almeida, L.T.; Ferraz, A.C.; da Silva, T.F.; Lima, R.L.S.; de Freitas Carvalho, M.M.; de Freitas Castro, T.; Carneiro, C.M.; de Mello Silva, B.; et al. Mayaro Virus Induction of Oxidative Stress is Associated With Liver Pathology in a Non-Lethal Mouse Model. Sci. Rep. 2019, 9, 15289. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Khan, M.; Alam, S.I.; Rao, P.V.L.; Parida, M. Differential proteome analysis of Chikungunya virus-infected new-born mice tissues reveal implication of stress, inflammatory and apoptotic pathways in disease pathogenesis. Proteomics 2011, 11, 1936–1951. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Jantzi, P.D.; Esham, D.L.; Spratt, H.; Kurosky, A.; Casola, A.; Garofalo, R.P. Viral-mediated Inhibition of Antioxidant Enzymes Contributes to the Pathogenesis of Severe Respiratory Syncytial Virus Bronchiolitis. Am. J. Respir. Crit. Care Med. 2011, 183, 1550–1560. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Ezzikouri, S.; Sanada, T.; Chi, H.; Hayashi, Y.; Rebbani, K.; Kitab, B.; Matsuu, A.; Miyoshi, N.; Hishima, T.; et al. Oxidative Stress and Immune Responses During Hepatitis C Virus Infection in Tupaia belangeri. Sci. Rep. 2017, 7, 9848. [Google Scholar] [CrossRef]
- Gil, L.; Martínez, G.; Tápanes, R.; Castro, O.; González, D.; Bernardo, L.; Vázquez, S.; Kourí, G.; Guzmán, M.G. Oxidative Stress in Adult Dengue Patients. Am. J. Trop. Med. Hyg. 2004, 71, 652–657. [Google Scholar] [CrossRef]
- Cavalheiro, M.G.; Costa, L.S.D.; Campos, H.S.; Alves, L.S.; Assunção-Miranda, I.; Poian, A.T.D.; Cavalheiro, M.G.; Costa, L.S.D.; Campos, H.S.; Alves, L.S.; et al. Macrophages as target cells for Mayaro virus infection: Involvement of reactive oxygen species in the inflammatory response during virus replication. An. Acad. Bras. Ciênc. 2016, 88, 1485–1499. [Google Scholar] [CrossRef]
- de Castro-Jorge, L.A.; de Carvalho, R.V.H.; Klein, T.M.; Hiroki, C.H.; Lopes, A.H.; Guimarães, R.M.; Fumagalli, M.J.; Floriano, V.G.; Agostinho, M.R.; Slhessarenko, R.D.; et al. The NLRP3 inflammasome is involved with the pathogenesis of Mayaro virus. PLOS Pathog. 2019, 15, e1007934. [Google Scholar] [CrossRef]
- Yang, Z.; Min, Z.; Yu, B. Reactive oxygen species and immune regulation. Int. Rev. Immunol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Foo, S.-S.; Rulli, N.E.; Taylor, A.; Sheng, K.-C.; Herrero, L.J.; Herring, B.L.; Lidbury, B.A.; Li, R.W.; Walsh, N.C.; et al. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc. Natl. Acad. Sci. USA 2014, 111, 6040–6045. [Google Scholar] [CrossRef] [PubMed]
- Rulli, N.E.; Rolph, M.S.; Srikiatkhachorn, A.; Anantapreecha, S.; Guglielmotti, A.; Mahalingam, S. Protection from Arthritis and Myositis in a Mouse Model of Acute Chikungunya Virus Disease by Bindarit, an Inhibitor of Monocyte Chemotactic Protein-1 Synthesis. J. Infect. Dis. 2011, 204, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Bengue, M.; Ferraris, P.; Baronti, C.; Diagne, C.T.; Talignani, L.; Wichit, S.; Liegeois, F.; Bisbal, C.; Nougairède, A.; Missé, D. Mayaro Virus Infects Human Chondrocytes and Induces the Expression of Arthritis-Related Genes Associated with Joint Degradation. Viruses 2019, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.R.; Russell, K.L.; Vasquez, C.; Barrera, R.; Tesh, R.B.; Salas, R.; Watts, D.M. Family Cluster of Mayaro Fever, Venezuela. Emerg. Infect. Dis. 2004, 10, 1304–1306. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M.; Nogueira, R.M.R.; Cavalcanti, S.M.B.; Schatzmayr, H.; da Rosa, A.T.; Figueiredo, L.T.M.; Nogueira, R.M.R.; Cavalcanti, S.M.B.; Schatzmayr, H.; da Rosa, A.T. Study of two different enzyme immunoassays for the detection of Mayaro virus antibodies. Mem. Inst. Oswaldo Cruz 1989, 84, 303–307. [Google Scholar] [CrossRef]
- Earnest, J.T.; Basore, K.; Roy, V.; Bailey, A.L.; Wang, D.; Alter, G.; Fremont, D.H.; Diamond, M.S. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J. Exp. Med. 2019, 216, 2282–2301. [Google Scholar] [CrossRef]
- da Rosa, A.P.T.; Vasconcelos, P.F.; da Rosa, J.F.T. An Overview of Arbovirology in Brazil and Neighbouring Countries; Instituto Evandro Chagas: Ananindeua, Brazil, 1998; ISBN 85-86784-01-X. [Google Scholar]
- Izurieta, R.O.; Macaluso, M.; Watts, D.M.; Tesh, R.B.; Guerra, B.; Cruz, L.M.; Galwankar, S.; Vermund, S.H. Hunting in the Rainforest and Mayaro Virus Infection: An emerging Alphavirus in Ecuador. J. Glob. Infect. Dis. 2011, 3, 317–323. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Karabatsos, N. Antigenic Relationships of Group a Arboviruses by Plaque Reduction Neutralization Testing. Am. J. Trop. Med. Hyg. 1975, 24, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Bozza, F.; Merino, X.J.M.; Pedroso, C.; de Oliveira Filho, E.F.; Moreira-Soto, A.; Schwalb, A.; de Lamballerie, X.; Netto, E.M.; Bozza, P.T.; et al. Robustness of Serologic Investigations for Chikungunya and Mayaro Viruses following Coemergence. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; el-Kafrawi, A.O.; Mahmud, M.I.A.-D.; da Rosa, A.P.T.; Bartz, C.R.; Brummer-Korvenkontio, M.; Haksohusodo, S.; Suharyono, W. Complex-specific immunoglobulin M antibody patterns in humans infected with alphaviruses. J. Clin. Microbiol. 1986, 23, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Martin, D.A.; Lanciotti, R.S. Detection of North American Eastern and Western Equine Encephalitis Viruses by Nucleic Acid Amplification Assays. J. Clin. Microbiol. 2003, 41, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Chung, B.Y.; Fleeton, M.N.; Atkins, J.F. Discovery of frameshifting in Alphavirus 6K resolves a 20-year enigma. Virol. J. 2008, 5, 108. [Google Scholar] [CrossRef]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef]
- Lavergne, A.; de Thoisy, B.; Lacoste, V.; Pascalis, H.; Pouliquen, J.-F.; Mercier, V.; Tolou, H.; Dussart, P.; Morvan, J.; Talarmin, A.; et al. Mayaro virus: Complete nucleotide sequence and phylogenetic relationships with other alphaviruses. Virus Res. 2006, 117, 283–290. [Google Scholar] [CrossRef]
- Powers, A.M.; Aguilar, P.V.; Chandler, L.J.; Brault, A.C.; Meakins, T.A.; Watts, D.; Russell, K.L.; Olson, J.; Vasconcelos, P.F.C.; Rosa, A.T.D.; et al. Genetic Relationships among Mayaro and Una Viruses Suggest Distinct Patterns of Transmission. Am. J. Trop. Med. Hyg. 2006, 75, 461–469. [Google Scholar] [CrossRef]
- Auguste, A.J.; Adams, A.P.; Arrigo, N.C.; Martinez, R.; da Rosa, A.P.A.T.; Adesiyun, A.A.; Chadee, D.D.; Tesh, R.B.; Carrington, C.V.F.; Weaver, S.C. Isolation and Characterization of Sylvatic Mosquito-Borne Viruses in Trinidad: Enzootic Transmission and a New Potential Vector of Mucambo Virus. Am. J. Trop. Med. Hyg. 2010, 83, 1262–1265. [Google Scholar] [CrossRef]
- Auguste, A.J.; Liria, J.; Forrester, N.L.; Giambalvo, D.; Moncada, M.; Long, K.C.; Morón, D.; de Manzione, N.; Tesh, R.B.; Halsey, E.S.; et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an Outbreak, Venezuela, 2010. Emerg. Infect. Dis. 2015, 21, 1742–1750. [Google Scholar] [CrossRef]
- Mota, M.T.O.; Vedovello, D.; Estofolete, C.; Malossi, C.D.; Araújo, J.P.; Nogueira, M.L. Complete Genome Sequence of Mayaro Virus Imported from the Amazon Basin to São Paulo State, Brazil. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; LeDuc, J.W. Mayaro virus disease. Arboviruses Epidemiol. Ecol. 1998, 3, 137–150. [Google Scholar]
- Hoch, A.L.; Peterson, N.E.; LeDuc, J.W.; Pinheiro, F.P. An Outbreak of Mayaro Virus Disease in Belterra, Brazil III. Entomological and Ecological Studies. Am. J. Trop. Med. Hyg. 1981, 30, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa, A.; Juliano, R.S.; Campos, Z.; Velez, J.; Nogueira, R.M.R.; Komar, N.; Pauvolid-Corrêa, A.; Juliano, R.S.; Campos, Z.; Velez, J.; et al. Neutralising antibodies for Mayaro virus in Pantanal, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Aitken, T.H.G.; Anderson, C.R. Virus Transmission Studies with Trinidadian Mosquitoes. Am. J. Trop. Med. Hyg. 1959, 8, 41–45. [Google Scholar] [CrossRef]
- Monath, T.P. Dengue: The risk to developed and developing countries. Proc. Natl. Acad. Sci. USA 1994, 91, 2395–2400. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Grimmer, G.H.; de Paula, V.S.; Figueiredo, L.T.M.; Braga, W.S.M.; Luz, S.L.B. Mayaro Virus Infection in Amazonia: A Multimodel Inference Approach to Risk Factor Assessment. PLoS Negl. Trop. Dis. 2012, 6, e1846. [Google Scholar] [CrossRef]
- Maia, L.M.S.; Bezerra, M.C.F.; Costa, M.C.S.; Souza, E.M.; Oliveira, M.E.B.; Ribeiro, A.L.M.; Miyazaki, R.D.; Slhessarenko, R.D. Natural vertical infection by dengue virus serotype 4, Zika virus and Mayaro virus in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus. Med. Vet. Entomol. 2019, 33, 437–442. [Google Scholar] [CrossRef]
- Gubler, D.J. The Global Emergence/Resurgence of Arboviral Diseases as Public Health Problems. Arch. Med. Res. 2002, 33, 330–342. [Google Scholar] [CrossRef]
- Bosio, C.F.; Thomas, R.E.; Grimstad, P.R.; Rai, K.S. Variation in the Efficiency of Vertical Transmission of Dengue-1 Virus by Strains of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1992, 29, 985–989. [Google Scholar] [CrossRef]
- Diallo, D.; Dia, I.; Diagne, C.T.; Gaye, A.; Diallo, M. Chapter 4—Emergences of Chikungunya and Zika in Africa. In Chikungunya and Zika Viruses; Higgs, S., Vanlandingham, D.L., Powers, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 87–133. ISBN 978-0-12-811865-8. [Google Scholar]
- de Toni Aquino da Cruz, L.C.; Serra, O.P.; Leal-Santos, F.A.; Ribeiro, A.L.M.; Slhessarenko, R.D.; dos Santos, M.A. Natural transovarial transmission of dengue virus 4 in Aedes aegypti from Cuiabá, State of Mato Grosso, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Paupy, C.; Dehecq, J.S.; Thiria, J.; Failloux, A.B.; Fontenille, D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite Paris Fr. 2008, 15, 3–13. [Google Scholar] [CrossRef]
- Mondet, B.; Vasconcelos, P.F.C.; Travassos da Rosa, A.P.A.; Travassos da Rosa, E.S.; Rodrigues, S.G.; Travassos da Rosa, J.F.S.; Bicout, D.J. Isolation of Yellow Fever Virus from Nulliparous Haemagogus (Haemagogus) janthinomys in Eastern Amazonia. Vector-Borne Zoonotic Dis. 2002, 2, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Junt, T.; Heraud, J.M.; Lelarge, J.; Labeau, B.; Talarmin, A. Determination of natural versus laboratory human infection with Mayaro virus by molecular analysis. Epidemiol. Infect. 1999, 123, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bugallo, G.; Piedra, L.A.; Rodriguez, M.; Bisset, J.A.; Lourenço-de-Oliveira, R.; Weaver, S.C.; Vasilakis, N.; Vega-Rúa, A. Vector-borne transmission and evolution of Zika virus. Nat. Ecol. Evol. 2019, 3, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental Transmission of Mayaro Virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Aitken, T.H.G.; Downs, W.G.; Anderson, C.R.; Spence, L.; Casals, J. Mayaro Virus Isolated from a Trinidadian Mosquito, Mansonia venezuelensis. Science 1960, 131, 986. [Google Scholar] [CrossRef]
- Karabatsos, N. International Catalogue of arthrOpod-Borne Viruses; American Society of Tropical Medicine and Hygiene for The Subcommittee on Information Exchange of the American Committee on Arthropod-borne Viruses: San Antonio, TX, USA, 1985; Volume 3. [Google Scholar]
- Groot, H.; Morales, A.; Vidales, H. Virus Isolations from Forest Mosquitoes in San Vicente de Chucuri, Colombia. Am. J. Trop. Med. Hyg. 1961, 10, 397–402. [Google Scholar] [CrossRef]
- Brustolin, M.; Pujhari, S.; Henderson, C.A.; Rasgon, J.L. Anopheles mosquitoes may drive invasion and transmission of Mayaro virus across geographically diverse regions. PLoS Negl. Trop. Dis. 2018, 12, e0006895. [Google Scholar] [CrossRef]
- Wiggins, K.; Eastmond, B.; Alto, B.W. Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes. Med. Vet. Entomol. 2018, 32, 436–442. [Google Scholar] [CrossRef]
- Smith, G.C.; Francy, D.B. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J. Am. Mosq. Control Assoc. 1991, 7, 89–93. [Google Scholar] [PubMed]
- Diop, F.; Alout, H.; Diagne, C.T.; Bengue, M.; Baronti, C.; Hamel, R.; Talignani, L.; Liegeois, F.; Pompon, J.; Vargas, R.E.M.; et al. Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus. Viruses 2019, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika—And now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e0005462. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using geographical information system and CLIMEX. Geospatial Health 2014, 8, 405–415. [Google Scholar] [CrossRef]
- Caminade, C.; Medlock, J.M.; Ducheyne, E.; McIntyre, K.M.; Leach, S.; Baylis, M.; Morse, A.P. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: Recent trends and future scenarios. J. R. Soc. Interface 2012, 9, 2708–2717. [Google Scholar] [CrossRef]
- Medlock, J.M.; Avenell, D.; Barrass, I.; Leach, S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J. Vector Ecol. 2006, 31, 292–304. [Google Scholar] [CrossRef]
- de Thoisy, B.; Gardon, J.; Salas, R.A.; Morvan, J.; Kazanji, M. Mayaro Virus in Wild Mammals, French Guiana. Emerg. Infect. Dis. 2003, 9, 1326–1329. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M. Emergent arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef]
- Rylands, A.B.; Mittermeier, R.A. The Diversity of the New World Primates (Platyrrhini): An Annotated Taxonomy. In South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation; Garber, P.A., Estrada, A., Bicca-Marques, J.C., Heymann, E.W., Strier, K.B., Eds.; Developments in Primatology: Progress and Prospects; Springer: New York, NY, USA, 2009; pp. 23–54. ISBN 978-0-387-78705-3. [Google Scholar]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef] [PubMed]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Brent, S.; Watts, A.G.; Hay, S.I.; Kulkarni, M.A.; Brownstein, J.S.; et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: A modelling study. Lancet Infect. Dis. 2016, 16, 1237–1245. [Google Scholar] [CrossRef]
- Stoddard, S.T.; Forshey, B.M.; Morrison, A.C.; Paz-Soldan, V.A.; Vazquez-Prokopec, G.M.; Astete, H.; Reiner, R.C.; Vilcarromero, S.; Elder, J.P.; Halsey, E.S. House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. USA 2013, 110, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Scott, T.W.; Lerdthusnee, K.; Coleman, R.C.; Costero, A.; Clark, G.G.; Jones, J.J.; Kitthawee, S.; Kittayapong, P.; Sithiprasasna, R.; et al. Dispersal of the Dengue Vector Aedes aegypti within and between Rural Communities. Am. J. Trop. Med. Hyg. 2005, 72, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Vanlandingham, D. Chikungunya Virus and Its Mosquito Vectors. Vector-Borne Zoonotic Dis. 2015, 15, 231–240. [Google Scholar] [CrossRef]
- Achee, N.L.; Gould, F.; Perkins, T.A.; Reiner, R.C., Jr.; Morrison, A.C.; Ritchie, S.A.; Gubler, D.J.; Teyssou, R.; Scott, T.W. A Critical Assessment of Vector Control for Dengue Prevention. PLoS Negl. Trop. Dis. 2015, 9, e0003655. [Google Scholar] [CrossRef]
- Izurieta, R.O.; Macaluso, M.; Watts, D.M.; Tesh, R.B.; Guerra, B.; Cruz, L.M.; Galwankar, S.; Vermund, S.H. Assessing Yellow Fever Risk in the Ecuadorian Amazon. J. Glob. Infect. Dis. 2009, 1, 7–13. [Google Scholar] [CrossRef]
- Esposito, D.L.A.; da Fonseca, B.A.L.; Esposito, D.L.A.; da Fonseca, B.A.L. Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Braz. J. Infect. Dis. 2017, 21, 540–544. [Google Scholar] [CrossRef]
- de Figueiredo, M.L.G.; Figueiredo, L.T.M. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev. Soc. Bras. Med. Trop. 2014, 47, 677–683. [Google Scholar] [CrossRef]
- Calisher, C.H.; Gutierrez, E.; Maness, K.; Lord, R.D. Isolation of Mayaro virus from a migrating bird captured in Louisiana in 1967. Bull. Pan Am. Health Organ. PAHO 1974, 8, 3. [Google Scholar]
- Weaver, S.C. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013, 21, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Travelling arboviruses: A historical perspective. Travel Med. Infect. Dis. 2019, 31, 101471. [Google Scholar] [CrossRef] [PubMed]
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.-J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Mavian, C.; Rife, B.D.; Dollar, J.J.; Cella, E.; Ciccozzi, M.; Prosperi, M.C.F.; Lednicky, J.; Morris, J.G.; Capua, I.; Salemi, M. Emergence of recombinant Mayaro virus strains from the Amazon basin. Sci. Rep. 2017, 7, 8718. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Black, W.; Eisen, L.; Flores, A.E.; García-Rejón, J.E.; Loroño-Pino, M.; Saavedra-Rodriguez, K. The intensifying storm: Domestication of Aedes aegypti, urbanization of arboviruses, and emerging insecticide resistance. In Global Health Impacts of Vector-Borne Diseases: Workshop Summary; Alison Mack, R., Forum on Microbial Threats, Board on Global Health, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine, Eds.; National Academies Press: Washington, DC, USA, 2016; pp. 111–142. [Google Scholar]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef]
| Country of Origin | Species | References |
|---|---|---|
| Brazil | Aedes aegypti | [28,89] |
| Panama | Sabethes spp. | [21] |
| Brazil | Haemagogus janthinomys | [20,83,84] |
| Colombia | Aedes serratus | [101] |
| Brazil | Cx. quinquefasciatus | [28] |
| Panama | Cx. vomerifer | [26] |
| Trinidad and Tobago | Mansonia venezuelensis | [99] |
| Colombia | Psorophora ferox | [101] |
| Colombia | Psorophora albipes | [101] |
| Country of Origin | Species | References |
|---|---|---|
| Iquitos, Peru | Ae. aegypti | [98,102,106] |
| Bora-Bora, French Polynesia | Ae. aegypti | [105] |
| Florida, USA | Ae. aegypti | [103] |
| Brazil | Ae. albopictus | [104] |
| La Reunion | Ae. albopictus | [105] |
| Florida, USA | Ae. albopictus | [103] |
| Trinidad and Tobago | Ae. scapularis | [86] |
| Virginia, USA | An. freeborni | [102] |
| Maryland, USA | An. gambiae | [102] |
| Virginia, USA | An. quadrimaculatus | [102] |
| Maryland, USA | An. stephensi | [102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. https://doi.org/10.3390/pathogens9090738
Diagne CT, Bengue M, Choumet V, Hamel R, Pompon J, Missé D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens. 2020; 9(9):738. https://doi.org/10.3390/pathogens9090738
Chicago/Turabian StyleDiagne, Cheikh Tidiane, Michèle Bengue, Valérie Choumet, Rodolphe Hamel, Julien Pompon, and Dorothée Missé. 2020. "Mayaro Virus Pathogenesis and Transmission Mechanisms" Pathogens 9, no. 9: 738. https://doi.org/10.3390/pathogens9090738
APA StyleDiagne, C. T., Bengue, M., Choumet, V., Hamel, R., Pompon, J., & Missé, D. (2020). Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens, 9(9), 738. https://doi.org/10.3390/pathogens9090738









