Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro
Abstract
:1. Introduction
2. Results
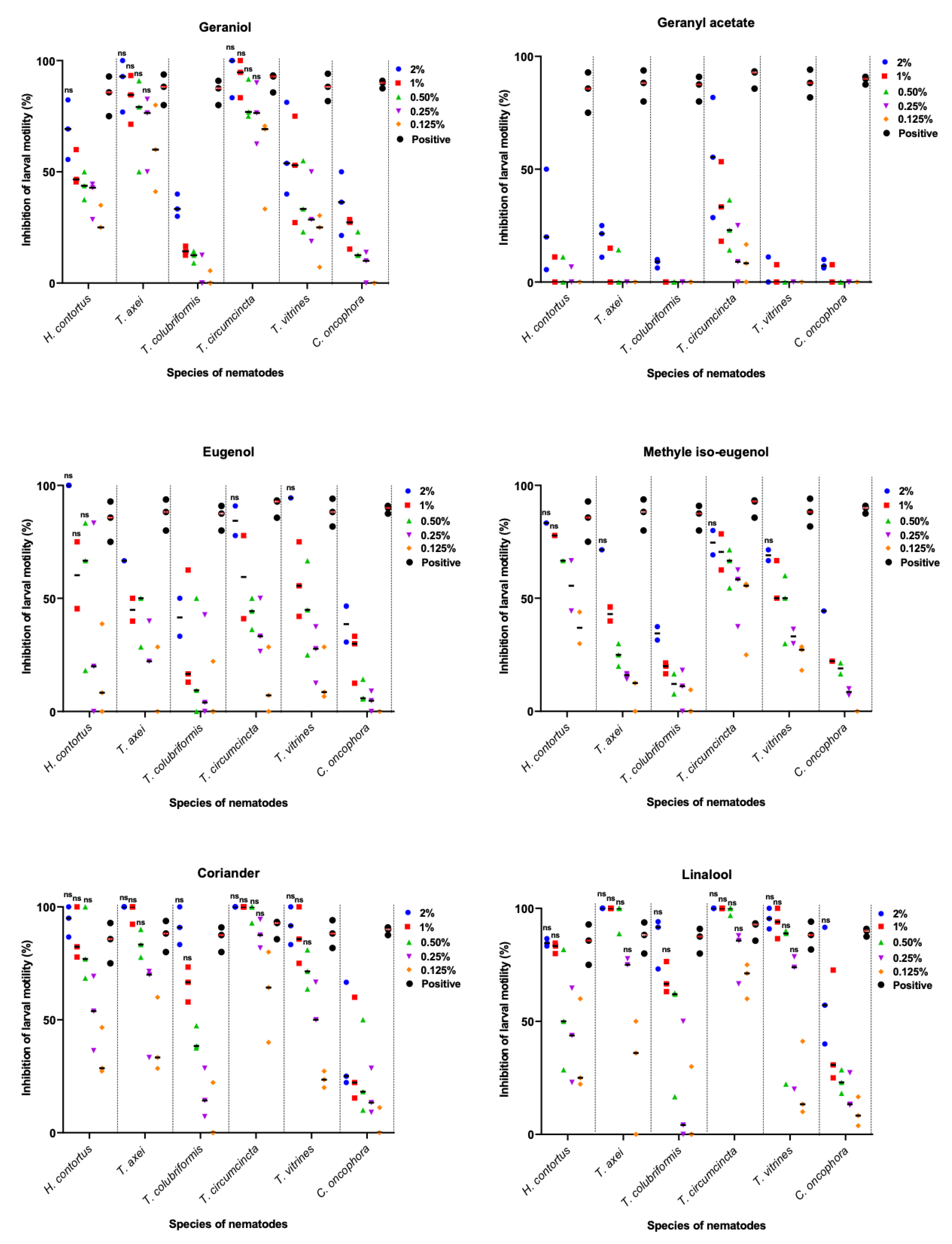
2.1. Efficacy of Essential Oil Extracts Using Larval Motility Assay
2.2. Optical Evaluation of the Feasibility of Fluorescent Stains
2.3. Optimization of the Fluorometric Assays
2.4. Efficacy of Coriander Essential Oil -Linalool Combination Based on Fluorometric Assays
2.5. Efficiency of Fluorescence Stains Versus Larval Motility Assay
2.6. Scanning Electron Microscopy (SEM)
2.7. Cytotoxic Effects of Coriander and Linalool
3. Discussion
4. Materials and Methods
4.1. Coriander Essential Oil and Pure Principles
4.2. Source of Nematode Larvae
4.3. Larval Motility Assay
4.4. Development of Fluorometric Microplate-based Assays
4.4.1. Optimization of the Fluorometric Microplate-based Assays
4.4.2. Efficacy of a Coriander Essential Oil and Five Pure Principles Using Fluorometric Assays
4.5. Scanning Electron Microscopy (SEM)
4.6. Cytotoxicity Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katiki, L.M.; Araujo, R.C.; Ziegelmeyer, L.; Gomes, A.C.P.; Gutmanis, G.; Rodrigues, L.; Bueno, M.S.; Verissimo, C.J.; Louvandini, H.; Ferreira, J.F.S.; et al. Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Exp. Parasitol. 2019, 197, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Dubois, O.; Allanic, C.; Charvet, C.L.; Guegnard, F.; Fevrier, H.; Thery-Kone, I.; Cortet, J.; Koch, C.; Bouvier, F.; Fassier, T.; et al. Lupin (Lupinus spp.) seeds exert anthelmintic activity associated with their alkaloid content. Sci. Rep. 2019, 9, 9070. [Google Scholar] [CrossRef] [PubMed]
- Baiak, B.H.B.; Lehnen, C.R.; da Rocha, R.A. Anthelmintic resistance in cattle: A systematic review and meta-analysis. Livestock Sci. 2018, 217, 127–135. [Google Scholar] [CrossRef]
- Muchiut, S.M.; Fernandez, A.S.; Steffan, P.E.; Riva, E.; Fiel, C.A. Anthelmintic resistance: Management of parasite refugia for Haemonchus contortus through the replacement of resistant with susceptible populations. Vet. Parasitol. 2018, 254, 43–48. [Google Scholar] [CrossRef]
- Kearney, P.E.; Murray, P.J.; Hoy, J.M.; Hohenhaus, M.; Kotze, A. The ‘Toolbox’ of strategies for managing Haemonchus contortus in goats: What’s in and what’s out. Vet. Parasitol. 2016, 220, 93–107. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.R.; Ramsay, A.; Hansen, T.V.A.; Ropiak, H.M.; Mejer, H.; Nejsum, P.; Mueller-Harvey, I.; Thamsborg, S.M. Anthelmintic activity of trans-cinnamaldehyde and A- and B-type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci. Rep. 2015, 5, 14791. [Google Scholar] [CrossRef] [Green Version]
- Alviano, D.S.; Barreto, A.L.; Dias Fde, A.; Rodrigues Ide, A.; Rosa Mdo, S.; Alviano, C.S.; Soares, R.M. Conventional therapy and promising plant-derived compounds against trypanosomatid parasites. Front. Microbiol. 2012, 3, 283. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Dai, J.; Yang, L.; Qiu, J. Anthelmintic activity of Arisaema franchetianum and Arisaema lobatum essential oils against Haemonchus contortus. J. Ethnopharmacol. 2013, 148, 311–316. [Google Scholar] [CrossRef]
- Cortes-Morales, J.A.; Olmedo-Juarez, A.; Trejo-Tapia, G.; Gonzalez-Cortazar, M.; Dominguez-Mendoza, B.E.; Mendoza-de Gives, P.; Zamilpa, A. In vitro ovicidal activity of Baccharis conferta Kunth against Haemonchus contortus. Exp. Parasitol. 2019, 197, 20–28. [Google Scholar] [CrossRef]
- Kumaran, A.M.; D’Souza, P.; Agarwal, A.; Bokkolla, R.M.; Balasubramaniam, M. Geraniol, the putative anthelmintic principle of Cymbopogon martinii. Phytother. Res. 2003, 17, 957. [Google Scholar] [CrossRef] [PubMed]
- Katiki, L.M.; Chagas, A.C.; Bizzo, H.R.; Ferreira, J.F.; Amarante, A.F. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet. Parasitol. 2011, 183, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiki, L.M.; Chagas, A.C.; Takahira, R.K.; Juliani, H.R.; Ferreira, J.F.; Amarante, A.F. Evaluation of Cymbopogon schoenanthus essential oil in lambs experimentally infected with Haemonchus contortus. Vet. Parasitol. 2012, 186, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, W.L.; Camurca-Vasconcelos, A.L.; Macedo, I.T.; dos Santos, J.M.; de Araujo-Filho, J.V.; Ribeiro Jde, C.; Pereira Vde, A.; Viana Dde, A.; de Paula, H.C.; Bevilaqua, C.M. In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet. Parasitol. 2015, 212, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Asha, M.K.; Prashanth, D.; Murali, B.; Padmaja, R.; Amit, A. Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia 2001, 72, 669–670. [Google Scholar] [CrossRef]
- Pessoa, L.M.; Morais, S.M.; Bevilaqua, C.M.; Luciano, J.H. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet. Parasitol. 2002, 109, 59–63. [Google Scholar] [CrossRef]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Verissimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef]
- Eguale, T.; Tilahun, G.; Debella, A.; Feleke, A.; Makonnen, E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacol. 2007, 110, 428–433. [Google Scholar] [CrossRef]
- Macedo, I.T.; de Oliveira, L.M.; Camurca-Vasconcelos, A.L.; Ribeiro, W.L.; dos Santos, J.M.; de Morais, S.M.; de Paula, H.C.; Bevilaqua, C.M. In vitro effects of Coriandrum sativum, Tagetes minuta, Alpinia zerumbet and Lantana camara essential oils on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2013, 22, 463–469. [Google Scholar] [CrossRef]
- Nordi, E.C.P.; Costa, R.L.D.; David, C.M.G.; Parren, G.A.E.; Freitas, A.C.B.; Lameirinha, L.P.; Katiki, L.M.; Bueno, M.S.; Quirino, C.R.; Gama, P.E.; et al. Supplementation of moist and dehydrated citrus pulp in the diets of sheep artificially and naturally infected with gastrointestinal nematodes on the parasitological parameters and performance. Vet. Parasitol. 2014, 205, 532–539. [Google Scholar] [CrossRef]
- Macedo, I.T.; Bevilaqua, C.M.; de Oliveira, L.M.; Camurca-Vasconcelos, A.L.; Vieira Lda, S.; Oliveira, F.R.; Queiroz-Junior, E.M.; Tome Ada, R.; Nascimento, N.R. Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet. Parasitol. 2010, 173, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Demeler, J.; Kuttler, U.; von Samson-Himmelstjerna, G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet. Parasitol. 2010, 170, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Partridge, F.A.; Brown, A.E.; Buckingham, S.D.; Willis, N.J.; Wynne, G.M.; Forman, R.; Else, K.J.; Morrison, A.A.; Matthews, J.B.; Russell, A.J.; et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs. Drug Resist. 2018, 8, 8–21. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Davey, M.W. A rapid colorimetric assay for the quantitation of the viability of free-living larvae of nematodes in vitro. Parasitol. Res. 2007, 101, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Wilkinson, A.R.; Paradis, B.D.; Lai, N. Rapid image-based cytometry for comparison of fluorescent viability staining methods. J. Fluoresc. 2012, 22, 1301–1311. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Mendes, T.A.; Bueno, L.L.; de Araujo, J.V.; Bartholomeu, D.C.; Fujiwara, R.T. A new methodology for evaluation of nematode viability. Biomed. Res. Int. 2015, 2015, 879263. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.R.; Olejnik, N.; Sprando, R.L. Toxicity ranking of heavy metals with screening method using adult Caenorhabditis elegans and propidium iodide replicates toxicity ranking in rat. Food. Chem. Toxicol. 2012, 50, 3280–3290. [Google Scholar] [CrossRef]
- Wimmersberger, D.; Tritten, L.; Keiser, J. Development of an in vitro drug sensitivity assay for Trichuris muris first-stage larvae. Parasit. Vectors 2013, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Satyal, P.; Setzer, W.N. Chemical compositions of commercial essential oils from Coriandrum sativum fruits and aerial parts. Nat. Prod. Commun. 2020, 15, 1934578X20933067. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621. [Google Scholar] [CrossRef]
- Abidi, A.; Sebai, E.; Dhibi, M.; Alimi, D.; Rekik, M.; B’Chir, F.; Maizels, R.M.; Akkari, H. Chemical analyses and anthelmintic effects of Artemisia campestris essential oil. Vet. Parasitol. 2018, 263, 59–65. [Google Scholar] [CrossRef]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; Franca, S.C.; Contini, S.; Chagas, A.C.S.; Beleboni, R.O. Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Wang, W.X.; Dai, J.L.; Zhu, L. In vitro anthelmintic activity of Zanthoxylum simulans essential oil against Haemonchus contortus. Vet. Parasitol. 2015, 211, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, W.L.; Macedo, I.T.; dos Santos, J.M.; de Oliveira, E.F.; Camurca-Vasconcelos, A.L.; de Paula, H.C.; Bevilaqua, C.M. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Exp. Parasitol. 2013, 135, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Long, E.; Wen, J.; Cao, L.; Zhu, C.; Hu, H.; Ruan, Y.; Okanurak, K.; Hu, H.; Wei, X.; et al. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis and inhibits infection of Schistosoma japonicum. Parasit. Vectors 2014, 7, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, M. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.). Br. Food. J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Egerton, J.R.; Eary, C.H.; Suhayda, D. The anthelmintic efficacy of ivermectin in experimentally infected cattle. Vet. Parasitol. 1981, 8, 59–70. [Google Scholar] [CrossRef]
- Egerton, J.R.; Birnbaum, J.; Blair, L.S.; Chabala, J.C.; Conroy, J.; Fisher, M.H.; Mrozik, H.; Ostlind, D.A.; Wilkins, C.A.; Campbell, W.C. 22, 23–Dihydroavermectin B1, A New Broad-Spectrum Antiparasitic Agent. Br. Vet. J. 1980, 136, 88–97. [Google Scholar] [CrossRef]
- Areskog, M.; Sollenberg, S.; Engström, A.; von Samson-Himmelstjerna, G.; Höglund, J. A controlled study on gastrointestinal nematodes from two Swedish cattle farms showing field evidence of ivermectin resistance. Parasit. Vectors 2014, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Landuyt, B.; Klaassen, H.; Geldhof, P.; Luyten, W. Screening of a drug repurposing library with a nematode motility assay identifies promising anthelmintic hits against Cooperia oncophora and other ruminant parasites. Vet. Parasitol. 2019, 265, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dai, J.L.; Yang, L.; Qiu, J. In vitro ovicidal and larvicidal activity of the essential oil of Artemisia lancea against Haemonchus contortus (Strongylida). Vet. Parasitol. 2013, 195, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Sriti Eljazi, J.; Bachrouch, O.; Salem, N.; Msaada, K.; Aouini, J.; Hammami, M.; Boushih, E.; Abderraba, M.; Limam, F.; Mediouni Ben Jemaa, J. Chemical composition and insecticidal activity of essential oil from coriander fruit against Tribolium castaenum, Sitophilus oryzae, and Lasioderma serricorne. Int. J. Food. Prop. 2017, 20, S2833–S2845. [Google Scholar] [CrossRef] [Green Version]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander essential oil and linalool-interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Lee, D.L.; Atkinson, H.J. Physiology of Nematodes, 2nd ed.; Macmillan Publishers Limited: London, UK, 1976. [Google Scholar] [CrossRef] [Green Version]
- Silva Soares, S.C.; de Lima, G.C.; Carlos Laurentiz, A.; Feboli, A.; Dos Anjos, L.A.; de Paula Carlis, M.S.; da Silva Filardi, R.; da Silva de Laurentiz, R. In vitro anthelmintic activity of grape pomace extract against gastrointestinal nematodes of naturally infected sheep. Int. J. Vet. Sci. Med 2018, 6, 243–247. [Google Scholar] [CrossRef]
- Andre, W.P.P.; Cavalcante, G.S.; Ribeiro, W.L.C.; Santos, J.; Macedo, I.T.F.; Paula, H.C.B.; Morais, S.M.; Melo, J.V.; Bevilaqua, C.M.L. Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Rev. Bras. Parasitol. Vet. 2017, 26, 323–330. [Google Scholar] [CrossRef]
- Santos, A.C.V.; Santos, F.O.; Lima, H.G.; Silva, G.D.D.; Uzeda, R.S.; Dias, E.R.; Branco, A.; Cardoso, K.V.; David, J.M.; Botura, M.B.; et al. In vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitology 2018, 145, 1884–1889. [Google Scholar] [CrossRef]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Ouellet, D.R.; Chiquette, J.; Chouinard, P.Y. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed Alfalfa silage or corn silage1. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Castro-Montoya, J.; Peiren, N.; Cone, J.W.; Zweifel, B.; Fievez, V.; De Campeneere, S. In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation. Livestock Sci. 2015, 180, 134–142. [Google Scholar] [CrossRef]
- Ritler, D.; Rufener, R.; Sager, H.; Bouvier, J.; Hemphill, A.; Lundstrom-Stadelmann, B. Development of a movement-based in vitro screening assay for the identification of new anti-cestodal compounds. PLoS Negl. Trop. Dis. 2017, 11, e0005618. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, S.I.; Jacob, J.; Sugihara, R.T.; Leinbach, I.L.; Klasner, I.H.; Kaluna, L.M.; Snook, K.A.; Howe, M.K.; Jacquier, S.H.; Lange, I.; et al. Validation of a death assay for Angiostrongylus cantonensis larvae (L3) using propidium iodide in a rat model (Rattus norvegicus). Parasitology 2019, 146, 1421–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, M.S.; Olsen, A.; Sampayo, J.N.; Lithgow, G.J. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2003, 35, 558–565. [Google Scholar] [CrossRef]
- MAFF (Ministry of Agriculture, Fisheries and Food). Manual of Veterinary Parasitological Laboratory Techniques, 3rd ed.; H.M. Stationery Office: London, UK, 1986.
- Thompson, G.; de Pomerai, D.I. Toxicity of short-chain alcohols to the nematode Caenorhabditis elegans: A comparison of endpoints. J. Biochem. Mol. Toxicol. 2005, 19, 87–95. [Google Scholar] [CrossRef]
- Ortega-Rivas, A.; Padrón, J.M.; Valladares, B.; Elsheikha, H.M. Acanthamoeba castellanii: A new high-throughput method for drug screening in vitro. Acta Top. 2016, 164, 95–99. [Google Scholar] [CrossRef]






| F.C. (%) | H. contortus | T. axei | T. colubriformis | T. circumcincta | T. vitrinus | C. oncophora | Mixed Infection |
|---|---|---|---|---|---|---|---|
| 2% | 91.25 (4.82) a | 98.80 (0.97) a | 94.65 (2.28) a | 100 a | 100 a | 24.58 (3.62) b | 84.88 (12.14) a |
| 1% | 87.36 (4.46) a | 98.71 (1.05) a | 81.11 (4.80) a | 100 a | 99 (0.76) a | 15.80 (2.69) b | 80.35 (13.28) a |
| 0.5% | 67.77 (4.03) b | 95.65 (3.55) a | 49.79 (4.02) b | 99 (0.82) a | 92 (0.64) a | 6.40 (2.71) b | 68.44 (14.64) a |
| 0.25% | 38 (3.89) b | 69 (8.35) b | 12.55 (5.23) b | 95.95 (1.73) a | 61.1 (4.53) b | 2.22 (1.81) b | 46.50 (14.55) b |
| 0.125% | 29.14 (3.31) b | 42.21 (9.04) b | 2.22 (1.81) b | 65.33 (6.42) b | 15 (1.80) b | 0.00 b | 25.67 (10.30) b |
| IC50 | 0.26 | 0.15 | 0.48 | 0.11 | 0.21 | 3.31 | 0.22 |
| R2 | 0.94 | 0.93 | 0.98 | 0.98 | 0.99 | 0.88 | 0.75 |
| +ve control | 87.18 (3.17) a | 87.80 (1.85) a | 91 (1.46) a | 85 (2.36) a | 93.27 (0.34) a | 94.55 (2.23) a | 89.81 (1.53) a |
| –ve control | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 0.00 b | 00.00 b | 00.00 b |
| F.C. (%) | H. contortus | T. axei | T. colubriformis | T. circumcincta | T. vitrinus | C. oncophora | Mixed Infection |
|---|---|---|---|---|---|---|---|
| 2% | 100 a | 100 a | 100 a | 100 a | 100 a | 76.1 (8.5) b | 100 a |
| 1% | 69.2 (13.4) a | 100 a | 91.6 (3.33) a | 100 a | 88.8 (6.6) a | 76.1 (4.2) b | 85.7 (7.1) a |
| 0.5% | 46.1 (11.5) b | 88.8 (6.6) a | 83.3 (8.33) a | 81.8 (7.2) a | 55.5 (8.8) b | 73.9 (2.8) b | 71.4 (1.43) b |
| 0.25% | 42.3 (11.5) b | 77.7 (13.1) b | 58.3 (5.2) b | 72.7 (8.1) a | 55.5 (5.5) b | 65.2 (4.5) b | 57.1 (2.49) b |
| 0.125% | 19.2 (1.9) b | 44.4 (16.3) b | 58.3 (1.6) b | 27.2 (9.1) b | 22.2 (3.3) b | 28.5 (4.8) b | 35.7 (7.4) b |
| IC50 (%) | 0.58 | 0.14 | 0.104 | 0.18 | 0.21 | 0.20 | 0.23 |
| R2 | 0.38 | 0.17 | 0.22 | 0.66 | 0.12 | 0.21 | 0.79 |
| +ve control | 92.3 (7.3) a | 88.7 (5.4) a | 83.3 (4.6) a | 72.7 (9.1) a | 88.8 (7.3) a | 100 a | 100 a |
| –ve control | 34.6 (3.8) b | 22.2 (5.7) b | 33.3 (8.3) b | 27.2 (7.2) b | 33.3 (3.4) b | 32.4 (2.7) b | 34.6 (1.3) b |
| F.C. (%) | H. contortus | T. axei | T. colubriformis | T. circumcincta | T. vitrinus | C. oncophora | Mixed Infection |
|---|---|---|---|---|---|---|---|
| 2% | 100 a | 100 a | 100 a | 100 a | 100 a | 78.26 (5.9) b | 100 a |
| 1% | 56.1 (6.8) b | 76.4 (4.7) a | 76.3 (3.8) a | 100 a | 84.6 (3.3) a | 72.46 (3.3) b | 73.0 (8.4) a |
| 0.5% | 39 (5.6) b | 58.8 (7.5) b | 34.2 (13.8) b | 40 (9.2) b | 46.1 (8.5) b | 69.5 (3.3) b | 42.3 (5.1) b |
| 0.25% | 39 (10.8) b | 58.8 (2.9) b | 31.5 (9.05) b | 40 (8.8) b | 34.6 (8.5) b | 55.1 (8.8) b | 34.6 (5.1) b |
| 0.125% | 2.4 (0.1) b | 41.1 (4.5) b | 15.7 (5.8) b | 6.6 (1.9) b | 30.7 (1.4) b | 50.7 (7.3) b | 11.5 (6.7) b |
| IC50 (%) | 0.58 | 0.25 | 0.52 | 0.41 | 0.38 | 0.42 | 0.49 |
| R2 | 0.37 | 0.07 | 0.35 | 0.12 | 0.14 | 0.09 | 0.75 |
| +ve control | 69.8 (3.2) a | 97.7 (1.17) a | 80.9 (8.5) a | 94.2 (2.6) a | 88.8 (4.07) a | 100 a | 89.8 (6.7) a |
| –ve control | 50.6 (4.3) b | 62.2 (7.5) b | 39.6 (3.1) b | 71.1 (3.4) b | 51.8 (1.8) b | 50.7 (7.2) b | 55.9 (5.1) b |
| F.C. (%) | H. contortus | T. axei | T. colubriformis | T. circumcincta | T. vitrinus | C. oncophora | Mixed Infection |
|---|---|---|---|---|---|---|---|
| 2% | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 30.7 (4.16) a | 0.00 a |
| 1% | 23.1 (3.2) a | 40 (5.7) a | 23.1 (7.6) a | 53.8 (1.5) b | 25 (2.0) a | 100 b | 40 (6.8) b |
| 0.5% | 46.1 (4.52) b | 60 (1.1) b | 69.2 (2.8) b | 69.2 (7.5) b | 80 (4.0) b | 53.8 (6.2) b | 66.6 (4.5) b |
| 0.25% | 69.2 (3.9) b | 70 (3.6) b | 100 b | 90 (2.5) b | 90 (6.1) b | 76.9 (8.3) b | 80 (4.5) b |
| 0.125% | 100 b | 100 b | 100 b | 100 b | 100 b | 76.9 (12.5) b | 100 b |
| IC50 (%) | 0.31 | 0.36 | 0.66 | 0.56 | 0.72 | 0.15 | 0.51 |
| R2 | 0.05 | 0.01 | 0.18 | 0.03 | 0.24 | 0.09 | 0.17 |
| +ve control | 30.7 (1.32) a | 52.6 (1.52) a | 48.7 (2.5) a | 30.7 (2) a | 30 (2.04) a | 45.8 (2.0) a | 26.6 (6.6) a |
| –ve control | 66.0 (3.7) b | 78.9 (5.2) b | 79.4 (5.3) b | 77.5 (5) b | 71.4 (4.0) b | 72.9 (2.0) b | 75 (2.2) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.E.; Cave, G.W.V.; Morrison, A.A.; Bartley, D.J.; Elsheikha, H.M. Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro. Pathogens 2020, 9, 740. https://doi.org/10.3390/pathogens9090740
Helal MA, Abdel-Gawad AM, Kandil OM, Khalifa MME, Cave GWV, Morrison AA, Bartley DJ, Elsheikha HM. Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro. Pathogens. 2020; 9(9):740. https://doi.org/10.3390/pathogens9090740
Chicago/Turabian StyleHelal, Mohamed A., Ahmed M. Abdel-Gawad, Omnia M. Kandil, Marwa M. E. Khalifa, Gareth W. V. Cave, Alison A. Morrison, David J. Bartley, and Hany M. Elsheikha. 2020. "Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro" Pathogens 9, no. 9: 740. https://doi.org/10.3390/pathogens9090740
APA StyleHelal, M. A., Abdel-Gawad, A. M., Kandil, O. M., Khalifa, M. M. E., Cave, G. W. V., Morrison, A. A., Bartley, D. J., & Elsheikha, H. M. (2020). Nematocidal Effects of a Coriander Essential Oil and Five Pure Principles on the Infective Larvae of Major Ovine Gastrointestinal Nematodes In Vitro. Pathogens, 9(9), 740. https://doi.org/10.3390/pathogens9090740






