Buffalopox Virus: An Emerging Virus in Livestock and Humans
Abstract
:1. Introduction
2. Virus Properties
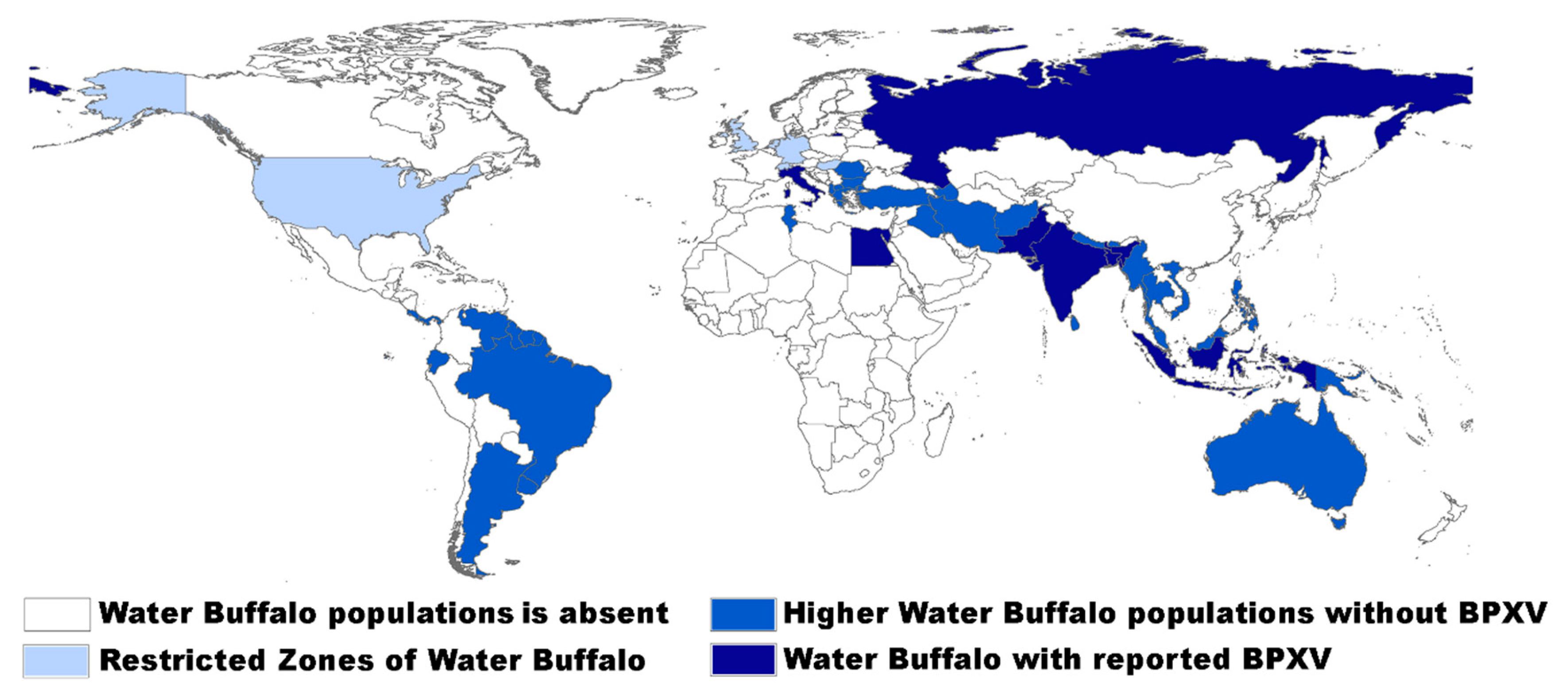
3. Epidemiology, Host Range, and Immune Response
4. Clinical Features
5. Diagnosis
6. Therapy and Prophylaxis
7. Future Aspects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. (Eds.) Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses Family Poxviridae; Elsevier: Amsterdam, The Nerterlands, 2012; pp. 291–309. [Google Scholar]
- Maqsood, M. Generalised buffalopox. Vet. Rec. 1958, 70, 321–322. [Google Scholar]
- Ramakrishna, M.; Ananthapadmanabham, K. An experimental study of virus of buffalopox. Indian Vet. J. 1957, 34, 23–30. [Google Scholar]
- Singh, I.; Singh, S. Isolation and characterization of the aetiologic agent of buffalopox. J. Res. Ludhiana 1967, 4, 440–448. [Google Scholar]
- Mathew, T. Virus study of pock disease among buffaloes. Indian J. Pathol. Bacteriol. 1967, 10, 101–102. [Google Scholar] [PubMed]
- Mayr, A.; Czerny, C.P. Buffalopox virus. In Virus Infections of Ruminants; Horinek, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 17–18. [Google Scholar] [CrossRef]
- Tantawi, H.H.; Fayed, A.A.; Shalaby, M.A. Isolation, cultivation and characterization of poxviruses from Egyptian water buffaloes. J. Egypt Vet. Med. Assoc. 1977, 37, 15–23. [Google Scholar]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO). Joint FAO/WHO Expert Committee on Zoonoses Third Report; FAO and WHO: Geneva, Switzerland, 1967. [Google Scholar]
- Singh, R.K.; Hosamani, M.; Balamurugan, V.; Bhanuprakash, V.; Rasool, T.J.; Yadav, M.P. Buffalopox: An emerging and re-emerging zoonosis. Anim. Health Res. Rev. 2007, 8, 105–114. [Google Scholar] [CrossRef]
- Yadav, S.; Hosamani, M.; Balamurugan, V.; Bhanuprakash, V.; Singh, R.K. Partial genetic characterization of viruses isolated from pox-like infection in cattle and buffaloes: Evidence of buffalo pox virus circulation in Indian cows. Arch. Virol. 2010, 155, 255–261. [Google Scholar] [CrossRef]
- Bloch, B.; Lal, S.M. A study of the ultrastructure of the buffalo pox virus. Acta Pathol. Microbiol. Scand. B 1975, 83, 191–200. [Google Scholar] [CrossRef]
- Chandra, R.; Rao, V.D.; Garg, S.K.; Singh, I.P. Comparative studies on cultivation of buffalo pox virus in pup kidney and chicken embryo fibroblast cell culture. Indian J. Exp. Biol. 1984, 22, 507–508. [Google Scholar]
- Goraya, M.U.; Qureshi, Z.U.; Abbas, M.; Ashraf, M.; Munir, M. Isolation of buffalo poxvirus from clinical case and variations in the genetics of the B5R gene over fifty passages. Virus Genes 2015, 51, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Varshney, A.; Bakshi, S.K.; Barua, S.; Bera, B.C.; Singh, R.K. Buffalo pox outbreak with atypical features: A word of caution and need for early intervention! Int. J. Dermatol. 2013, 52, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Riyesh, T.; Karuppusamy, S.; Bera, B.C.; Barua, S.; Virmani, N.; Yadav, S.; Vaid, R.K.; Anand, T.; Bansal, M.; Malik, P.; et al. Laboratory-acquired buffalopox virus infection, India. Emerg. Infect. Dis. 2014, 20, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Hosamani, M.; Balamurugan, V.; Satheesh, C.C.; Shingal, K.R.; Tatwarti, S.B.; Bambal, R.G.; Ramteke, V.; Yadav, M.P. An outbreak of buffalopox in buffalo (Bubalus bubalis) dairy herds in Aurangabad, India. Rev. Sci. Tech. 2006, 25, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Dumbell, K.; Richardson, M. Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State, India between 1985 and 1987. Arch. Virol. 1993, 128, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Balamurugan, V.; Hosamani, M.; De, U.K.; Chandra, B.M.; Krishnappa, M.P.G. B5r gene based sequence analysis of Indian buffalopox virus isolates in relation to other orthopoxviruses. Acta Virol. 2007, 51, 47–50. [Google Scholar]
- Singh, R.K.; Hosamani, M.; Balamurugan, V.; Satheesh, C.C.; Rasool, T.J.; Yadav, M.P. Comparative sequence analysis of envelope protein genes of Indian buffalopox virus isolates. Arch. Virol. 2006, 151, 1995–2005. [Google Scholar] [CrossRef]
- Afrough, B.; Zafar, A.; Hasan, R.; Hewson, R. Complete Genome Sequence of Buffalopox Virus. Genome Announc. 2018, 6, e00444–e004518. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.D.; Mauldin, M.R.; Nyayanit, D.A.; Albariño, C.G.; Sarkale, P.; Shete, A.; Guerrero, L.W.; Nakazawa, Y.; Nichol, S.T.; Mourya, D.T. Isolation and phylogenomic analysis of buffalopox virus from human and buffaloes in India. Virus Res. 2020, 277, 197836. [Google Scholar] [CrossRef]
- Czerny, C.-P. Orthopoxviruses—Plagues of Mankind, Strategists in Immune Evasion, Teachers in Vaccination. In Zoonoses—Infections Affecting Humans and Animals: Focus on Public Health Aspects; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 497–525. [Google Scholar] [CrossRef]
- Baxby, D.; Hill, B.J. Characteristics of a new poxvirus isolated from Indian buffaloes. Arch. Gesamte Virusforsch 1971, 35, 70–79. [Google Scholar] [CrossRef]
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988; p. 1460. [Google Scholar]
- Bera, B.C.; Shanmugasundaram, K.; Barua, S.; Anand, T.; Riyesh, T.; Vaid, R.K.; Virmani, N.; Bansal, M.; Shukla, B.N.; Malik, P.; et al. Sequence and phylogenetic analysis of host-range (E3L, K3L, and C7L) and structural protein (B5R) genes of buffalopox virus isolates from buffalo, cattle, and human in India. Virus Genes 2012, 45, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.M.; Singh, I.P. Buffalopox-a review. Trop. Anim. Health Prod. 1977, 9, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Bhanuprakash, V.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Yogisharadhya, R.; Gandhale, P.; Reddy, K.V.; Damle, A.S.; Kher, H.N.; Chandel, B.S.; et al. Zoonotic infections of buffalopox in India. Zoonoses Public Health 2010, 57, e149–e155. [Google Scholar] [CrossRef] [PubMed]
- Gujarati, R.; Reddy Karumuri, S.R.; Babu, T.N.; Janardhan, B. A case report of buffalopox: A zoonosis of concern. Indian J. Derm. Venereol. Leprol. 2019, 85, 348. [Google Scholar] [CrossRef]
- Gurav, Y.K.; Raut, C.G.; Yadav, P.D.; Tandale, B.V.; Sivaram, A.; Pore, M.D.; Basu, A.; Mourya, D.T.; Mishra, A.C. Buffalopox outbreak in humans and animals in Western Maharashtra, India. Prev. Vet. Med. 2011, 100, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Marinaik, C.B.; Venkatesha, M.D.; Gomes, A.R.; Reddy, P.; Nandini, P.; Byregowda, S.M. Isolation and molecular characterization of zoonotic Buffalopox virus from skin lesions of humans in India. Int. J. Dermatol. 2018, 57, 590–592. [Google Scholar] [CrossRef]
- Nedunchelliyan, S.; Reddy, D.S.; Venkataraman, K.S. Buffalo pox infection in man. Indian J. Public Health 1992, 36, 57. [Google Scholar]
- Singh, R.K.; Balamurugan, V.; Bhanuprakash, V.; Venkatesan, G.; Hosamani, M. Emergence and reemergence of vaccinia-like viruses: Global scenario and perspectives. Indian J. Virol. 2012, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Balamurugan, V.; Hosamani, M.; Kallesh, D.J.; Bhanuprakash, V. Sequence analysis of C18L gene of buffalopox virus: PCR strategy for specific detection and differentiation of buffalopox from orthopoxviruses. J. Virol. Methods 2008, 154, 146–153. [Google Scholar] [CrossRef]
- Kolhapure, R.M.; Deolankar, R.P.; Tupe, C.D.; Raut, C.G.; Basu, A.; Dama, B.M.; Pawar, S.D.; Joshi, M.V.; Padbidri, V.S.; Goverdhan, M.K.; et al. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J. Med. Res. 1997, 106, 441–446. [Google Scholar]
- Zafar, A.; Swanepoel, R.; Hewson, R.; Nizam, M.; Ahmed, A.; Husain, A.; Grobbelaar, A.; Bewley, K.; Mioulet, V.; Dowsett, B.; et al. Nosocomial buffalopoxvirus infection, Karachi, Pakistan. Emerg. Infect. Dis. 2007, 13, 902–904. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Perkus, M.E.; Goebel, S.J.; Davis, S.W.; Johnson, G.P.; Limbach, K.; Norton, E.K.; Paoletti, E. Vaccinia virus host range genes. Virology 1990, 179, 276–286. [Google Scholar] [CrossRef]
- Seet, B.T.; Johnston, J.B.; Brunetti, C.R.; Barrett, J.W.; Everett, H.; Cameron, C.; Sypula, J.; Nazarian, S.H.; Lucas, A.; McFadden, G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003, 21, 377–423. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Watson, J.C.; Jacobs, B.L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1992, 89, 4825–4829. [Google Scholar] [CrossRef] [Green Version]
- Guerra, S.; Caceres, A.; Knobeloch, K.P.; Horak, I.; Esteban, M. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog. 2008, 4, e1000096. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.V.; Elroy-Stein, O.; Jagus, R.; Moss, B.; Kaufman, R.J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 1992, 66, 1943–1950. [Google Scholar] [CrossRef] [Green Version]
- Gubser, C.; Hue, S.; Kellam, P.; Smith, G.L. Poxvirus genomes: A phylogenetic analysis. J. Gen. Virol. 2004, 85, 105–117. [Google Scholar] [CrossRef]
- Najera, J.L.; Gomez, C.E.; Domingo-Gil, E.; Gherardi, M.M.; Esteban, M. Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene. J. Virol. 2006, 80, 6033–6047. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Jiang, C.; Arsenio, J.; Dick, K.; Cao, J.; Xiang, Y. Vaccinia virus K1L and C7L inhibit antiviral activities induced by type I interferons. J. Virol. 2009, 83, 10627–10636. [Google Scholar] [CrossRef] [Green Version]
- Payne, L.G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 1980, 50, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, S.N.; Kotwal, G.J.; Moss, B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffe, E.J.; Isaacs, S.N.; Moss, B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 1993, 67, 4732–4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, M.; Siva Sankar, M.S.; Bhanuprakash, V.; Venkatesan, G.; Bora, D.P.; Yogisharadhya, R.; Balamurugan, V. Real time PCR: A rapid tool for potency estimation of live attenuated camelpox and buffalopox vaccines. Biologicals 2012, 40, 92–95. [Google Scholar] [CrossRef]
- Ghosh, T.; Arora, R.; Sehgal, C.; Ray, S.; Wattal, B. An investigation of buffalopox outbreak in animals and human beings in Dhulia District (Maharashtra State). 2. Epidemiological studies. J. Commun. Dis. 1977, 9, 93–101. [Google Scholar]
- Damle, A.S.; Gaikwad, A.A.; Patwardhan, N.S.; Duthade, M.M.; Sheikh, N.S.; Deshmukh, D.G. Outbreak of human buffalopox infection. J. Glob. Infect. Dis. 2011, 3, 187–188. [Google Scholar] [CrossRef]
- Czerny, C.P.; Johann, S.; Holzle, L.; Meyer, H. Epitope detection in the envelope of intracellular naked orthopox viruses and identification of encoding genes. Virology 1994, 200, 764–777. [Google Scholar] [CrossRef]
- Anand Kumar, P.; Butchaiah, G. Partial antigenic characterization of buffalopox virus. Vet. Res. Commun. 2004, 28, 543–552. [Google Scholar] [CrossRef]
- Kumar, A.; Yogisharadhya, R.; Bhanuprakash, V.; Venkatesan, G.; Shivachandra, S.B. Structural analysis and immunogenicity of recombinant major envelope protein (rA27L) of buffalopox virus, a zoonotic Indian vaccinia-like virus. Vaccine 2015, 33, 5396–5405. [Google Scholar] [CrossRef]
- Kumar, A.; Yogisharadhya, R.; Venkatesan, G.; Bhanuprakash, V.; Shivachandra, S.B. Immunogenicity and protective efficacy of recombinant major envelope protein (rH3L) of buffalopox virus in animal models. Antivir. Res. 2016, 126, 108–116. [Google Scholar] [CrossRef]
- Czerny, C.P.; Eis-Hubinger, A.M.; Mayr, A.; Schneweis, K.E.; Pfeiff, B. Animal poxviruses transmitted from cat to man: Current event with lethal end. Zent. Vet. B 1991, 38, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Eis-Hubinger, A.M.; Gerritzen, A.; Schneweis, K.E.; Pfeiff, B.; Pullmann, H.; Mayr, A.; Czerny, C.P. Fatal cowpox-like virus infection transmitted by cat. Lancet 1990, 336, 880. [Google Scholar] [CrossRef]
- Umesha, K.; Sarojini, B.K.; Darshan Raj, C.G.; Bhanuprakash, V.; Yogisharadhya, R.; Raghavendra, R.; Khan, M.T.H. In vitro and in silico biological studies of novel thiazolo[3,2-a]pyrimidine-6-carboxylate derivatives. Med. Chem. Res. 2013, 23, 168–180. [Google Scholar] [CrossRef]
- Kumar, R.; Khandelwal, N.; Chander, Y.; Riyesh, T.; Tripathi, B.N.; Kashyap, S.K.; Barua, S.; Maherchandani, S.; Kumar, N. MNK1 inhibitor as an antiviral agent suppresses buffalopox virus protein synthesis. Antivir. Res. 2018, 160, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hobday, T.L.; Rao, A.R.; Kempe, C.H.; Downie, A.W. Comparison of dried vaccine with fresh Indian buffalo-calf lymph in revaccination against smallpox. Bull. World Health Organ. 1961, 25, 69–71. [Google Scholar]
- Meyer, H. Summary Report on First, Second and Third Generation Smallpox Vaccines; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Kumar, A.; Yogisharadhya, R.; Venkatesan, G.; Bhanuprakash, V.; Pandey, A.B.; Shivachandra, S.B. Co-administration of recombinant major envelope proteins (rA27L and rH3L) of buffalopox virus provides enhanced immunogenicity and protective efficacy in animal models. Antivir. Res. 2017, 141, 174–178. [Google Scholar] [CrossRef]
- Damaso, C.R.; Esposito, J.J.; Condit, R.C.; Moussatche, N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; dos Santos, J.R.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef]
- de Souza Trindade, G.; da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.; Borges, A.S.; Peiro, J.R.; Pituco, E.M.; Bonjardim, C.A.; Ferreira, P.C.; et al. Aracatuba virus: A vaccinialike virus associated with infection in humans and cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef]
- Springer, Y.P.; Hsu, C.H.; Werle, Z.R.; Olson, L.E.; Cooper, M.P.; Castrodale, L.J.; Fowler, N.; McCollum, A.M.; Goldsmith, C.S.; Emerson, G.L.; et al. Novel Orthopoxvirus Infection in an Alaska Resident. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 1737–1741. [Google Scholar] [CrossRef]
- Brown, K.; Leggat, P.A. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vora, N.M.; Li, Y.; Geleishvili, M.; Emerson, G.L.; Khmaladze, E.; Maghlakelidze, G.; Navdarashvili, A.; Zakhashvili, K.; Kokhreidze, M.; Endeladze, M.; et al. Human infection with a zoonotic orthopoxvirus in the country of Georgia. N. Engl. J. Med. 2015, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Cardeti, G.; Gruber, C.E.M.; Eleni, C.; Carletti, F.; Castilletti, C.; Manna, G.; Rosone, F.; Giombini, E.; Selleri, M.; Lapa, D.; et al. Fatal Outbreak in Tonkean Macaques Caused by Possibly Novel Orthopoxvirus, Italy, January 2015 (1). Emerg. Infect. Dis. 2017, 23, 1941–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanave, G.; Dowgier, G.; Decaro, N.; Albanese, F.; Brogi, E.; Parisi, A.; Losurdo, M.; Lavazza, A.; Martella, V.; Buonavoglia, C.; et al. Novel Orthopoxvirus and Lethal Disease in Cat, Italy. Emerg. Infect. Dis. 2018, 24, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltom, K.H.; Samy, A.M.; Abd El Wahed, A.; Czerny, C.-P. Buffalopox Virus: An Emerging Virus in Livestock and Humans. Pathogens 2020, 9, 676. https://doi.org/10.3390/pathogens9090676
Eltom KH, Samy AM, Abd El Wahed A, Czerny C-P. Buffalopox Virus: An Emerging Virus in Livestock and Humans. Pathogens. 2020; 9(9):676. https://doi.org/10.3390/pathogens9090676
Chicago/Turabian StyleEltom, Kamal H., Abdallah M. Samy, Ahmed Abd El Wahed, and Claus-Peter Czerny. 2020. "Buffalopox Virus: An Emerging Virus in Livestock and Humans" Pathogens 9, no. 9: 676. https://doi.org/10.3390/pathogens9090676
APA StyleEltom, K. H., Samy, A. M., Abd El Wahed, A., & Czerny, C.-P. (2020). Buffalopox Virus: An Emerging Virus in Livestock and Humans. Pathogens, 9(9), 676. https://doi.org/10.3390/pathogens9090676






