Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine
Abstract
1. Introduction
2. Results
2.1. Humoral Immune Response of Living and Non-Living Anthrax Vaccines in Cattle
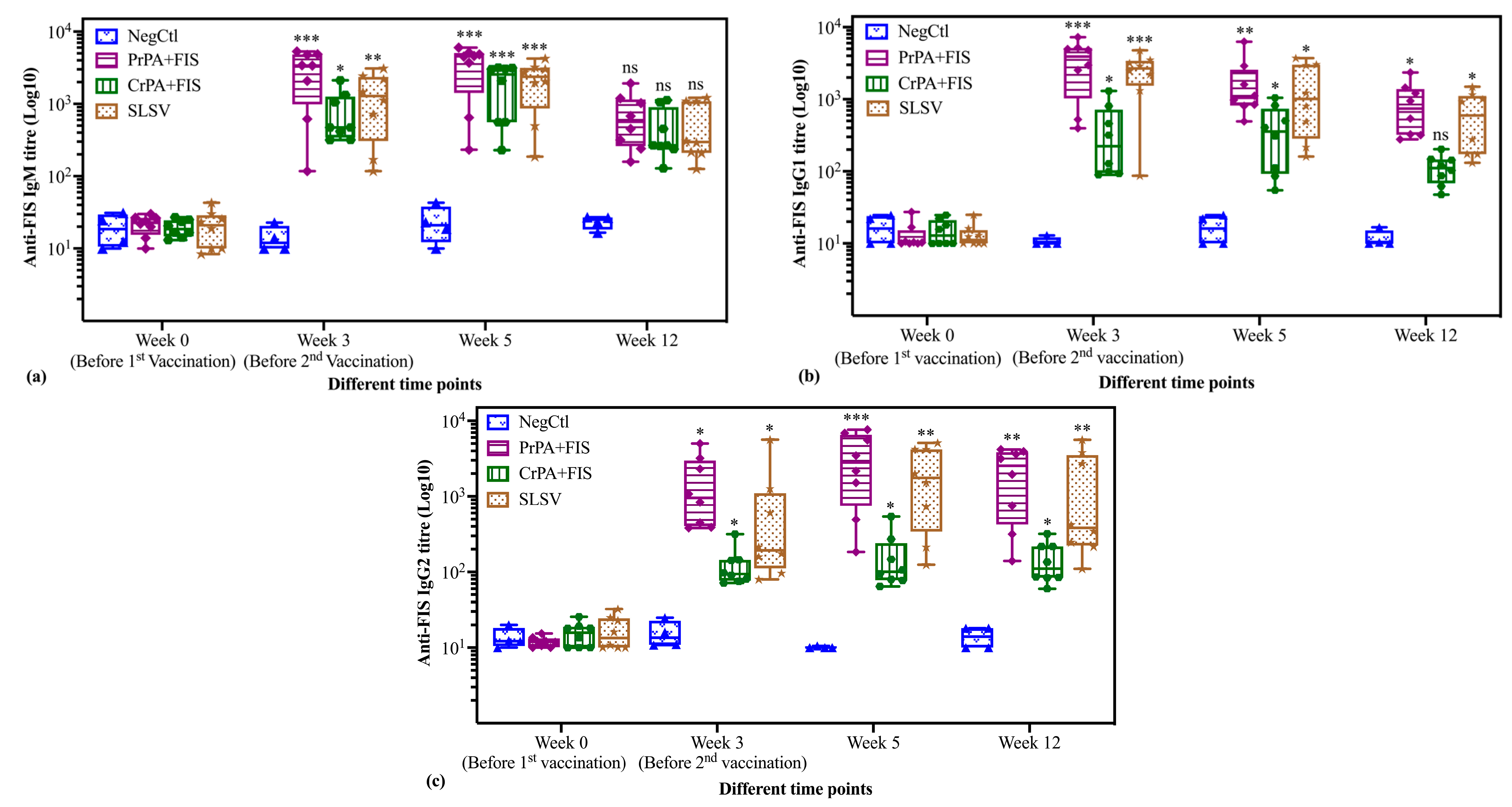
2.2. Immunoglobulins IgM and IgG Subclasses Titres
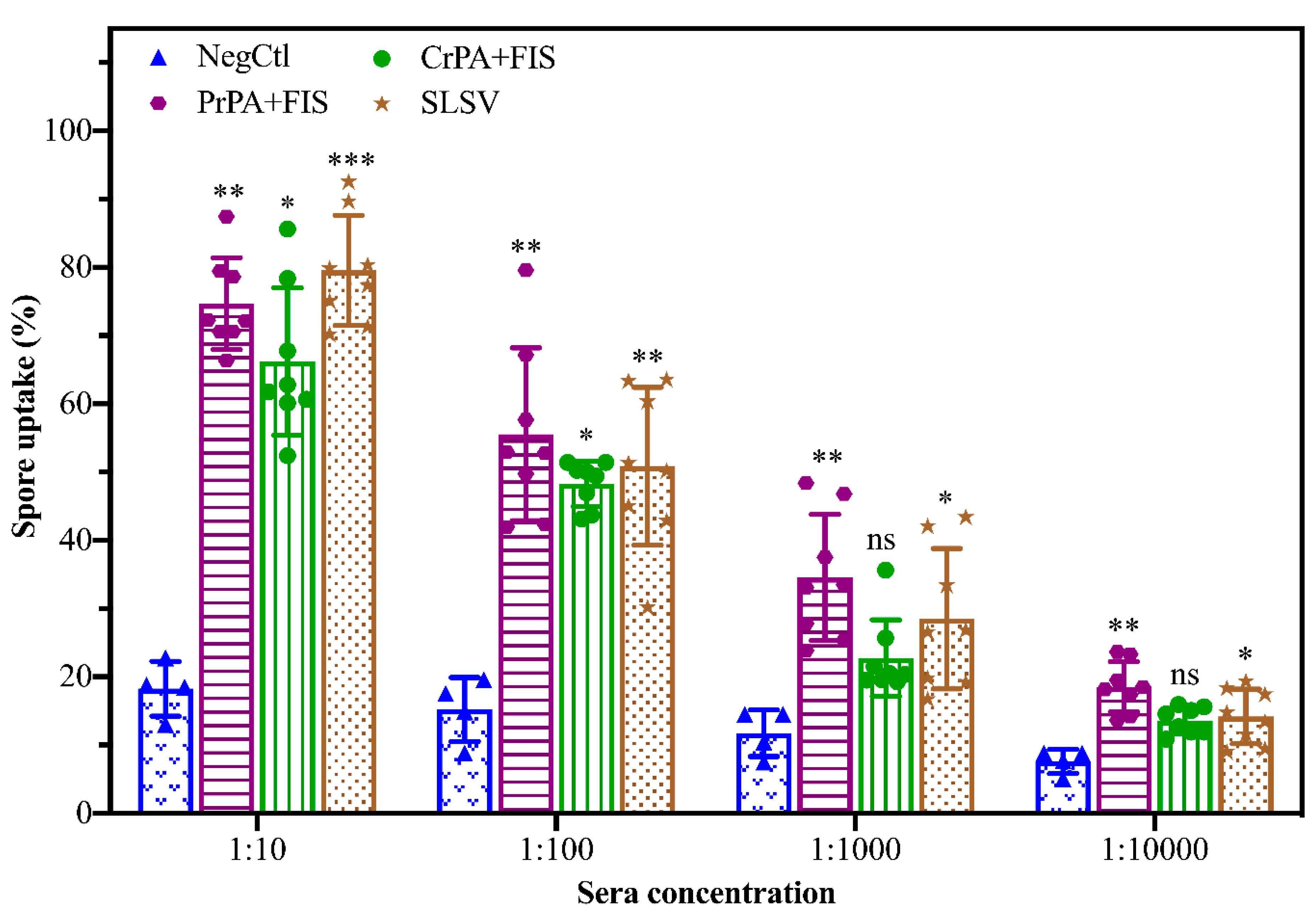
2.3. Opsonising Ability of Vaccine-Induced Antibodies
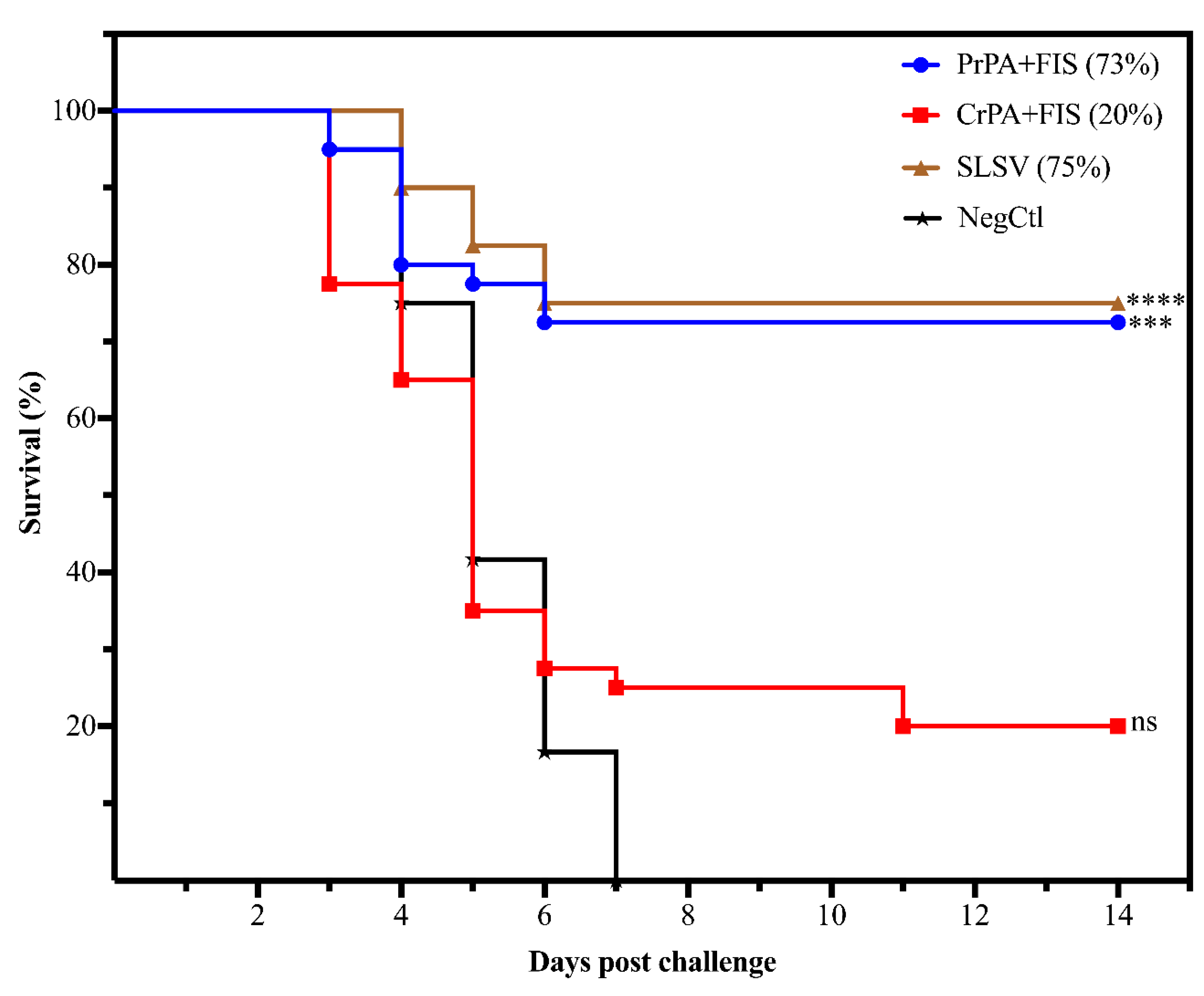
2.4. Protection Conferred on A/J Mice by Antibodies from Cattle Immune Sera
3. Discussion
4. Materials and Methods
4.1. Recombinant Protein Expression and Purification
4.2. Formalin-Inactivated Spores (FIS) Preparation
4.3. Formulation of Non-Living Anthrax Vaccine
4.4. Immunization Animal Experiment and Passive Mouse Protection Tests
4.5. Serum Immunoglobulin Titre Determination
4.6. Toxin Neutralization Assay (TNA)
4.7. Opsonophagocytic Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hambleton, P.; Carman, J.A.; Melling, J. Anthrax: The disease in relation to vaccines. Vaccine 1984, 2, 125–132. [Google Scholar] [CrossRef]
- Beyer, W.; Turnbull, P. Anthrax in animals. Mol. Asp. Med. 2009, 30, 481–489. [Google Scholar] [CrossRef]
- Cote, C.K.; Kaatz, L.; Reinhardt, J.; Bozue, J.; Tobery, A.S.; Bassett, D.A.; Sanz, P.; Darnell, C.S.; Alem, F.; O’Brien, D.A.; et al. Characterization of a multi-component anthrax vaccine designed to target the initial stages of infection as well as toxaemia. J. Med. Microbiol. 2012, 61, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Koehler, T. Bacillus anthracis genetics and virulence gene regulation, in Anthrax. Curr. Top. Microbiol. Immunol. 2002, 271, 143–164. [Google Scholar] [CrossRef]
- Guidi-Rontani, C.; Weber-Levy, M.; Labruyère, E.; Mock, M. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 1999, 31, 9–17. [Google Scholar] [CrossRef]
- Lacy, D.B.; Lin, H.C.; Melnyk, R.A.; Schueler-Furman, O.; Reither, L.; Cunningham, K.; Baker, D.; Collier, R.J. A model of anthrax toxin lethal factor bound to protective antigen. Proc. Natl. Acad. Sci. USA 2005, 102, 16409–16414. [Google Scholar] [CrossRef]
- Leppla, S.H. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar] [CrossRef]
- Mogridge, J.; Cunningham, K.; Lacy, D.B.; Mourez, M.; Collier, J.R. Biological Sciences—Microbiology—The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc. Natl. Acad. Sci. USA 2002, 99, 7045–7048. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.J.; Uhde, B.K.; Shadomy, V.S.; Messonnier, N. Conference report on public health and clinical guidelines for anthrax. Emerg. Infect. Dis. 2008, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Sterne, M. The use of anthrax vaccines prepared from avirulent (uncapsulated) variants of Bacillus anthracis. Onderstepoort J. Vet. 1939, 13, 307–312. [Google Scholar]
- Ndumnego, O.C.; Köhler, S.M.; Crafford, J.E.; van Heerden, H.; Beyer, W. Comparative analysis of the immunologic response induced by the Sterne 34F2 live spore Bacillus anthracis vaccine in a ruminant model. Vet. Immunol. Immunopathol. 2016, 178, 14–21. [Google Scholar] [CrossRef]
- Fasanella, A.; Tonello, F.; Garofolo, G.; Muraro, L.; Carattoli, A.; Adone, R.; Muntecucco, C. Protective activity and immunogenicity of two recombinant anthrax vaccines for veterinary use. Vaccine 2008, 26, 5684–5688. [Google Scholar] [CrossRef] [PubMed]
- Sterne, M. The immunization of laboratory animals against anthrax. Onderstepoort J. Vet. 1939, 13, 25. [Google Scholar]
- Turnbull, P.C. Anthrax vaccines: Past, present and future. Vaccine 1991, 9, 533–539. [Google Scholar] [CrossRef]
- Turnbull, P.C.B. Anthrax in Humans and Animals: Decisions on Treatment and/or Vaccination of Livestock; WHO: Geneva, Swtzerland, 2008; pp. 89–100. [Google Scholar]
- Ndumnego, O.C.; Köhler, S.M.; Crafford, J.E.; Beyer, W.; van Heerden, H. Immunogenicity of anthrax recombinant peptides and killed spores in goats and protective efficacy of immune sera in A/J mouse model. Sci. Rep. 2018, 8, 16937. [Google Scholar] [CrossRef]
- Brossier, F.; Levy, M.; Mock, M. Anthrax spores make an essential contribution to vaccine efficacy. Infect. Immun. 2002, 70, 661–664. [Google Scholar] [CrossRef]
- Hahn, U.K.; Alex, M.; Czerny, C.-P.; Böhm, R.; Beyer, W. Protection of mice against challenge with Bacillus anthracis STI spores after DNA vaccination. Int. J. Med. Microbiol. 2004, 294, 35–44. [Google Scholar] [CrossRef]
- Hahn, U.K.; Boehm, R.; Beyer, W. DNA vaccination against anthrax in mice—Combination of anti-spore and anti-toxin components. Vaccine 2006, 24, 4569–4571. [Google Scholar] [CrossRef]
- Little, S.F.; Knudson, G.B. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect. Immun. 1986, 52, 509–512. [Google Scholar] [CrossRef]
- Parreiras, P.M.; Sirota, L.A.; Wagner, L.D.; Menzies, S.L.; Arciniega, J.L. Comparability of ELISA and toxin neutralization to measure immunogenicity of Protective Antigen in mice, as part of a potency test for anthrax vaccines. Vaccine 2009, 27, 4537–4542. [Google Scholar] [CrossRef]
- Ribot, W.; Powell, B.S.; Ivins, B.E.; Little, S.F.; Johnson, W.M.; Hoover, T.A.; Norris, S.L.; Adamovicz, J.J.; Friedlander, A.M.; Andrews, G.P. Comparative vaccine efficacy of different isoforms of recombinant protective antigen against Bacillus anthracis spore challenge in rabbits. Vaccine 2006, 24, 3469–3476. [Google Scholar] [CrossRef]
- Vance, D.J.; Rong, Y.; Brey, R.N.; Mantis, N.J. Combination of two candidate subunit vaccine antigens elicits protective immunity to ricin and anthrax toxin in mice. Vaccine 2015, 33, 417–421. [Google Scholar] [CrossRef][Green Version]
- Verma, A.; Burns, D.L. Improving the stability of recombinant anthrax protective antigen vaccine. Vaccine 2018, 36, 6379–6382. [Google Scholar] [CrossRef]
- Koehler, S.M.; Buyuk, F.; Celebi, O.; Demiraslan, H.; Doganay, M.; Sahin, M.; Moehring, J.; Ndumnego, O.C.; Otlu, S.; van Heerden, H.; et al. Protection of farm goats by combinations of recombinant peptides and formalin inactivated spores from a lethal Bacillus anthracis challenge under field conditions. BMC Vet. Res. 2017, 13, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Saxena, A.; Rai, A.; Bhatnagar, R. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J. 2010, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, J. Comparison of adjuvants for stimulation of HI antibody to SIV. In Proceedings of the 19th IPVS Congress, Copenhage, Denmark, 16–19 July 2006. [Google Scholar]
- Shabana, W.; Ismail, H.M.; Mossad, W. Using Emulsigen-D as Recent Adjuvant in Trivalent Foot and Mouth Disease Vaccine. Glob. J. Med. Res. 2018, 18, 1–9. [Google Scholar]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef]
- Nicol, J.; Sterne, M.L.M. The effect of large scale active immunization against anthrax. J. S. Afr. Vet. Assoc. 1942, 13, 53–63. [Google Scholar]
- Webster, A. Inhibiting effect of antibiotics on anthrax vaccination. Aust. Vet. J. 1973, 49, 545. [Google Scholar] [CrossRef]
- Ascough, S.; Ingram, R.J.; Chu, K.K.; Reynolds, C.J.; Musson, J.A.; Doganay, M.; Metan, G.; Metan, G.; Ozkul, Y.; Baillie, L.; et al. Anthrax lethal factor as an immune target in humans and transgenic mice and the impact of HLA polymorphism on CD4+ T cell immunity. PLoS Pathog. 2014, 10, e1004085. [Google Scholar] [CrossRef]
- Brahmbhatt, T.N.; Darnell, S.C.; Carvalho, H.M.; Sanz, P.; Kang, T.J.; Bull, R.L.; Rasmussen, S.B.; Cross, A.S.; O’Brien, A.D. Recombinant exosporium protein BclA of Bacillus anthracis is effective as a booster for mice primed with suboptimal amounts of protective antigen. Infect. Immun. 2007, 75, 5240–5247. [Google Scholar] [CrossRef]
- Duverger, A.; Carré, J.M.; Jee, J.; Leppla, S.H.; Cormet-Boyaka, E.; Tang, W.J.; Tomé, D.; Boyaka, P.N. Contributions of edema factor and protective antigen to the induction of protective immunity by Bacillus anthracis edema toxin as an intranasal adjuvant. J. Immunol. 2010, 185, 5943–5952. [Google Scholar] [CrossRef]
- Enkhtuya, J.; Kawamoto, K.; Kobayashi, Y.; Uchida, I.; Rana, N.; Makino, S.; Makino, S. Significant passive protective effect against anthrax by antibody to Bacillus anthracis inactivated spores that lack two virulence plasmids. Microbiology 2006, 152, 3103–3110. [Google Scholar] [CrossRef]
- Gauthier, Y.P.; Tournier, J.N.; Paucod, J.C.; Corre, J.P.; Mock, M.; Goossens, P.L.; Vidal, D.R. Efficacy of a vaccine based on protective antigen and killed spores against experimental inhalational anthrax. Infect. Immun. 2009, 77, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Das, S.; Somani, V.; Makam, S.S.; Joseph, K.J.; Bhatnagar, R. A bivalent protein r-PB, comprising PA and BclA immunodominant regions for comprehensive protection against Bacillus anthracis. Sci. Rep. 2018, 8, 7242. [Google Scholar] [CrossRef]
- Majumder, S.; Majumder, S.; Das, S.; Somani, K.V.; Makam, S.S.; Joseph, K.J.; Bhatnagar, R. A bivalent protein r-PAbxpB comprising PA domain IV and exosporium protein BxpB confers protection against B. anthracis spores and toxin. Front. Immunol. 2019, 10, 498. [Google Scholar] [CrossRef]
- Little, S.F.; Ivin, B.E.; Friedlander, A.M. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 1997, 65, 5171–5175. [Google Scholar] [CrossRef]
- Little, S.F.; Leppla, S.H.; Cora, E. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 1988, 56, 1807–1813. [Google Scholar] [CrossRef]
- Vergis, J.M.; Cote, C.K.; Bozue, J.; Alem, F.; Ventura, C.L.; Welkos, S.L.; O’Brien, A.D. Immunization of mice with formalin-inactivated spores from avirulent Bacillus cereus strains provides significant protection from challenge with Bacillus anthracis Ames. Clin. Vaccine Immunol. 2013, 20, 56–65. [Google Scholar] [CrossRef]
- Mittenbühler, K.; v d Esche, U.; Heinevetter, L.; Bessler, W.G.; Huber, M. Lipopeptides: Adjuvanticity in conventional and genetic immunization. FEMS Immunol. Med. Microbiol. 2003, 37, 193–200. [Google Scholar] [CrossRef][Green Version]
- Welkos, S.L.; Friedlander, A.M. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 1988, 5, 127–139. [Google Scholar] [CrossRef]
- Crowe, S.R.; Ash, L.L.; Engler, R.J.; Ballard, J.D.; Harley, J.B.; Farris, A.D.; James, J.A. Select human anthrax protective antigen epitope-specific antibodies provide protection from lethal toxin challenge. J. Infect. Dis. 2010, 202, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Antibody-based defense strategies against biological weapons. ASM News 2005, 71, 28–33. [Google Scholar]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees): Anthrax. Ch. 2.1.1; Office International des Épizooties: Paris, France, 2012; Volume 1, pp. 555–574. [Google Scholar]
- Williamson, E.D.; Hodgson, I.; Walker, N.J.; Topping, A.W.; Duchars, M.G.; Mott, M.J.; Estep, J.; LeButt, C.; Flick-Smith, C.H.; Jones, E.H.; et al. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 2005, 73, 5978–5987. [Google Scholar] [CrossRef]
- Welkos, S.; Little, S.; Friedlander, A.; Fritz, D.; Fellows, P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 2001, 147, 1677–1685. [Google Scholar] [CrossRef]
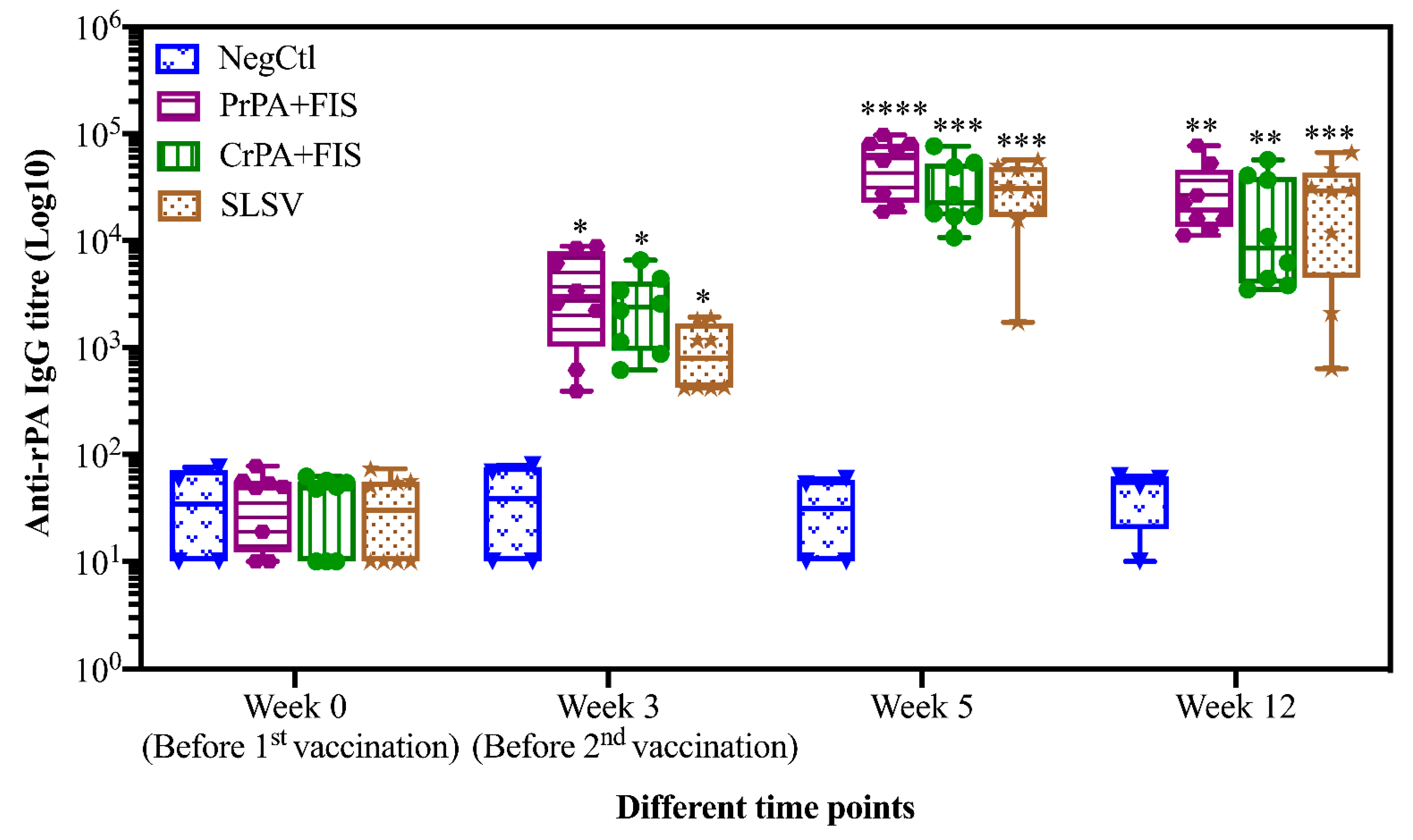
| Anti-rPA (PrPA+FIS) | Anti-rPA (CrPA+FIS) | Anti-rPA (SLSV) | Anti-FIS (PrPA+FIS) | Anti-FIS (CrPA+FIS) | Anti-FIS (SLSV) | TNA (PrPA+FIS) | TNA (CrPA+FIS) | TNA (SLSV) | |
|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation | 0.8196 * | 0.1237 ns | 0.9239 ** | 0.7883 * | 0.3836 ns | 0.8518 ** | 0.8588 ** | 0.4564 ns | 0.8778 ** |
| Significance (2-tailed) | 0.0128 | 0.7704 | 0.0010 | 0.0201 | 0.3482 | 0.0073 | 0.0063 | 0.2556 | 0.0042 |
| Vaccine Groups and Cattle Number (n) | Vaccine and Dose | Cattle Vaccination and Sampling Schedule | A/J Mice Used in Passive Mouse Challenge (n) | |||
|---|---|---|---|---|---|---|
| Wk 0 | Wk 3 | Wk 5 | Wk 12 | |||
| SLSV (n = 8) | SLSV vaccine with Anthravax® (108 spores) | ± | ± | + | + | 5 mice/serum sample (n = 40) |
| PrPA+FIS (n = 8) | Purified rPA (75 µg) + FIS (108 spores) + Emulsigen-D®/Alhydrogel® adjuvants (33% v/v) | ± | ± | + | + | 5 mice/serum sample (n = 40) |
| CrPA+FIS (n = 8) | Crude rPA (75 µg) + FIS (108 spores) + Emulsigen-D®/Alhydrogel® adjuvants (33% v/v) | ± | ± | + | + | 5 mice/serum sample (n = 40) |
| NegCtl (n = 4) | Emulsigen-D®/Alhydrogel® adjuvants/saline (33% v/v) | ± | ± | + | + | 3 mice/serum sample (n = 12) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jauro, S.; C. Ndumnego, O.; Ellis, C.; Buys, A.; Beyer, W.; van Heerden, H. Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine. Pathogens 2020, 9, 557. https://doi.org/10.3390/pathogens9070557
Jauro S, C. Ndumnego O, Ellis C, Buys A, Beyer W, van Heerden H. Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine. Pathogens. 2020; 9(7):557. https://doi.org/10.3390/pathogens9070557
Chicago/Turabian StyleJauro, Solomon, Okechukwu C. Ndumnego, Charlotte Ellis, Angela Buys, Wolfgang Beyer, and Henriette van Heerden. 2020. "Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine" Pathogens 9, no. 7: 557. https://doi.org/10.3390/pathogens9070557
APA StyleJauro, S., C. Ndumnego, O., Ellis, C., Buys, A., Beyer, W., & van Heerden, H. (2020). Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine. Pathogens, 9(7), 557. https://doi.org/10.3390/pathogens9070557






