Mechanisms of Oncogenesis by HTLV-1 Tax
Abstract
1. Introduction
2. HTLV-1 Genomic Structure and Modes of Entry
3. Oncogenic Functions and Regulation of Viral Gene Expression by HTLV-1 Tax
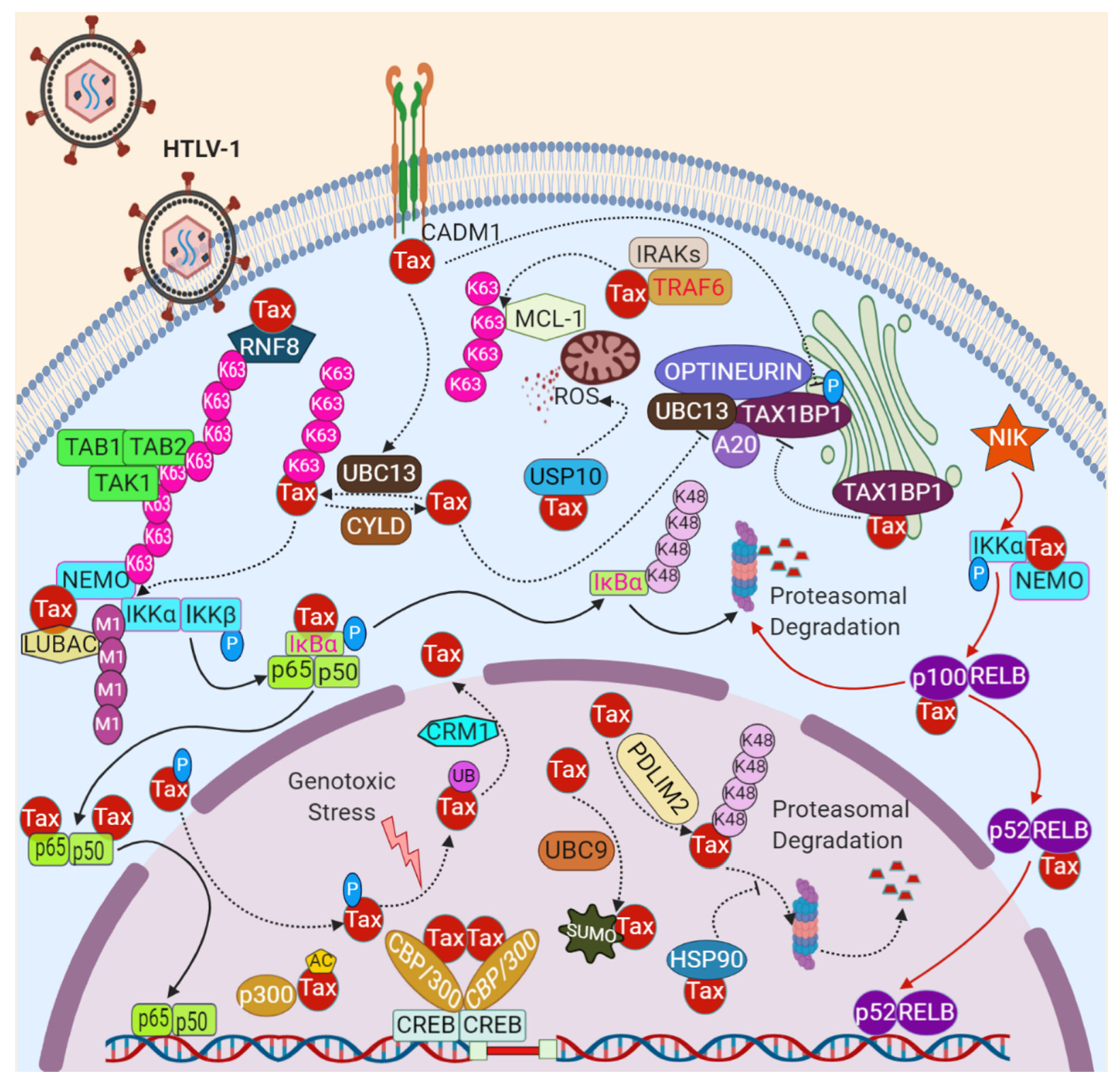
4. HTLV-1 Tax Activation of NF-κB
5. Post-translational Modifications (PTMs) of Tax and Its Contribution to Cellular Transformation
5.1. Tax Ubiquitination
5.2. Tax SUMOylation
5.3. Tax Phosphorylation
5.4. Tax Acetylation
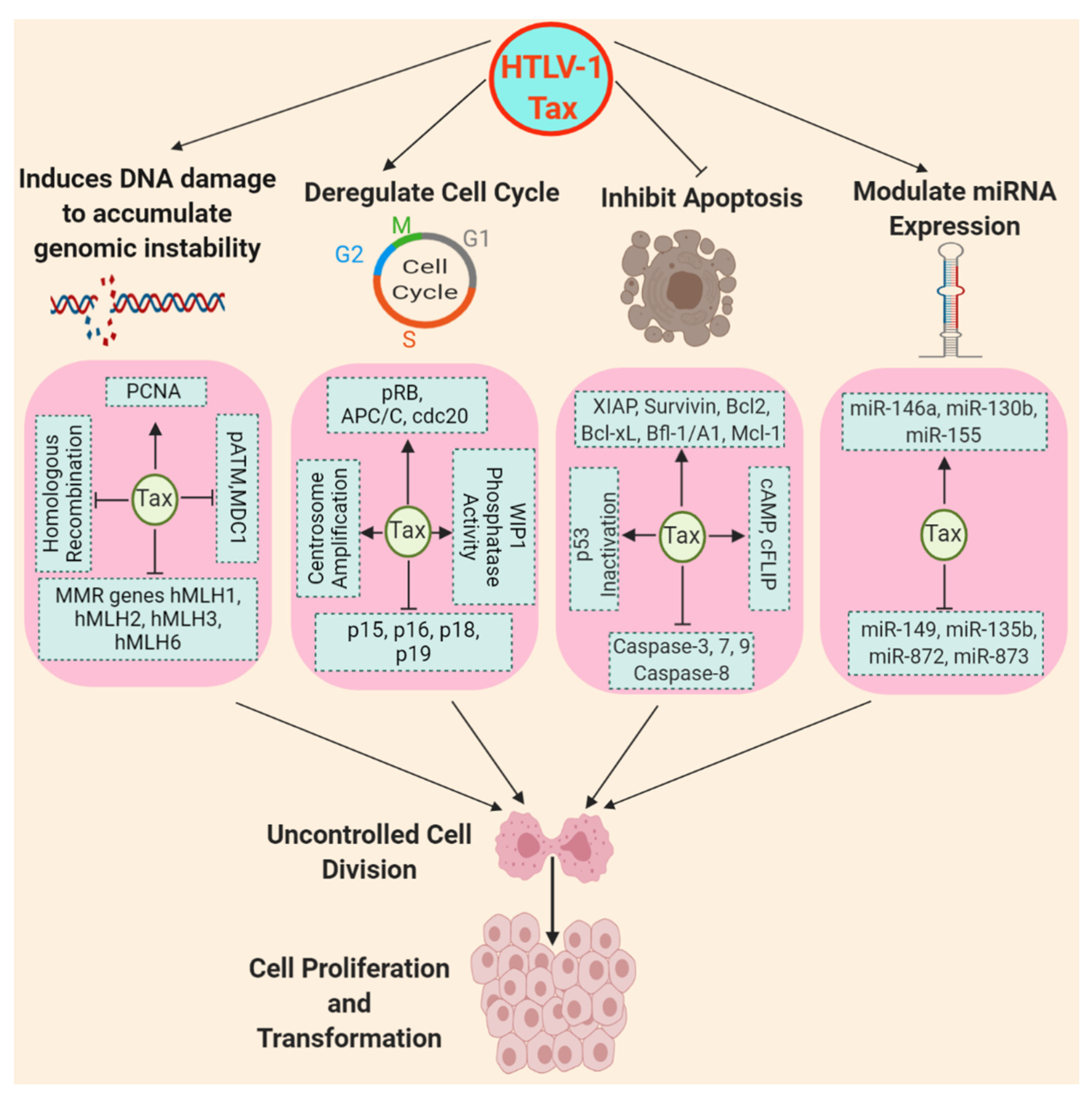
6. HTLV-1-mediated Inhibition of Apoptosis
6.1. The Imbalance between Pro- and Anti-apoptotic Proteins
6.2. Impaired Caspase Signaling and Compromised Death Receptor Signaling
7. HTLV-1 Deregulation of Cell Cycle and DNA Repair
8. Tax Modulation of miRNA Expression for T cell Proliferation and Transformation
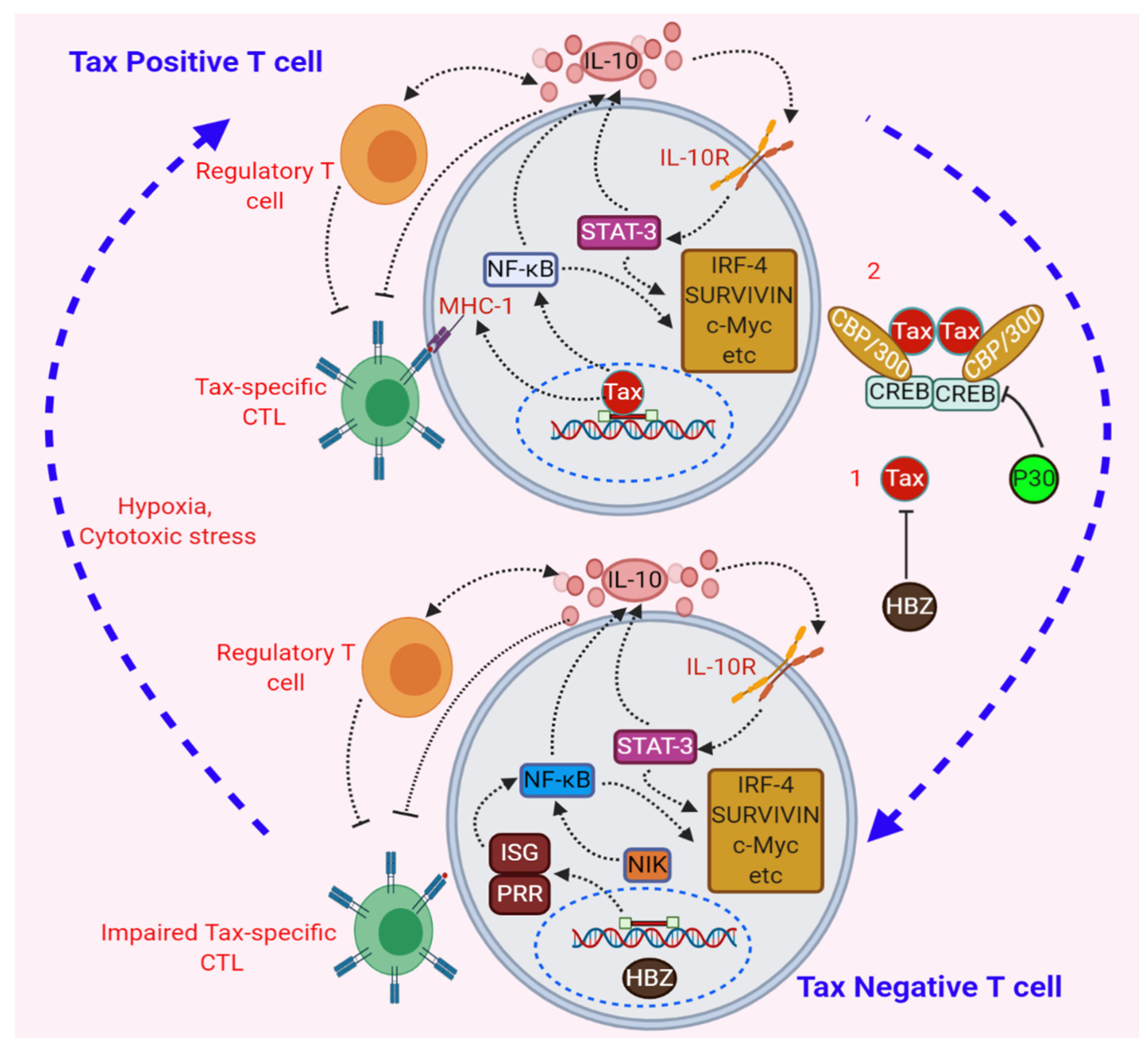
9. Negative Regulation of Tax through Its Repression by Viral and Cellular genes
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30. [Google Scholar] [CrossRef]
- Kalyanaraman, V.; Sarngadharan, M.; Robert-Guroff, M.; Miyoshi, I.; Golde, D.; Gallo, R. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 1982, 218, 571–573. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Prosser, A.T.; Lebreton, M.; Mpoudi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. USA 2005, 102, 7994–7999. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Ramos, J.C.; Tobinai, K. A Review of New Findings in Adult T-cell Leukemia-Lymphoma: A Focus on Current and Emerging Treatment Strategies. Adv. Ther. 2018, 35, 135–152. [Google Scholar] [CrossRef]
- Martin, F.; Taylor, G.P.; Jacobson, S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev. Clin. Immunol. 2014, 10, 1531–1546. [Google Scholar] [CrossRef]
- Martinez, M.P.; Al-Saleem, J.; Green, P.L. Comparative virology of HTLV-1 and HTLV-2. Retrovirology 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, W.; Kashiwagi, S.; Ikematsu, H.; Hayashi, J.; Nomura, H.; Okochi, K. Intrafamilial Transmission of Adult T Cell Leukemia Virus. J. Infect. Dis. 1986, 154, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Khabbaz, R.F.; Murphy, E.L.; Hermansen, S.; Roberts, C.; Lal, R.; Heneine, W.; Wright, D.; Matijas, L.; Thomson, R.; et al. Male-to-Female Transmission of Human T-Cell Lymphotropic Virus Types I and II: Association with Viral Load. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 12, 193–201. [Google Scholar] [CrossRef]
- Murphy, E.L.; Figueroa, J.P.; Gibbs, W.N.; Brathwaite, A.; Holding-Cobham, M.; Waters, D.; Cranston, B.; Hanchard, B.; Blattner, W.A. Sexual Transmission of Human T-Lymphotropic Virus Type I (HTLV-I). Ann. Intern. Med. 1989, 111, 555. [Google Scholar] [CrossRef] [PubMed]
- Ureta-Vidal, A.; Angelin-Duclos, C.; Tortevoye, P.; Murphy, E.; Jolly, N.; Joubert, M.; Carles, G.; Moreau, J.-P.; Gessain, A. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. Int. J. Cancer 1999, 82, 832–836. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Pham, H.; Woodman, R.J.; Pepperill, C.; A Taylor, K. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med. J. Aust. 2016, 205, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Demontis, M.A.; Sadiq, M.T.; Golz, S.; Taylor, G.P. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J. Med. Virol. 2015, 87, 2130–2134. [Google Scholar] [CrossRef]
- Melamed, A.; Laydon, D.J.; Gillet, N.A.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. Genome-wide Determinants of Proviral Targeting, Clonal Abundance and Expression in Natural HTLV-1 Infection. PLoS Pathog. 2013, 9, e1003271. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Zahoor, M.A.; Zhi, H.; Ho, Y.K.; Giam, C.Z. Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex. PLoS Pathog. 2014, 10, e1004040. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W.; O’Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, Prognostic Factors, Treatment, and Response Criteria of Adult T-Cell Leukemia-Lymphoma: A Proposal From an International Consensus Meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef]
- Richardson, J.H.; Edwards, A.J.; Cruickshank, J.K.; Rudge, P.; Dalgleish, A.G. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 1990, 64, 5682–5687. [Google Scholar] [CrossRef] [PubMed]
- Franchini, G.; Mann, D.L.; Popovic, M.; Zicht, R.R.; Gallo, R.C.; Wong-Staal, F. HTLV-I infection of T and B cells of a patient with adult T-cell leukemia-lymphoma (ATLL) and transmission of HTLV-I from B cells to normal T cells. Leuk. Res. 1985, 9, 1305–1314. [Google Scholar] [CrossRef]
- De Castro-Amarante, M.F.; Pise-Masison, C.A.; McKinnon, K.; Parks, R.W.; Galli, V.; Omsland, M.; Andresen, V.; Massoud, R.; Brunetto, G.; Caruso, B.; et al. Human T Cell Leukemia Virus Type 1 Infection of the Three Monocyte Subsets Contributes to Viral Burden in Humans. J. Virol. 2015, 90, 2195–2207. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Huang, Y.K.; Bertolette, D.C.; Ruscetti, F.W. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4+ T cells. Nat. Med. 2008, 14, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Igakura, T.; Núñez, L.; Grosjean, M.; Cartajena, I. Spread of HTLV-I Between Lymphocytes by Virus-Induced Polarization of the Cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef]
- Gross, C.; Thoma-Kress, A.K. Molecular Mechanisms of HTLV-1 Cell-to-Cell Transmission. Viruses 2016, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Pique, C.; Jones, K.S. Pathways of cell-cell transmission of HTLV-1. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Pais-Correia, A.-M.; Sachse, M.; Guadagnini, S.; Robbiati, V.; Lasserre, R.; Gessain, A.; Gout, O.; Alcover, A.; Thoulouze, M.-I. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 2009, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Van Prooyen, N.; Gold, H.; Andresen, V.; Schwartz, O.; Jones, K.; Ruscetti, F.; Lockett, S.; Gudla, P.; Venzon, D.; Franchini, G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 20738–20743. [Google Scholar] [CrossRef] [PubMed]
- Nejmeddine, M.; Barnard, A.L.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R.M. Human T-lymphotropic Virus, Type 1, Tax Protein Triggers Microtubule Reorientation in the Virological Synapse. J. Biol. Chem. 2005, 280, 29653–29660. [Google Scholar] [CrossRef] [PubMed]
- Nejmeddine, M.; Negi, V.S.; Mukherjee, S.; Tanaka, Y.; Orth, K.; Taylor, G.P.; Bangham, C.R.M. HTLV-1–Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood 2009, 114, 1016–1025. [Google Scholar] [CrossRef]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan Sulfate Proteoglycans Mediate Attachment and Entry of Human T-Cell Leukemia Virus Type 1 Virions into CD4+ T Cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef]
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Heveker, N.; Ruscetti, F.W.; Perret, G.; Jones, K.S.; et al. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 2009, 113, 5176–5185. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.-M.; Blot, V.; Janvier, S.; Arnulf, B.; Van Endert, P.; Heveker, N.; Pique, C.; et al. Neuropilin-1 Is Involved in Human T-Cell Lymphotropic Virus Type 1 Entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.-L. The Ubiquitous Glucose Transporter GLUT-1 Is a Receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef]
- Kannian, P.; Green, P.L. Human T Lymphotropic Virus Type 1 (HTLV-1, Molecular Biology and Oncogenesis. Viruses 2010, 2, 2037–2077. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, I.; Rende, F.; D’Agostino, D.M.; Ciminale, V. Converging Strategies in Expression of Human Complex Retroviruses. Viruses 2011, 3, 1395–1414. [Google Scholar] [CrossRef] [PubMed]
- Ciminale, V.; Pavlakis, G.N.; Derse, D.; Cunningham, C.P.; Felber, B.K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: Novel mRNAs and proteins produced by HTLV type I. J. Virol. 1992, 66. [Google Scholar] [CrossRef]
- Cavanagh, M.-H.; Landry, S.; Audet, B.; Arpin-André, C.; Hivin, P.; Paré, M.-E.; Thête, J.; Wattel, E.; Marriott, S.J.; Mesnard, J.-M.; et al. HTLV-I antisense transcripts initiating in the 3’LTR are alternatively spliced and polyadenylated. Retrovirology 2006, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Rende, F.; Cavallari, I.; Andresen, V.; Valeri, V.W.; D’Agostino, D.M.; Franchini, G.; Ciminale, V. Identification of novel monocistronic HTLV-1 mRNAs encoding functional Rex isoforms. Retrovirology 2015, 12, 58. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Martin, J.L.; Mueller, J.D.; Mansky, L.M. Morphology and ultrastructure of retrovirus particles. AIMS Biophys. 2015, 2, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, K.H.; Zhang, W.; Grigsby, I.F.; Johnson, J.L.; Chen, Y.; Mueller, J.D.; Mansky, L.M. New Insights into HTLV-1 Particle Structure, Assembly, and Gag-Gag Interactions in Living Cells. Viruses 2011, 3, 770–793. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.O.; Martin, J.L.; Mueller, J.D.; Zhang, W.; Mansky, L.M. New insights into retroviral Gag–Gag and Gag–membrane interactions. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Konvalinka, J.; Kräusslich, H.-G.; Müller, B. Retroviral proteases and their roles in virion maturation. Virology 2015, 479, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.T.; Nicot, C. Overview on HTLV-1 p12, p8, p30, p13: Accomplices in persistent infection and viral pathogenesis. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Moles, R.; Sarkis, S.; Galli, V.; Omsland, M.; Purcell, D.F.J.; Yurick, D.; Khoury, G.; Pise-Masison, C.A.; Franchini, G. p30 protein: A critical regulator of HTLV-1 viral latency and host immunity. Retrovirology 2019, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.-T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef]
- Bartoe, J.T.; Albrecht, B.; Collins, N.D.; Robek, M.D.; Ratner, L.; Green, P.L.; Lairmore, M.D. Functional Role of pX Open Reading Frame II of Human T-Lymphotropic Virus Type 1 in Maintenance of Viral Loads In Vivo. J. Virol. 2000, 74, 1094–1100. [Google Scholar] [CrossRef]
- Silverman, L.R.; Phipps, A.J.; Montgomery, A.; Ratner, L.; Lairmore, M.D. Human T-Cell Lymphotropic Virus Type 1 Open Reading Frame II-Encoded p30II Is Required for In Vivo Replication: Evidence of In Vivo Reversion. J. Virol. 2004, 78, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Unge, T.; Solomin, L.; Mellini, M.; Derse, D.; Felber, B.K.; Pavlakis, G.N. The Rex regulatory protein of human T-cell lymphotropic virus type I binds specifically to its target site within the viral RNA. Proc. Natl. Acad. Sci. USA 1991, 88, 7145–7149. [Google Scholar] [CrossRef]
- D’Agostino, D.M.; Cavallari, I.; Romanelli, M.G.; Ciminale, V. Post-transcriptional Regulation of HTLV Gene Expression: Rex to the Rescue. Front. Microbiol. 2019, 10, 1958. [Google Scholar] [CrossRef]
- Gaudray, G.; Gachon, F.; Basbous, J.; Biard-Piechaczyk, M.; Devaux, C.; Mesnard, J.-M. The Complementary Strand of the Human T-Cell Leukemia Virus Type 1 RNA Genome Encodes a bZIP Transcription Factor That Down-Regulates Viral Transcription. J. Virol. 2002, 76, 12813–12822. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.-I.; Yoshida, M.; Matsuoka, M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725. [Google Scholar] [CrossRef]
- Arnold, J.; Zimmerman, B.; Li, M.; Lairmore, M.D.; Green, P.L. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood 2008, 112, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Nosaka, K.; Yasunaga, J.-I.; Maeda, M.; Mueller, N.; Okayama, A.; Matsuoka, M. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2005, 2, 64. [Google Scholar] [CrossRef]
- Currer, R.; Van Duyne, R.; Jaworski, E.; Guendel, I.; Sampey, G.; Das, R.; Narayanan, A.; Kashanchi, F. HTLV Tax: A Fascinating Multifunctional Co-Regulator of Viral and Cellular Pathways. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Giam, C.-Z.; Semmes, O.J. HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma—A Tale of Two Proteins: Tax and HBZ. Viruses 2016, 8, 161. [Google Scholar] [CrossRef]
- Grossman, W.J.; Kimata, J.T.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 1995, 92, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Nerenberg, M.; Hinrichs, S.; Reynolds, R.; Khoury, G.; Jay, G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987, 237, 1324–1329. [Google Scholar] [CrossRef]
- Rauch, D.; Gross, S.; Harding, J.; Niewiesk, S.; Lairmore, M.; Piwnica-Worms, D.; Ratner, L. Imaging spontaneous tumorigenesis: Inflammation precedes development of peripheral NK tumors. Blood 2009, 113, 1493–1500. [Google Scholar] [CrossRef]
- Kwon, H.; Ogle, L.; Benitez, B.; Bohuslav, J.; Montaño, M.; Felsher, D.W.; Greene, W.C. Lethal Cutaneous Disease in Transgenic Mice Conditionally Expressing Type I Human T Cell Leukemia Virus Tax. J. Biol. Chem. 2005, 280, 35713–35722. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takahashi, C.; Yamaoka, S.; Nosaka, T.; Maki, M.; Hatanaka, M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Robek, M.D.; Ratner, L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 1999, 73, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Tsunetsugu-Yokota, Y.; Katano, H.; Takahashi, H.; et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 2006, 12, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ono, H.; Shimotohno, K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood 1995, 86, 4243–4249. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Tripp, A.; Lairmore, M.D.; Crawford, L.; Sieburg, M.; Ramos, J.C.; Harrington, W.; Beilke, M.A.; Feuer, G. Adult T-cell leukemia/lymphoma development in HTLV-1–infected humanized SCID mice. Blood 2010, 115, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Fujikawa, D.; Watanabe, T.; Uchimaru, K. HTLV-1-Mediated Epigenetic Pathway to Adult T-Cell Leukemia–Lymphoma. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.W.P.; Iha, H.; Iwanaga, Y.; Bittner, M.; Chen, Y.; Jiang, Y.; Gooden, G.; Trent, J.M.; Meltzer, P.; Jeang, K.-T.; et al. Genome-wide expression changes induced by HTLV-1 Tax: Evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-κB activation. Oncogene 2001, 20, 4484–4496. [Google Scholar] [CrossRef] [PubMed]
- Pise-Masison, C.A.; Radonovich, M.; Dohoney, K.; Morris, J.C.; O’Mahony, D.; Lee, M.-J.; Trepel, J.; Waldmann, T.A.; Janik, J.E.; Brady, J.N. Gene expression profiling of ATL patients: Compilation of disease-related genes and evidence for TCF4 involvement in BIRC5 gene expression and cell viability. Blood 2009, 113, 4016–4026. [Google Scholar] [CrossRef]
- Fujikawa, D.; Nakagawa, S.; Hori, M.; Kurokawa, N.; Soejima, A.; Nakano, K.; Yamochi, T.; Nakashima, M.; Kobayashi, S.; Tanaka, Y.; et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood 2016, 127, 1790–1802. [Google Scholar] [CrossRef]
- Zhao, L.J.; Giam, C.Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 1992, 89, 7070–7074. [Google Scholar] [CrossRef]
- Franklin, A.A.; Kubik, M.F.; Uittenbogaard, M.N.; Brauweiler, A.; Utaisincharoen, P.; Matthews, M.A.; Dynan, W.S.; Hoeffler, J.P.; Nyborg, J.K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB). J. Biol. Chem. 1993, 268, 21225–21231. [Google Scholar]
- Yin, M.J.; Gaynor, R.B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell. Biol. 1996, 16, 3156–3168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwok, R.P.S.; Laurance, M.E.; Lundblad, J.R.; Goldman, P.S.; Shih, H.-M.; Connor, L.M.; Marriott, S.J.; Goodman, R.H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 1996, 380, 642–646. [Google Scholar] [CrossRef]
- Sharma, N.; Nyborg, J.K. The coactivators CBP/p300 and the histone chaperone NAP1 promote transcription-independent nucleosome eviction at the HTLV-1 promoter. Proc. Natl. Acad. Sci. USA 2008, 105, 7959–7963. [Google Scholar] [CrossRef] [PubMed]
- Easley, R.; Carpio, L.; Guendel, I.; Klase, Z.; Choi, S.; Kehn-Hall, K.; Brady, J.N.; Kashanchi, F. Human T-Lymphotropic Virus Type 1 Transcription and Chromatin-Remodeling Complexes. J. Virol. 2010, 84, 4755–4768. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Lavorgna, A.; Sehgal, M.; Gao, L.; Ginwala, R.; Sagar, D.; Harhaj, E.W.; Khan, Z.K. Myocyte enhancer factor (MEF)-2 plays essential roles in T-cell transformation associated with HTLV-1 infection by stabilizing complex between Tax and CREB. Retrovirology 2015, 12, 23. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Pise-Masison, C.A.; Linton, R.; Park, H.U.; Schiltz, R.L.; Sartorelli, V.; Brady, J.N. Tax Relieves Transcriptional Repression by Promoting Histone Deacetylase 1 Release from the Human T-Cell Leukemia Virus Type 1 Long Terminal Repeat. J. Virol. 2004, 78, 6735–6743. [Google Scholar] [CrossRef]
- Kamoi, K.; Yamamoto, K.; Misawa, A.; Miyake, A.; Ishida, T.; Tanaka, Y.; Mochizuki, M.; Watanabe, T. SUV39H1 interacts with HTLV-1 Tax and abrogates Tax transactivation of HTLV-1 LTR. Retrovirology 2006, 3, 5. [Google Scholar] [CrossRef]
- Alefantis, T.; Barmak, K.; Harhaj, E.W.; Grant, C.; Wigdahl, B. Characterization of a Nuclear Export Signal within the Human T Cell Leukemia Virus Type I Transactivator Protein Tax. J. Biol. Chem. 2003, 278, 21814–21822. [Google Scholar] [CrossRef]
- Burton, M.; Upadhyaya, C.D.; Maier, B.; Hope, T.J.; Semmes, O.J. Human T-Cell Leukemia Virus Type 1 Tax Shuttles between Functionally Discrete Subcellular Targets. J. Virol. 2000, 74, 2351–2364. [Google Scholar] [CrossRef]
- Smith, M.R.; Greene, W.C. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology 1992, 187, 316–320. [Google Scholar] [CrossRef]
- Lavorgna, A.; Harhaj, E.W. Regulation of HTLV-1 tax stability, cellular trafficking and NF-kappaB activation by the ubiquitin-proteasome pathway. Viruses 2014, 6, 3925–3943. [Google Scholar] [CrossRef] [PubMed]
- Lodewick, J.; Lamsoul, I.; Bex, F. Move or Die: The Fate of the Tax Oncoprotein of HTLV-1. Viruses 2011, 3, 829–857. [Google Scholar] [CrossRef]
- Alefantis, T.; Mostoller, K.; Jain, P.; Harhaj, E.; Grant, C.; Wigdahl, B. Secretion of the Human T Cell Leukemia Virus Type I Transactivator Protein Tax. J. Biol. Chem. 2005, 280, 17353–17362. [Google Scholar] [CrossRef] [PubMed]
- Gatza, M.L.; Marriott, S.J. Genotoxic Stress and Cellular Stress Alter the Subcellular Distribution of Human T-Cell Leukemia Virus Type 1 Tax through a CRM1-Dependent Mechanism. J. Virol. 2006, 80, 6657–6668. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, Y.; Nasr, R.; Journo, C.; Mahieux, R.; Pique, C.; Bazarbachi, A. The Multifaceted Oncoprotein Tax. Adv. Cancer Res. 2012, 113, 85–120. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Henkel, T.; Zabel, U.; Van Zee, K.; Müller, J.M.; Fanning, E.; A Baeuerle, P. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell 1992, 68, 1121–1133. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Mohanty, S.; Kumar, A.; Das, P.; Sahu, S.K.; Choudhuri, T. Multi-targeted therapy of everolimus in Kaposi’s sarcoma associated herpes virus infected primary effusion lymphoma. Apoptosis 2017, 22, 1098–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, S.; Minassian, A.; Li, J.; Feng, P. Recent advances on viral manipulation of NF-kappaB signaling pathway. Curr. Opin. Virol. 2015, 15, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; Van Dam, P. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Siekevitz, M.; Feinberg, M.B.; Holbrook, N.; Wong-Staal, F.; Greene, W.C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. USA 1987, 84, 5389–5393. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Giam, C.Z. NF-kappaB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma. FEBS J. 2018, 285, 3324–3336. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Fujii, M.; Ikeda, S.; Yamada, Y.; Tomonaga, M.; Ballard, D.W.; Yamamoto, N. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 1999, 93, 2360–2368. [Google Scholar] [PubMed]
- Sun, S.C.; Elwood, J.; Béraud, C.; Greene, W.C. Human T-cell leukemia virus type I Tax activation of NF-kappa B/Rel involves phosphorylation and degradation of I kappa B alpha and RelA (p65)-mediated induction of the c-rel gene. Mol. Cell. Biol. 1994, 14, 7377–7384. [Google Scholar] [CrossRef]
- Geleziunas, R.; Ferrell, S.; Lin, X.; Mu, Y.; Cunningham, E.T., Jr.; Grant, M.; Connelly, M.A.; Hambor, J.E.; Marcu, K.B.; Greene, W.C. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol. Cell Biol. 1998, 18, 5157–5165. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Sun, S.C. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 1999, 274, 22911–22914. [Google Scholar] [CrossRef]
- Chu, Z.L.; Shin, Y.A.; Yang, J.M.; DiDonato, J.A.; Ballard, D.W. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 1999, 274, 15297–15300. [Google Scholar] [CrossRef]
- Petropoulos, L.; Hiscott, J. Association between HTLV-1 Tax and I kappa B alpha is dependent on the I kappa B alpha phosphorylation state. Virology 1998, 252, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Fujisawa, J.; Suzuki, T.; Ueda, K.; Muramatsu, M.; Tsuboi, A.; Arai, N.; Yoshida, M. Transcriptional activator Tax of HTLV-1 binds to the NF-kappa B precursor p105. Oncogene 1992, 7, 1737–1742. [Google Scholar]
- Rousset, R.; Desbois, C.; Bantignies, F.; Jalinot, P. Effects on NF-kappa B1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature 1996, 381, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, J.; Lacoste, J.; Pepin, N.; Rice, N.; Hiscott, J. Overproduction of NFKB2 (lyt-10) and c-Rel: A mechanism for HTLV-I Tax-mediated trans-activation via the NF-kappa B signalling pathway. Oncogene 1994, 9, 841–852. [Google Scholar]
- Good, L.; Sun, S.C. Persistent activation of NF-kappa B/Rel by human T-cell leukemia virus type 1 tax involves degradation of I kappa B beta. J. Virol. 1996, 70, 2730–2735. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirai, H.; Yoshida, M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-kappa B p65 and c-Rel proteins bound to the NF-kappa B binding site and activates transcription. Oncogene 1994, 9, 3099–3105. [Google Scholar]
- Bex, F.; McDowall, A.; Burny, A.; Gaynor, R. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-kappaB proteins. J. Virol. 1997, 71, 3484–3497. [Google Scholar] [CrossRef]
- Bex, F.; Yin, M.-J.; Burny, A.; Gaynor, R.B. Differential Transcriptional Activation by Human T-Cell Leukemia Virus Type 1 Tax Mutants Is Mediated by Distinct Interactions with CREB Binding Protein and p300. Mol. Cell. Boil. 1998, 18, 2392–2405. [Google Scholar] [CrossRef]
- Xiao, G.; Cvijic, M.E.; Fong, A.; Harhaj, E.W.; Uhlik, M.T.; Waterfield, M.; Sun, S.C. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: Evidence for the involvement of IKKalpha. EMBO J. 2001, 20, 6805–6815. [Google Scholar] [CrossRef]
- Xiao, G.; Fong, A.; Sun, S.C. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 2004, 279, 30099–30105. [Google Scholar] [CrossRef]
- Yamagishi, M.; Nakano, K.; Miyake, A.; Yamochi, T.; Kagami, Y.; Tsutsumi, A.; Matsuda, Y.; Sato-Otsubo, A.; Muto, S.; Utsunomiya, A.; et al. Polycomb-mediated loss of miR-31 activates NIK-dependent NF-kappaB pathway in adult T cell leukemia and other cancers. Cancer Cell 2012, 21, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Minoda, Y.; Yoshida, R.; Yoshida, H.; Iha, H.; Kobayashi, T.; Yoshimura, A.; Takaesu, G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 2008, 365, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, S.C. Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 2007, 8, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-K.; Zhi, H.; Bowlin, T.; Dorjbal, B.; Philip, S.; Zahoor, M.A.; Shih, H.-M.; Semmes, O.J.; Schaefer, B.; Glover, J.N.M.; et al. HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation. PLoS Pathog. 2015, 11, e1005102. [Google Scholar] [CrossRef]
- Shibata, Y.; Tokunaga, F.; Goto, E.; Komatsu, G.; Gohda, J.; Saeki, Y.; Tanaka, K.; Takahashi, H.; Sawasaki, T.; Inoue, S.; et al. HTLV-1 Tax Induces Formation of the Active Macromolecular IKK Complex by Generating Lys63- and Met1-Linked Hybrid Polyubiquitin Chains. PLoS Pathog. 2017, 13, e1006162. [Google Scholar] [CrossRef]
- Choi, Y.B.; Harhaj, E.W. HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation. PLoS Pathog. 2014, 10, e1004458. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qu, Z.; Yan, P.; Ishikawa, C.; Aqeilan, R.I.; Rabson, A.B.; Xiao, G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-kappaB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 2011, 117, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, A.; Matsuoka, M.; Harhaj, E.W. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-kappaB activation and T-cell transformation. PLoS Pathog. 2014, 10, e1004418. [Google Scholar] [CrossRef] [PubMed]
- Peloponese, J.-M.; Yasunaga, J.-I.; Kinjo, T.; Watashi, K.; Jeang, K.-T. Peptidylproline cis-trans-Isomerase Pin1 Interacts with Human T-Cell Leukemia Virus Type 1 Tax and Modulates Its Activation of NF-κB. J. Virol. 2009, 83, 3238–3248. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishida, T.; Nakano, K.; Yamagishi, M.; Yamochi, T.; Tanaka, Y.; Furukawa, Y.; Nakamura, Y.; Watanabe, T. SMYD3 interacts with HTLV-1 Tax and regulates subcellular localization of Tax. Cancer Sci. 2010, 102, 260–266. [Google Scholar] [CrossRef]
- Fu, D.X.; Kuo, Y.L.; Liu, B.Y.; Jeang, K.T.; Giam, C.Z. Human T-lymphotropic virus type I tax activates I-kappa B kinase by inhibiting I-kappa B kinase-associated serine/threonine protein phosphatase 2A. J. Biol Chem. 2003, 278, 1487–1493. [Google Scholar] [CrossRef]
- Shembade, N.; Harhaj, N.S.; Parvatiyar, K.; Copeland, N.G.; A Jenkins, N.; E Matesic, L.; Harhaj, E.W. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 2008, 9, 254–262. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Dixit, V.M. Regulation of NF-kappaB by deubiquitinases. Immunol Rev. 2012, 246, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Shembade, N.; Pujari, R.; Harhaj, N.S.; Abbott, D.W.; Harhaj, E.W. The kinase IKKalpha inhibits activation of the transcription factor NF-kappaB by phosphorylating the regulatory molecule TAX1BP1. Nat. Immunol. 2011, 12, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Shembade, N.; Ma, A.; Harhaj, E.W. Inhibition of NF-κB Signaling by A20 Through Disruption of Ubiquitin Enzyme Complexes. Science 2010, 327, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Journo, C.; Filipe, J.; About, F.; Chevalier, S.A.; Afonso, P.V.; Brady, J.N.; Flynn, D.; Tangy, F.; Israel, A.; Vidalain, P.O.; et al. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009, 5, e1000521. [Google Scholar] [CrossRef] [PubMed]
- Schwob, A.; Teruel, E.; Dubuisson, L.; Lormieres, F.; Verlhac, P.; Abudu, Y.P.; Gauthier, J.; Naoumenko, M.; Cloarec-Ung, F.M.; Faure, M.; et al. SQSTM-1/p62 potentiates HTLV-1 Tax-mediated NF-kappaB activation through its ubiquitin binding function. Sci. Rep. 2019, 9, 16014. [Google Scholar] [CrossRef]
- Pujari, R.; Hunte, R.; Thomas, R.; van der Weyden, L.; Rauch, D.; Ratner, L.; Nyborg, J.K.; Ramos, J.C.; Takai, Y.; Shembade, N. Human T-cell leukemia virus type 1 (HTLV-1) tax requires CADM1/TSLC1 for inactivation of the NF-kappaB inhibitor A20 and constitutive NF-kappaB signaling. PLoS Pathog. 2015, 11, e1004721. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhang, M.; Sun, S.-C. Mutual regulation between deubiquitinase CYLD and retroviral oncoprotein Tax. Cell Biosci. 2011, 1, 27. [Google Scholar] [CrossRef]
- Chiari, E.; Lamsoul, I.; Lodewick, J.; Chopin, C.; Bex, F.; Pique, C. Stable Ubiquitination of Human T-Cell Leukemia Virus Type 1 Tax Is Required for Proteasome Binding. J. Virol. 2004, 78, 11823–11832. [Google Scholar] [CrossRef]
- Péloponèse, J.-M.; Iha, H.; Yedavalli, V.R.K.; Miyazato, A.; Li, Y.; Haller, K.; Benkirane, M.; Jeang, K.-T. Ubiquitination of Human T-Cell Leukemia Virus Type 1 Tax Modulates Its Activity. J. Virol. 2004, 78, 11686–11695. [Google Scholar] [CrossRef]
- Shembade, N.; Harhaj, N.S.; Yamamoto, M.; Akira, S.; Harhaj, E.W. The Human T-Cell Leukemia Virus Type 1 Tax Oncoprotein Requires the Ubiquitin-Conjugating Enzyme Ubc13 for NF-κB Activation. J. Virol. 2007, 81, 13735–13742. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, N.S.; Sun, S.C.; Harhaj, E.W. Activation of NF-kappaB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IkappaB kinase. J. Biol. Chem. 2007, 282, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, Y.; Nasr, R.; Favre-Bonvin, A.; El-Sabban, M.; Renault, N.; Giron, M.-L.; Setterblad, N.; El Hajj, H.; Chiari, E.; Mikati, A.G.; et al. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene 2007, 27, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Gatza, M.L.; Dayaram, T.; Marriott, S.J. Ubiquitination of HTLV-I Tax in response to DNA damage regulates nuclear complex formation and nuclear export. Retrovirology 2007, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Fu, J.; Qu, Z.; Li, S.; Tanaka, T.; Grusby, M.J.; Xiao, G. PDLIM2 suppresses human T-cell leukemia virus type I Tax-mediated tumorigenesis by targeting Tax into the nuclear matrix for proteasomal degradation. Blood 2009, 113, 4370–4380. [Google Scholar] [CrossRef]
- Fu, J.; Yan, P.; Li, S.; Qu, Z.; Xiao, G. Molecular determinants of PDLIM2 in suppressing HTLV-I Tax-mediated tumorigenesis. Oncogene 2010, 29, 6499–6507. [Google Scholar] [CrossRef]
- Yan, P.; Qu, Z.; Ishikawa, C.; Mori, N.; Xiao, G. Human T-Cell Leukemia Virus Type I-Mediated Repression of PDZ-LIM Domain-Containing Protein 2 Involves DNA Methylation But Independent of the Viral Oncoprotein Tax. Neoplasia 2009, 11, 1036–1041. [Google Scholar] [CrossRef]
- Gao, L.; Harhaj, E.W. HSP90 protects the human T-cell leukemia virus type 1 (HTLV-1) tax oncoprotein from proteasomal degradation to support NF-kappaB activation and HTLV-1 replication. J. Virol. 2013, 87, 13640–13654. [Google Scholar] [CrossRef]
- Ikebe, E.; Kawaguchi, A.; Tezuka, K.; Taguchi, S.; Hirose, S.; Matsumoto, T.; Mitsui, T.; Senba, K.; Nishizono, A.; Hori, M.; et al. Oral administration of an HSP90 inhibitor, 17-DMAG, intervenes tumor-cell infiltration into multiple organs and improves survival period for ATL model mice. Blood Cancer J. 2013, 3, e132. [Google Scholar] [CrossRef]
- Yasunaga, J.; Lin, F.C.; Lu, X.; Jeang, K.T. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J. Virol. 2011, 85, 6212–6219. [Google Scholar] [CrossRef]
- Lamsoul, I.; Lodewick, J.; Lebrun, S.; Brasseur, R.; Burny, A.; Gaynor, R.B.; Bex, F. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell Biol. 2005, 25, 10391–10406. [Google Scholar] [CrossRef]
- Kfoury, Y.; Setterblad, N.; El-Sabban, M.; Zamborlini, A.; Dassouki, Z.; El Hajj, H.; Hermine, O.; Pique, C.; de The, H.; Saib, A.; et al. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 2011, 117, 190–199. [Google Scholar] [CrossRef]
- Pene, S.; Waast, L.; Bonnet, A.; Benit, L.; Pique, C. A non-SUMOylated tax protein is still functional for NF-kappaB pathway activation. J. Virol. 2014, 88, 10655–10661. [Google Scholar] [CrossRef]
- Nasr, R.; Chiari, E.; El-Sabban, M.; Mahieux, R.; Kfoury, Y.; Abdulhay, M.; Yazbeck, V.; Hermine, O.; de The, H.; Pique, C.; et al. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood 2006, 107, 4021–4029. [Google Scholar] [CrossRef]
- Bex, F.; Murphy, K.; Wattiez, R.; Burny, A.; Gaynor, R.B. Phosphorylation of the Human T-Cell Leukemia Virus Type 1 Transactivator Tax on Adjacent Serine Residues Is Critical for Tax Activation. J. Virol. 1999, 73, 738–745. [Google Scholar] [CrossRef]
- Durkin, S.S.; Ward, M.D.; Fryrear, K.A.; Semmes, O.J. Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J. Biol. Chem. 2006, 281, 31705–31712. [Google Scholar] [CrossRef]
- Lodewick, J.; Lamsoul, I.; Polania, A.; Lebrun, S.; Burny, A.; Ratner, L.; Bex, F. Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-κB pathway. Virology 2009, 386, 68–78. [Google Scholar] [CrossRef]
- Lodewick, J.; Sampaio, C.; Boxus, M.; Rinaldi, A.-S.; Coulonval, K.; Willems, L.; Roger, P.; Bex, F. Acetylation at lysine 346 controls the transforming activity of the HTLV-1 Tax oncoprotein in the Rat-1 fibroblast model. Retrovirology 2013, 10, 75. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Good, L.; Xiao, G.; Sun, S.-C. Gene expression profiles in HTLV-I-immortalized T cells: Deregulated expression of genes involved in apoptosis regulation. Oncogene 1999, 18, 1341–1349. [Google Scholar] [CrossRef]
- Kawakami, A.; Nakashima, T.; Sakai, H.; Urayama, S.; Yamasaki, S.; Hida, A.; Tsuboi, M.; Nakamura, H.; Ida, H.; Migita, K.; et al. Inhibition of caspase cascade by HTLV-I tax through induction of NF-kappaB nuclear translocation. Blood 1999, 94, 3847–3854. [Google Scholar] [CrossRef]
- Kamihira, S.; Yamada, Y.; Hirakata, Y.; Tomonaga, M.; Sugahara, K.; Hayashi, T.; Dateki, N.; Harasawa, H.; Nakayama, K. Aberrant expression of caspase cascade regulatory genes in adult T-cell leukaemia: Survivin is an important determinant for prognosis. Br. J. Haematol. 2001, 114, 63–69. [Google Scholar] [CrossRef]
- Kawakami, H.; Tomita, M.; Matsuda, T.; Ohta, T.; Tanaka, Y.; Fujii, M.; Hatano, M.; Tokuhisa, T.; Mori, N. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int. J. Cancer 2005, 115, 967–974. [Google Scholar] [CrossRef]
- Mori, N.; Yamada, Y.; Hata, T.; Ikeda, S.; Yamasaki, Y.; Tomonaga, M.; Yamamoto, N. Expression of Survivin in HTLV-I-Infected T-Cell Lines and Primary ATL Cells. Biochem. Biophys. Res. Commun. 2001, 282, 1110–1113. [Google Scholar] [CrossRef]
- Mitobe, Y.; Yasunaga, J.-I.; Furuta, R.; Matsuoka, M. HTLV-1 bZIP Factor RNA and Protein Impart Distinct Functions on T-cell Proliferation and Survival. Cancer Res. 2015, 75, 4143–4152. [Google Scholar] [CrossRef]
- Zhang, M.; Griner, L.A.M.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Retrovirology 2015, 12, O42. [Google Scholar] [CrossRef][Green Version]
- Macaire, H.; Riquet, A.; Moncollin, V.; Biemont-Trescol, M.C.; Duc Dodon, M.; Hermine, O.; Debaud, A.L.; Mahieux, R.; Mesnard, J.M.; Pierre, M.; et al. Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J. Biol. Chem. 2012, 287, 21357–21370. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020, 2020, 100672. [Google Scholar] [CrossRef]
- Mukai, R.; Ohshima, T. Enhanced Stabilization of MCL1 by the Human T-Cell Leukemia Virus Type 1 bZIP Factor Is Modulated by Blocking the Recruitment of Cullin 1 to the SCF Complex. Mol. Cell. Boil. 2016, 36, 3075–3085. [Google Scholar] [CrossRef]
- Higuchi, M.; Takahashi, M.; Tanaka, Y.; Fujii, M. Downregulation of proapoptotic Bim augments IL-2-independent T-cell transformation by human T-cell leukemia virus type-1 Tax. Cancer Med. 2014, 3, 1605–1614. [Google Scholar] [CrossRef]
- Tanaka, A.; Yasunaga, J.-I.; Takai, K.; Matsuoka, M. HTLV-1 bZIP Factor Suppresses Apoptosis by Attenuating the Function of FoxO3a and Altering Its Localization. Cancer Res. 2013, 74, 188–200. [Google Scholar] [CrossRef]
- Pise-Masison, C.A.; Jeong, S.-J.; Brady, J.N. Human T cell leukemia virus type 1: The role of Tax in leukemogenesis. Arch. Immunol. Ther. Exp. 2005, 53, 283–296. [Google Scholar]
- Jeong, S.J.; Radonovich, M.; Brady, J.N.; Pise-Masison, C.A. HTLV-I Tax induces a novel interaction between p65/RelA and p53 that results in inhibition of p53 transcriptional activity. Blood 2004, 104, 1490–1497. [Google Scholar] [CrossRef]
- Pise-Masison, C.A.; Mahieux, R.; Jiang, H.; Ashcroft, M.; Radonovich, M.; Duvall, J.; Guillerm, C.; Brady, J.N. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol. Cell Biol. 2000, 20, 3377–3386. [Google Scholar] [CrossRef]
- Ariumi, Y.; Kaida, A.; Lin, J.-Y.; Hirota, M.; Masui, O.; Yamaoka, S.; Taya, Y.; Shimotohno, K. HTLV-1 Tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 2000, 19, 1491–1499. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchida-Toita, M.; Yoshida, M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene 1999, 18, 4137–4143. [Google Scholar] [CrossRef]
- Tawara, M.; Hogerzeil, S.J.; Yamada, Y.; Takasaki, Y.; Soda, H.; Hasegawa, H.; Murata, K.; Ikeda, S.; Imaizumi, Y.; Sugahara, K.; et al. Impact of p53 aberration on the progression of Adult T-cell Leukemia/Lymphoma. Cancer Lett. 2006, 234, 249–255. [Google Scholar] [CrossRef]
- Nicot, C. Tumor Suppressor Inactivation in the Pathogenesis of Adult T-Cell Leukemia. J. Oncol. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Vajente, N.; Trevisan, R.; Saggioro, D. HTLV-1 Tax protein cooperates with Ras in protecting cells from apoptosis. Apoptosis 2008, 14, 153–163. [Google Scholar] [CrossRef]
- Song, C.; Wang, W.; Li, M.; Liu, Y.; Zheng, D. Tax1 enhances cancer cell proliferation via Ras-Raf-MEK-ERK signaling pathway. IUBMB Life 2009, 61, 685–692. [Google Scholar] [CrossRef]
- Thoma-Kress, A.K.; Schneider, G.; Pichler, K.; Kalmer, M.; Fleckenstein, B.; Grassmann, R. Elevated Cyclic AMP Levels in T Lymphocytes Transformed by Human T-Cell Lymphotropic Virus Type 1. J. Virol. 2010, 84, 8732–8742. [Google Scholar] [CrossRef]
- Yoshita, M.; Higuchi, M.; Takahashi, M.; Oie, M.; Tanaka, Y.; Fujii, M. Activation of mTOR by human T-cell leukemia virus type 1 Tax is important for the transformation of mouse T cells to interleukin-2-independent growth. Cancer Sci. 2011, 103, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Futsch, N.; Prates, G.; Mahieux, R.; Casseb, J.; Dutartre, H. Cytokine Networks Dysregulation during HTLV-1 Infection and Associated Diseases. Viruses 2018, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Shirakawa, F.; Shimizu, H.; Murakami, S.; Oda, S.; Yamamoto, K.; Eto, S. Transcriptional regulation of the human interleukin-6 gene promoter in human T-cell leukemia virus type I-infected T-cell lines: Evidence for the involvement of NF-kappa B. Blood 1994, 84, 2904–2911. [Google Scholar] [CrossRef]
- Horiuchi, S.; Yamamoto, N.; Dewan, Z.; Takahashi, Y.; Yamashita, A.; Yoshida, T.; Nowell, M.; Richards, P.; Jones, S.A.; Yamamoto, N. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R, Shedding of soluble IL-6R and activation of STAT3 signaling. Int. J. Cancer 2006, 119, 823–830. [Google Scholar] [CrossRef]
- Good, L.; Maggirwar, S.B.; Sun, S.C. Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 1996, 15, 3744–3750. [Google Scholar] [CrossRef]
- Leung, K.; Nabel, G.J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature 1988, 333, 776–778. [Google Scholar] [CrossRef]
- Chung, H.-K.; Young, H.A.; Goon, P.K.C.; Heidecker, G.; Princler, G.L.; Shimozato, O.; Taylor, G.P.; Bangham, C.R.M.; Derse, D. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 2003, 102, 4130–4136. [Google Scholar] [CrossRef]
- Silbermann, K.; Schneider, G.; Grassmann, R. Stimulation of interleukin-13 expression by human T-cell leukemia virus type 1 oncoprotein Tax via a dually active promoter element responsive to NF-kappaB and NFAT. J. Gen. Virol. 2008, 89 (Pt 11), 2788–2798. [Google Scholar] [CrossRef]
- Fung, M.M.; Chu, Y.-L.; Fink, J.L.; Wallace, A.; McGuire, K.L. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene 2005, 24, 4624–4633. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Leo, E.; Stennicke, H.R.; Welsh, K.; Salvesen, G.S.; Reed, J.C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999, 18, 5242–5251. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Renatus, M.; Schwarzenbacher, R.; Zhou, Q.; Sun, C.; Fesik, S.W.; Liddington, R.C.; Salvesen, G.S. Structural basis for the inhibition of caspase-3 by XIAP. Cell 2001, 104, 791–800. [Google Scholar] [CrossRef]
- Sugahara, K.; Hayashi, T.; Dateki, N.; Hirakata, Y.; Harasawa, H.; Tomonaga, M.; Yamada, Y.; Kamihira, S. Possible Attenuation of Fas-Mediated Signaling by Dominant Expression of Caspase-8 Aberrant Isoform in Adult T-cell Leukemia Cells. Int. J. Hematol. 2002, 76, 50–54. [Google Scholar] [CrossRef]
- Krueger, A.; Fas, S.C.; Giaisi, M.; Bleumink, M.; Merling, A.; Stumpf, C.; Baumann, S.; Holtkotte, D.; Bosch, V.; Krammer, P.H.; et al. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP). Blood 2006, 107, 3933–3939. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujisawa, J.; Reth, M.; Yonehara, S. Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-kappaB. Genes Cells 2006, 11, 177–191. [Google Scholar] [CrossRef]
- Daniel, S.; Arvelo, M.B.; Patel, V.I.; Longo, C.R.; Shrikhande, G.; Shukri, T.; Mahiou, J.; Sun, D.W.; Mottley, C.; Grey, S.T.; et al. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood 2004, 104, 2376–2384. [Google Scholar] [CrossRef]
- Laherty, C.D.; Perkins, N.D.; Dixit, V.M. Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor kappa B. J. Boil. Chem. 1993, 268, 5032–5039. [Google Scholar]
- Saitoh, Y.; Hamano, A.; Mochida, K.; Kakeya, A.; Uno, M.; Tsuruyama, E.; Ichikawa, H.; Tokunaga, F.; Utsunomiya, A.; Watanabe, T.; et al. A20 targets caspase-8 and FADD to protect HTLV-I-infected cells. Leukemia 2016, 30, 716–727. [Google Scholar] [CrossRef]
- Miyake, H.; Suzuki, T.; Hirai, H.; Yoshida, M. Trans-activator Tax of Human T-Cell Leukemia Virus Type 1 Enhances Mutation Frequency of the Cellular Genome. Virology 1999, 253, 155–161. [Google Scholar] [CrossRef]
- Haller, K.; Wu, Y.; Derow, E.; Schmitt, I.; Jeang, K.-T.; Grassmann, R. Physical Interaction of Human T-Cell Leukemia Virus Type 1 Tax with Cyclin-Dependent Kinase 4 Stimulates the Phosphorylation of Retinoblastoma Protein. Mol. Cell. Boil. 2002, 22, 3327–3338. [Google Scholar] [CrossRef]
- Ohtani, K.-I.; Iwanaga, R.; Arai, M.; Huang, Y.; Matsumura, Y.; Nakamura, M. Cell Type-specific E2F Activation and Cell Cycle Progression Induced by the Oncogene Product Tax of Human T-cell Leukemia Virus Type I. J. Boil. Chem. 2000, 275, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, I.; Thebault, S.; Sardet, C.; Devaux, C.; Mesnard, J.-M. Activation of E2F-mediated Transcription by Human T-cell Leukemia Virus Type I Tax Protein in a p16INK4A-negative T-cell Line. J. Boil. Chem. 1998, 273, 23598–23604. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ono, H.; Shimotohno, K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: Possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 1996, 12, 1645–1652. [Google Scholar] [PubMed]
- Hatta, Y.; Hirama, T.; Miller, C.W.; Yamada, Y.; Tomonaga, M.; Koeffler, H.P. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood 1995, 85, 2699–2704. [Google Scholar] [CrossRef]
- Low, K.G.; Dorner, L.F.; Fernando, D.B.; Grossman, J.; Jeang, K.T.; Comb, M.J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J. Virol. 1997, 71, 1956–1962. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitao, S.; Matsushime, H.; Yoshida, M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996, 15, 1607–1614. [Google Scholar] [CrossRef]
- Suzuki, T.; Narita, T.; Uchida-Toita, M.; Yoshida, M. Down-regulation of the INK4 Family of Cyclin-Dependent Kinase Inhibitors by Tax Protein of HTLV-1 through Two Distinct Mechanisms. Virology 1999, 259, 384–391. [Google Scholar] [CrossRef]
- Uchida, T.; Kinoshita, T.; Murate, T.; Saito, H.; Hotta, T. CDKN2 (MTS1/p16INK4A) gene alterations in adult T-cell leukemia/lymphoma. Leuk. Lymphoma 1998, 29, 27–35. [Google Scholar] [CrossRef]
- Iwanaga, R.; Ohtani, K.; Hayashi, T.; Nakamura, M. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 2001, 20, 2055–2067. [Google Scholar] [CrossRef]
- Kehn-Hall, K.; De La Fuente, C.; Strouss, K.; Berro, R.; Jiang, H.; Brady, J.; Mahieux, R.; Pumfery, A.; Bottazzi, M.E.; Kashanchi, F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene 2004, 24, 525–540. [Google Scholar] [CrossRef]
- Laptenko, O.; Prives, C. Transcriptional regulation by p53: One protein, many possibilities. Cell Death Differ. 2006, 13, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.H.; Farhadi, A.; Wang, X.F.; Robb, M.L.; Birx, D.L.; Kim, J.H. Human T-cell leukemia virus type 1 Tax activates cyclin-dependent kinase inhibitor p21/Waf1/Cip1 expression through a p53-independent mechanism: Inhibition of cdk2. Int. J. Cancer 2003, 107, 603–611. [Google Scholar] [CrossRef]
- Kehn, K.; Deng, L.; de la Fuente, C.; Strouss, K.; Wu, K.; Maddukuri, A.; Baylor, S.; Rufner, R.; Pumfery, A.; Bottazzi, M.E.; et al. The role of cyclin D2 and p21/waf1 in human T-cell leukemia virus type 1 infected cells. Retrovirology 2004, 1, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dayaram, T.; Lemoine, F.J.; Donehower, L.A.; Marriott, S.J. Activation of WIP1 Phosphatase by HTLV-1 Tax Mitigates the Cellular Response to DNA Damage. PLoS ONE 2013, 8, e55989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yam, C.H.; Fung, T.K.; Poon, R.Y. Cyclin A in cell cycle control and cancer. Cell Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, H.H.; A Cherian, M.; Green, P.L.; Ratner, L. Inducible nitric oxide synthase mediates DNA double strand breaks in Human T-Cell Leukemia Virus Type 1-induced leukemia/lymphoma. Retrovirology 2015, 12, 71. [Google Scholar] [CrossRef]
- Kibler, K.V.; Jeang, K.-T. CREB/ATF-Dependent Repression of Cyclin A by Human T-Cell Leukemia Virus Type 1 Tax Protein. J. Virol. 2001, 75, 2161–2173. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Giam, C.Z. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 2006, 25, 1741–1752. [Google Scholar] [CrossRef]
- Nitta, T.; Kanai, M.; Sugihara, E.; Tanaka, M.; Sun, B.; Nagasawa, T.; Sonoda, S.; Saya, H.; Miwa, M. Centrosome amplification in adult T-cell leukemia and human T-cell leukemia virus type 1 Tax-induced human T cells. Cancer Sci. 2006, 97, 836–841. [Google Scholar] [CrossRef]
- Péloponèse, J.-M.; Haller, K.; Miyazato, A.; Jeang, K.-T. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc. Natl. Acad. Sci. USA 2005, 102, 18974–18979. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Chandhasin, C.; Ducu, R.I.; Berkovich, E.; Kastan, M.B.; Marriott, S.J. Human T-Cell Leukemia Virus Type 1 Tax Attenuates the ATM-Mediated Cellular DNA Damage Response. J. Virol. 2008, 82, 6952–6961. [Google Scholar] [CrossRef] [PubMed]
- Belgnaoui, S.M.; Fryrear, K.A.; Nyalwidhe, J.O.; Guo, X.; Semmes, O.J. The Viral Oncoprotein Tax Sequesters DNA Damage Response Factors by Tethering MDC1 to Chromatin. J. Boil. Chem. 2010, 285, 32897–32905. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Buehring, G.C. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: Role of tax gene. J. Natl. Cancer Inst. 1999, 91, 933–942. [Google Scholar] [CrossRef]
- Kao, S.Y.; Marriott, S.J. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax Protein. J. Virol. 1999, 73, 4299–4304. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.J.; Kao, S.-Y.; Marriott, S.J. Suppression of DNA Repair by HTLV Type 1 Tax Correlates with Taxtrans-Activation of Proliferating Cell Nuclear Antigen Gene Expression. AIDS Res. Hum. Retrovir. 2000, 16, 1623–1627. [Google Scholar] [CrossRef]
- Ressler, S.; Morris, G.F.; Marriott, S.J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 1997, 71, 1181–1190. [Google Scholar] [CrossRef]
- Morimoto, H.; Tsukada, J.; Kominato, Y.; Tanaka, Y. Reduced expression of human mismatch repair genes in adult T-cell leukemia. Am. J. Hematol. 2005, 78, 100–107. [Google Scholar] [CrossRef]
- Baydoun, H.H.; Bai, X.T.; Shelton, S.; Nicot, C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS ONE 2012, 7, e42226. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Lepelletier, Y.; Hermine, O.; Nicot, C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 2009, 113, 4914–4917. [Google Scholar] [CrossRef] [PubMed]
- Fochi, S.; Ciminale, V.; Trabetti, E.; Bertazzoni, U.; D’Agostino, D.M.; Zipeto, D.; Romanelli, M.G. NF-kappaB and MicroRNA Deregulation Mediated by HTLV-1 Tax and HBZ. Pathogens 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Moles, R.; Nicot, C. The Emerging Role of miRNAs in HTLV-1 Infection and ATLL Pathogenesis. Viruses 2015, 7, 4047–4074. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Schneider, G.; Grassmann, R. MicroRNA miR-146a and further oncogenesis-related cellular microRNAs are dysregulated in HTLV-1-transformed T lymphocytes. Retrovirology 2008, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, K.; Corradin, A.; Zanovello, P.; Amadori, A.; Bronte, V.; Ciminale, V.; D’Agostino, D. Role of microRNAs in HTLV-1 infection and transformation. Mol. Asp. Med. 2010, 31, 367–382. [Google Scholar] [CrossRef]
- Yeung, M.L.; Yasunaga, J.-I.; Bennasser, Y.; Dusetti, N.; Harris, D.T.; Ahmad, N.; Matsuoka, M.; Jeang, K.-T. Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res. 2008, 68, 8976–8985. [Google Scholar] [CrossRef]
- Van Duyne, R.; Guendel, I.; Klase, Z.; Narayanan, A.; Coley, W.; Jaworski, E.; Roman, J.; Popratiloff, A.; Mahieux, R.; Kehn-Hall, K.; et al. Localization and Sub-Cellular Shuttling of HTLV-1 Tax with the miRNA Machinery. PLoS ONE 2012, 7, e40662. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M. MicroRNA miR-146a is induced by HTLV-1 tax and increases the growth of HTLV-1-infected T-cells. Int. J. Cancer 2012, 130. [Google Scholar] [CrossRef]
- Tomita, M. Important Roles of Cellular MicroRNA miR-155 in Leukemogenesis by Human T-Cell Leukemia Virus Type 1 Infection. ISRN Microbiol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Quann, K.; Pandya, D.; Singh, S.; Khan, Z.K.; Jain, P. HTLV-1 Tax Mediated Downregulation of miRNAs Associated with Chromatin Remodeling Factors in T Cells with Stably Integrated Viral Promoter. PLoS ONE 2012, 7, e34490. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.A.; Brinkmann, M.M. Strategies for immune evasion by human tumor viruses. Curr. Opin. Virol. 2018, 32, 30–39. [Google Scholar] [CrossRef]
- Hanon, E.; Hall, S.; Taylor, G.P.; Saito, M.; Davis, R.; Tanaka, Y.; Usuku, K.; Osame, M.; Weber, J.N.; Bangham, C.R. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 2000, 95, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Okayama, A.; Chen, Y.-M.A.; Thchibana, N.; Shioiri, S.; Lee, T.-H.; Tsuda, K.; Essex, M. High Incidence of Antibodies to HTLV-I tax in Blood Relatives of Adult T Cell Leukemia Patients. J. Infect. Dis. 1991, 163, 47–52. [Google Scholar] [CrossRef]
- Zhi, H.; Yang, L.; Kuo, Y.L.; Ho, Y.K.; Shih, H.M.; Giam, C.Z. NF-kappaB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011, 7, e1002025. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Higuchi, M.; Makokha, G.N.; Matsuki, H.; Yoshita, M.; Tanaka, Y.; Fujii, M. HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 2013, 122, 715–725. [Google Scholar] [CrossRef]
- Kinjo, T.; Ham-Terhune, J.; Peloponese, J.M., Jr.; Jeang, K.T. Induction of reactive oxygen species by human T-cell leukemia virus type 1 tax correlates with DNA damage and expression of cellular senescence marker. J. Virol. 2010, 84, 5431–5437. [Google Scholar] [CrossRef]
- Kulkarni, A.; Bangham, C.R.M. HTLV-1: Regulating the Balance Between Proviral Latency and Reactivation. Front. Microbiol. 2018, 9, 449. [Google Scholar] [CrossRef]
- Kulkarni, A.; Mateus, M.; Thinnes, C.C.; McCullagh, J.S.; Schofield, C.J.; Taylor, G.P.; Bangham, C.R.M. Glucose Metabolism and Oxygen Availability Govern Reactivation of the Latent Human Retrovirus HTLV-1. Cell Chem. Biol. 2017, 24, 1377–1387.e3. [Google Scholar] [CrossRef]
- Kulkarni, A.; Taylor, G.P.; Klose, R.J.; Schofield, C.J.; Bangham, C.R. Histone H2A monoubiquitylation and p38-MAPKs regulate immediate-early gene-like reactivation of latent retrovirus HTLV-1. JCI Insight 2018, 3, e123196. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.; Yasunaga, J.-I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef]
- Nicot, C.; Dundr, M.; Johnson, J.M.; Fullen, J.R.; Alonzo, N.; Fukumoto, R.; Princler, G.L.; Derse, D.; Misteli, T.; Franchini, G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 2004, 10, 197–201. [Google Scholar] [CrossRef]
- Zhang, W.; Nisbet, J.W.; Albrecht, B.; Ding, W.; Kashanchi, F.; Bartoe, J.T.; Lairmore, M.D. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J. Virol. 2001, 75, 9885–9895. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, I.; Lewis, M.R.; Polakowski, N.; Hivin, P.; Cavanagh, M.-H.; Thébault, S.; Barbeau, B.; Nyborg, J.K.; Mesnard, J.-M. Human T-Cell Leukemia Virus Type 1 (HTLV-1) bZIP Protein Interacts with the Cellular Transcription Factor CREB To Inhibit HTLV-1 Transcription. J. Virol. 2006, 81, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.; Ratner, L. The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA. Virology 2011, 410, 307–315. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shaffer, A.L., 3rd; Ceribelli, M.; Zhang, M.; Wright, G.; Huang, D.W.; Xiao, W.; Powell, J.; Petrus, M.N.; Yang, Y.; et al. Targeting the HTLV-I-Regulated BATF3/IRF4 Transcriptional Network in Adult T Cell Leukemia/Lymphoma. Cancer Cell 2018, 34, 286–297.e10. [Google Scholar] [CrossRef]
- Saitoh, Y.; Yamamoto, N.; Dewan, M.Z.; Sugimoto, H.; Martinez Bruyn, V.J.; Iwasaki, Y.; Matsubara, K.; Qi, X.; Saitoh, T.; Imoto, I.; et al. Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed-Sternberg cells. Blood 2008, 111, 5118–5129. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.-I.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Wong, R.W.J.; Tan, T.K.; Amanda, S.; Ngoc, P.C.T.; Leong, W.Z.; Tan, S.H.; Asamitsu, K.; Hibi, Y.; Ueda, R.; Okamoto, T.; et al. Feed-forward regulatory loop driven by IRF4 and NF-kappaB in adult T-cell leukemia/lymphoma. Blood 2020, 135, 934–947. [Google Scholar] [CrossRef]
- Kinpara, S.; Ito, S.; Takahata, T.; Saitoh, Y.; Hasegawa, A.; Kijiyama, M.; Utsunomiya, A.; Masuda, M.; Miyazaki, Y.; Matsuoka, M.; et al. Involvement of double-stranded RNA-dependent protein kinase and antisense viral RNA in the constitutive NFkappaB activation in adult T-cell leukemia/lymphoma cells. Leukemia 2015, 29, 1425–1429. [Google Scholar] [CrossRef]
- Mordechai, E.; Kon, N.; Henderson, E.E.; Suhadolnik, R.J. Activation of the interferon-inducible enzymes, 2’,5’-oligoadenylate synthetase and PKR by human T-cell leukemia virus type I Rex-response element. Virology 1995, 206, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Gill, P.S.; Mougdil, T.; Murakami, S.; Eto, S.; Prager, D. Interleukin-10 gene expression in adult T-cell leukemia. Blood 1996, 88, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Hakeem, M.A.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed]
- Takamori, A.; Hasegawa, A.; Utsunomiya, A.; Maeda, Y.; Yamano, Y.; Masuda, M.; Shimizu, Y.; Tamai, Y.; Sasada, A.; Zeng, N.; et al. Functional impairment of Tax-specific but not cytomegalovirus-specific CD8+ T lymphocytes in a minor population of asymptomatic human T-cell leukemia virus type 1-carriers. Retrovirology 2011, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Sawada, L.; Nagano, Y.; Hasegawa, A.; Kanai, H.; Nogami, K.; Ito, S.; Sato, T.; Yamano, Y.; Tanaka, Y.; Masuda, T.; et al. IL-10-mediated signals act as a switch for lymphoproliferation in Human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways. PLoS Pathog. 2017, 13, e1006597. [Google Scholar] [CrossRef]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.-I.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and epigenetic inactivation oftax gene in adult T-cell leukemia cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Zhang, Y.; Zhang, H.; Cheng, H. The autophagy molecule Beclin 1 maintains persistent activity of NF-kappaB and Stat3 in HTLV-1-transformed T lymphocytes. Biochem. Biophys. Res. Commun. 2015, 465, 739–745. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, S.; Harhaj, E.W. Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens 2020, 9, 543. https://doi.org/10.3390/pathogens9070543
Mohanty S, Harhaj EW. Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens. 2020; 9(7):543. https://doi.org/10.3390/pathogens9070543
Chicago/Turabian StyleMohanty, Suchitra, and Edward W. Harhaj. 2020. "Mechanisms of Oncogenesis by HTLV-1 Tax" Pathogens 9, no. 7: 543. https://doi.org/10.3390/pathogens9070543
APA StyleMohanty, S., & Harhaj, E. W. (2020). Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens, 9(7), 543. https://doi.org/10.3390/pathogens9070543




