Early-Onset HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
Abstract
1. Introduction
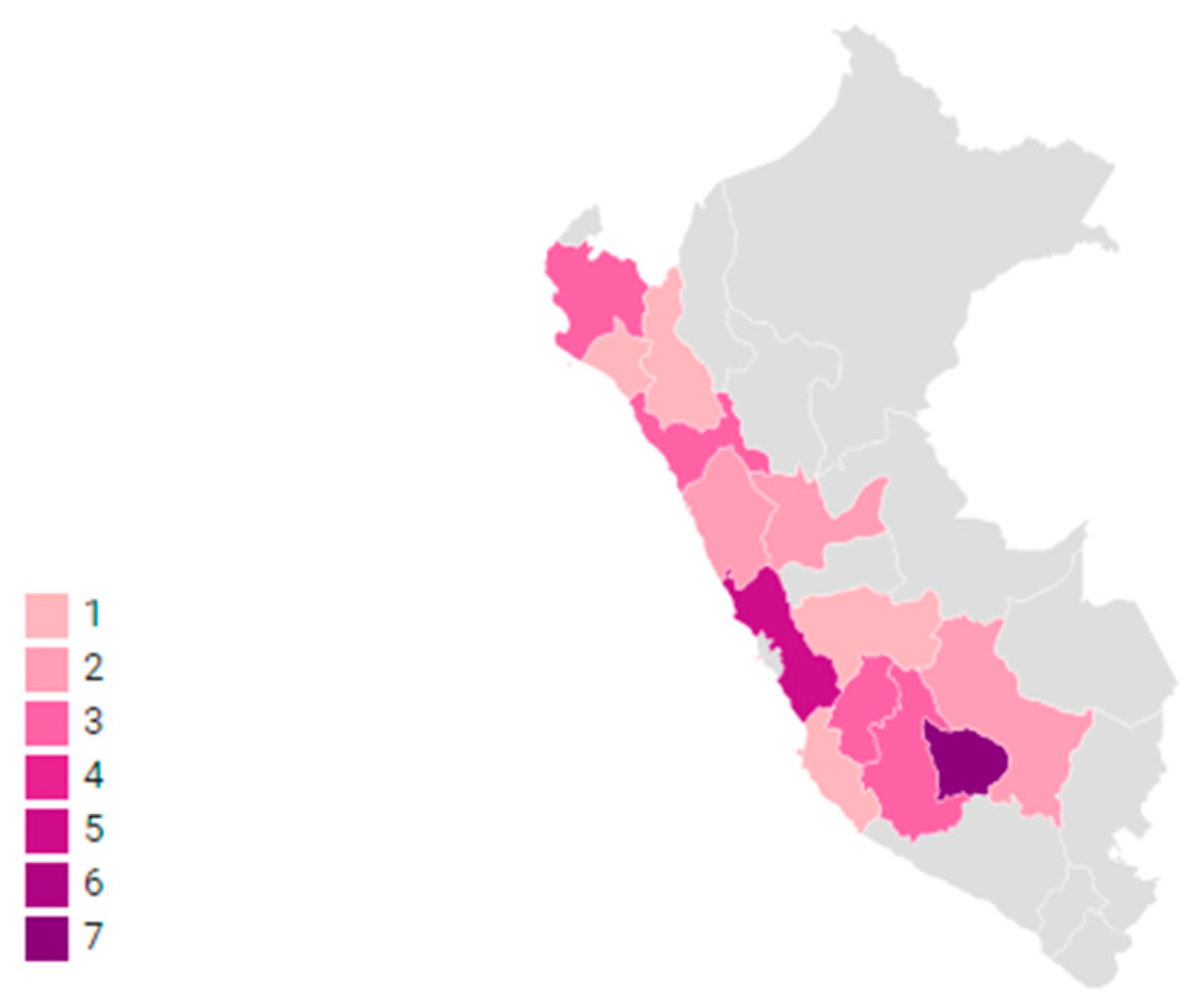
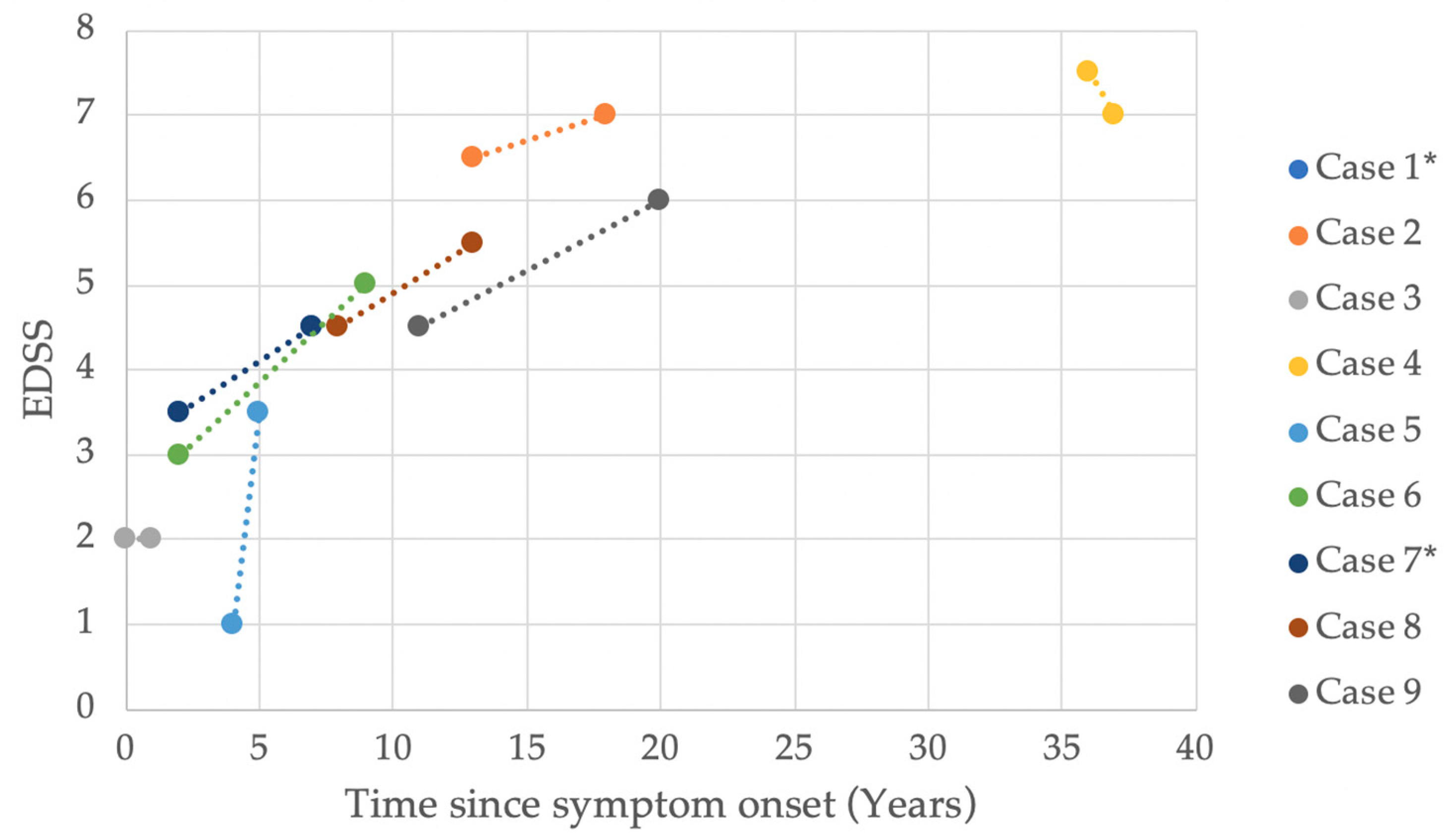
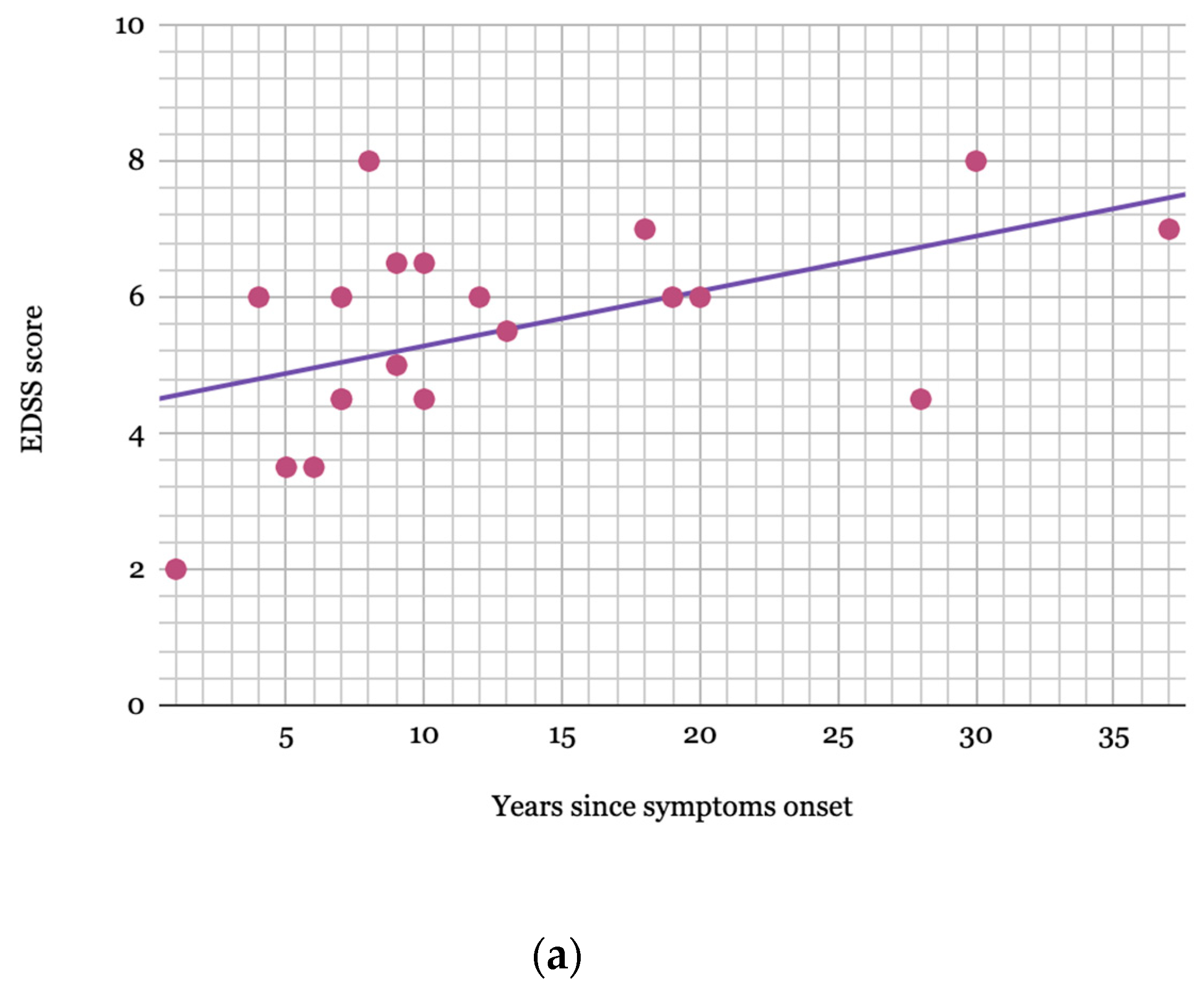
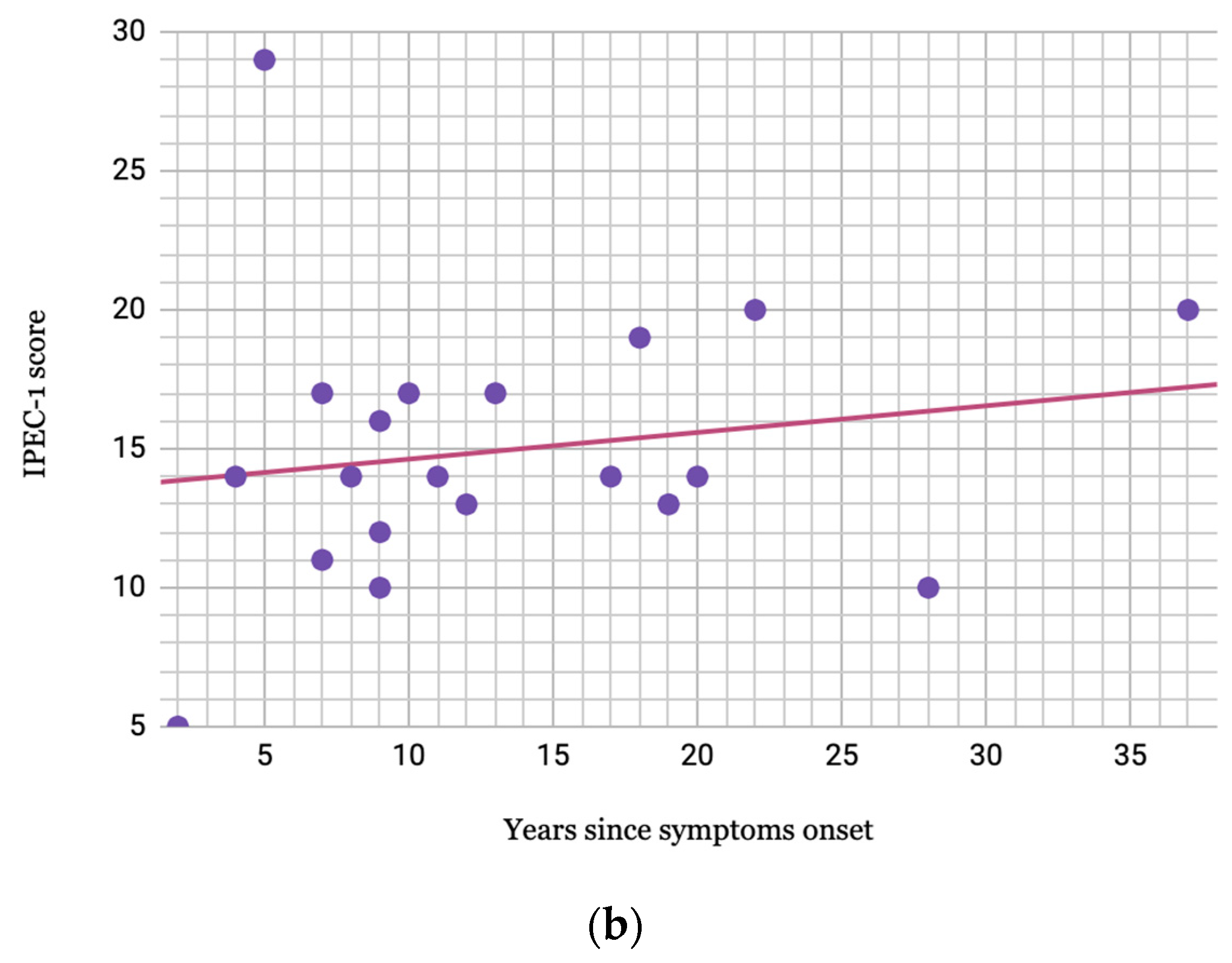
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Quispe, N.C.S.; Feria, E.B.; Santos-Fortuna, E.D.L.; Caterino-de-Araujo, A. Confirming the presence of HTLV-1 infection and the absence of HTLV-2 in blood donors from Arequipa, Peru. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 25–29. [Google Scholar] [CrossRef]
- Schierhout, G.; McGregor, S.; Gessain, A.; Einsiedel, L.; Martinello, M.; Kaldor, J. Association between HTLV-1 infection and adverse health outcomes: A systematic review and meta-analysis of epidemiological studies. Lancet Infect. Dis. 2020, 20, 133–143. [Google Scholar] [CrossRef]
- Araujo, A.Q.C.; Silva, M.T.T. The HTLV-1 neurological complex. Lancet Neurol. 2006, 5, 1068–1076. [Google Scholar] [CrossRef]
- Yamano, Y.; Sato, T. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front. Microbiol. 2012, 3, 389. [Google Scholar] [CrossRef]
- Martin, F.; Taylor, G.P.; Jacobson, S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev. Clin. Immunol. 2014, 10, 1531–1546. [Google Scholar] [CrossRef]
- Takatani, M.; Crispim, M.E.; Fraiji, N.; Stefani, M.M.A.; Kiesslich, D. Clinical and laboratory features of HTLV-I asymptomatic carriers and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis from the Brazilian Amazon. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e5. [Google Scholar] [CrossRef]
- Nakagawa, M.; Izumo, S.; Ijichi, S.; Kubota, H.; Arimura, K.; Kawabata, M.; Osame, M. HTLV-I-associated myelopathy: Analysis of 213 patients based on clinical features and laboratory findings. J. Neurovirol. 1995, 1, 50–61. [Google Scholar] [CrossRef]
- Martins, J.V.P.; Baptista, A.F.; Araújo, A.D.Q.C. Quality of life in patients with HTLV-I associated myelopathy/tropical spastic paraparesis. Arq. Neuropsiquiatr. 2012, 70, 257–261. [Google Scholar] [CrossRef][Green Version]
- Varandas, C.M.N.; da Silva, J.L.S.; Primo, J.R.L.; de Oliveira, M.D.F.S.P.; Moreno-Carvalho, O.; Farre, L.; Bittencourt, A.L. Early Juvenile Human T-cell Lymphotropic Virus Type-1-Associated Myelopathy/Tropical Spastic Paraparesis: Study of 25 Patients. Clin. Infect. Dis. 2018, 67, 1427–1433. [Google Scholar] [CrossRef]
- Oliveira, P.D.; Kachimarek, A.C.; Bittencourt, A.L. Early Onset of HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) and Adult T-cell Leukemia/Lymphoma (ATL): Systematic Search and Review. J. Trop. Pediatr. 2018, 64, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, A.L.; Primo, J.; Oliveira, M.F.P. de Manifestations of the human T-cell lymphotropic virus type I infection in childhood and adolescence. J. Pediatr. 2006, 82, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Katamine, S.; Miyata, H.; Tsuji, Y.; Yamabe, T.; Miyamoto, T. Primary prevention of HTLV-1 in Japan. Leukemia 1997, 11 (Suppl. 3), 57–59. [Google Scholar] [CrossRef]
- Carneiro-Proietti, A.B.F.; Amaranto-Damasio, M.S.; Leal-Horiguchi, C.F.; Bastos, R.H.C.; Seabra-Freitas, G.; Borowiak, D.R.; Ribeiro, M.A.; Proietti, F.A.; Ferreira, A.S.D.; Martins, M.L. Mother-to-Child Transmission of Human T-Cell Lymphotropic Viruses-1/2: What We Know, and What Are the Gaps in Understanding and Preventing This Route of Infection. J. Pediatric. Infect. Dis. Soc. 2014. [Google Scholar] [CrossRef]
- Rosadas, C.; Taylor, G.P. Mother-to-Child HTLV-1 Transmission: Unmet Research Needs. Front. Microbiol. 2019, 10, 999. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Goga, A.E.; Waitt, C.; Gessain, A.; Taylor, G.P.; Rollins, N.; Abrams, E.J.; Lyall, E.H.; Van de Perre, P. Transmission of CMV, HTLV-1, and HIV through breastmilk. Lancet Child Adolesc. Health 2019, 3, 264–273. [Google Scholar] [CrossRef]
- Murphy, E.L.; Hanchard, B.; Figueroa, J.P.; Gibbs, W.N.; Lofters, W.S.; Campbell, M.; Goedert, J.J.; Blattner, W.A. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer 1989, 43, 250–253. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Moody, J.; Verdonck, K.; Cabada, M.M.; González, E.; Van Dooren, S.; Vandamme, A.-M.; Terashima, A.; Vermund, S.H. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev. Panam. Salud Publica 2007, 22, 223–230. [Google Scholar] [CrossRef]
- Boostani, R.; Sadeghi, R.; Sabouri, A.; Ghabeli-Juibary, A. Human T-lymphotropic virus type I and breastfeeding; systematic review and meta-analysis of the literature. Iran J. Neurol. 2018, 17, 174–179. [Google Scholar] [CrossRef]
- Wiktor, S.Z.; Pate, E.J.; Rosenberg, P.S.; Barnett, M.; Palmer, P.; Medeiros, D.; Maloney, E.M.; Blattner, W.A. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J. Hum. Virol. 1997, 1, 37–44. [Google Scholar]
- Takezaki, T.; Tajima, K.; Ito, M.; Ito, S.; Kinoshita, K.; Tachibana, K.; Matsushita, Y. Short-term breast-feeding may reduce the risk of vertical transmission of HTLV-I. The Tsushima ATL Study Group. Leukemia 1997, 11 (Suppl. 3), 60–62. [Google Scholar]
- Oxhorn, P.; Jouve-Martín, J.R. Inequality and Inclusion in Latin America. Lat. Am. Res. Rev. 2017, 52, 203–207. [Google Scholar] [CrossRef]
- Bartholomew, C.; Jack, N.; Edwards, J.; Charles, W.; Corbin, D.; Cleghorn, F.R.; Blattner, W.A. HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Hum. Virol. 1998, 1, 302–305. [Google Scholar]
- Eusebio-Ponce, E.; Candel, F.J.; Anguita, E. Human T-Cell Lymphotropic Virus Type 1 and associated diseases in Latin America. Trop. Med. Int. Health 2019, 24, 934–953. [Google Scholar] [CrossRef]
- Alarcón, J.O.; Friedman, H.B.; Montano, S.M.; Zunt, J.R.; Holmes, K.K.; Quinnan, G.V., Jr. High endemicity of human T-cell lymphotropic virus type 1 among pregnant women in peru. J. Acquir. Immune Defic. Syndr. 2006, 42, 604–609. [Google Scholar] [CrossRef]
- Sanchez-Palacios, C.; Gotuzzo, E.; Vandamme, A.-M.; Maldonado, Y. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int. J. Infect. Dis. 2003, 7, 132–137. [Google Scholar] [CrossRef]
- INEI. Perú: Migraciones Internas 1993–2007; INEI: Lima, Peru, 2009. [Google Scholar]
- Alvarez, C.; Gotuzzo, E.; Vandamme, A.-M.; Verdonck, K. Family Aggregation of Human T-Lymphotropic Virus 1-Associated Diseases: A Systematic Review. Front. Microbiol. 2016, 7, 1674. [Google Scholar] [CrossRef]
- Alvarez, C.; Verdonck, K.; Tipismana, M.; Gotuzzo, E. A Peruvian family with a high burden of HTLV-1-associated myelopathy/tropical spastic paraparesis. BMJ Case Rep. 2015. [Google Scholar] [CrossRef]
- Nozuma, S.; Matsuura, E.; Matsuzaki, T.; Watanabe, O.; Kubota, R.; Izumo, S.; Takashima, H. Familial clusters of HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS ONE 2014, 9, e86144. [Google Scholar] [CrossRef]
- Champs, A.P.S.; Passos, V.M.D.A.; Barreto, S.M.; Vaz, L.S.; Ribas, J.G.R. [HTLV-1 associated myelopathy: Clinical and epidemiological profile in a 10-year case series study]. Rev. Soc. Bras. Med. Trop. 2010, 43, 668–672. [Google Scholar] [CrossRef]
- Jeffery, K.J.; Usuku, K.; Hall, S.E.; Matsumoto, W.; Taylor, G.P.; Procter, J.; Bunce, M.; Ogg, G.S.; Welsh, K.I.; Weber, J.N.; et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA 1999, 96, 3848–3853. [Google Scholar] [CrossRef]
- Artal, F.J.C.; Mesquita, H.M.; da Silveira Ribeiro, L. Manifestaciones neurológicas y discapacidad en pacientes que padecen mielopatía asociada al HTLV-I. Neurología: Publicación oficial de la Sociedad Española de Neurología 2008, 23, 78–84. [Google Scholar]
- Olindo, S.; Cabre, P.; Lézin, A.; Merle, H.; Saint-Vil, M.; Signate, A.; Bonnan, M.; Chalon, A.; Magnani, L.; Cesaire, R.; et al. Natural history of human T-lymphotropic virus 1-associated myelopathy: A 14-year follow-up study. Arch. Neurol. 2006, 63, 1560–1566. [Google Scholar] [CrossRef]
- Bangham, C.R.M.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Primers. 2015, 1, 15012. [Google Scholar] [CrossRef]
- Castro, N.M.; Rodrigues, W., Jr.; Freitas, D.M.; Muniz, A.; Oliveira, P.; Carvalho, E.M. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: Evidence of urinary manifestations in large group of HTLV-I carriers. Urology 2007, 69, 813–818. [Google Scholar] [CrossRef]
- Okajima, R.; Oliveira, A.C.P.; Smid, J.; Casseb, J.; Sanches, J.A., Jr. High prevalence of skin disorders among HTLV-1 infected individuals independent of clinical status. PLoS Negl. Trop. Dis. 2013, 7, e2546. [Google Scholar] [CrossRef]
- Amano, M.; Setoyama, M.; Grant, A.; Kerdel, F.A. Human T-lymphotropic virus 1 (HTLV-1) infection--dermatological implications. Int. J. Dermatol. 2011, 50, 915–920. [Google Scholar] [CrossRef]
- Silva, J.L.S.D.; Primo, J.R.L.; De Oliveira, M.D.F.S.P.; Batista, E.D.S.; Moreno-Carvalho, O.; Farré, L.; Bittencourt, A.L. Clustering of HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) and infective dermatitis associated with HTLV-1 (IDH) in Salvador, Bahia, Brazil. J. Clin. Virol. 2013, 58, 482–485. [Google Scholar] [CrossRef]
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.; Izumo, S.; et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: High proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef]
- Best, I.; Adaui, V.; Verdonck, K.; González, E.; Tipismana, M.; Clark, D.; Gotuzzo, E.; Vanham, G. Proviral load and immune markers associated with human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Peru. Clin. Exp. Immunol. 2006, 146, 226–233. [Google Scholar] [CrossRef]
- Takenouchi, N.; Yamano, Y.; Usuku, K.; Osame, M.; Izumo, S. Usefulness of proviral load measurement for monitoring of disease activity in individual patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 2003, 9, 29–35. [Google Scholar] [CrossRef]
- Ueno, S.; Umeki, K.; Takajo, I.; Nagatomo, Y.; Kusumoto, N.; Umekita, K.; Morishita, K.; Okayama, A. Proviral loads of human T-lymphotropic virus Type 1 in asymptomatic carriers with different infection routes. Int. J. Cancer 2012, 130, 2318–2326. [Google Scholar] [CrossRef]
- Nagai, M.; Yamano, Y.; Brennan, M.B.; Mora, C.A.; Jacobson, S. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 2001, 50, 807–812. [Google Scholar] [CrossRef]
- Primo, J.; Siqueira, I.; Nascimento, M.C.F.; Oliveira, M.F.; Farre, L.; Carvalho, E.M.; Bittencourt, A.L. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. Braz. J. Med. Biol. Res. 2009, 42, 761–764. [Google Scholar] [CrossRef]
- Otero-Romero, S.; Sastre-Garriga, J.; Comi, G.; Hartung, H.-P.; Soelberg Sørensen, P.; Thompson, A.J.; Vermersch, P.; Gold, R.; Montalban, X. Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper. Mult. Scler. 2016, 22, 1386–1396. [Google Scholar] [CrossRef]
- Kataoka, A.; Imai, H.; Inayoshi, S.; Tsuda, T. Intermittent high-dose vitamin C therapy in patients with HTLV-I associated myelopathy. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1213–1216. [Google Scholar] [CrossRef]
- De Castro-Costa, C.M.; Araújo, A.Q.C.; Barreto, M.M.; Takayanagui, O.M.; Sohler, M.P.; da Silva, E.L.M.; de Paula, S.M.B.; Ishak, R.; Ribas, J.G.R.; Rovirosa, L.C.; et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res. Hum. Retroviruses 2006, 22, 931–935. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Schmidt, F.; Oliveira, A.L.; Araujo, A. Development and Validation of a Neurological Disability Scale for Patients with HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP): The IPEC-1 Scale (P03.258). Neurology 2012, 78. [Google Scholar] [CrossRef]
| Demographic/Clinical Characteristics (Total = 38) | n (%) 1 |
|---|---|
| Female | 25 (66) |
| HTLV-1 diagnosis made due to HAM/TSP symptoms | 32 (84) |
| Duration of breastfeeding >1 year (n = 34) | 27 (79) |
| History of blood transfusion | 6 (16) |
| Patients with family members tested for HTLV-1 | 31 (82) |
| Mothers with confirmed HTLV-1 diagnosis | 22 (71) |
| Patients with other family members with HTLV-1 infection | 17 (55) |
| A family member with walking difficulties attributable to HAM/TSP | 14 (37) |
| Mothers diagnosed with HAM/TSP | 10 (26) |
| Age of onset of HAM/TSP symptoms, years, median (IQR) | 14 (7) |
| Female patients | 14 (5) |
| Male patients | 16 (6) |
| Duration of symptoms prior to diagnosis, years, median (IQR) | 6 (7) |
| Follow-up duration, years, median (IQR) | 4.5 (13) |
| Dermatologic Manifestations (Total = 38) | n (%) 1 |
|---|---|
| Infective dermatitis | 15 (40) |
| Scabies | 10 (26) |
| Onychomycosis | 14 (37) |
| Warts | 9 (24) |
| Seborrheic dermatitis | 3 (8) |
| Herpes zoster | 2 (5) |
| Other 2 | 5 (13) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwalb, A.; Pérez-Muto, V.; Cachay, R.; Tipismana, M.; Álvarez, C.; Mejía, F.; González-Lagos, E.; Gotuzzo, E. Early-Onset HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Pathogens 2020, 9, 450. https://doi.org/10.3390/pathogens9060450
Schwalb A, Pérez-Muto V, Cachay R, Tipismana M, Álvarez C, Mejía F, González-Lagos E, Gotuzzo E. Early-Onset HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Pathogens. 2020; 9(6):450. https://doi.org/10.3390/pathogens9060450
Chicago/Turabian StyleSchwalb, Alvaro, Valeria Pérez-Muto, Rodrigo Cachay, Martín Tipismana, Carolina Álvarez, Fernando Mejía, Elsa González-Lagos, and Eduardo Gotuzzo. 2020. "Early-Onset HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis" Pathogens 9, no. 6: 450. https://doi.org/10.3390/pathogens9060450
APA StyleSchwalb, A., Pérez-Muto, V., Cachay, R., Tipismana, M., Álvarez, C., Mejía, F., González-Lagos, E., & Gotuzzo, E. (2020). Early-Onset HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Pathogens, 9(6), 450. https://doi.org/10.3390/pathogens9060450






