Hepatic Stellate Cells and Hepatocytes as Liver Antigen-Presenting Cells during B. abortus Infection
Abstract
1. Introduction
2. Results
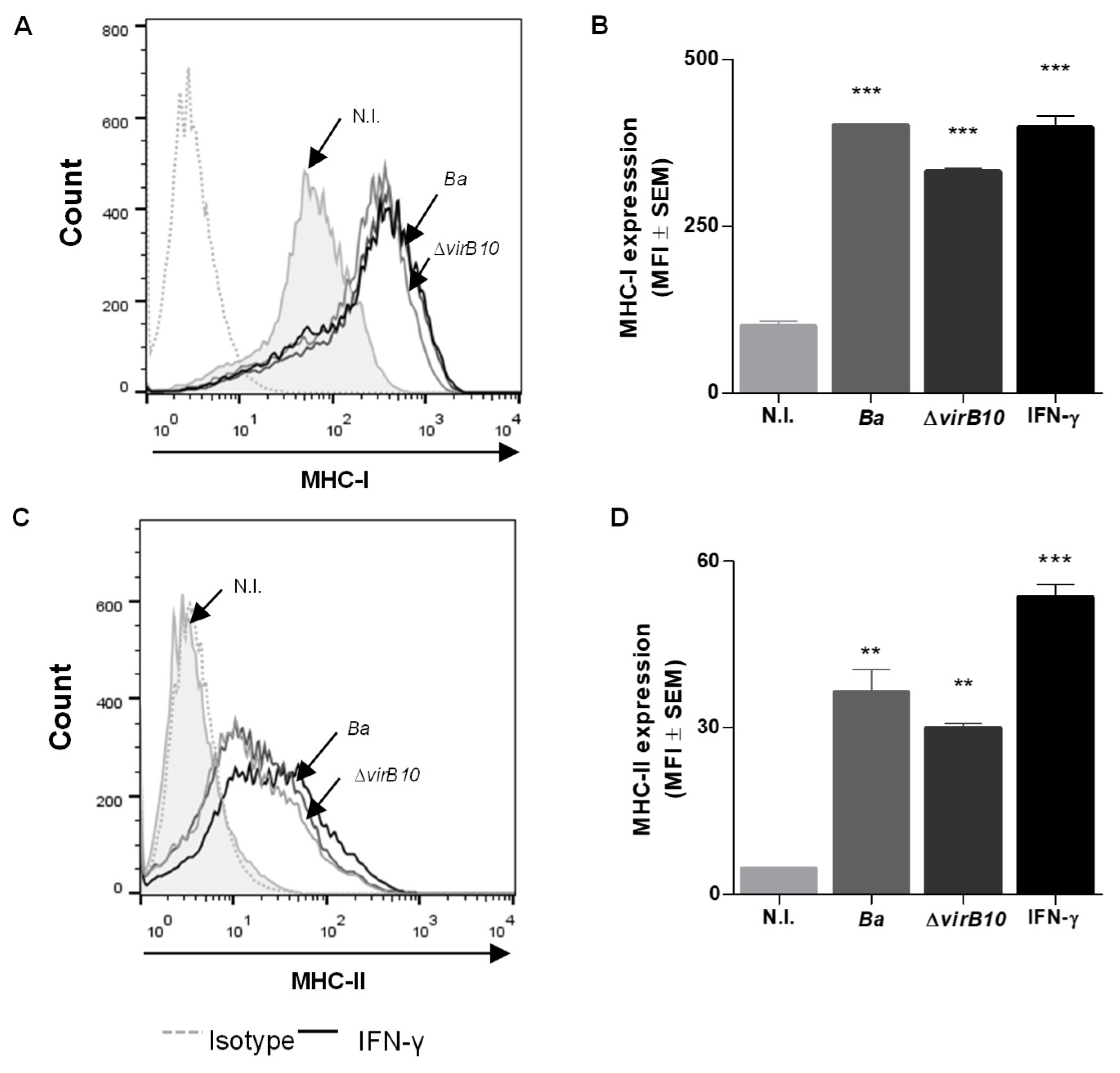
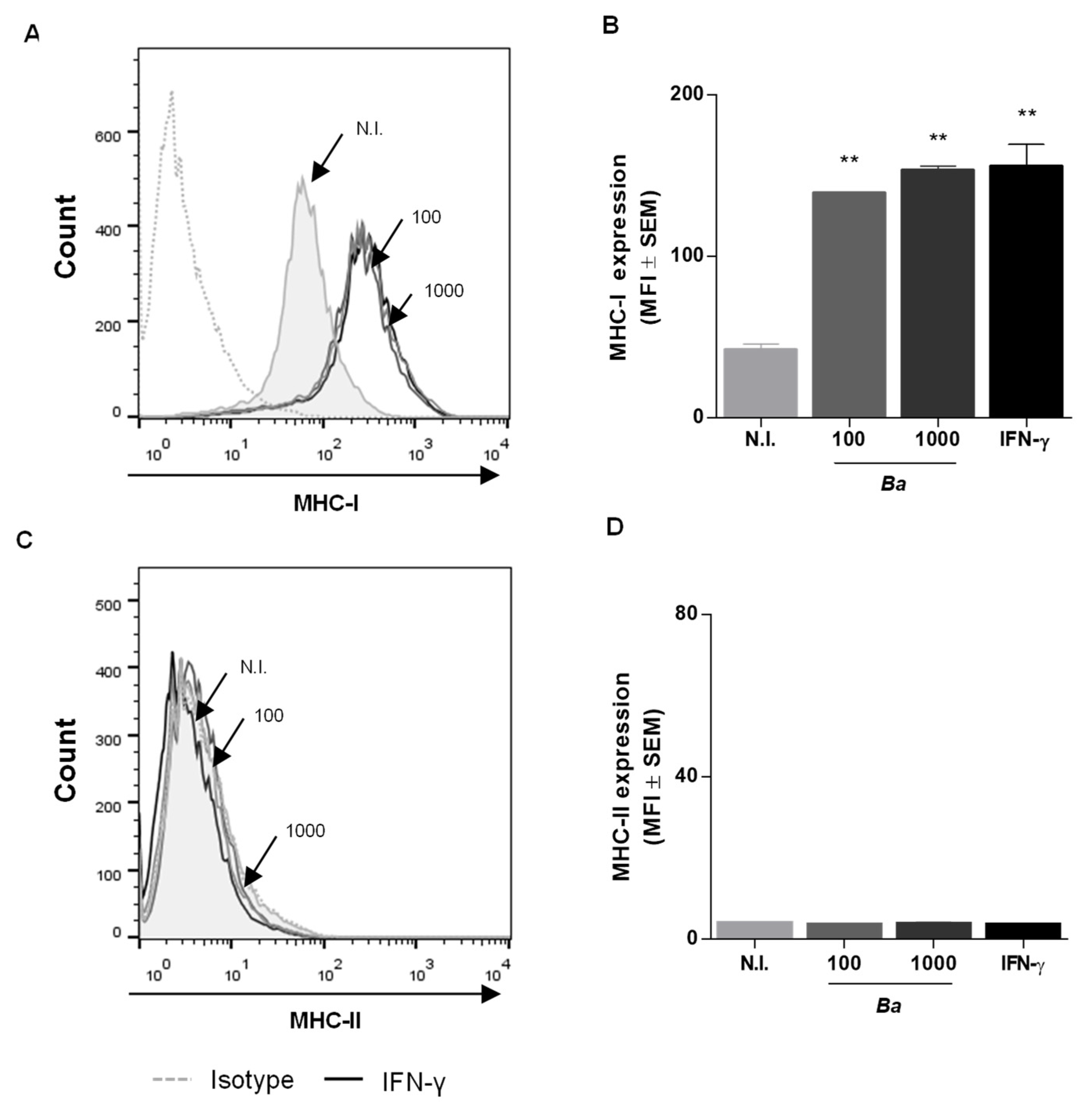
2.1. B. abortus Infection Induces MHC-I and -II Expression in LX-2 Cells via a T4SS-Independent Mechanism
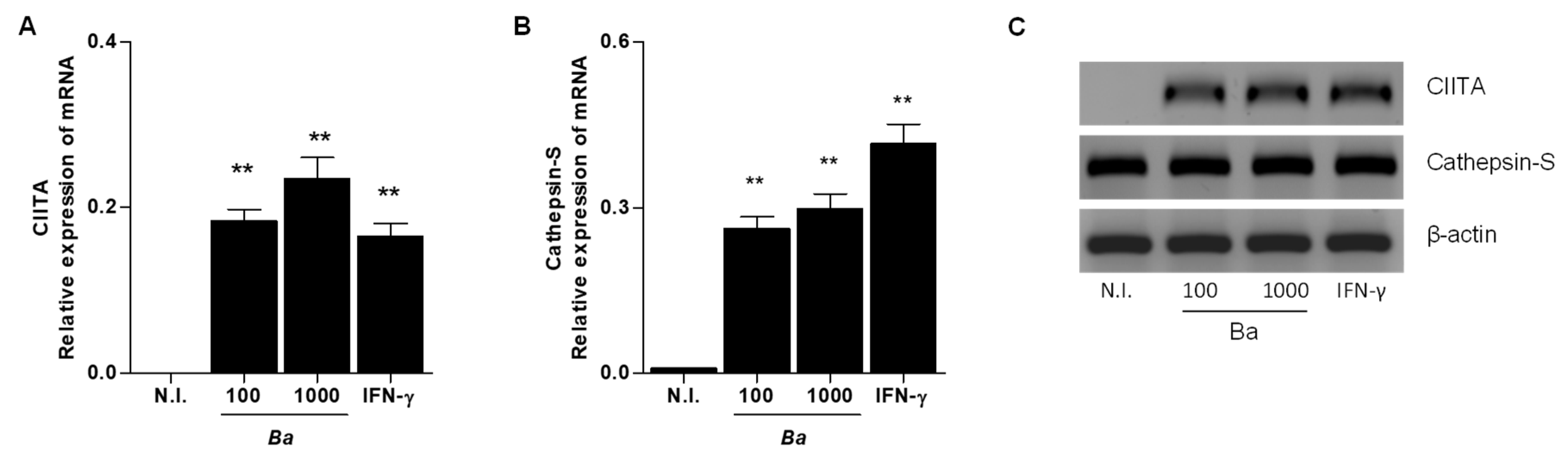
2.2. B. abortus Infection Induce CIITA and Cathepsin S Transcription in LX-2 cells
2.3. B. abortus Infection Does not Induce the Expression of Costimulatory Molecules CD80, CD86 and CD40
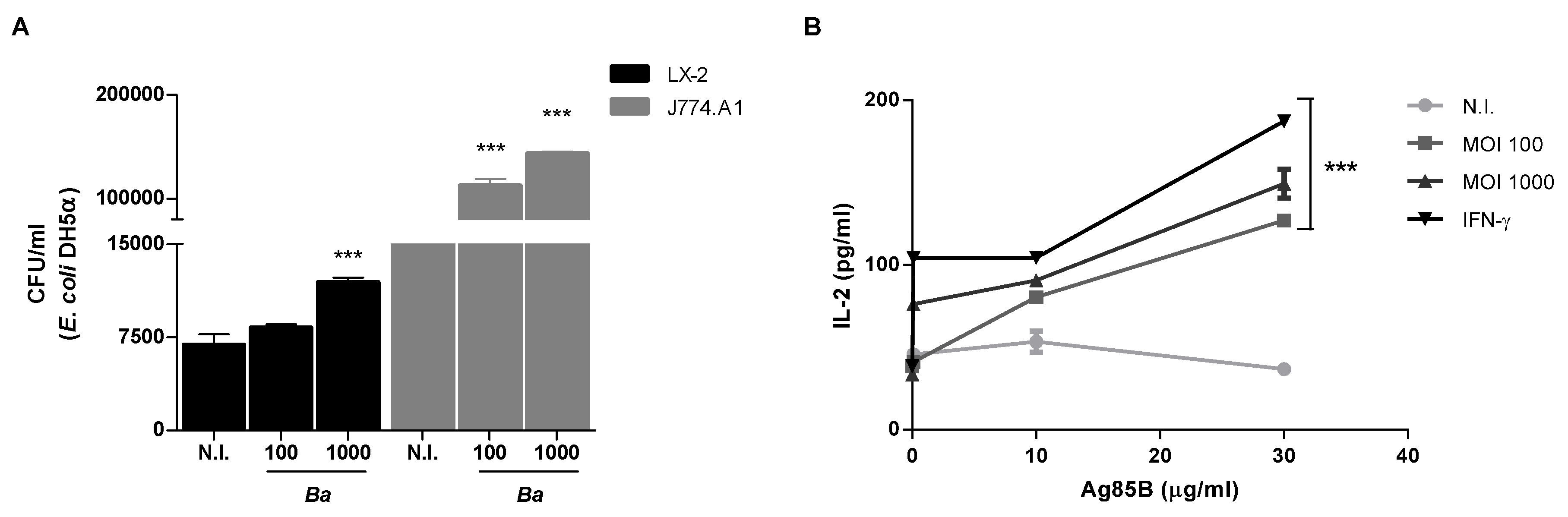
2.4. B. abortus Increase the Phagocytic Capability of LX-2 Cells
2.5. B. abortus Induces MHC-II-Restricted Antigen Processing and Presentation by LX-2 Cells
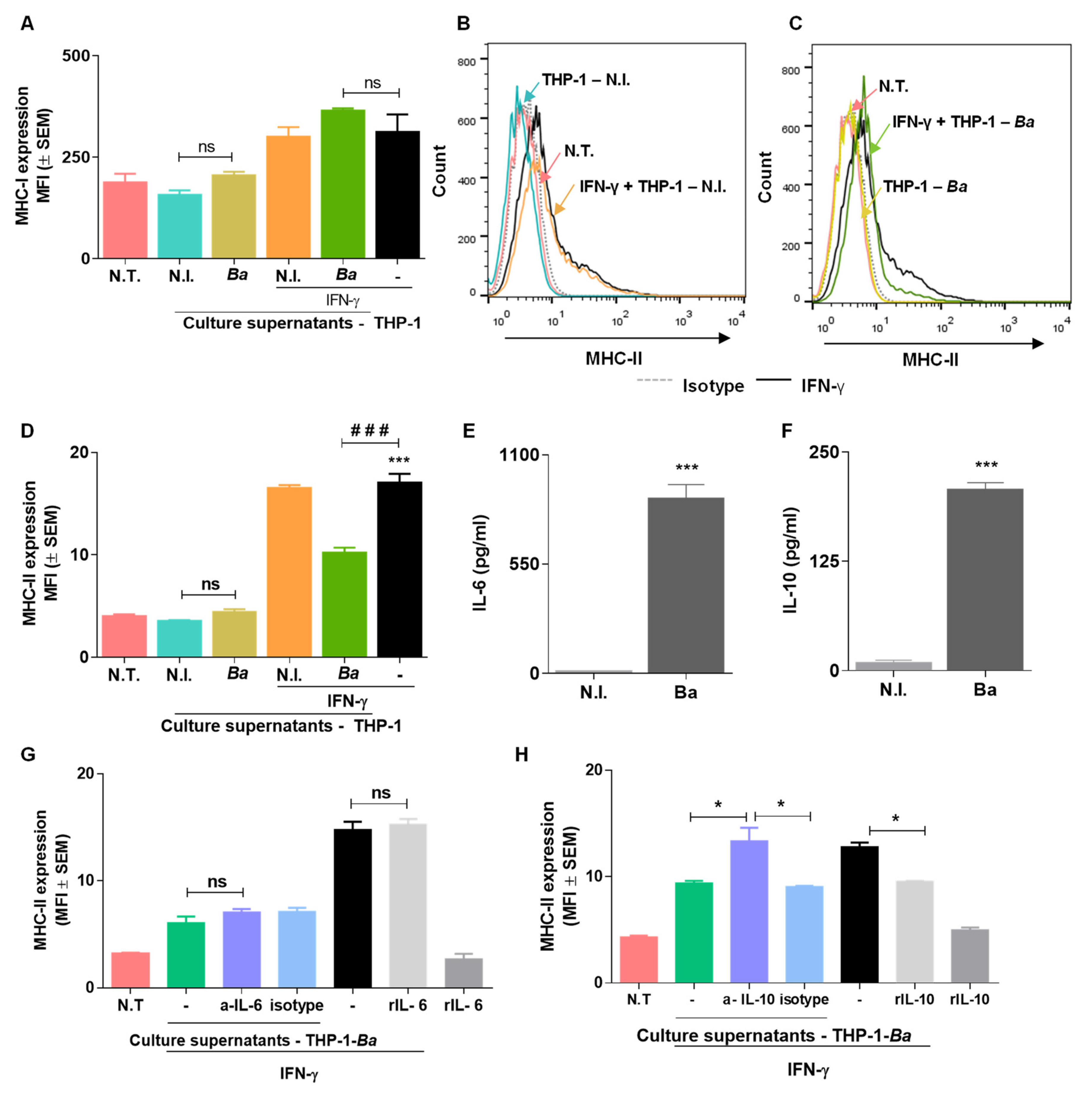
2.6. Culture Supernatants from B. abortus Infected THP-I Cells Do not Induce MHC-I and MHC-II Expression by LX2 Cells
2.7. Culture Supernatants from B. abortus Infected THP-I Monocytes Inhibit MHC-II Expression Induced by IFN-γ in an IL-10 Dependent Mechanism.
2.8. B. abortus Infection Induces MHC-I Expression in HepG2 Cells but Does not Alter MHC-II Levels
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture
4.2. Cell Culture
4.3. Cellular Infection
4.4. Flow Cytometry
4.5. Cytokine ELISA
4.6. Phagocytosis Assays
4.7. mRNA Preparation and RT-qPCR
4.8. Ag Processing and Presentation Assays
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pappas, G.; Akritidis, N.; Bosilkovski, M.; Tsianos, E. Brucellosis. N. Engl. J. Med. 2005, 352, 2325–2336. [Google Scholar] [CrossRef]
- Hayoun, M.A.; Smith, M.E.; Shorman, M. Brucellosis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kelly, A.M.; Golden-Mason, L.; Traynor, O.; Geoghegan, J.; McEntee, G.; Hegarty, J.E.; O’Farrelly, C. Changes in hepatic immunoregulatory cytokines in patients with metastatic colorectal carcinoma: Implications for hepatic anti-tumour immunity. Cytokine 2006, 35, 171–179. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.M. Gastrointestinal brucellosis. In Madkour’s Brucellosis, 2nd ed.; Madkour, M.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 150–158. [Google Scholar]
- Akritidis, N.; Tzivras, M.; Delladetsima, I.; Stefanaki, S.; Moutsopoulos, H.M.; Pappas, G. The liver in brucellosis. Clin. Gastroenterol. Hepatol. 2007, 5, 1109–1112. [Google Scholar] [CrossRef]
- Heller, T.; Belard, S.; Wallrauch, C.; Carretto, E.; Lissandrin, R.; Filice, C.; Brunetti, E. Patterns of hepatosplenic brucella abscesses on cross-sectional imaging: A review of clinical and imaging features. Am. J. Trop Med. Hyg. 2015, 93, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009, 27, 147–163. [Google Scholar] [CrossRef]
- Arriola Benitez, P.C.; Scian, R.; Comerci, D.J.; Serantes, D.R.; Vanzulli, S.; Fossati, C.A.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus induces collagen deposition and MMP-9 down-modulation in hepatic stellate cells via TGF-beta1 production. Am. J. Pathol. 2013, 183, 1918–1927. [Google Scholar] [CrossRef]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Winau, F.; Hegasy, G.; Weiskirchen, R.; Weber, S.; Cassan, C.; Sieling, P.A.; Modlin, R.L.; Liblau, R.S.; Gressner, A.M.; Kaufmann, S.H. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 2007, 26, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Dunham, R.M.; Thapa, M.; Velazquez, V.M.; Elrod, E.J.; Denning, T.L.; Pulendran, B.; Grakoui, A. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 2013, 190, 2009–2016. [Google Scholar] [CrossRef]
- Roop, R.M., II; Bellaire, B.H.; Valderas, M.W.; Cardelli, J.A. Adaptation of the Brucellae to their intracellular niche. Mol. Microbiol. 2004, 52, 621–630. [Google Scholar] [CrossRef]
- McCormick, P.J.; Martina, J.A.; Bonifacino, J.S. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA 2005, 102, 7910–7915. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, P.; Cassataro, J.; Delpino, M.V.; Zwerdling, A.; Pasquevich, K.A.; Garcia Samartino, C.; Wallach, J.C.; Fossati, C.A.; Giambartolomei, G.H. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 2008, 76, 250–262. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Delpino, M.V.; Pozner, R.G.; Velasquez, L.N.; Cassataro, J.; Giambartolomei, G.H. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8(+) T cell responses. Cell. Microbiol. 2013, 15, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Delpino, M.V.; Barrionuevo, P.; Scian, R.; Fossati, C.A.; Baldi, P.C. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J. Hepatol. 2010, 53, 145–154. [Google Scholar] [CrossRef]
- Herkel, J.; Jagemann, B.; Wiegard, C.; Lazaro, J.F.; Lueth, S.; Kanzler, S.; Blessing, M.; Schmitt, E.; Lohse, A.W. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology 2003, 37, 1079–1085. [Google Scholar] [CrossRef]
- Golden-Mason, L.; Douek, D.C.; Koup, R.A.; Kelly, J.; Hegarty, J.E.; O’Farrelly, C. Adult human liver contains CD8pos T cells with naive phenotype, but is not a site for conventional alpha beta T cell development. J. Immunol. 2004, 172, 5980–5985. [Google Scholar] [CrossRef] [PubMed]
- Giuffre, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Croce, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef] [PubMed]
- Comerci, D.J.; Martinez-Lorenzo, M.J.; Sieira, R.; Gorvel, J.P.; Ugalde, R.A. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 2001, 3, 159–168. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, P.; Ficht, T.A.; Rice-Ficht, A.; Rossetti, C.A.; Adams, L.G. Pathogenesis and immunobiology of brucellosis: Review of Brucella-host interactions. Am. J. Pathol. 2015, 185, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Vinas, O.; Bataller, R.; Sancho-Bru, P.; Gines, P.; Berenguer, C.; Enrich, C.; Nicolas, J.M.; Ercilla, G.; Gallart, T.; Vives, J.; et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 2003, 38, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.C.; Chen, C.H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef]
- Ebrahimkhani, M.R.; Mohar, I.; Crispe, I.N. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology 2011, 54, 1379–1387. [Google Scholar] [CrossRef]
- Garrett, W.S.; Chen, L.M.; Kroschewski, R.; Ebersold, M.; Turley, S.; Trombetta, S.; Galan, J.E.; Mellman, I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 2000, 102, 325–334. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Grakoui, A. B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells. Hepatology 2010, 52, 2177–2185. [Google Scholar] [CrossRef]
- Charles, R.; Chou, H.S.; Wang, L.; Fung, J.J.; Lu, L.; Qian, S. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation 2013, 96, 17–24. [Google Scholar] [CrossRef]
- Reith, W.; LeibundGut-Landmann, S.; Waldburger, J.M. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 2005, 5, 793–806. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N.; Nekrep, N.; Tosi, G.; Esserman, L.; Peterlin, B.M. MHC class II enhanceosome: How is the class II transactivator recruited to DNA-bound activators? Int. Immunol. 2003, 15, 467–475. [Google Scholar] [CrossRef]
- Gobin, S.J.; Peijnenburg, A.; Keijsers, V.; van den Elsen, P.J. Site alpha is crucial for two routes of IFN gamma-induced MHC class I transactivation: The ISRE-mediated route and a novel pathway involving CIITA. Immunity 1997, 6, 601–611. [Google Scholar] [CrossRef]
- Gobin, S.J.; Peijnenburg, A.; van Eggermond, M.; van Zutphen, M.; van den Berg, R.; van den Elsen, P.J. The RFX complex is crucial for the constitutive and CIITA-mediated transactivation of MHC class I and beta2-microglobulin genes. Immunity 1998, 9, 531–541. [Google Scholar] [CrossRef][Green Version]
- Gobin, S.J.; van Zutphen, M.; Westerheide, S.D.; Boss, J.M.; van den Elsen, P.J. The MHC-specific enhanceosome and its role in MHC class I and beta(2)-microglobulin gene transactivation. J. Immunol. 2001, 167, 5175–5184. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.E.; Lu, S.; Humphreys, R.E. Cathepsin B cleavage of Ii from class II MHC alpha- and beta-chains. J. Immunol. 1991, 146, 3877–3880. [Google Scholar]
- Bevec, T.; Stoka, V.; Pungercic, G.; Dolenc, I.; Turk, V. Major histocompatibility complex class II-associated p41 invariant chain fragment is a strong inhibitor of lysosomal cathepsin L. J. Exp. Med. 1996, 183, 1331–1338. [Google Scholar] [CrossRef]
- Fineschi, B.; Sakaguchi, K.; Appella, E.; Miller, J. The proteolytic environment involved in MHC class II-restricted antigen presentation can be modulated by the p41 form of invariant chain. J. Immunol. 1996, 157, 3211–3215. [Google Scholar]
- Riese, R.J.; Mitchell, R.N.; Villadangos, J.A.; Shi, G.P.; Palmer, J.T.; Karp, E.R.; De Sanctis, G.T.; Ploegh, H.L.; Chapman, H.A. Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Investig. 1998, 101, 2351–2363. [Google Scholar] [CrossRef]
- Nakagawa, T.; Roth, W.; Wong, P.; Nelson, A.; Farr, A.; Deussing, J.; Villadangos, J.A.; Ploegh, H.; Peters, C.; Rudensky, A.Y. Cathepsin L: Critical role in Ii degradation and CD4 T cell selection in the thymus. Science 1998, 280, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Maubach, G.; Lim, M.C.; Kumar, S.; Zhuo, L. Expression and upregulation of cathepsin S and other early molecules required for antigen presentation in activated hepatic stellate cells upon IFN-gamma treatment. Biochim. Biophys. Acta 2007, 1773, 219–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arriola Benitez, P.C.; Pesce Viglietti, A.I.; Gomes, M.T.R.; Oliveira, S.C.; Quarleri, J.F.; Giambartolomei, G.H.; Delpino, M.V. Brucella abortus infection elicited hepatic stellate cell-mediated fibrosis through inflammasome-dependent IL-1beta production. Front. Immunol. 2019, 10, 3036. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.T.; Campos, P.C.; Oliveira, F.S.; Corsetti, P.P.; Bortoluci, K.R.; Cunha, L.D.; Zamboni, D.S.; Oliveira, S.C. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J. Immunol. 2013, 190, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.P.; Moreno, E. Brucella intracellular life: From invasion to intracellular replication. Vet. Microbiol. 2002, 90, 281–297. [Google Scholar] [CrossRef]
- Kohler, S.; Michaux-Charachon, S.; Porte, F.; Ramuz, M.; Liautard, J.P. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 2003, 11, 215–219. [Google Scholar] [CrossRef]
- Zhan, Y.; Cheers, C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 1993, 61, 4899–4901. [Google Scholar] [CrossRef]
- Dornand, J.; Gross, A.; Lafont, V.; Liautard, J.; Oliaro, J.; Liautard, J.P. The innate immune response against Brucella in humans. Vet. Microbiol. 2002, 90, 383–394. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Hop, H.T.; Reyes, A.W.B.; Huy, T.X.N.; Arayan, L.T.; Min, W.; Lee, H.J.; Rhee, M.H.; Chang, H.H.; Kim, S. Interleukin 10 suppresses lysosome-mediated killing of Brucella abortus in cultured macrophages. J. Biol. Chem. 2018, 293, 3134–3144. [Google Scholar] [CrossRef]
- Giambartolomei, G.H.; Scian, R.; Acosta-Rodriguez, E.; Fossati, C.A.; Delpino, M.V. Brucella abortus-infected macrophages modulate T lymphocytes to promote osteoclastogenesis via IL-17. Am. J. Pathol. 2012, 181, 887–896. [Google Scholar] [CrossRef]
- Kitamura, H.; Kamon, H.; Sawa, S.; Park, S.J.; Katunuma, N.; Ishihara, K.; Murakami, M.; Hirano, T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity 2005, 23, 491–502. [Google Scholar] [CrossRef]
- Fumeaux, T.; Pugin, J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am. J. Respir. Crit. Care Med. 2002, 166, 1475–1482. [Google Scholar] [CrossRef]
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; De Simone, E.A.; Vanzulli, S.I.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immun. 2011, 79, 3619–3632. [Google Scholar] [CrossRef]
- Gaikwad, S.; Agrawal-Rajput, R. Lipopolysaccharide from rhodobacter sphaeroides attenuates microglia-mediated inflammation and phagocytosis and directs regulatory T cell response. Int. J. Inflam. 2015, 2015, 361326. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriola Benitez, P.C.; Pesce Viglietti, A.I.; Elizalde, M.M.; Giambartolomei, G.H.; Quarleri, J.F.; Delpino, M.V. Hepatic Stellate Cells and Hepatocytes as Liver Antigen-Presenting Cells during B. abortus Infection. Pathogens 2020, 9, 527. https://doi.org/10.3390/pathogens9070527
Arriola Benitez PC, Pesce Viglietti AI, Elizalde MM, Giambartolomei GH, Quarleri JF, Delpino MV. Hepatic Stellate Cells and Hepatocytes as Liver Antigen-Presenting Cells during B. abortus Infection. Pathogens. 2020; 9(7):527. https://doi.org/10.3390/pathogens9070527
Chicago/Turabian StyleArriola Benitez, Paula Constanza, Ayelén Ivana Pesce Viglietti, María Mercedes Elizalde, Guillermo Hernán Giambartolomei, Jorge Fabián Quarleri, and María Victoria Delpino. 2020. "Hepatic Stellate Cells and Hepatocytes as Liver Antigen-Presenting Cells during B. abortus Infection" Pathogens 9, no. 7: 527. https://doi.org/10.3390/pathogens9070527
APA StyleArriola Benitez, P. C., Pesce Viglietti, A. I., Elizalde, M. M., Giambartolomei, G. H., Quarleri, J. F., & Delpino, M. V. (2020). Hepatic Stellate Cells and Hepatocytes as Liver Antigen-Presenting Cells during B. abortus Infection. Pathogens, 9(7), 527. https://doi.org/10.3390/pathogens9070527





