Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response
Abstract
1. Introduction
2. Results
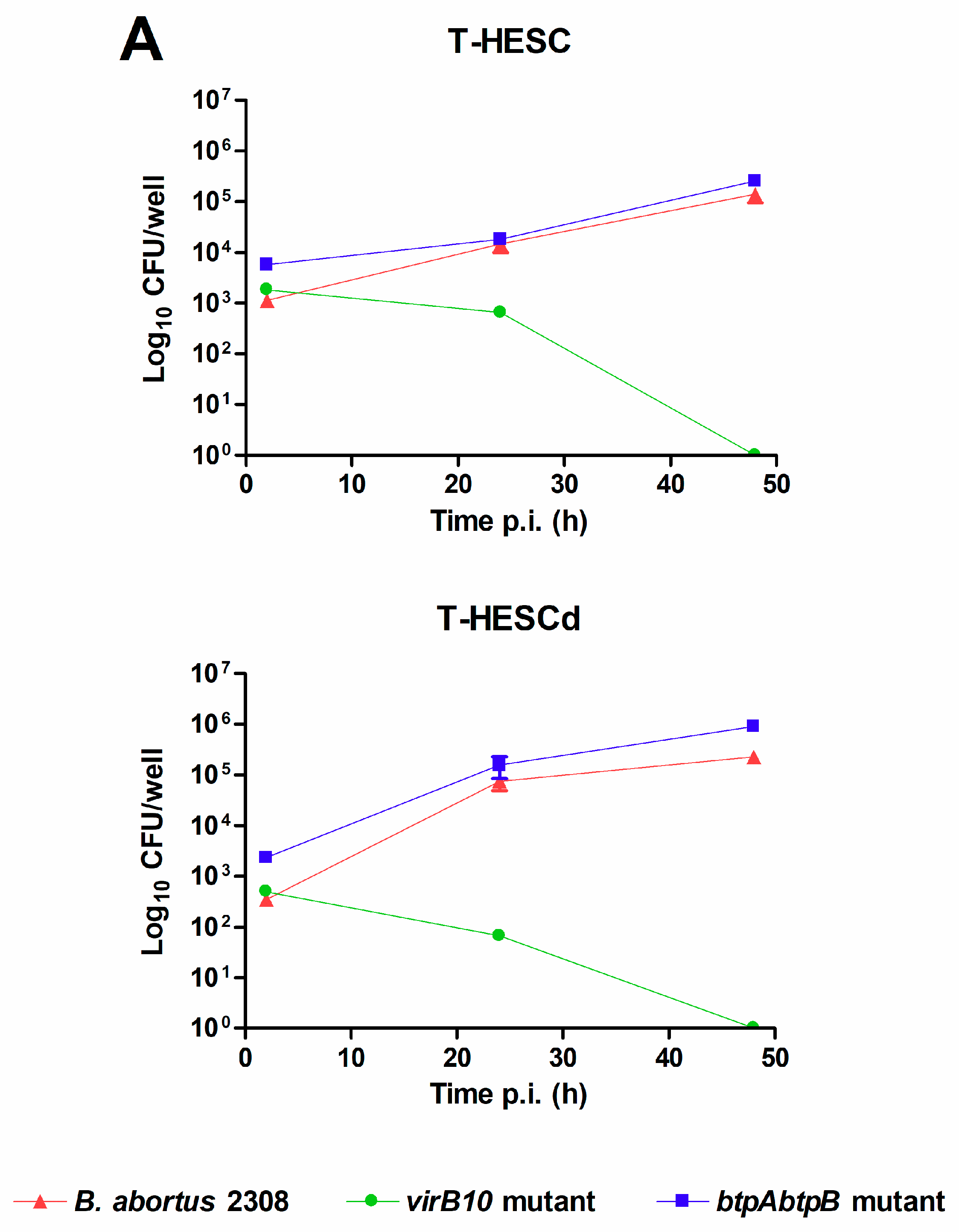
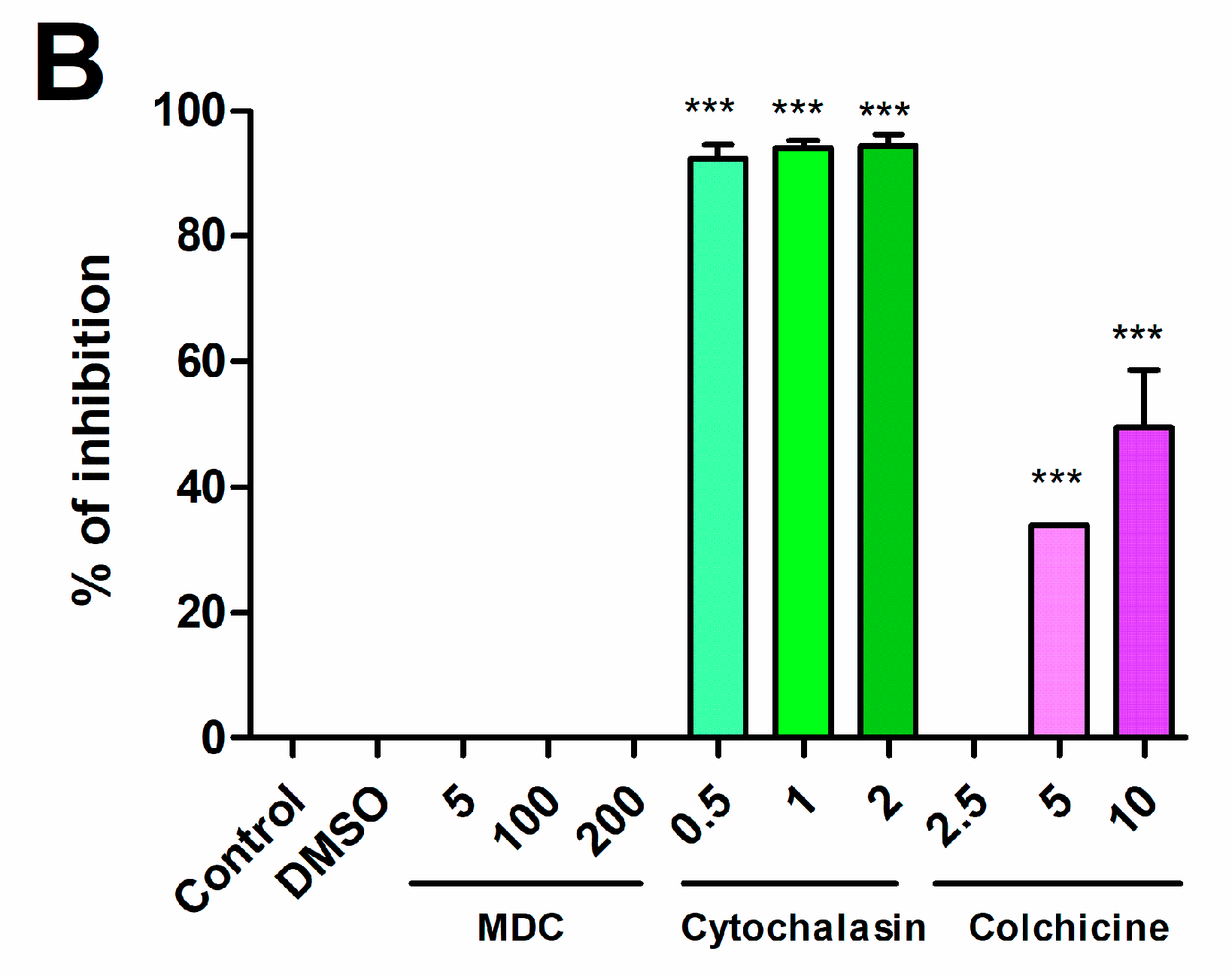
2.1. Brucella abortus Infects and Replicates in Both Decidualized and Non-Decidualized T-HESC Cells
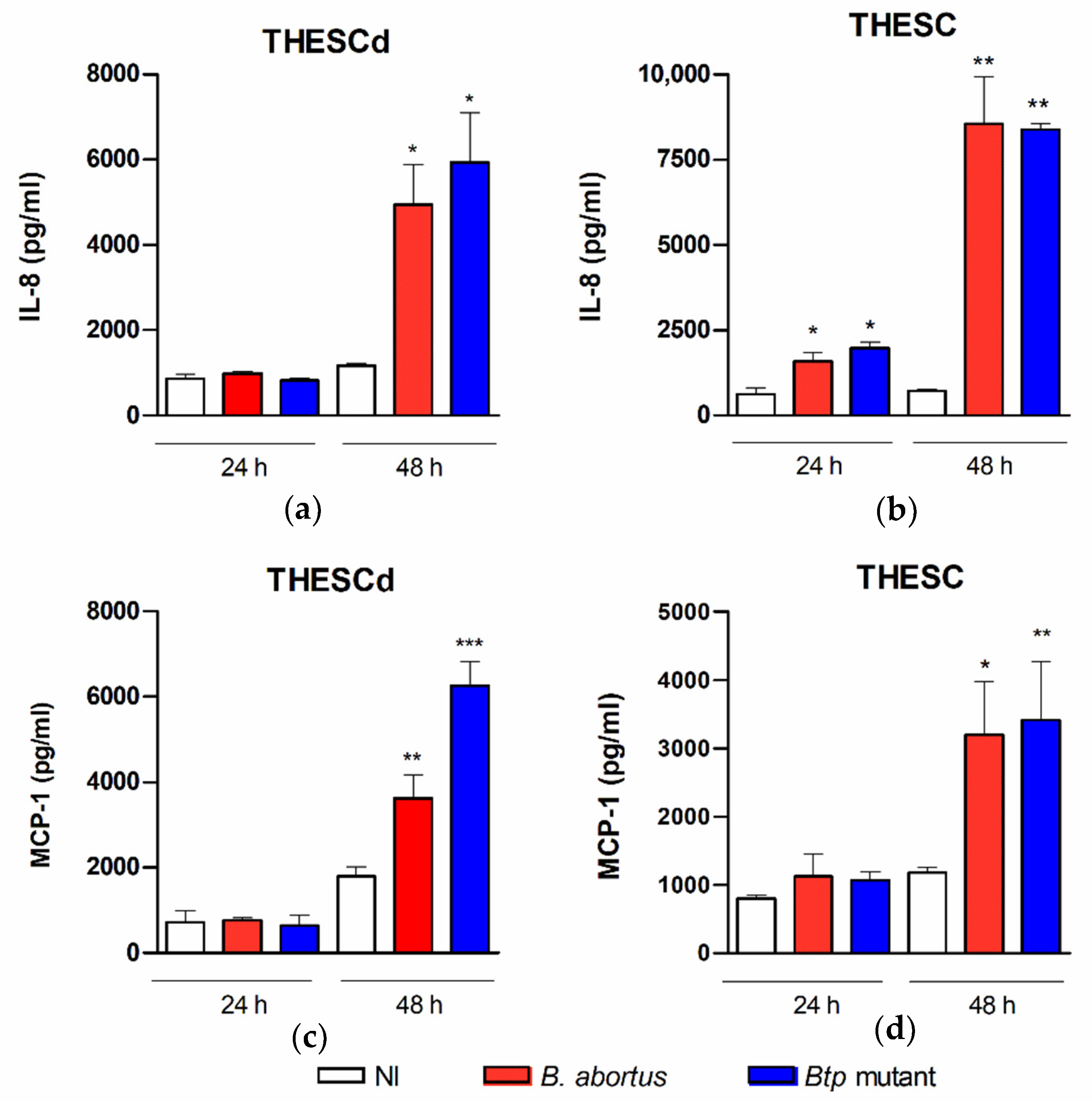
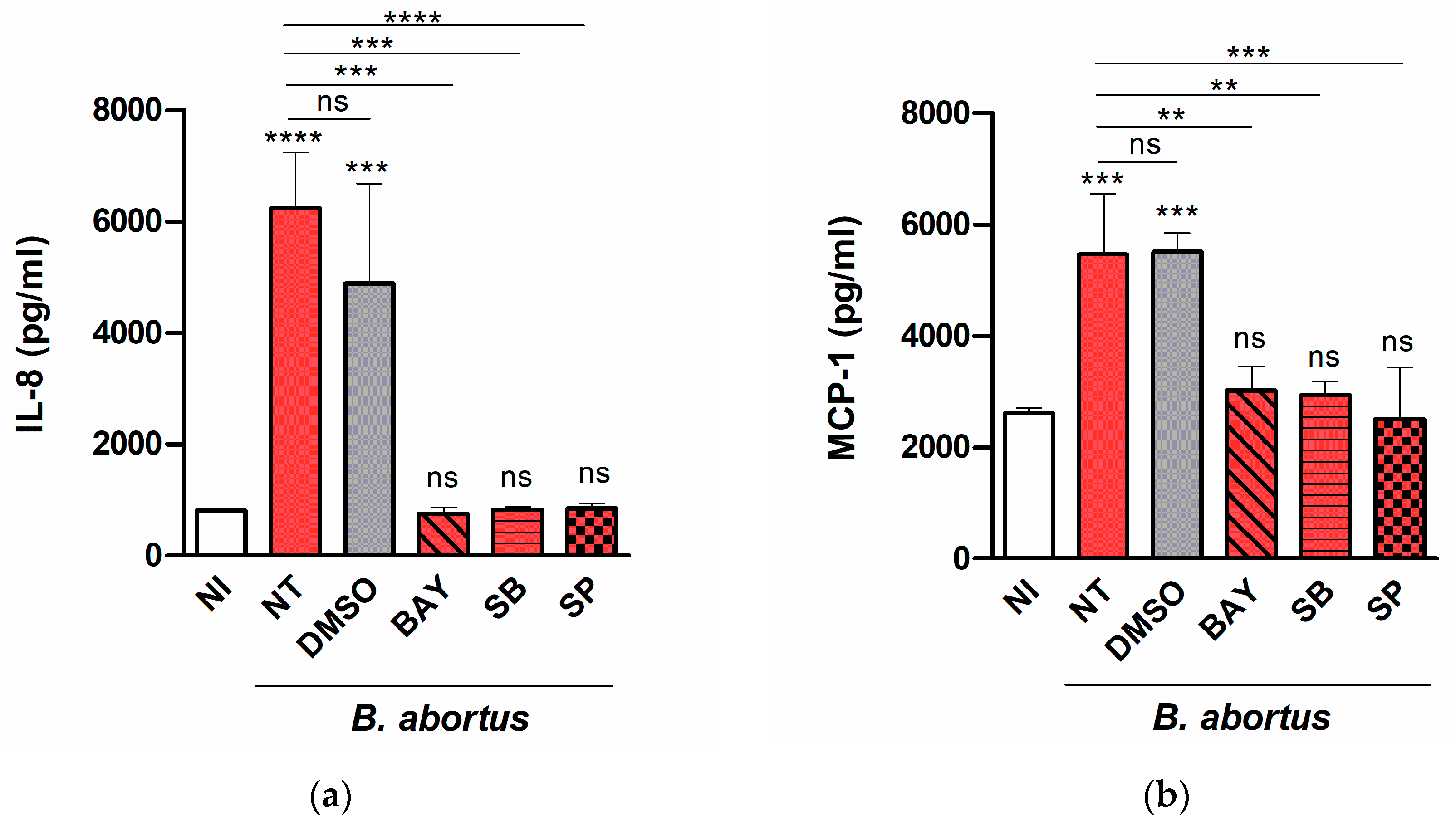
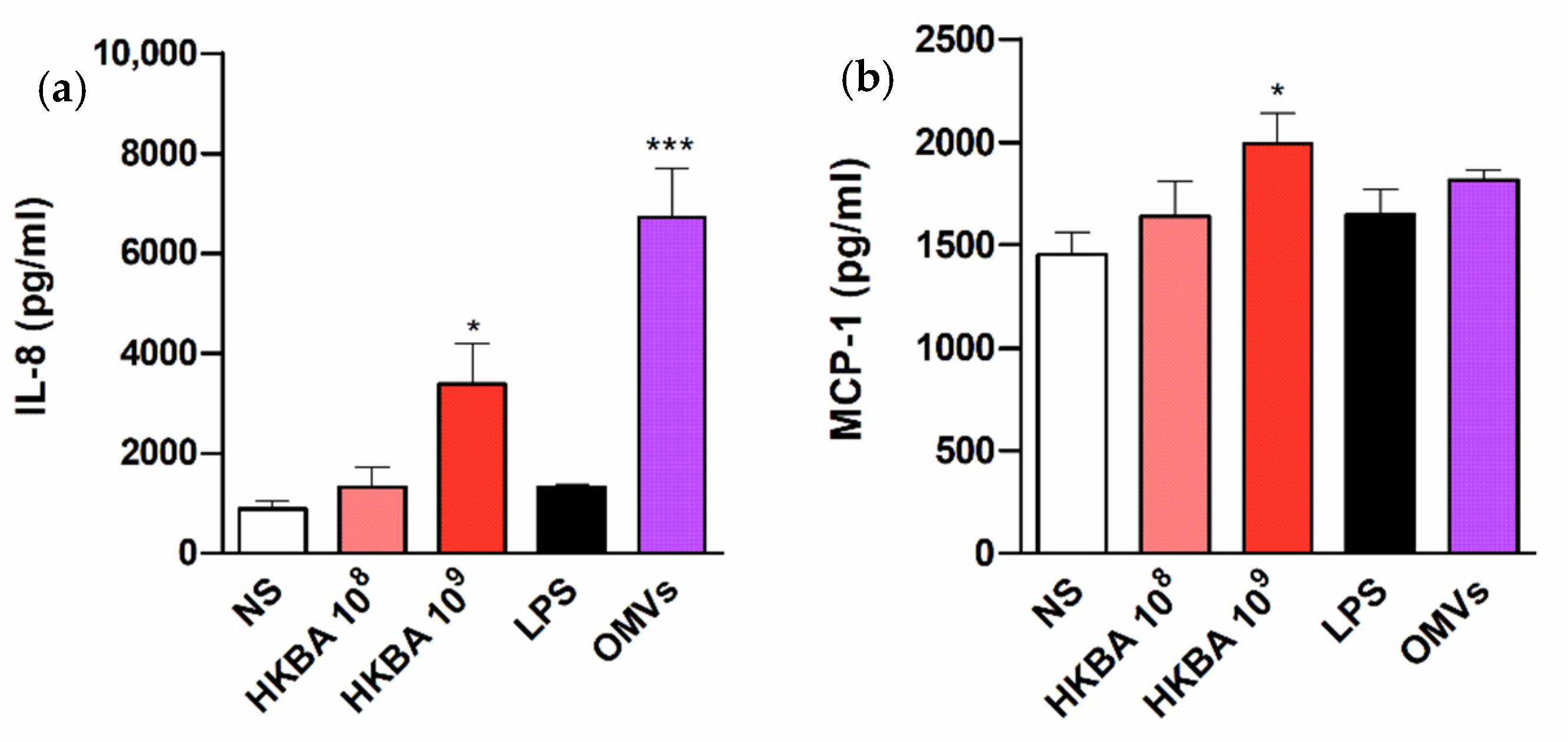
2.2. B. abortus Infection Induces the Secretion of Proinflammatory Chemokines in T-HESC Cells
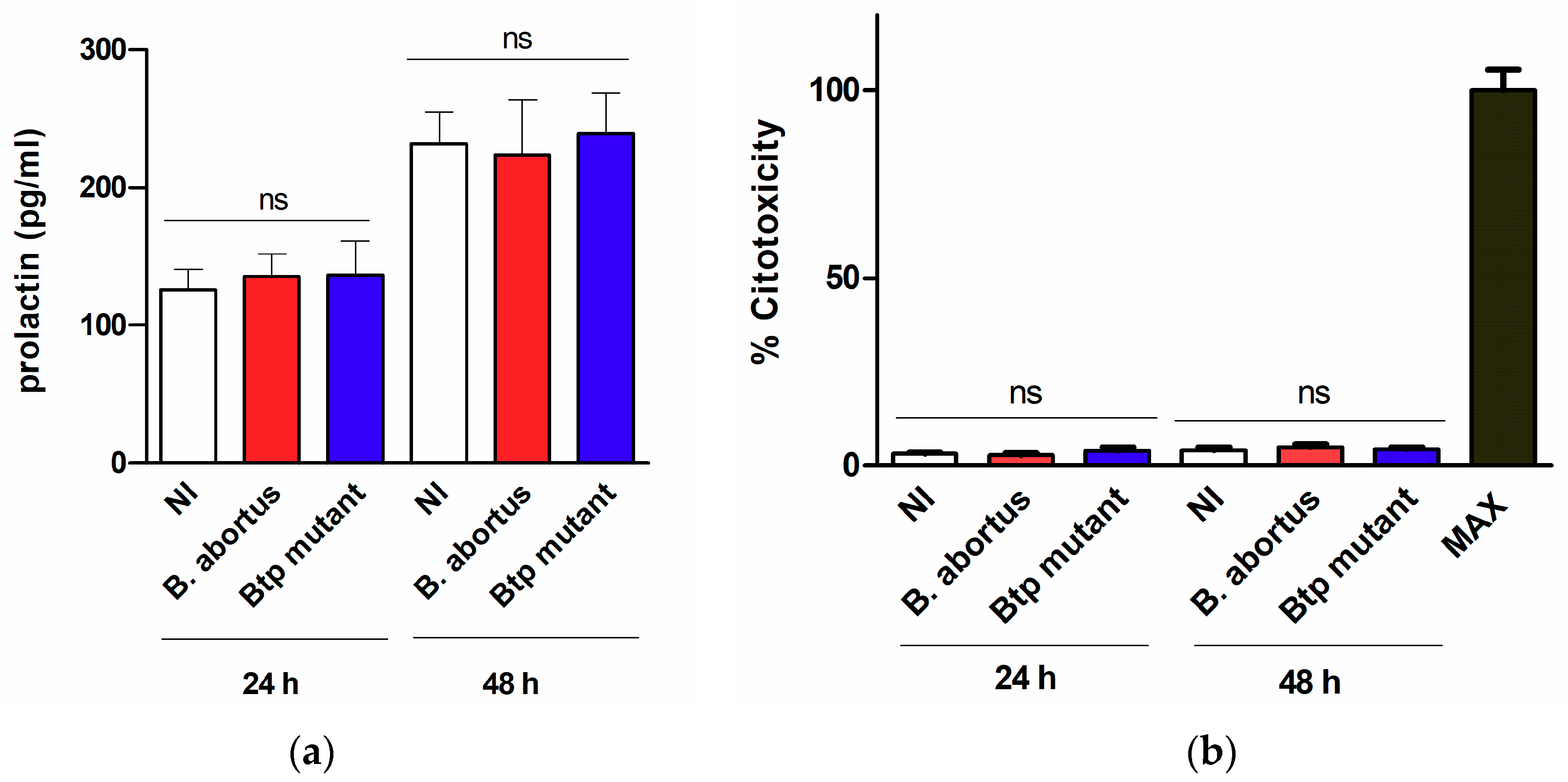
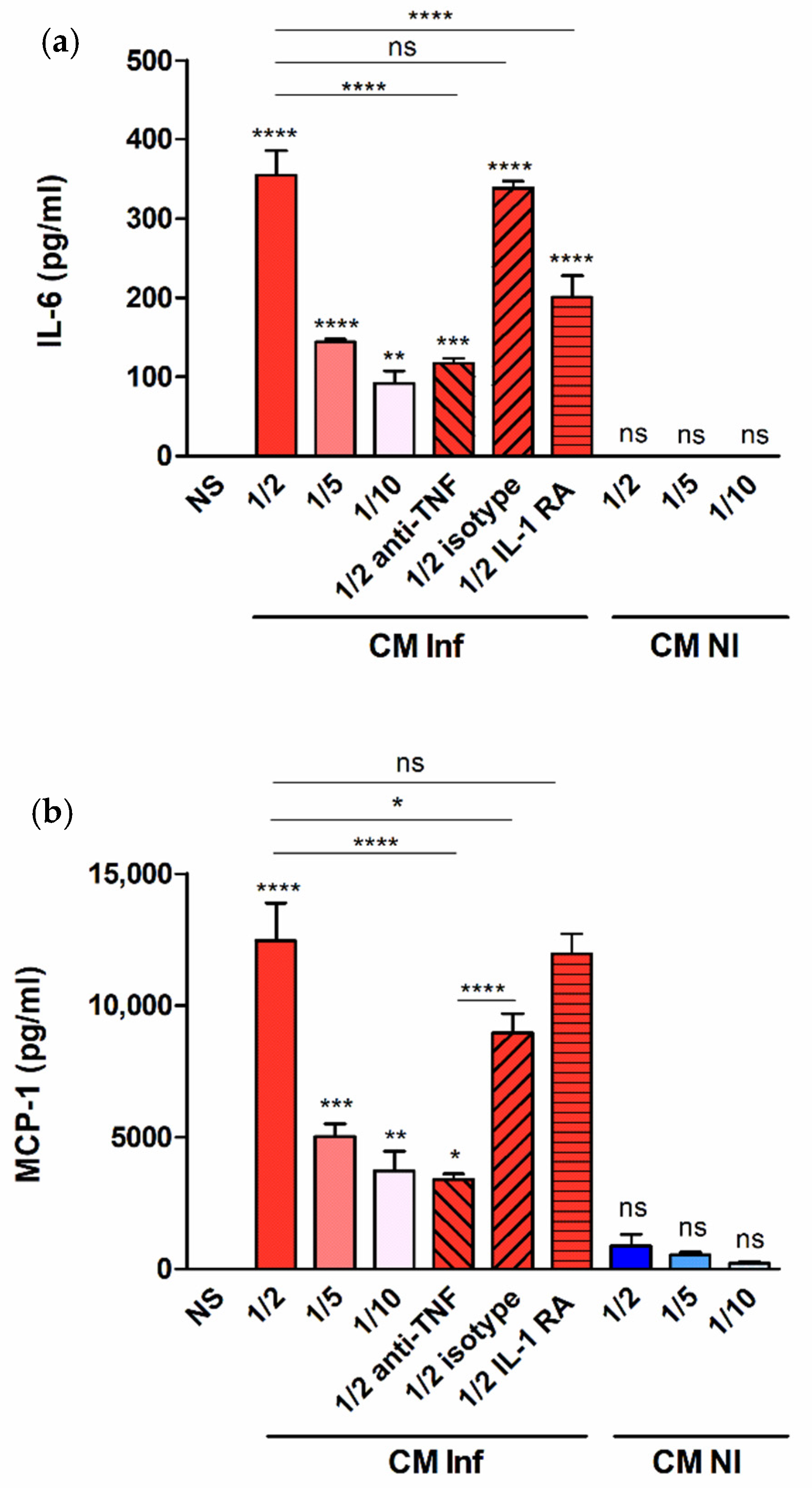
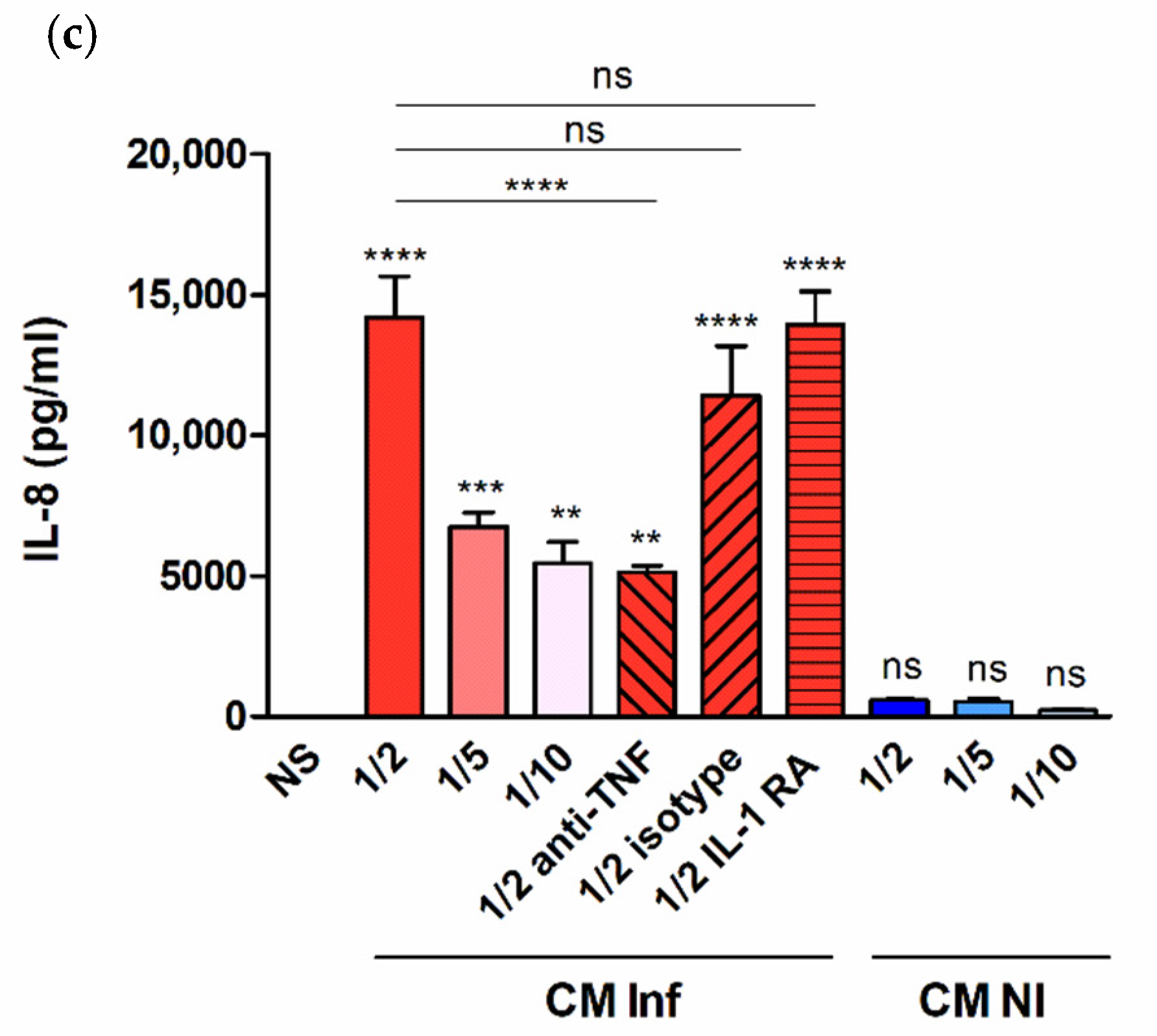
2.3. Factors Produced by Brucella-Infected Macrophages Stimulate Proinflammatory Responses in Decidualized T-HESC Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell lines
4.3. Monocyte Isolation and Macrophage Differentiation
4.4. Bacterial Strains and Growth Conditions
4.5. Isolation of Outer Membrane Vesicles
4.6. Stimulation of T-HESC Cells with Brucella antigens
4.7. Cellular Infections
4.8. Evaluation of Cytotoxicity
4.9. Internalization Pathways
4.10. Stimulation of T-HESC with Conditioned Media (CM) from Brucella-Infected Macrophages
4.11. Inhibition of Signaling Pathways
4.12. Measurement of Cytokines and Chemokines
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Pappas, G.; Akritidis, N.; Bosilkovski, M.; Tsianos, E. Brucellosis. N. Engl. J. Med. 2005, 352, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, L.E.; Kenney, R.M. Canine abortion caused by Brucella canis. J. Am. Vet. Med. Assoc. 1968, 152, 605–616. [Google Scholar]
- Carvalho, A.V.; Mol, J.P.S.; Xavier, M.N.; Paixão, T.A.; Lage, A.P.; Santos, R.L. Pathogenesis of bovine brucellosis. Vet. J. 2010, 184, 146–155. [Google Scholar]
- Bildfell, R.J.; Thomson, G.W.; Haines, D.M.; McEwen, B.J.; Smart, N. Coxiella burnetii infection is associated with placentitis in cases of bovine abortion. J. Vet. Diagn. Invest. 2000, 12, 419–425. [Google Scholar] [CrossRef]
- Buxton, D.; Anderson, I.E.; Longbottom, D.; Livingstone, M.; Wattegedera, S.; Entrican, G. Ovine chlamydial abortion: Characterization of the inflammatory immune response in placental tissues. J. Comp. Pathol. 2002, 127, 133–141. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Robinson, N.; Sandhu, J.K.; Finlay, B.B.; Sad, S.; Krishnan, L. Salmonella enterica Serovar Typhimurium-Induced Placental Inflammation and Not Bacterial Burden Correlates with Pathology and Fatal Maternal Disease. Infect. Immun. 2010, 78, 2292–2301. [Google Scholar] [CrossRef]
- Lulu, A.R.; Araj, G.F.; Khateeb, M.I.; Mustafa, M.Y. Human Brucellosis in Kuwait: A Prospective Study of 400 Cases. Q J. Med. 1988, 66, 39–54. [Google Scholar]
- Sarram, M.; Feiz, J.; Foruzandeh, M.; Gazanfarpour, P. Intrauterine fetal infection with Brucella melitensis as a possible cause of second-trimester abortion. Am. J. Obstet. Gynecol. 1974, 119, 657–660. [Google Scholar] [CrossRef]
- Makhseed, M.; Harouny, A.; Araj, G.; Moussa, M.A.; Sharma, P. Obstetric and gynecologic implication of brucellosis in Kuwait. J. Perinatol. 1998, 18, 196–199. [Google Scholar]
- Khan, M.Y.; Mah, M.W.; Memish, Z.A. Brucellosis in Pregnant Women. Clin. Infect. Dis. 2001, 32, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.G.; Ferrero, M.C.; Hielpos, M.S.; Fossati, C.A.; Baldi, P.C. Proinflammatory Response of Human Trophoblastic Cells to Brucella abortus Infection and upon Interactions with Infected Phagocytes. Biol. Reprod. 2016, 94, 48. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, S.P.; Chevrier, N.; Lacerda, T.L.S.; Ben Amara, A.; Gerart, S.; Gorvel, V.A.; De Chastellier, C.; Blasco, J.M.; Mege, J.L.; Gorvel, J.P. Pathogenic brucellae replicate in human trophoblasts. J. Infect. Dis. 2013, 207, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef]
- Vigliani, M.B.; Bakardjiev, A.I. Intracellular organisms as placental invaders. Fetal Matern. Med. Rev. 2014, 25, 332–338. [Google Scholar] [CrossRef]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O.D. Decidualization of the human endometrial stromal cell: An enigmatic transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef]
- Anders, A.P.; Gaddy, J.A.; Doster, R.S.; Aronoff, D.M. Current concepts in maternal-fetal immunology: Recognition and response to microbial pathogens by decidual stromal cells. Am. J. Reprod. Immunol. 2017, 77, e12623. [Google Scholar] [CrossRef]
- Castro-Leyva, V.; Zaga-Clavellina, V.; Espejel-Nuñez, A.; Vega-Sanchez, R.; Flores-Pliego, A.; Reyes-Muñoz, E.; Giono-Cerezo, S.; Nava-Salazar, S.; Espino y Sosa, S.; Estrada-Gutierrez, G. Decidualization Mediated by Steroid Hormones Modulates the Innate Immunity in Response to Group B Streptococcal Infection in vitro. Gynecol. Obstet. Invest. 2017, 82, 592–600. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Schust, D.J. Review: The Immunomodulatory Roles of Macrophages at the Maternal-Fetal Interface. Reprod. Sci. 2010, 17, 209–218. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Oreshkova, T.; Dimitrov, R.; Mourdjeva, M. A Cross-Talk of Decidual Stromal Cells, Trophoblast, and Immune Cells: A Prerequisite for the Success of Pregnancy. Am. J. Reprod. Immunol. 2012, 68, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M.; Hansen, K.A.; Winterton, E.; Klinkova, O.; Drappeau, D.; Mark-Kappeler, C.J. Reciprocal Communication Between Endometrial Stromal Cells and Macrophages. Reprod. Sci. 2010, 17, 809–822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogers, L.M.; Anders, A.P.; Doster, R.S.; Gill, E.A.; Gnecco, J.S.; Holley, J.M.; Randis, T.M.; Ratner, A.J.; Gaddy, J.A.; Osteen, K.; et al. Decidual stromal cell-derived PGE 2 regulates macrophage responses to microbial threat. Am. J. Reprod. Immunol. 2018, 80, e13032. [Google Scholar] [CrossRef] [PubMed]
- Celli, J. Surviving inside a macrophage: The many ways of Brucella. Res. Microbiol. 2006, 157, 93–98. [Google Scholar] [CrossRef]
- Zhan, Y.; Cheers, C. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect. Immun. 1995, 63, 720–723. [Google Scholar] [CrossRef]
- Covert, J.; Mathison, A.J.; Eskra, L.; Banai, M.; Splitter, G. Brucella melitensis, B. neotomae and B. ovis elicit common and distinctive macrophage defense transcriptional responses. Exp. Biol. Med. 2009, 234, 1450–1467. [Google Scholar] [CrossRef]
- Pagani, I.; Ghezzi, S.; Ulisse, A.; Rubio, A.; Turrini, F.; Garavaglia, E.; Candiani, M.; Castilletti, C.; Ippolito, G.; Poli, G.; et al. Human Endometrial Stromal Cells Are Highly Permissive To Productive Infection by Zika Virus. Sci. Rep. 2017, 7, 44286. [Google Scholar] [CrossRef]
- Lu, Y.; Bocca, S.; Anderson, S.; Wang, H.; Manhua, C.; Beydoun, H.; Oehninger, S. Modulation of the Expression of the Transcription Factors T-Bet and GATA-3 in Immortalized Human Endometrial Stromal Cells (HESCs) by Sex Steroid Hormones and cAMP. Reprod. Sci. 2013, 20, 699–709. [Google Scholar] [CrossRef]
- Gellersen, B.; Wolf, A.; Kruse, M.; Schwenke, M.; Bamberger, A.-M. Human Endometrial Stromal Cell-Trophoblast Interactions: Mutual Stimulation of Chemotactic Migration and Promigratory Roles of Cell Surface Molecules CD82 and CEACAM11. Biol. Reprod. 2013, 88, 80. [Google Scholar] [CrossRef]
- Grasso, E.; Gori, S.; Soczewski, E.; Fernández, L.; Gallino, L.; Vota, D.; Martínez, G.; Irigoyen, M.; Ruhlmann, C.; Lobo, T.F.; et al. Impact of the Reticular Stress and Unfolded Protein Response on the inflammatory response in endometrial stromal cells. Sci. Rep. 2018, 8, 12274. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Mor, G.; Alvero, A.; Guller, S.; Schatz, F.; Sapi, E.; Rahman, M.; Caze, R.; Qumsiyeh, M.; Lockwood, C.J. A Novel Immortalized Human Endometrial Stromal Cell Line with Normal Progestational Response. Endocrinology 2004, 145, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Sieira, R.; Comerci, D.J.; Sanchez, D.O.; Ugalde, R.A. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 2000, 182, 4849–4855. [Google Scholar] [CrossRef] [PubMed]
- Comerci, D.J.; Martínez-Lorenzo, M.J.; Sieira, R.; Gorvel, J.P.; Ugalde, R.A. Essential role of the virB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 2001, 3, 159–168. [Google Scholar] [CrossRef]
- Salcedo, S.P.; Marchesini, M.I.; Degos, C.; Terwagne, M.; Von Bargen, K.; Lepidi, H.; Herrmann, C.K.; Santos Lacerda, T.L.; Imbert, P.R.C.; Pierre, P.; et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front. Cell. Infect. Microbiol. 2013, 3, 28. [Google Scholar] [CrossRef]
- Hielpos, M.S.; Ferrero, M.C.; Fernández, A.G.; Falivene, J.; Vanzulli, S.; Comerci, D.J.; Baldi, P.C. Btp Proteins from Brucella abortus modulate the lung innate immune response to infection by the respiratory route. Front. Immunol. 2017, 8, 1011. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Moreno, E.; Gorvel, J. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2000, 2, 829–835. [Google Scholar] [CrossRef]
- Detilleux, P.G.; Deyoe, B.L.; Cheville, N.F. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am. J. Vet. Res. 1991, 52, 1658–1664. [Google Scholar]
- Lockwood, C.J.; Kumar, P.; Krikun, G.; Kadner, S.; Dubon, P.; Critchley, H.; Schatz, F. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: Implications for long-term progestin-only contraceptive-induced bleeding. J. Clin. Endocrinol. Metab. 2004, 89, 1467–1475. [Google Scholar] [CrossRef][Green Version]
- Hielpos, M.S.; Ferrero, M.C.; Fernández, A.G.; Bonetto, J.; Giambartolomei, G.H.; Fossati, C.A.; Baldi, P.C. CCL20 and beta-defensin 2 production by human lung epithelial cells and macrophages in response to Brucella abortus infection. PLoS ONE 2015, 10, e0140408. [Google Scholar] [CrossRef]
- Miraglia, M.C.; Scian, R.; Samartino, C.G.; Barrionuevo, P.; Rodriguez, A.M.; Ibañez, A.E.; Coria, L.M.; Velásquez, L.N.; Baldi, P.C.; Cassataro, J.; et al. Brucella abortus induces TNF-α-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases. J. Neuroinflamm. 2013, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.C.; Bregante, J.; Delpino, M.V.; Barrionuevo, P.; Fossati, C.A.; Giambartolomei, G.H.; Baldi, P.C. Proinflammatory response of human endothelial cells to Brucella infection. Microbes Infect. 2011, 13, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; De Simone, E.A.; Vanzulli, S.I.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immun. 2011, 79, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.C.; Fossati, C.A.; Baldi, P.C. Direct and monocyte-induced innate immune response of human lung epithelial cells to Brucella abortus infection. Microbes Infect. 2010, 12, 736–747. [Google Scholar] [CrossRef]
- Giambartolomei, G.H.; Zwerdling, A.; Cassataro, J.; Bruno, L.; Fossati, C.A.; Philipp, M.T. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 2004, 173, 4635–4642. [Google Scholar] [CrossRef]
- Velasco, J.; Bengoechea, J.A.; Brandenburg, K.; Lindner, B.; Seydel, U.; González, D.; Zähringer, U.; Moreno, E.; Moriyón, I. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect. Immun. 2000, 68, 3210–3218. [Google Scholar] [CrossRef]
- Grasso, E.; Gori, S.; Paparini, D.; Soczewski, E.; Fernández, L.; Gallino, L.; Salamone, G.; Martinez, G.; Irigoyen, M.; Ruhlmann, C.; et al. VIP induces the decidualization program and conditions the immunoregulation of the implantation process. Mol. Cell. Endocrinol. 2018, 460, 63–72. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Pollak, C.N.; Delpino, M.V.; Fossati, C.A.; Baldi, P.C. Outer Membrane Vesicles from Brucella abortus Promote Bacterial Internalization by Human Monocytes and Modulate Their Innate Immune Response. PLoS ONE 2012, 7, e50214. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavattieri, L.; Ferrero, M.C.; Alonso Paiva, I.M.; Sotelo, A.D.; Canellada, A.M.; Baldi, P.C. Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response. Pathogens 2020, 9, 369. https://doi.org/10.3390/pathogens9050369
Zavattieri L, Ferrero MC, Alonso Paiva IM, Sotelo AD, Canellada AM, Baldi PC. Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response. Pathogens. 2020; 9(5):369. https://doi.org/10.3390/pathogens9050369
Chicago/Turabian StyleZavattieri, Lucía, Mariana C. Ferrero, Iván M. Alonso Paiva, Agustina D. Sotelo, Andrea M. Canellada, and Pablo C. Baldi. 2020. "Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response" Pathogens 9, no. 5: 369. https://doi.org/10.3390/pathogens9050369
APA StyleZavattieri, L., Ferrero, M. C., Alonso Paiva, I. M., Sotelo, A. D., Canellada, A. M., & Baldi, P. C. (2020). Brucella abortus Proliferates in Decidualized and Non-Decidualized Human Endometrial Cells Inducing a Proinflammatory Response. Pathogens, 9(5), 369. https://doi.org/10.3390/pathogens9050369





