Prevalence and Epitope Recognition of Anti-Trypanosoma cruzi Antibodies in Two Procyonid Species: Implications for Host Resistance
Abstract
1. Introduction
2. Results
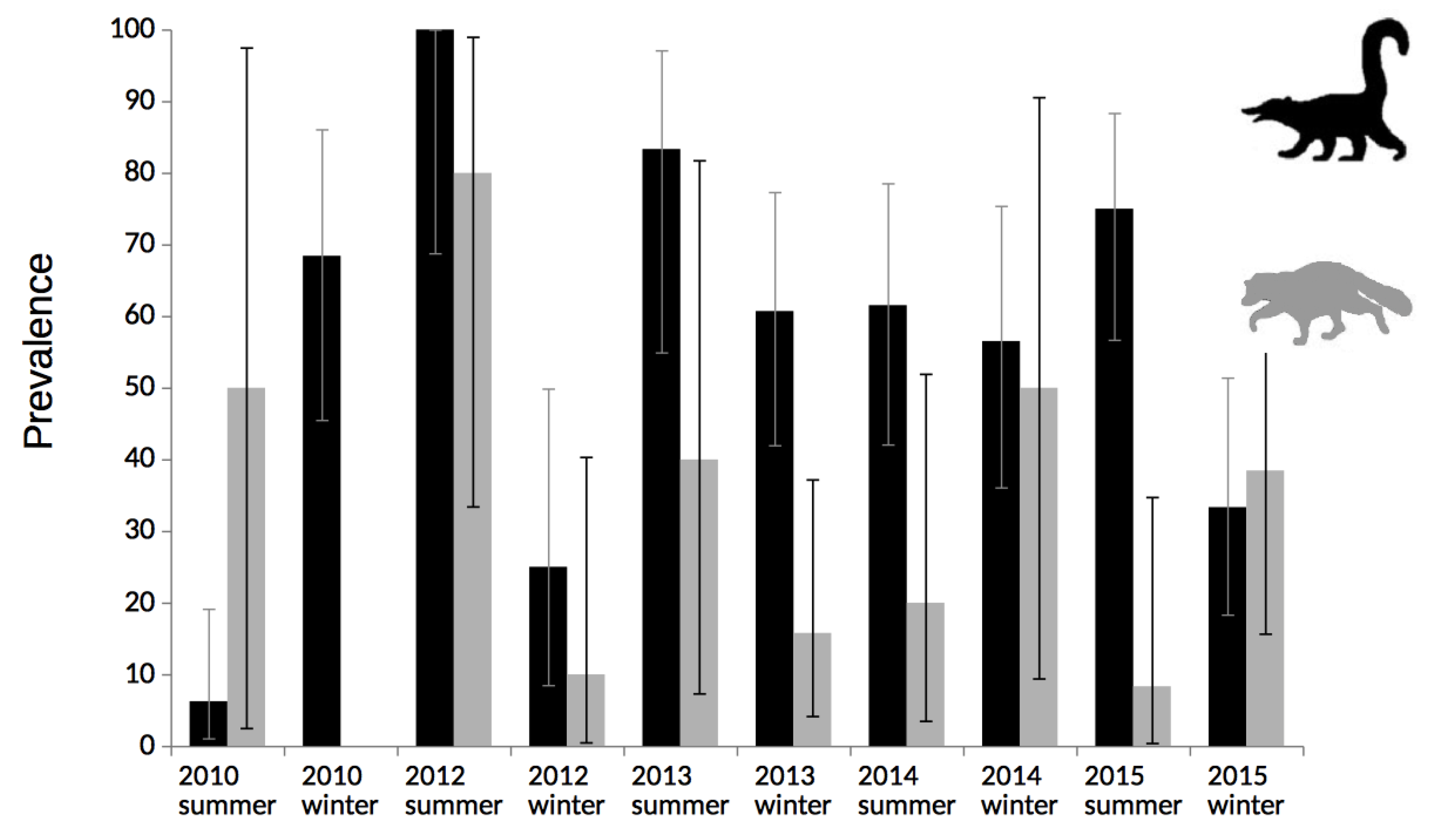
2.1. Seroprevalence
2.2. Antibodies Persistence
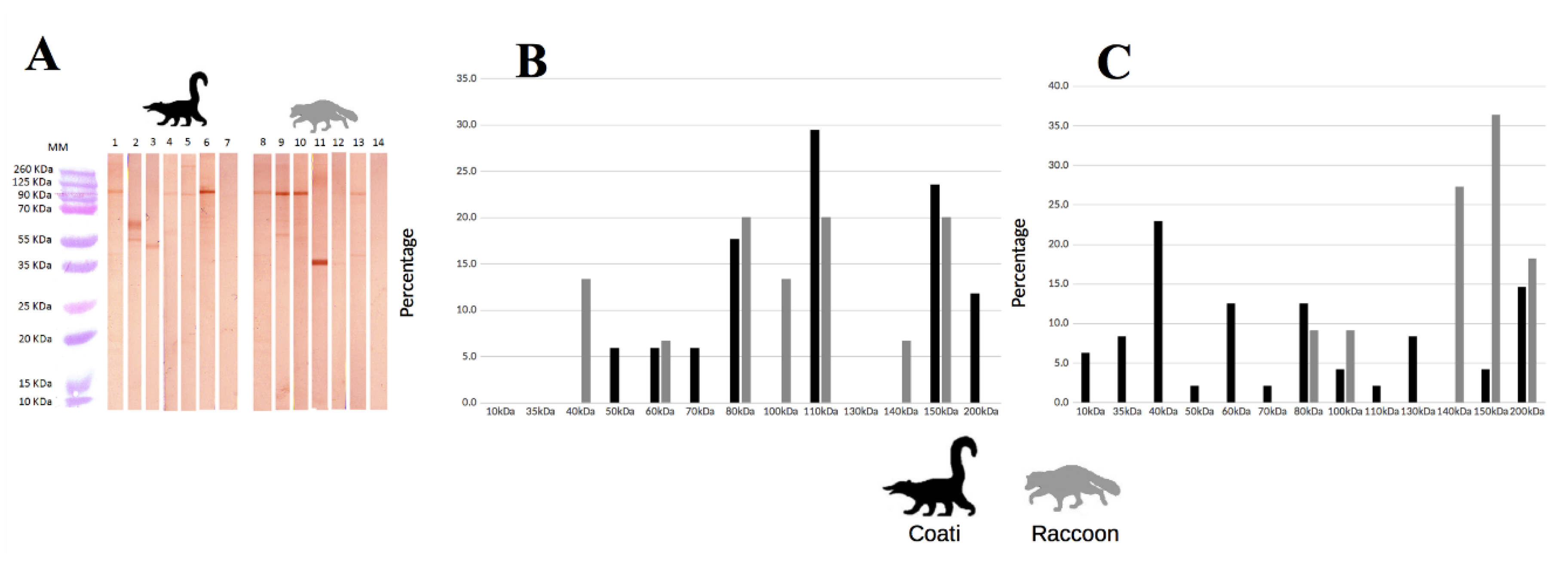
2.3. Epitope Recognition
3. Discussion
4. Materials and Methods
4.1. Capture and Animal Sampling
4.2. Serological Test
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Control of Chagas Disease, Second Report of the WHO Expert Committee; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Scott, M.E. The impact of infection and disease on animal populations: Implications for conservation biology. Conserv. Biol. 1998, 2, 40–56. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.G.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Carabarin-Lima, A.; González-Vázquez, M.C.; Rodríguez-Morales, O.; Baylón-Pacheco, L.; Rosales-Encina, J.L.; Reyes-López, P.A.; Arce-Fonseca, M. Chagas disease (American tripanosomiasis) in Mexico: An update. Acta Trop. 2013, 127, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bosseno, M.F.; Garcia, L.S.; Baunaure, F.; Gastelum, E.M.; Gutierrez, M.S.; Kasten, F.L.; Dumonteil, E.; Breniere, S.F. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am. J. Trop. Med. Hyg. 2006, 74, 303–305. [Google Scholar] [CrossRef]
- Breniere, S.F.; Bosseno, M.F.; Magallon-Gastelum, E.; Castillo Ruvalcaba, E.G.; Gutierrez, M.S.; Montano Luna, E.C.; Basulto, J.T.; Mathieu-Daude, F.; Walter, A.; Lozano-Kasten, F. Peridomestic colonization of Triatoma longipennis (Hemiptera, Reduviidae) and Triatoma barberi (Hemiptera, Reduviidae) in a rural community with active transmission of Trypanosoma cruzi in jalisco state Mexico. Acta Trop. 2007, 101, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ligonio, A.; Torres-Montero, J.; López-Monteon, A.; Dumonteil, E. Extensive diversity of Trypanosoma cruzi discrete typing units circulating in Triatoma dimidiata from central Veracruz, Mexico. Infect. Genet. Evol. 2012, 12, 1341–1343. [Google Scholar] [CrossRef]
- Ibañez-Cervantes, G.; Martínez-Ibarra, A.; Nogueda-Torres, B.; López-Orduña, E.; Alonso, A.L.; Perea, C.; Maldonado, T.; Hernández, J.M.; León-Avila, G. Identification by Q-PCR of Trypanosoma Cruzi Lineage and Determination of Blood Meal Sources in Triatomine Gut Samples in México. Parasitol. Int. 2013, 62, 36–43. [Google Scholar] [CrossRef]
- Monteón, V.; Triana-Chávez, O.; Mejía-Jaramillo, A.; Pennignton, P.; Ramos-Ligonio, A.; Acosta, K.; Lopez, R. Circulation of Tc Ia discrete type unit Trypanosoma cruzi in Yucatan Mexico. J. Parasit. Dis. 2016, 40, 550–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martínez-Hernández, F.; Rendón-Franco, E.; Gama-Campillo, L.M.; Villanueva-García, C.; Romero-Valdovinos, M.; Maravilla, P.; Villalobos, G. Follow up of natural infection with Trypanosoma cruzi in two mammals species, Nasua narica and Procyon lotor (Carnivora: Procyonidae): Evidence of infection control? Parasit. Vectors 2014, 7, 405. [Google Scholar] [CrossRef]
- López-Cancino, S.A.; Tun-Ku, E.; De la Cruz-Felix, H.K.; Ibarra-Cerdeña, C.N.; Izeta-Alberdi, A.; Pech-May, A.; Mazariegos-Hidalgo, J.C.; Valdez-Tah, A.; Ramsey, J.M. Landscape ecology of Trypanosoma cruzi in the southern Yucatan Peninsula. Acta Trop. 2015, 151, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; das Chagas Xavier, S.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Macedo, G.C.; Barreto, W.T.G.; Oliveira-Santos, L.G.R.; Garcia, C.M.; Mourão, G.M.; de Oliveira, P.G.E.; Marino, E.D.; Andre, M.R.; Perles, L.; et al. Outcomes of Trypanosoma cruzi and Trypanosoma evansi infections on health of Southern coati (Nasua nasua), crab-eating fox (Cerdocyon thous), and ocelot (Leopardus pardalis) in the Brazilian Pantanal. PLoS ONE 2018, 13, e0201357. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.L.; Roque, A.L.; de Lima, J.S.; Cheida, C.C.; Lemos, F.G.; de Azevedo, F.C.; Arrais, R.C.; Bilac, D.; Herrera, H.M.; Mourão, G.; et al. Trypanosoma cruzi infection in neotropical wild carnivores (Mammalia: Carnivora): At the top of the T. cruzi transmission chain. PLoS ONE 2013, 8, 67463. [Google Scholar] [CrossRef] [PubMed]
- Herrera, H.M.; Lisboa, C.V.; Pinho, A.P.; Olifiers, N.; Bianchi, R.C.; Rocha, F.L.; Jansen, A.M. The coati (Nasua nasua, Carnivora, Procyonidae) as a reservoir host for the main lineages of Trypanosoma cruzi in the Pantanal region, Brazil. Trans. R. Soc. Trop Med. Hyg. 2008, 102, 1133–1139. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; López-Díaz, O.; Bello-Bedoy, R.; Villalobos, G.; Muñoz-García, C.I.; Alejandre-Aguilar, R.; Córdoba-Aguilar, A.; Gutiérrez-Cabrera, A.; Suzán, G.; Villanueva-García, C.; et al. Possible differences in the effects of Trypanosoma cruzi on blood cells and serum protein of two wildlife reservoirs. Vector Borne Zoonotic Dis. 2016, 16, 709–716. [Google Scholar] [CrossRef]
- Cardoso, M.S.; Reis-Cunha, J.L.; Bartholomeu, D.C. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front. Immunol. 2016, 6, 659. [Google Scholar] [CrossRef]
- Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; Dándrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in small mammals of an agroecosystem in Central Brazil: Another piece in the puzzle of parasite transmission in an anthropogenic landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]
- Damodar, T.; Mani, R.S.; Prathyusha, P.V. Utility of rabies neutralizing antibody detection in cerebrospinal fluid and serum for ante-mortem diagnosis of human rabies. PLoS Negl. Trop. Dis. 2019, 13, e0007128. [Google Scholar] [CrossRef]
- D’Ávila, D.A.; Galvao, L.M.C.; Sousa, G.R.; Britto, C.; Moreira, O.C.; Chiari, E. Monitoring the parasite load in chronic Chagas disease patients: Comparison between blood culture and quantitative real time PCR. PLoS ONE 2018, 13, e0208133. [Google Scholar] [CrossRef]
- Risueño, J.; Bermejo, E.; Muñoz-García, C.I.; Chitimia, I.; del Río, L.; García-Martínez, J.D.; Goyena, E.; Fisa, R.; Riera, C.; Jiménez-Montalbán, P.; et al. Comparative value of microscopy, serology and real time PCR in the diagnosis of asymptomatic canine Leishmania infantum infection. An. Vet. Murcia 2012, 28, 33–41. [Google Scholar] [CrossRef][Green Version]
- Chitimia, L.; Muñoz-García, C.I.; Sánchez-Velasco, D.; Lizana, V.; del Río, L.; Murcia, L.; Fisa, R.; Riera, C.; Giménez-ont, P.; Jímenez-Montalbán, P.; et al. Cryptic Leishmaniosis by Leishmania infantum, a feature of canines only? A study of natural infection in wild rabbits, humans and dogs in southern Spain. Vet. Parasitol. 2011, 181, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas’ Disease in the United State. Clin. Microbiol. Rev. 2011, 24, 655–681. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Guerrero-Ros, I.; Ma, Y.; dos Santos, F.M.; Scherer, P.E.; Gordillo, R.; Horta, A.; Macian, F.; Weiss, L.M.; Huang, H. Induction of effective immunity against Trypanosoma cruzi. Infec. Immun. 2020, 88, e00908–e00919. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Romero, N.F.; Aréchiga-Ceballos, N.; Emerson, G.L.; Martínez-Martínez, F.O.; Doty, J.B.; Nakazawa, Y.J.; Rendón-Franco, E.; Muñoz-García, C.I.; Villanueva-García, C.; Ramírez-Cid, C.; et al. Endemic orthopoxvirus circulating in procyonids in Mexico. J. Wildl. Dis. 2016, 52, 609–615. [Google Scholar] [CrossRef]
- Araújo, F.M.G.D.; Bahia, M.T.; Magalhães, N.M.D.; Martins-Filho, O.A.; Veloso, V.M.; Carneiro, C.M.; Lana, M.D. Follow-up of experimental chronic Chagas’ disease in dogs: Use of polymerase chain reaction (PCR) compared with parasitological and serological methods. Acta Trop. 2002, 81, 21–31. [Google Scholar] [CrossRef]
- Carvalho, C.M.E.; Andrade, M.C.R.; Xavier, S.S.; Mangia, R.H.R.; Britto, C.C.; Jansen, A.M.; Fernandes, O.; Lannes-Vieira, J.; Bonecini-Almeida, M.D.G. Chronic Chagas’ disease in rhesus monkeys (Macaca mulata): Evaluation of parasitemia, serology, electrocardiography, and radiology. Am. J. Trop. Med. Hyg. 2003, 68, 683–691. [Google Scholar] [CrossRef]
- Oladiran, A.; Belosevic, M. Immune evasion strategies of trypanosomes: A review. J. Parasitol. 2012, 98, 284–292. [Google Scholar] [CrossRef]
- Kurup, S.P.; Tarleton, R.L. Perpetual expression of PAMPs necessary for optimal immune control and clearance of a persistent pathogen. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Obst, R.; van Santen, H.M.; Mathis, D.; Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J. Exp. Med. 2005, 201, 1555–1565. [Google Scholar] [CrossRef]
- Rangel-Flores, H.; Sanchez, B.; Mendoza-Duarte, J.; Barnabé, C.; Breniére, F.; Ramos, C.; Espinoza, B. Serological and parasitology demonstration of Trypanosoma cruzi infections in an urban area of central Mexico: Correlation with electrocardiographic alterations. Am. J. Trop. Med. Hyg. 2001, 65, 887–895. [Google Scholar] [CrossRef][Green Version]
- Maeda, F.Y.; Clemente, T.M.; Macedo, S.; Cortez, C.; Yoshida, N. Host cell invasion and oral infection by Trypanosoma cruzi strain of genetic groups TcI and TcIV from chagasic patients. Parasite Vectors 2016, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Ruiz, R.C.; Araya, J.E.; Da Silveira, J.F.; Yoshida, N. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect. Immun. 1993, 61, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Neira, I.; Silva, F.A.; Cortez, M.; Yoshida, N. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect. Immun. 2003, 71, 557–561. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cortez, M.; Ferreira, D.; Yoshida, N. Interaction with host factors exacerbate Trypanosoma cruzi cell invasion capacity upon oral infection. Int. J. Parasitol. 2007, 37, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortiz, A. Estudio Epidemiológico de Trichinella sp y Toxoplasma gondii en Poblaciones de Prociónidos y Animales Sinantrópicos de Tabasco, México. Master’s Thesis, Universidad Nacional Autonoma de México, Mexico City, Mexico, 2016. [Google Scholar]
- Authié, E. Trypanosomiasis and trypanotolerance in cattle: A role for congopain? Parasitol. Today 1994, 10, 360–364. [Google Scholar] [CrossRef]
- Brossas, J.Y.; Gulin, J.E.N.; Bisio, M.M.C.; Chapelle, M.; Marinach-Patrice, C.; Bordessoules, M.; Palazon, R.G.; Vion, J.; Paris, L.; Altcheh, J.; et al. Secretome analysis of Trypanosoma cruzi by proteomics studies. PLoS ONE 2017, 12, e0185504. [Google Scholar] [CrossRef]
- Parodi-Talice, A.; Durán, R.; Arrambide, N.; Prieto, V.; Piñeyro, M.D.; Pritsch, O.; Cayota, A.; Cerveñansky, C.; Robello, C. Proteome analysis of the causative agent of Chagas disease: Trypanosoma cruzi. Int. J. Parasitol. 2004, 34, 881–886. [Google Scholar] [CrossRef]
- Rima, B.K.; Duffy, N.; Mitchell, W.J.; Summers, B.A.; Appel, M.J.G. Correlation between humoral immune responses and presence of virus in the CNS in dogs experimentally infected with canine distemper virus. Arch. Virol. 1991, 121, 1–8. [Google Scholar] [CrossRef]
- Rendón-Franco, E.; López-Díaz, O.; Rodríguez-Espinosa, O.; Rojas-Serranía, N.; Rodríguez-Cabo-Mercado, R.; Moreno-Altamirano, M.M.; Muñoz-García, C.I.; Villanueva-García, C.; Aguilar-Setién, A. Comparative leucocyte populations between two sympatric carnivores (Nasua narica and Procyon lotor). Conserv. Physiol. 2019, 7, coz050. [Google Scholar] [CrossRef]
- Goding, J.W. Use of staphylococcal protein A as an immunological reagent. J. Immunol. Methods 1978, 20, 241–253. [Google Scholar] [CrossRef]
- Rangel-Gamboa, L.; López-García, L.; Moreno-Sánchez, F.; Hoyo-Ulloa, I.; Vega-Mémije, M.E.; Mendoza-Bazán, N.; Romero-Valdovinos, M.; Olivo-Díaz, A.; Villalobos, G.; Martínez-Hernández, F. Trypanosoma cruzi infection associated with atypical clinical manifestations during the acute phase of the Chagas disease. Parasit. Vectors 2019, 30, 506. [Google Scholar] [CrossRef] [PubMed]
| Category | Positive (n) | Prevalence (%) | CI 95% | p-Value | OR | CI95% |
|---|---|---|---|---|---|---|
| Coatis | 115(222) | 51.8 | 45.2–58.3 | |||
| Raccoons | 23(81) | 28.3 | 19.3–38.9 | 0.00 | 2.70 | 1.56–4.74 1 |
| Coati female | 73(139) | 52.5 | 44.2–60.7 | |||
| Coati male | 42(83) | 50.6 | 39.9–61.2 | 0.44 | 1.07 | 0.62–1.86 |
| Raccoon female | 13(47) | 27.6 | 16.3–41.6 | |||
| Raccoon male | 10(33) | 30.3 | 16.5–47.4 | 0.49 | 0.88 | 0.32–2.40 |
| Coati adult | 103(194) | 53 | 46.0–60.0 | |||
| Coati young | 12(28) | 42.8 | 25.6–61.4 | 0.20 | 1.50 | 0.67–3.43 |
| Raccoon adult | 20(61) | 32.7 | 21.9–45.2 | |||
| Raccoon young | 3(19) | 15.7 | 4.1–37.2 | 0.12 | 2.57 | 0.71–12.19 |
| Coati summer | 58(106) | 54.7 | 45.1–64.0 | |||
| Coati winter | 57(116) | 49.1 | 40.1–58.2 | 0.24 | 1.24 | 0.73–2.12 |
| Raccoon summer | 11(35) | 31.4 | 17.7–48.0 | |||
| Raccoon winter | 12(46) | 26 | 14.9–40.1 | 0.39 | 1.29 | 0.48–3.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, G.; Muñoz-García, C.I.; Rodríguez-Cabo-Mercado, R.; Mendoza-Bazán, N.; Hernández-Ortiz, A.; Villanueva-García, C.; Martínez-Hernández, F.; Rendón-Franco, E. Prevalence and Epitope Recognition of Anti-Trypanosoma cruzi Antibodies in Two Procyonid Species: Implications for Host Resistance. Pathogens 2020, 9, 464. https://doi.org/10.3390/pathogens9060464
Villalobos G, Muñoz-García CI, Rodríguez-Cabo-Mercado R, Mendoza-Bazán N, Hernández-Ortiz A, Villanueva-García C, Martínez-Hernández F, Rendón-Franco E. Prevalence and Epitope Recognition of Anti-Trypanosoma cruzi Antibodies in Two Procyonid Species: Implications for Host Resistance. Pathogens. 2020; 9(6):464. https://doi.org/10.3390/pathogens9060464
Chicago/Turabian StyleVillalobos, Guiehdani, Claudia I. Muñoz-García, Roberto Rodríguez-Cabo-Mercado, Nancy Mendoza-Bazán, Adrián Hernández-Ortiz, Claudia Villanueva-García, Fernando Martínez-Hernández, and Emilio Rendón-Franco. 2020. "Prevalence and Epitope Recognition of Anti-Trypanosoma cruzi Antibodies in Two Procyonid Species: Implications for Host Resistance" Pathogens 9, no. 6: 464. https://doi.org/10.3390/pathogens9060464
APA StyleVillalobos, G., Muñoz-García, C. I., Rodríguez-Cabo-Mercado, R., Mendoza-Bazán, N., Hernández-Ortiz, A., Villanueva-García, C., Martínez-Hernández, F., & Rendón-Franco, E. (2020). Prevalence and Epitope Recognition of Anti-Trypanosoma cruzi Antibodies in Two Procyonid Species: Implications for Host Resistance. Pathogens, 9(6), 464. https://doi.org/10.3390/pathogens9060464