Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape
Abstract
:1. Introduction
2. Results
2.1. Small Mammal Fauna Composition
2.2. Trypanosomatid Infection
2.3. Serological Diagnosis
3. Discussion
4. Materials and Methods
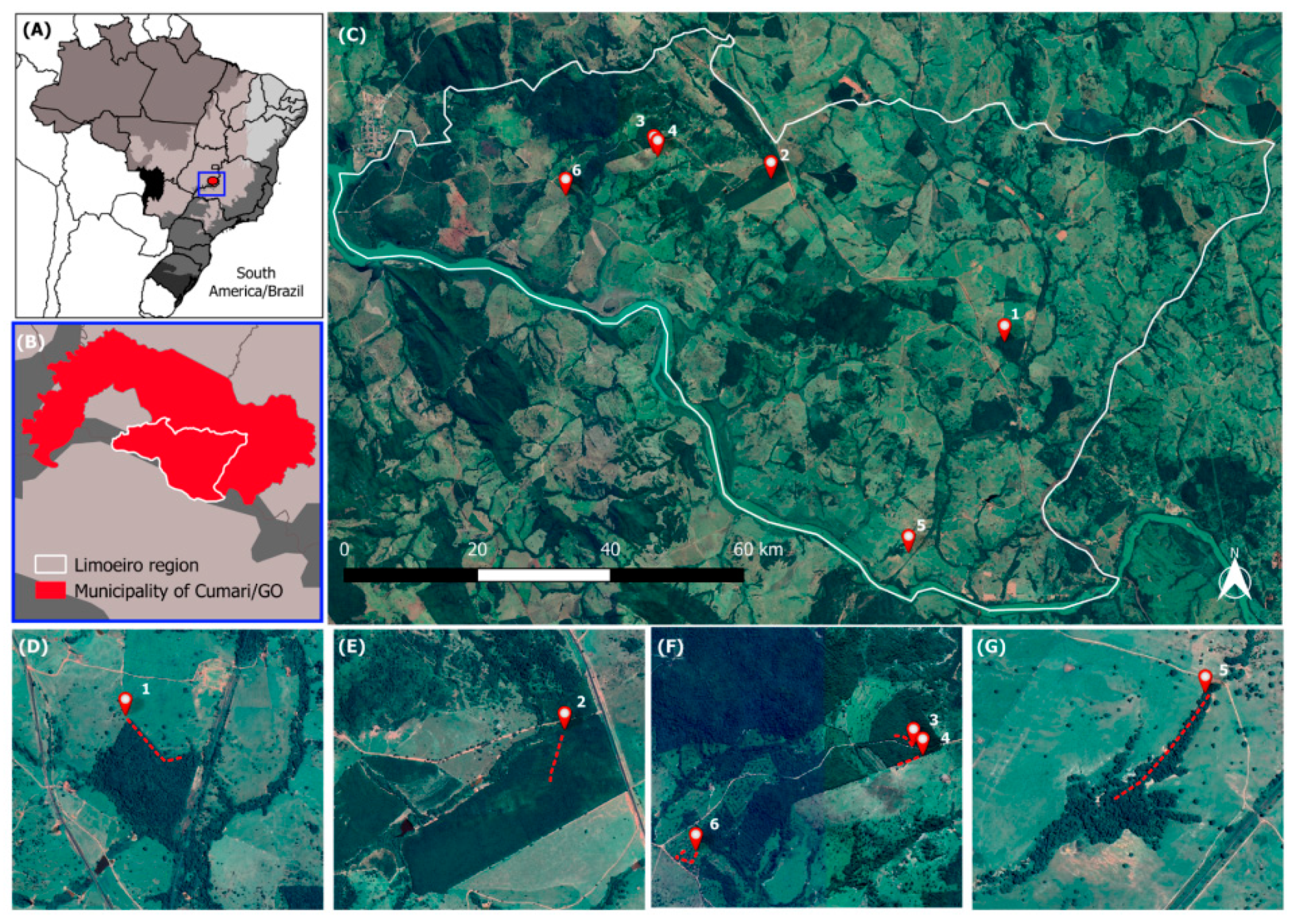
4.1. Study Area
4.2. Small Wild Mammal Capture and Identification
Field Procedures
4.3. Infection Diagnosis Procedures
4.4. Molecular Diagnosis and Characterization
4.5. Statistical Analysis
4.6. Ethics Statement
4.7. Map Construction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Leishmaniasis: Background Information. A Brief History of the Diseases; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Jansen, A.M.; Roque, A.L.R. Domestic and wild mammalian reservoir. In American Trypanosomiasis Chagas Disease—100 Years of Research, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: London, UK, 2010; pp. 249–276. [Google Scholar]
- Zingales, B.; Andrade, S.G.; Briones, M.R.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Marcili, A.; Lima, L.; Cavazzana, M.; Junqueira, A.C.; Veludo, H.H.; Maia Da, S.F.; Campaner, M.; Paiva, F.; Nunes, V.L.; Teixeira, M.M. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology 2009, 136, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors. 2018, 6, 502. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Manual de Vigilância e Controle da Leishmaniose Visceral/Ministério da Saúde; Editora do Ministério da Saúde: Brasília, Federal Disctric, Brazil, 2006; 120p. [Google Scholar]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef]
- Fraga, J.; Montalvo, A.M.; de Doncker, S.; Dujardin, J.C.; Van der Auwera, G. Phylogeny of Leishmania species based on the heat-shock protein 70 gene. Infect. Genet. Evol. 2010, 10, 238–245. [Google Scholar] [CrossRef]
- Silveira, F.T.; Souza, A.A.A.; Lainson, R.; Shaw, J.J.; Braga, R.R.; Ishikawa, E.A.Y. Cutaneous leishmaniasis in the Amazon Region: Natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominidae) by Leishmania lainsoni in Pará State, Brazil. Mem. Inst. Oswaldo Cruz. 1991, 86, 127–130. [Google Scholar] [CrossRef]
- Rangel, E.; Lainson, R. Flebotomíneos do Brasil; Editora da Fiocruz: Rio de Janeiro, Brasil, 2006; p. 360. [Google Scholar]
- Lainson, R. Espécies neotropicais de Leishmania: Uma breve revisão histórica sobre sua descoberta, ecologia e taxonomia. Rev. Pan-Amaz. Saúde. 2010, 2, 13–32. [Google Scholar]
- Cantanhêde, L.M.; Mattos, C.B.; Ronconi, C.S.; Filgueira, C.P.B.; Silva Júnior, C.F.S.; Limeira, C.; Silva, H.P.J.; Ferreira, G.E.M.; Porrozzi, R.; Ferreira, R.G.M.; et al. First report of Leishmania (Viannia) lindenbergi causing tegumentary leishmaniasis in the Brazilian western Amazon region. Parasite 2019, 26, 1–5. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Ratter, J.T. Vegetation physiognomies and woody flora of the cerrado biome. In The Cerrados of Brazil: Ecology and Natural History of Neotropical Savanna; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 91–120. [Google Scholar]
- Mittermeier, R.A.; Gil, R.P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions, 2nd ed.; University of Chicago Press: Boston, MA, USA, 2005; p. 200. [Google Scholar]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Carvalho, F.M.V.; Marco-Júnior, P.; Ferreira, L.G. The Cerrado into-pieces: Habitat fragmentation as a function of landscape use in the savannas of central Brazil. Biol. Cons. 2009, 142, 1329–1403. [Google Scholar] [CrossRef]
- Paglia, A.P.; Fonseca, G.A.B.; Rylands, A.B.; Herrmann, G.; Aguiar, L.M.S.; Chiarello, A.G.; Leite, Y.L.R.; Costa, L.P.; Siciliano, S.; Kierulff, M.C.M.; et al. Lista anotada dos mamíferos do Brasil. In Occasional Papers in Conservation Biology, 2nd ed.; Conservação Internacional: Belo Horizonte, Brasil, 2012; p. 88. [Google Scholar]
- Roque, A.L.R.; Jansen, A.M. Importância dos animais domésticos sentinelas na identificação de áreas de risco de emergência de doença de Chagas. Rev. Soc. Bras. Med. Trop. 2008, 41, 191–193. [Google Scholar]
- Facure, K.G.; Giaretta, A.A.; Monteiro-Filho, E.L.A. Food habits of the crab-eating fox, Cerdocyon thous, in an altitudinal forest of the Mantiqueira Range, southeastern Brazil. Mammalia 2003, 67, 503–511. [Google Scholar] [CrossRef]
- Kotviski, B.M.; Giaretta, K.G.F.; Azevedo, F.C.; Freitas-Junior, M.C.; Lemos, F.G. Trophic niche overlap and resource partitioning among wild canids in an anthropized neotropical ecotone. J. Neotrop. Mamm. 2019, in press. [Google Scholar]
- Rocha, F.L.; Roque, A.L.R.; de Lima, J.S.; Cheida, C.C.; Lemos, F.G.; Azevedo, F.C.; Arrais, R.C.; Bilac, D.; Herrera, H.M.; Mourão, G.; et al. Trypanosoma cruzi Infection in Neotropical Wild Carnivores (Mammalia: Carnivora): At the Top of the T. cruzi Transmission Chain. PLoS ONE 2013, 8, e67463. [Google Scholar] [CrossRef]
- Barros, J.H.S.; Xavier, S.C.C.; Bilac, D.; Lima, V.S.; Dario, M.A.; Jansen, A.M. Identification of novel mammalian hosts and Brazilian biome geographic distribution of Trypanosoma cruzi TcIII and TcIV. Acta Trop. 2017, 172, 173–179. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Lima, L.; Xavier, S.C.C.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Int. J. Parasitol. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Macclearn, D.; Kohler, J.; Mcgowan, K.J.; Cedeño, E.; Carbone, L.G.; Miller, D. Arboreal and Terrestrial Mammal Trapping on Gigante Peninsula, Barro Colorado Nature Monument, Panama. Biotropica. 1994, 26, 208–213. [Google Scholar] [CrossRef]
- Santos-Filho, M.; Silva, D.J.; Sanaiotti, T.M. Variação sazonal na riqueza e na abundância de pequenos mamíferos, na estrutura da floresta e na disponibilidade de artrópodes em fragmentos florestais no Mato Grosso, Brasil. Biota Neotrop. 2008, 8, 115–121. [Google Scholar] [CrossRef]
- Vieira, M.V. Dynamics of a rodent assemblage in a Cerrado of Southeast Brazil. Rev. Bras. Biol. 1996, 57, 99–107. [Google Scholar]
- Quental, T.B.; Fernandez, F.A.S.; Dias, A.T.C.; Rocha, F.S. Population dynamics of the marsupial Micoureus demerarae in small fragments of Atlantic Coastal Forest in Brazil. J. Trop. Ecol. 2001, 17, 339–352. [Google Scholar] [CrossRef]
- Mello, D.A. Estudo populacional de algumas espécies de roedores do Cerrado (Norte do Município de Formosa, Goiás). Rev. Bras. Biol. 1980, 40, 843–860. [Google Scholar]
- Alho, C.J.R.; Strüssmann, C.; Volpe, M.; Sonoda, F.; Marques, A.A.B.; Schneider, M.; Santos Junior, T.S.; Marque, S.R. Conservação da Biodiversidade da Bacia do Alto Paraguai—Monitoramento da Fauna Sob Impacto Ambiental; Editora UNIDERP: Campo Grande, Brasil, 2003; p. 449. [Google Scholar]
- Reis, N.R.; Peracchi, A.L.; Pedro, W.A.; Lima, I.P. Mamíferos do Brasil; Biblioteca Central da Universidade Estadual de Londrina: Paraná, Brasil, 2006; p. 439. [Google Scholar]
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Llewellyn, M.S.; Lewis, M.D.; Yeo, M.; Baleela, R.; Fitzpatrick, S.; Gaunt, M.W.; Mauricio, I.L. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: Looking back and to the future. Parasitology 2009, 136, 1509–1528. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef]
- Lima, V.S.; Xavier, S.C.C.; Maldonado, I.F.R.; Roque, A.L.R.; Vicente, A.C.P.; Jansen, A.M. Expanding the knowledge of the geographic distribution of Trypanosoma cruzi TcII and TcV/TcVI genotypes in the Brazilian Amazon. PLoS ONE. 2014, 9, e116137. [Google Scholar] [CrossRef]
- Herrera, C.P.; Licon, M.H.; Nation, C.S.; Jameson, S.B.; Wesson, D.M. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors 2015, 8, 123. [Google Scholar] [CrossRef]
- Miles, M.A.; Povoa, M.M.; de Souza, A.A.; Lainson, R.; Shaw, J.J.; Ketteridge, D.S. Chagas’s disease in the Amazon Basin: Ii. The distribution of Trypanosoma cruzi zymodemes 1 and 3 in Para’ State, north Brazil. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 667–674. [Google Scholar] [CrossRef]
- Lisboa, C.V.; Xavier, S.C.; Herrera, H.M.; Jansen, A.M. The ecology of the Trypanosoma cruzi transmission cycle: Dispersion of zymodeme 3 (Z3) in wild hosts from Brazilian biomes. Vet. Parasitol. 2009, 165, 19–24. [Google Scholar] [CrossRef]
- Madeira, M.F.; Almeida, A.B.; Barros, J.H.; Oliveira, T.S.; Sousa, V.R.; Alves, A.S.; Miranda, L.F.; Schubach, A.O.; Marzochi, M.C. Trypanosoma caninum, a new parasite described in dogs in Brazil: Aspects of natural infection. J. Parasitol. 2014, 100, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Capewell, P.; Cren-Travaillé, C.; Marchesi, F.; Johnston, P.; Clucas, C.; Benson, R.A.; Gorman, T.A.; Calvo-Alvarez, E.; Crouzols, A.; Jouvion, G.; et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Francisco, A.F.; Jayawardhana, S.; Langston, H.; Taylor, M.C.; Kelly, J.M. Imaging the development of chronic Chagas disease after oral transmission. Sci Rep. 2018, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.P.; Cortez, M.; Maeda, F.Y.; Fernandes, M.C.; Haapalainen, E.F.; Yoshida, N.; Mortara, R.A. Unique behavior of Trypanosoma dionisii interacting with mammalian cells: Invasion, intracellular growth and nuclear localization. Acta Trop. 2009, 110, 65–74. [Google Scholar] [CrossRef]
- Dario, M.A.; Rodrigues, M.S.; Barros, J.H.; Xavier, S.C.; D’Andrea, P.S.; Roque, A.L.R.; Jansen, A.M. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espírito Santo state, Brazil). Parasit. Vectors 2016, 9, 477. [Google Scholar] [CrossRef]
- De Araújo, V.A.; Boité, M.C.; Cupolillo, E.; Jansen, A.M.; Roque, A.L. Mixed infection in the anteater Tamandua tetradactyla (Mammalia: Pilosa) from Pará State, Brazil: Trypanosoma cruzi, T. rangeli and Leishmania infantum. Parasitology 2013, 140, 455–460. [Google Scholar] [CrossRef]
- Stoco, P.H.; Talavera-López, C.W.G.; Gerber, A.; Zaha, A.; Thompson, C.E. Genome of the Avirulent Human-Infective Trypanosome—Trypanosoma rangeli. PLoS Negl. Trop. Dis. 2014, 8, 1–17. [Google Scholar] [CrossRef]
- Quaresma, P.F.; Rego, F.D.; Botelho, H.A.; da Silva, S.R.; Moura Junior, A.J.; Neto, R.G.T.; Madeira, F.M.; Carvalho, M.B.; Paglia, A.P.; Melo, M.N.; et al. Wild, synanthropic and domestic hosts of Leishmania in an endemic area of cutaneous leishmaniasis in Minas Gerais State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 579–585. [Google Scholar] [CrossRef]
- Arias, J.R.; Naiff, R.D.; Miles, M.A.; de Souza, A.A. The opossum, Didelphis marsupialis (Marsupialia: Didelphidae), as a reservoir host of Leishmania braziliensis guyanensis in the Amazon Basin of Brazil. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 537–541. [Google Scholar] [CrossRef]
- Dedet, J.P.; Gay, F.; Chatenay, G. Isolation of Leishmania species from wild mammals in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 613–615. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Pirmez, C.; Pires, M.Q.; Brazil, R.P.; Pacheco, R.S. PCR-based diagnosis for detection of Leishmania in skin and blood of rodents from an endemic area of cutaneous and visceral leishmaniasis in Brazil. Vet. Parasitol. 2005, 129, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Spotin, A.; Parvizi, P. Comparative study of viscerotropic pathogenicity of Leishmania major amastigotes and promastigotes based on identification of mitochondrial and nucleus sequences. Parasitol. Res. 2015, 115, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Ishikawa, E.A.Y.; Silveira, F.T. American visceral leishmaniasis: Wild animal hosts. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 630–663. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Xavier, S.C.; da Rocha, M.G.; Duarte, A.C.; D’Andrea, P.S. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. [Google Scholar] [CrossRef]
- Lessa, L.G.; Costa, F.N. Diet and seed dispersal by five marsupials (Didelphimorphia, Didelphidae) in a Brazilian cerrado reserve. Mamm. Biol. 2010, 75, 10–16. [Google Scholar] [CrossRef]
- Roman, F.; Iñiguez, A.M.; Yeo, M.; Jansen, A.M. Multilocus sequence typing: Genetic diversity in Trypanosoma cruzi I (TcI) isolates from Brazilian didelphids. Parasit Vectors 2018, 11, 107. [Google Scholar] [CrossRef]
- Lemos, F.G. Ecologia e Conservação da Raposa-do-Campo (Lycalopex vetulus) e Suas Interações com Canídeos Simpátricos em Áreas Antropizadas de Cerrado do Brasil Central. Ph.D. Thesis, Universidade Federal de Uberlândia, UFU, Brasil, 2016. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes Gonçalves, J.L.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Bonvicino, C.R.; Otazu, I.B.; D’andrea, P.S. Karyologic evidence of diversification of the genus Thrichomys (Rodentia, Echimyidae). Cytogenet Genome Res. 2002, 97, 200–204. [Google Scholar] [CrossRef]
- Campos, M.P.; Silva, D.A.; Madeira, M.F.; Júnior, A.A.M.V.; Figueiredo, F.B. First autochthonous case of canine visceral leishmaniasis in Volta Redonda, Rio de Janeiro, Brazil. Ver. Bras. Parasitol. Vet. 2013, 3, 424–426. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 1626. [Google Scholar]
- Camargo, M.E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preseved culture forms of Trypanosoma cruzi in a slide test. Ver. Inst. Med. Trop. S. Paulo. 1966, 8, 227–235. [Google Scholar]
- Jansen, A.M.; Moriearty, P.L.; Castro, B.G.; Deane, M.P. Trypanosoma cruzi in the opossum Didelphis marsupialis: An indirect fluorescent antibody test for the diagnosis and follow-up of natural and experimental infections. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 474–477. [Google Scholar] [CrossRef]
- Roque, A.L.; Xavier, S.C.; Gerhardt, M.; Silva, M.F.; Lima, V.S.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Pará State, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013, 193, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, O.; Santos, S.S.; Cupolillo, E.; Mendonca, B.; Derre, R.; Junqueira, A.C.V.; Santos, L.C.; Sturm, N.R.; Naiff, R.D.; Barret, T.V.; et al. A Mini exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 97–99. [Google Scholar] [CrossRef]
- Noyes, H.A.; Stevens, J.R.; Teixeria, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binney in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef]
- Smith, A.; Clark, P.; Averis, S.; Lymbery, A.J.; Wayne, A.F.; Morris, K.D.; Tompson, R.C. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoroidae). Parasitology 2008, 135, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Breniere, S.F.; Barnabe, C. Further interest of miniexon multiplex PCR for a rapid typing of Trypanosoma cruzi DTU groups. Infect. Genet. Evol. 2011, 11, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Westenberger, S.J.; Barnabe, C.; Campbell, D.A.; Sturm, N.R. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics 2005, 171, 527–543. [Google Scholar] [CrossRef]
- Dario, M.A.; Moratelli, R.; Schwabl, P.; Jansen, A.M.; Llewellyn, M.S. Small subunit ribosomal metabarcoding reveals extraordinary trypanosomatid diversity in Brazilian bats. PLoS Negl. Trop. Dis. 2017, 11, 1–15. [Google Scholar] [CrossRef]
- Degrave, W.; Fernandes, O.; Campbell, D.; Bozza, M.; Lopes, U. Use of molecular probes and PCR for detection and typing of Leishmania—A mini-review. Mem. Inst. Oswaldo Cruz. 1994, 89, 463–469. [Google Scholar] [CrossRef]
- Cássia-Pires, R.; Boité, M.C.; D’Andrea, P.S.; Herrera, H.M.; Cupolillo, E.; Jansen, A.M.; Roque, A.L.R. Distinct Leishmania Species Infecting Wild Caviomorph Rodents (Rodentia: Hystricognathi) from Brazil. PLoS Negl. Trop. Dis. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Graça, G.C.; Volpini, A.C.; Romero, G.A.S.; Neto, M.P.O.; Hueb, M.; Porrozi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem. Inst. Oswaldo Cruz. 2012, 107, 664–667. [Google Scholar] [CrossRef] [PubMed]
| Order | Species | Expedition | |||
|---|---|---|---|---|---|
| 1st Dry and Cold | 2nd Wet and Hot | 3rd Dry and Cold | 4th Wet and Hot | ||
| Rodentia | Calomys tener | 6 | - | 2 | - |
| Calomys expulsus | 6 | - | 24 | - | |
| Rhipidomys macrurus | 2 | 2 | 3 | 2 | |
| Hylaeamys megacephalus | 1 | 1 | 2 | - | |
| Oligoryzomys mattogrossae | 1 | - | - | - | |
| Oecomys cleberi | 1 | 1 | 7 | 6 | |
| Necromys lasiurus | - | - | 1 | - | |
| Oligoryzomys nigripes | - | - | 2 | - | |
| Total rodents | 17 | 4 | 41 | 8 | |
| Didelphimorphia | Gracilinanus agilis | 17 | 1 | 30 | 22 |
| Didelphis albiventris | - | 2 | 1 | 1 | |
| Total marsupials | 17 | 3 | 31 | 23 | |
| Total of captures (n = 144)/expedition (n = 4) | 34 | 7 | 72 | 31 | |
| Expeditions/Captured Animals | 1st Expedition/34 | 2nd Expedition/7 | 3rd Expedition/72 | 4th Expedition/31 | |||
|---|---|---|---|---|---|---|---|
| Positive parasitological/molecular diagnosis | Fresh blood examination | 3 | 1 * | 3 | 3 | ||
| Hemoculture | - | 1 * | - | - | |||
| Skin, spleen or liver culture | - | - | 2 SKINS | - | |||
| Skin, spleen or liver in ethanol for kDNA-PCR | - | - | - | 4 LIVERS | |||
| Parasite identification | TcI (n = 2) and TcI/Z3 (Discrete Typing Unit - DTU TcIII/IV) (n = 1) | TcI (n = 1) | Blood: TcI/TcIV (n = 2) TcI/Z3 (DTU TcIII/IV) (n = 1) | Skin: T. rangeli (n = 1) T. dionisii (n = 1) ** | Blood: Not amplified (n = 3) | Liver: Leishmania spp. (n = 2) L. braziliensis (n = 1) L. guyanensis (n = 1) ** | |
| Mammal species | G. agilis (n = 3) | G. agilis (n = 1) | G. agilis (n = 3) | Didelphis albiventris (T. rangeli) Oecomys cleberi (T. dionisii) | G. agilis (n = 3) | C. expulsus and O. nigripes (n = 2 Leishmania spp.) G. agilis (L. braziliensis and L. guyanensis) | |
| ID of Sample | Mini Exon/Restriction Fragment Length Polymorphism Results | Similarity to genBank Sequences by the 18S rDNA Target (Coverage/Identity—%) * |
|---|---|---|
| LBCE 15978 | DTU TcI | T cruzi TcI-Reverse Stranded: 96/78.86% |
| LBCE 15979 | DTU TcI/Z3 | T cruzi TcIII/TcV-Reverse Stranded: 100/83.65% |
| LBCE 15980 | DTU TcI | T. cruzi TcI-Forward Stranded: 95/86.11% T. cruzi TcI-Reverse Stranded: 99/95.64% |
| LBCE 18574 | DTU TcI | T cruzi TcII-Foward Stranded: 97/76.38% |
| LBCE 18583 ** | DTU TcI/TcIV | T. cruzi TcIV-Forward Stranded: 100/68.03% T. cruzi TcIV-Reverse Stranded: 98/73.19% |
| LBCE 18584 ** | DTUTcI/TcIV | T. cruzi TcIV-Forward Stranded: 99/72.20% T. cruzi TcIV-Reverse Stranded: 93/74.26% |
| LBCE 18586 ** | DTU TcI/Z3 | T cruzi TcII-Reverse Stranded: 100/100% |
| Infection by | 1st Expedition | 2nd Expedition | 3rd Expedition | 4th Expedition | ||||
|---|---|---|---|---|---|---|---|---|
| Rodents | Marsupials | Rodents | Marsupials | Rodents | Marsupials | Rodents | Marsupials | |
| T. cruzi (Positive) | 1/7 (14.3%) | 4/14 (28.6%) | 1/4 (25%) | 2/3 (66.7%) | 1/37 (2.7%) | 4/30 (13.3%) | 0/7 (0%) | 0/20 (0%) |
| Leishmania spp. (Positive) | 0/7 (0%) | 1/14 (7.1%) | 0/4 (0%) | 0/3 (0%) | 2/37 (5.4%) | 1/30 (3.3%) | 0/7 (0%) | 0/20 (0%) |
| Mixed infection | 2/7 (28.6%) | 3/14 (21.4%) | 0/4 (0%) | 1/3 (33.3%) | 1/37 (2.7%) | 2/30 (6.6%) | 0/7 (0%) | 0/20 (0%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; D'Andrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens 2019, 8, 190. https://doi.org/10.3390/pathogens8040190
Brandão EMV, Xavier SCC, Carvalhaes JG, D'Andrea PS, Lemos FG, Azevedo FC, Cássia-Pires R, Jansen AM, Roque ALR. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens. 2019; 8(4):190. https://doi.org/10.3390/pathogens8040190
Chicago/Turabian StyleBrandão, Elida M. V., Samanta C. C. Xavier, Jeiel G. Carvalhaes, Paulo S. D'Andrea, Frederico G. Lemos, Fernanda C. Azevedo, Renata Cássia-Pires, Ana M. Jansen, and André L. R. Roque. 2019. "Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape" Pathogens 8, no. 4: 190. https://doi.org/10.3390/pathogens8040190
APA StyleBrandão, E. M. V., Xavier, S. C. C., Carvalhaes, J. G., D'Andrea, P. S., Lemos, F. G., Azevedo, F. C., Cássia-Pires, R., Jansen, A. M., & Roque, A. L. R. (2019). Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens, 8(4), 190. https://doi.org/10.3390/pathogens8040190







