Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba
Abstract
:1. Introduction
2. Results
2.1. Reactivation of A. culbertsoni Virulence
2.2. Schwann Cell Culture
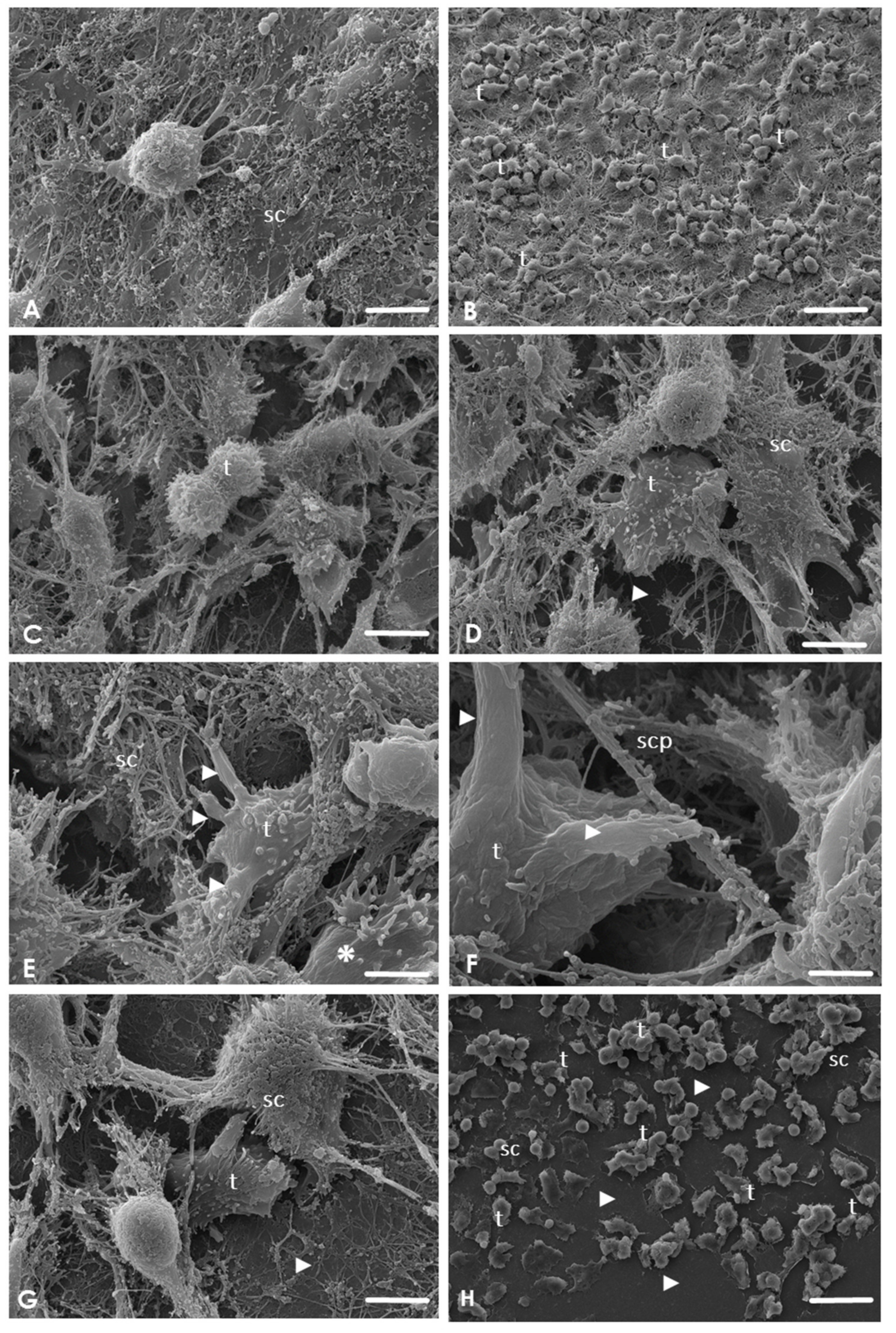
2.3. Analysis of the Interaction of A. culbertsoni Trophozoites with SC through SEM
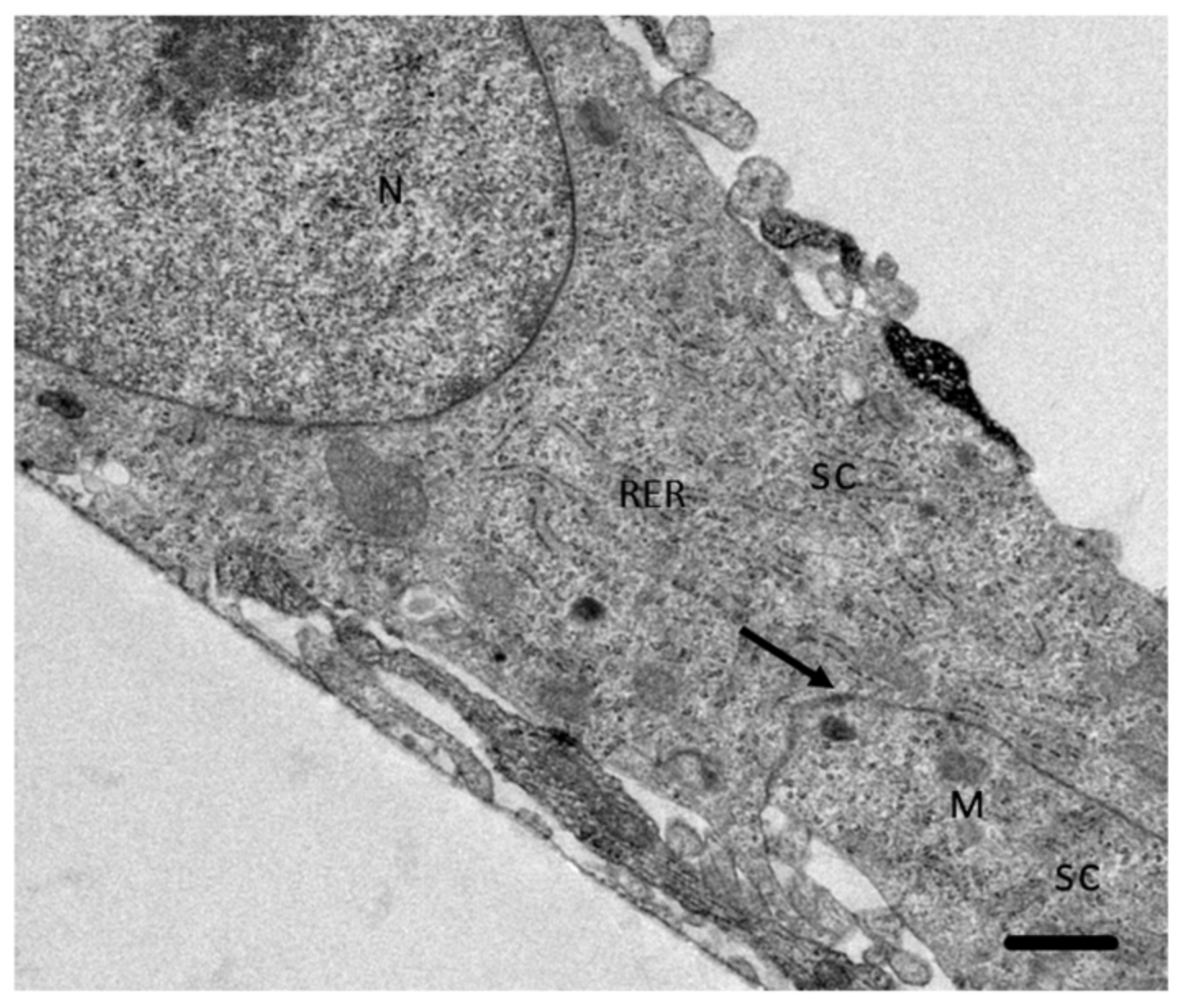
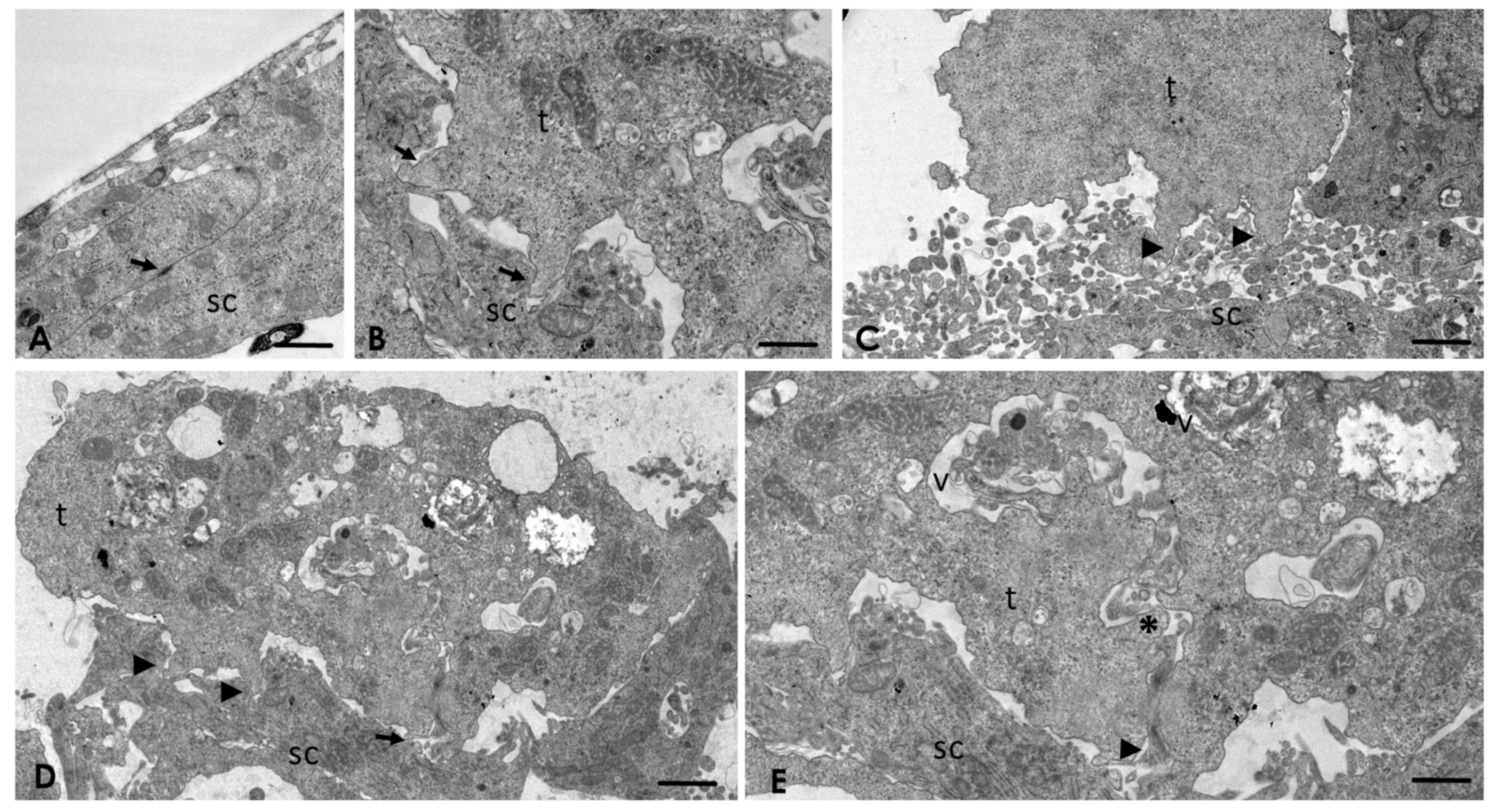
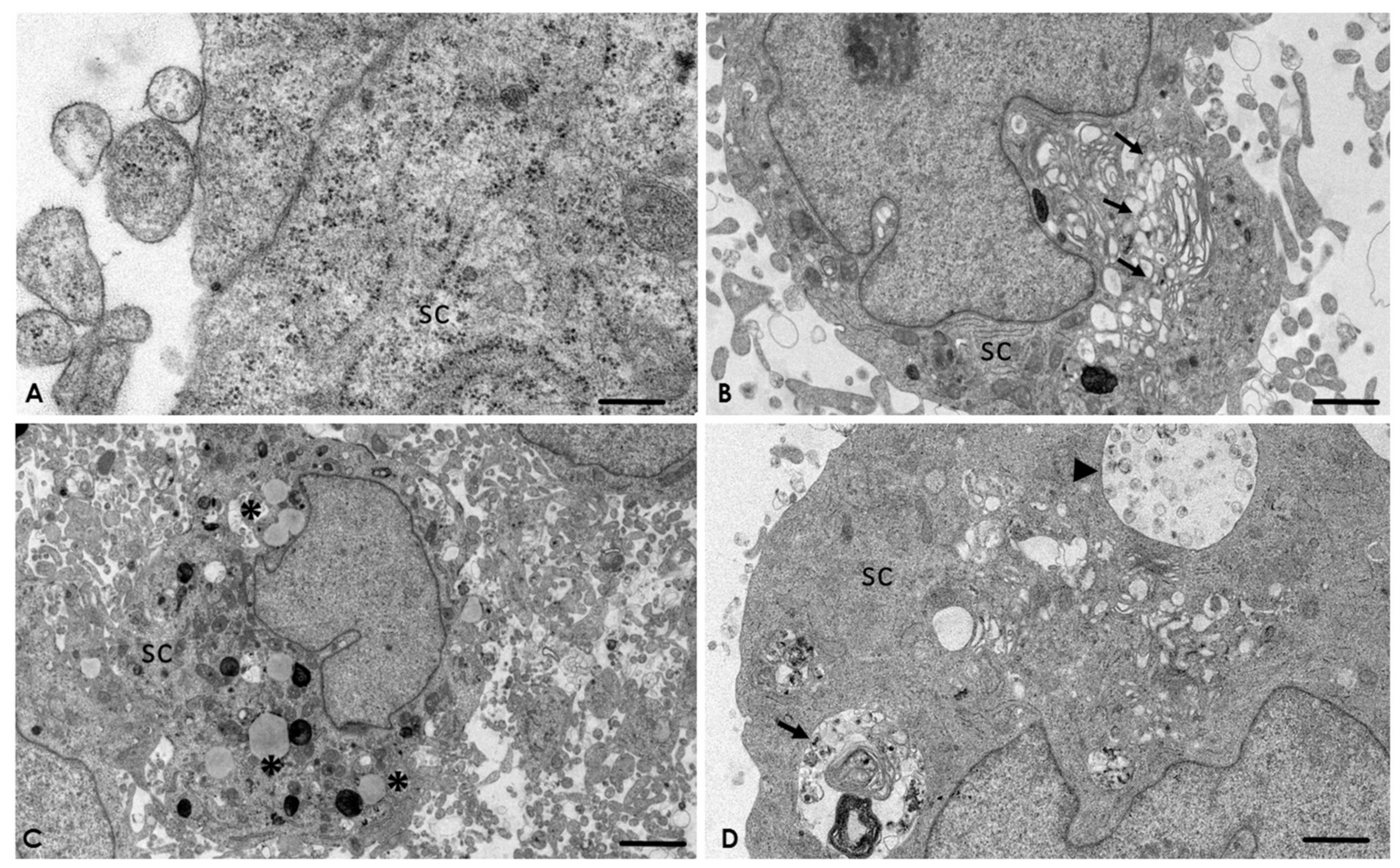
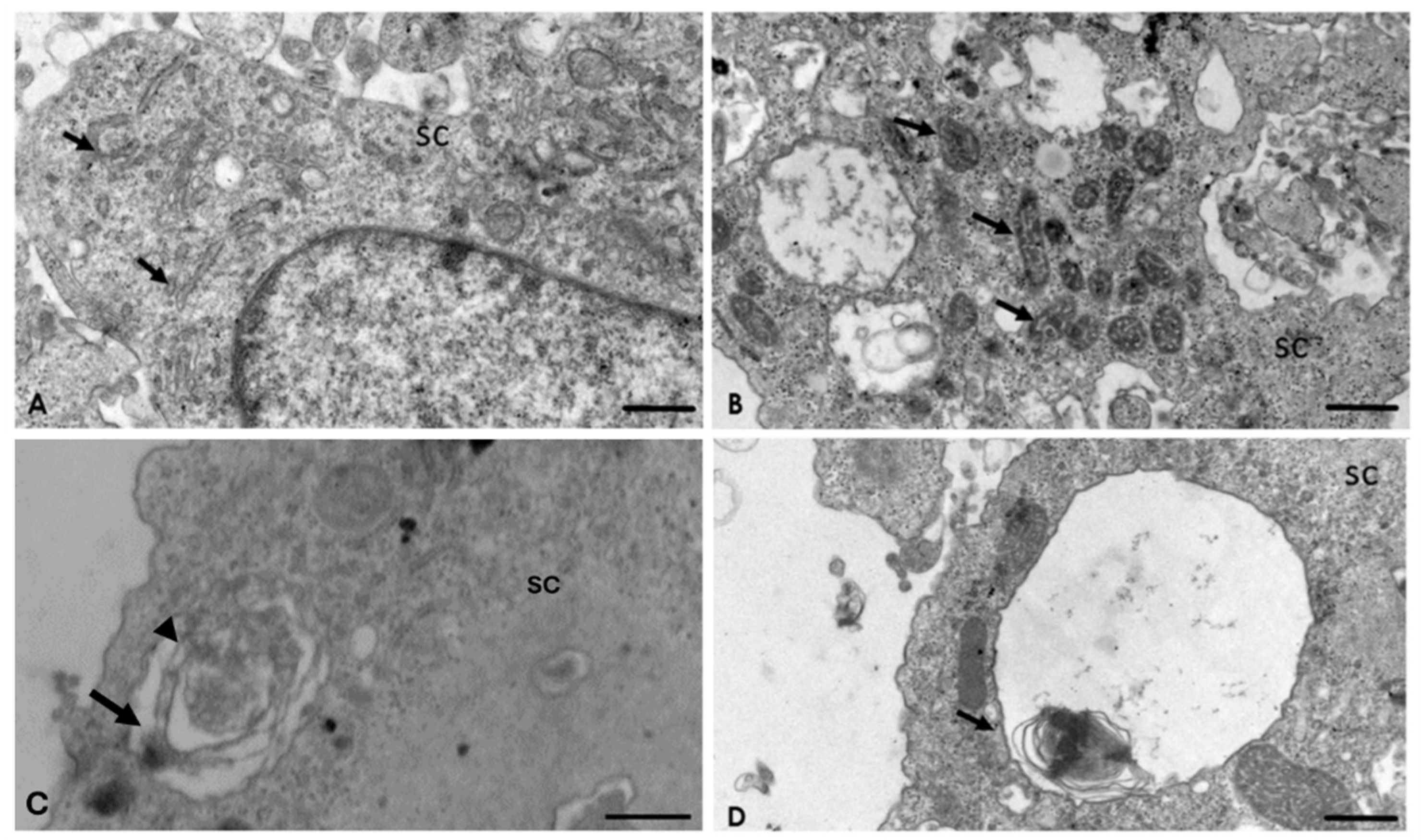
2.4. Analysis of the Interaction of A. culbertsoni Trophozoites with SC through TEM
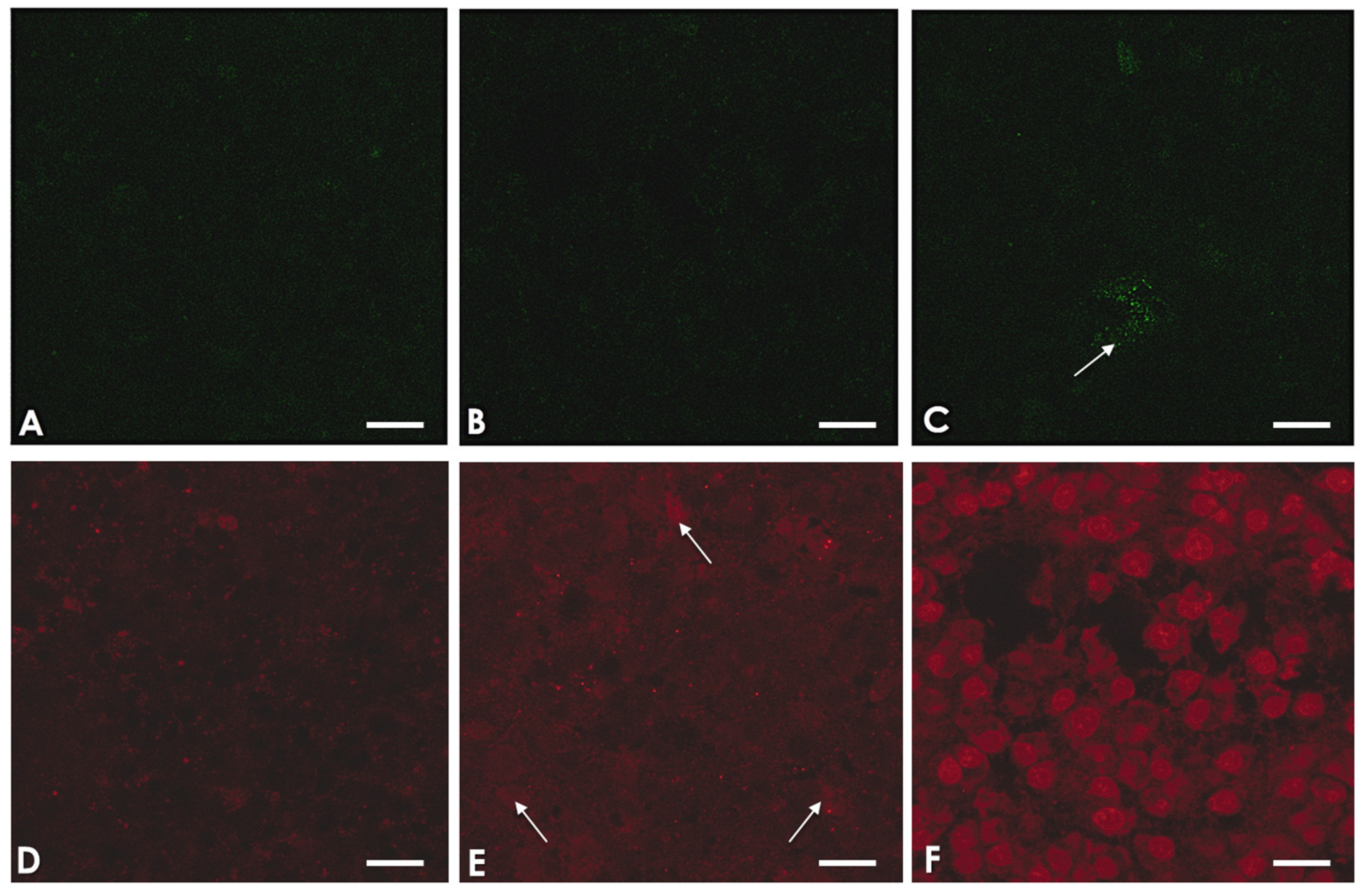
2.5. A. culbertsoni Induces Necrosis but Not Apoptosis in SC
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Amoeba Strain
5.2. Reactivation of A. culbertsoni Virulence
5.3. Schwann Cells Culture
5.4. Interaction of A. culbertsoni Trophozoites with Schwann Cell
5.5. Scanning Electron Microscopy
5.6. Transmission Electron Microscopy
5.7. Confocal Microscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bursle, E.; Robson, J. Free living amoebae and human disease. Microbiol. Aust. 2016, 37, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in Granulomatous Amebic Encephalitis. Exp. Parasitol. 2019, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S. Infections with free-living amebae. Hand. Clin. Neurol. 2013, 114, 153–168. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; Hernández-Martínez, D.; Sánchez-Rocha, R.; Cárdenas-Lemus, U.; Salinas-Lara, C.; Méndez-Cruz, A.R.; Colín-Barenque, L.; Aley-Medina, P.; Espinosa-Villanueva, J.; Moreno-Fierros, L.; et al. In vivo CNS infection model of Acanthamoeba genotype T4: The early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol. Res. 2017, 116, 725–733. [Google Scholar] [CrossRef]
- Ferrante, A. Free-living amoebae: Pathogenicity and immunity. Parasite Immunol. 1991, 13, 31–47. [Google Scholar] [CrossRef]
- Khan, N.A.; Siddiqui, R. Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int. J. Parasitol. 2009, 39, 1611–1616. [Google Scholar] [CrossRef]
- Alves, D.S.; Moraes, A.S.; Alves, L.M.; Gurgel-Gonçalves, R.; Lino Junior, R.S.; Cuba-Cuba, C.A.; Vinaud, M.C. Experimental infection of T4 Acanthamoeba genotype determines the pathogenic potential. Parasitol. Res. 2016, 115, 3435–3440. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; Sánchez-Rocha, R.; Hernández-Martínez, D.; Romero-Grijalva, M.; Salinas-Lara, C.; Rodríguez-Sosa, M.; Juárez-Avelar, I.; Salazar-Villatoro, L.; González-Robles, A.; Méndez-Cruz, A.R.; et al. Type 2 diabetes mellitus BALB/c mice are more susceptible to granulomatous amoebic encephalitis: Immunohistochemical study. Exp. Parasitol. 2017, 183, 150–159. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Cristóbal-Ramos, A.R.; González-Lázaro, M.; Salinas-Moreno, E.; Méndez-Cruz, R.; Sánchez-Cornejo, M.; De la Torre-González, E.; Martínez-Palomo, A. Acanthamoeba castellanii: Morphological analysis of the interaction with human cornea. Exp. Parasitol. 2010, 126, 73–78. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Lorenzo-Morales, J.; Cristóbal-Ramos, A.R.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Méndez Cruz, A.R.; Martínez-Palomo, A. Reevaluating the Role of Acanthamoeba Proteases in Tissue Invasion: Observation of Cytopathogenic Mechanisms on MDCK Cell Monolayers and Hamster Corneal Cells. Biomed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Flores-Maldonado, C.; González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Gallardo, J.M.; González-Lázaro, M.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Lorenzo-Morales, J.; Martínez-Palomo, A. Acanthamoeba (T4) trophozoites cross the MDCK epithelium without cell damage but increase paracellular permeability and transepithelial resistance by modifying tight junction composition. Exp. Parasitol. 2017, 183, 69–75. [Google Scholar] [CrossRef]
- Alizadeh, H.; Pidherney, M.S.; McCulley, J.P.; Niederkorn, J.Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic Free-Living Amoeba. Infect. Immun. 1994, 62, 1298–1303. [Google Scholar] [CrossRef] [Green Version]
- Pettit, D.A.; Williamson, J.; Cabral, G.A.; Marciano-Cabral, F. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: A transmission and scanning electron microscopy study. J. Parasitol. 1996, 82, 769–777. [Google Scholar] [CrossRef]
- Chusattayanond, A.D.; Boonslip, S.; Kasisit, J.; Boonmee, A.; Warit, S. Thai Acanthamoeba isolate (T4) induced apoptotic death in neuroblastoma cells via the Bax-mediated pathway. Parasitol. Int. 2010, 59, 512–516. [Google Scholar] [CrossRef]
- Shin, H.J.; Cho, M.S.; Jung, S.Y.; Kim, H.I.; Park, S.; Seo, J.H.; Yoo, J.C.; Im, K.I. Cytopathic changes in rat microglial cells induced by pathogenic Acanthamoeba culbertsoni: Morphology and cytokine release. Clin. Diagn. Lab. Immunol. 2001, 8, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Cho, M.S.; Kim, H.I.; Lee, M.; Park, S.; Sohn, S.; Im, K.I. Apoptosis of primary-culture rat microgial cells induced by pathogenic Acanthamoeba spp. Clin. Diagn. Lab. Immunol. 2000, 7, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Marciano-Cabral, F.; Ludwick, C.; Puffenbarger, R.A.; Cabral, G.A. Differential stimulation of microglial pro-inflammatory cytokines by Acanthamoeba culbertsoni versus Acanthamoeba castellanii. Eukaryot. Microbiol. 2004, 51, 462–469. [Google Scholar] [CrossRef]
- Alsam, S.; Kim, K.S.; Stins, M.; Rivas, A.O.; Sissons, J.; Khan, N.A. Acanthamoeba interactions with human brain microvascular endothelial cells. Microb. Pathog. 2003, 35, 235–241. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba invasion of the central nervous system. Int. J. Parasitol. 2007, 37, 131–138. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Li, X.; Jamaluddin, M.F.B.; Hondermarck, H. Proteomic Profile of Human Schwann Cells. Proteomics 2019, 20, e1900294. [Google Scholar] [CrossRef]
- González-Robles, A.; González-Lázaro, M.; Omaña-Molina, M.; Martínez-Palomo, A. Acanthamoeba castellanii: Endocytic Structures Involved in the Ingestion of Diverse Target Elements. Acta Protozool. 2009, 48, 327–332. [Google Scholar]
- Marciano-Cabral, F.; Cabral, G. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Microbiol. 2007, 51, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Culbertson, C.G.; Smith, J.W.; Minner, J.R. Acanthamoeba: Observations on animal pathogenicity. Science 1958, 127, 1506. [Google Scholar] [CrossRef]
- Arroyo, E.J.; Scherer, S.S. The molecular organization of myelinating Schwann cells. In The Biology of Schwann Cells: Development, Differentiation and Immunomodulation, 1st ed.; Armati, P., Ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 37–54. [Google Scholar]
- John, D.T.; Cole, T.B.; Marciano-Cabral, F. Sucker-Like Structures on the Pathogenic Amoeba Naegleria fowleri. Appl. Environ. Microbiol. 1984, 47, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Palomo, A.; González-Robles, A.; Chávez, B.; Orozco, E.; Fernández-Castelo, S.; Cervantes, A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J. Protozool. 1985, 32, 166–175. [Google Scholar] [CrossRef]
- González-Robles, A.; Lázaro-Haller, A.G.; Espinoza-Cantellano, M.; Anaya-Vázquez, F.; Martínez-Palomo, A. Trichomonas vaginalis: Ultrastructural bases of the cytophatic effect. J. Eukaryot. Microbiol. 1995, 42, 641–651. [Google Scholar] [CrossRef]
- Betanzos, A.; Bañuelos, C.; Orozco, E. Host Invasion by Pathogenic Amoebae: Epithelial Disruption by Parasite Proteins. Genes. 2019, 10, 618. [Google Scholar] [CrossRef] [Green Version]
- Burattini, S.; Falcieri, E. Analysis of cell death by electron microscopy. Methods Mol. Biol. 2013, 1004, 77–89. [Google Scholar] [CrossRef]
- Yonekawa, T.; Thorburn, A. Autophagy and Cell Death. Essays Biochem. 2013, 55, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Höhn, A.; Grune, T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, G. Lipofuscin, lipofuscin-like pigments and autofluorescence. Eur. J. Histochem. 2015, 59, 72–73. [Google Scholar] [CrossRef] [Green Version]
- Eskelinen, E.L. Autophagy: Supporting cellular and organismal homeostasis by self-eating. Int. J. Biochem. Cell Biol. 2019, 111, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskelinen, E.L. Maturation of autophagic vacuoles in Mammalian cells. Autophagy 2005, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinet, W.; Timmermans, J.P.; De Meyer, G.R. Methods to assess autophagy in situ—Transmission electron microscopy versus immunohistochemistry. Methods Enzymol. 2014, 543, 89–114. [Google Scholar] [CrossRef]
- Eskelinen, E.L.; Reggiori, F.; Baba, M.; Kovacs, A.L.; Seglen, P.O. Seeing is believing: The impact of electron microscopy on autophagy reserch. Autophagy 2011, 7, 935–956. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Uno, T.; Goto, T.; Zhang, W.; Hill, J.M.; Ohashi, Y. Pathogenic Acanthamoeba induces apoptosis of human corneal epithelial cells. Jpn. J. Ophthalmol. 2004, 48, 23–29. [Google Scholar] [CrossRef]
- Sissons, J.; Kim, K.S.; Stins, M.; Jayasekera, S.; Alsam, S.; Khan, N.A. Acanthamoeba castellanii induces host cell death via Phosphatidylinositol 3-kinasa-dependent mechanism. Infect. Immun. 2005, 73, 2704–2708. [Google Scholar] [CrossRef] [Green Version]
- Takaoka-Sugihara, N.; Yamagami, S.; Yokoo, S.; Matsubara, M.; Yagita, K. Cytophatic effect of Acanthamoeba on human corneal fibroblasts. Mol. Vis. 2012, 18, 2221–2228. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Huerta, S.; Goulet, E.J.; Huerta-Yepez, S.; Livingston, E.H. Screening and detection of apoptosis. J. Surg. Res. 2007, 139, 143–156. [Google Scholar] [CrossRef]
- Neal, J.W.; Gasque, P. The role of primary infection of Schwann cells in the aetiology of infective inflammatory neuropathies. J. Infect. 2016, 73, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.G.; Smith, J.W.; Cohen, H.K.; Minner, J.R. Experimental Infection of Mice and Monkeys by Acanthamoeba. Am. J. Pathol. 1959, 35, 185–197. [Google Scholar] [PubMed]
- Luo, X.; Chen, B.; Zheng, R.; Lin, P.; Li, J.; Chen, H. Hydrogen peroxide induces apoptosis through the mitochondrial pathway in rat Schwann cells. Neurosci. Lett. 2010, 485, 60–64. [Google Scholar] [CrossRef] [PubMed]
| Mice | Day of Death (d)/Sacrifice (s) | Organs | |
|---|---|---|---|
| Brain | Lungs | ||
| 1 | 7 (d) | + | + |
| 2 | 7 (d) | + | + |
| 3 | 21 (s) | − | + |
| 4 | 21 (s) | − | + |
| 5 | 21 (s) | − | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelan-Ramírez, I.; Salazar-Villatoro, L.; Chávez-Munguía, B.; Salinas-Lara, C.; Sánchez-Garibay, C.; Flores-Maldonado, C.; Hernández-Martínez, D.; Anaya-Martínez, V.; Ávila-Costa, M.R.; Méndez-Cruz, A.R.; et al. Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba. Pathogens 2020, 9, 458. https://doi.org/10.3390/pathogens9060458
Castelan-Ramírez I, Salazar-Villatoro L, Chávez-Munguía B, Salinas-Lara C, Sánchez-Garibay C, Flores-Maldonado C, Hernández-Martínez D, Anaya-Martínez V, Ávila-Costa MR, Méndez-Cruz AR, et al. Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba. Pathogens. 2020; 9(6):458. https://doi.org/10.3390/pathogens9060458
Chicago/Turabian StyleCastelan-Ramírez, Ismael, Lizbeth Salazar-Villatoro, Bibiana Chávez-Munguía, Citlaltepetl Salinas-Lara, Carlos Sánchez-Garibay, Catalina Flores-Maldonado, Dolores Hernández-Martínez, Verónica Anaya-Martínez, María Rosa Ávila-Costa, Adolfo René Méndez-Cruz, and et al. 2020. "Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba" Pathogens 9, no. 6: 458. https://doi.org/10.3390/pathogens9060458






