Local Lung Immune Response to Mycobacterium bovis Challenge after BCG and M. bovis Heat-Inactivated Vaccination in European Badger (Meles meles)
Abstract
1. Introduction
2. Results
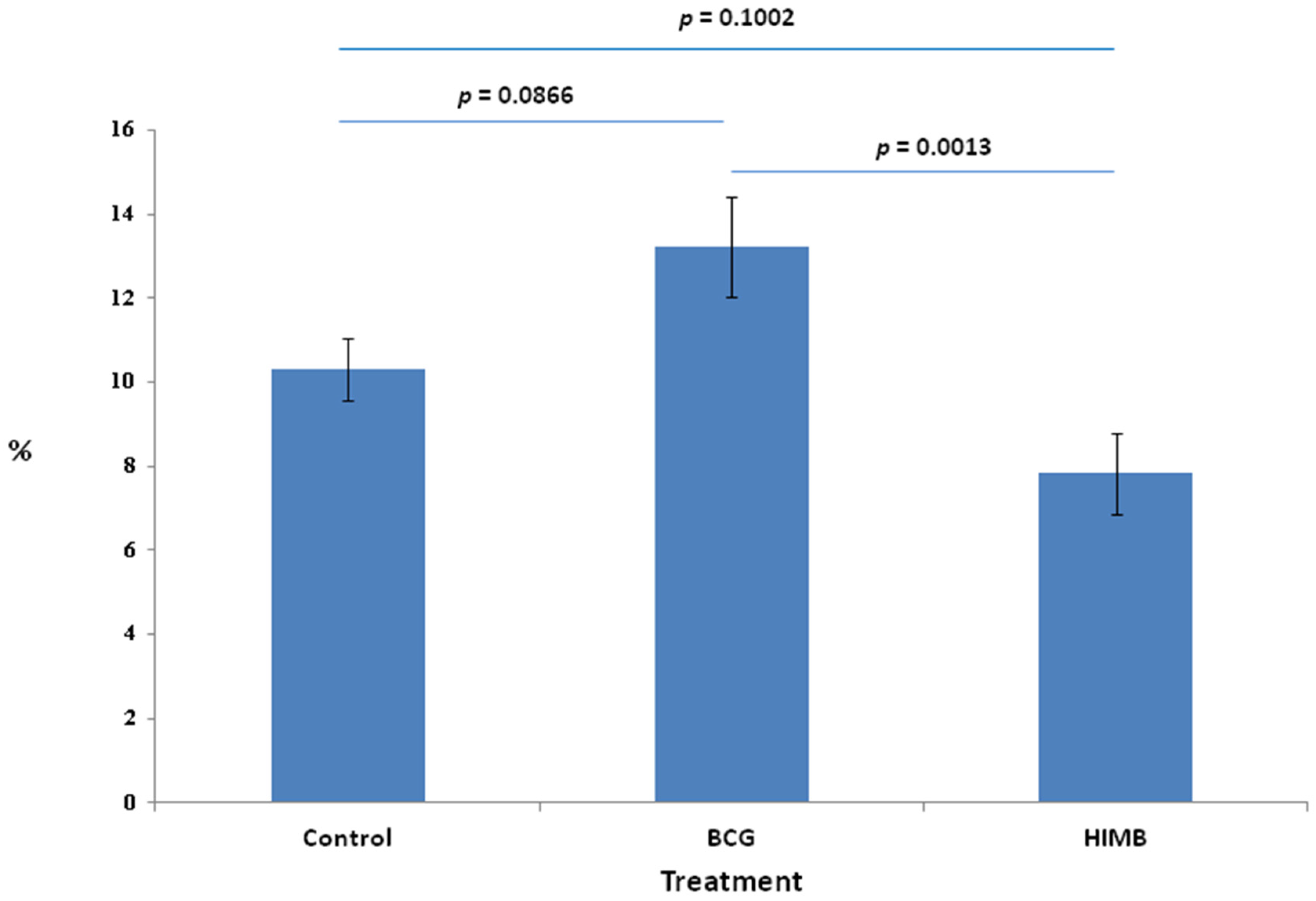
2.1. Proportion of Total Cells in Treatment Groups
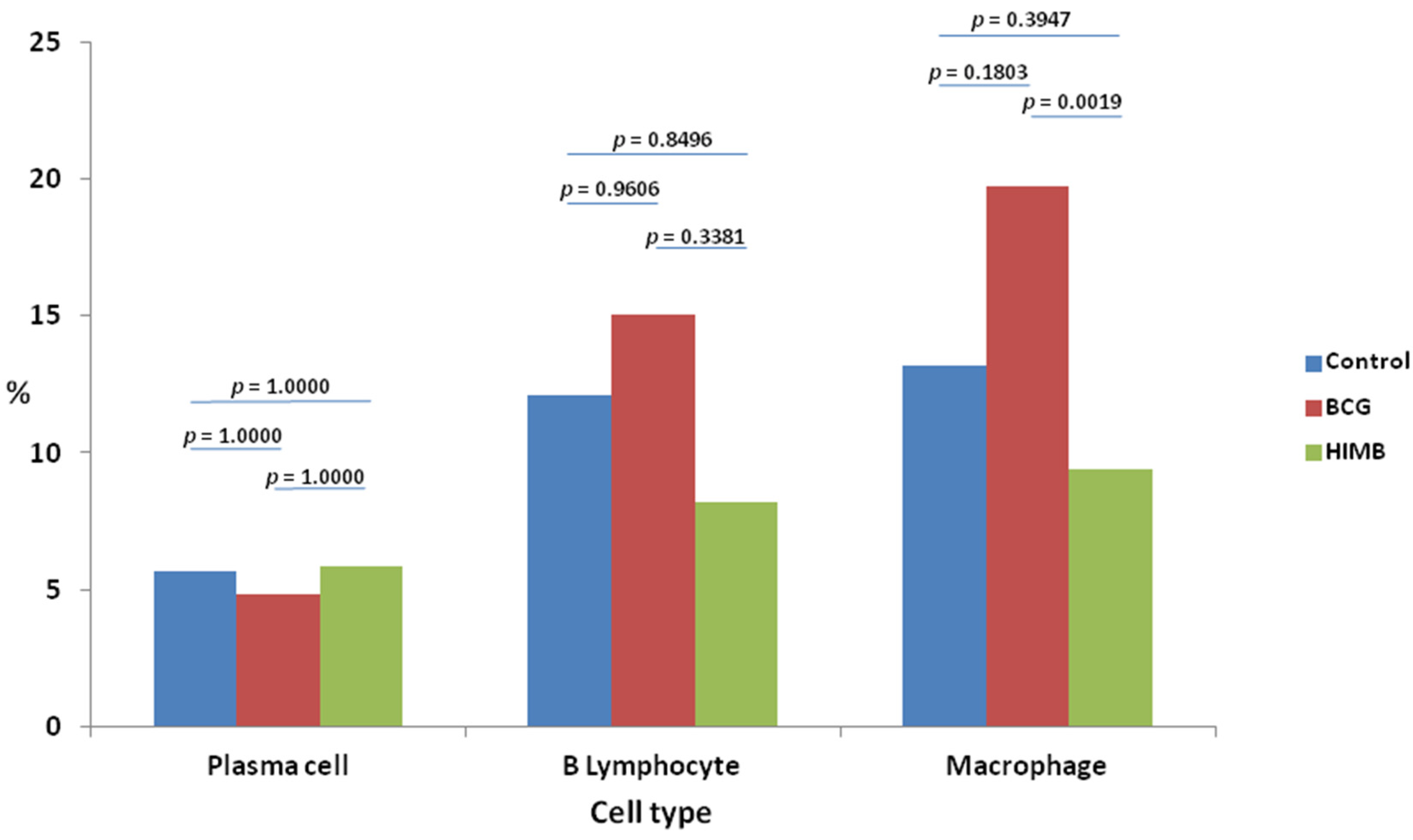
2.2. Cell Types and Differences within Treatment Groups
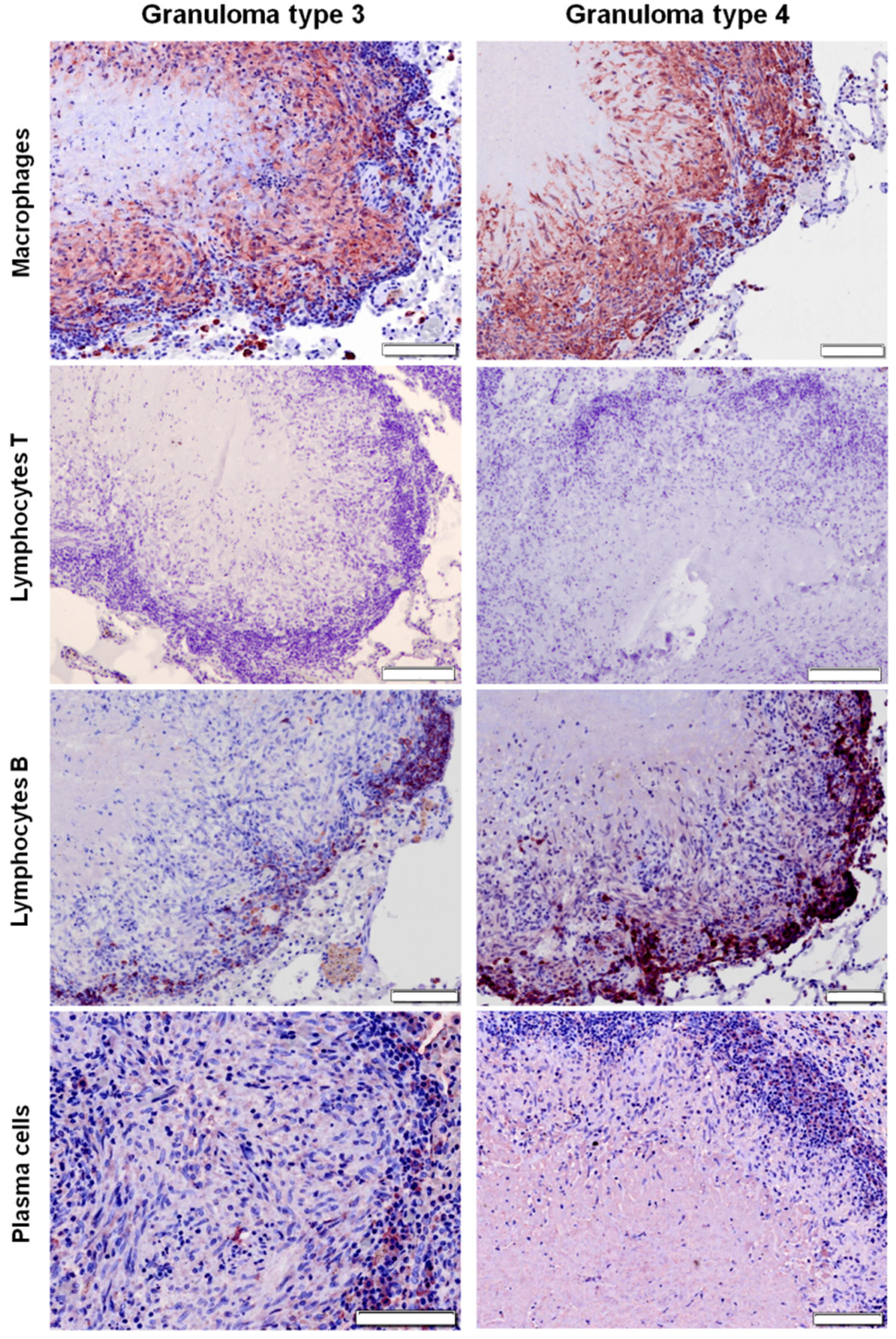
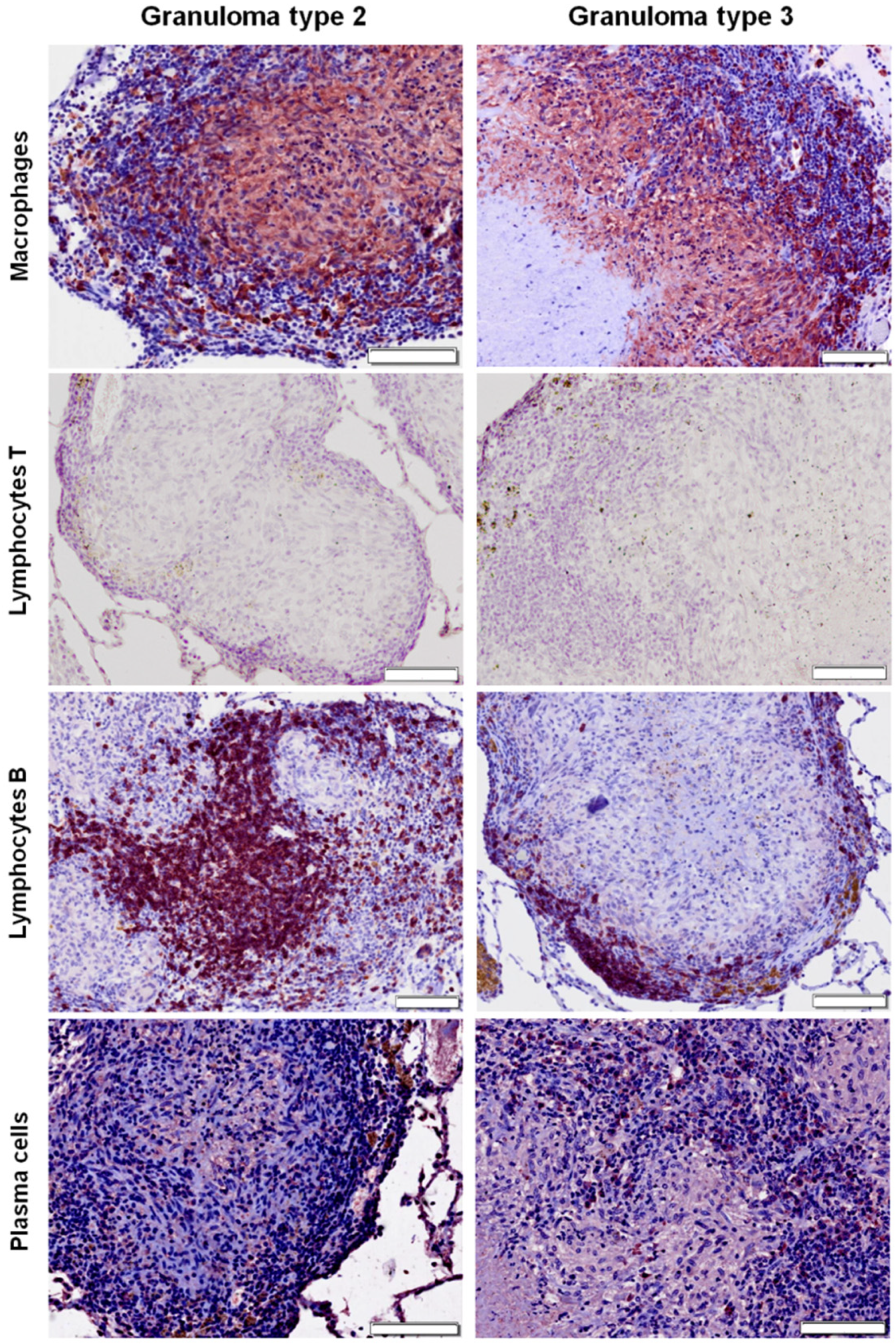
2.3. Distribution of Cells according to Granuloma Type (1 to 4)
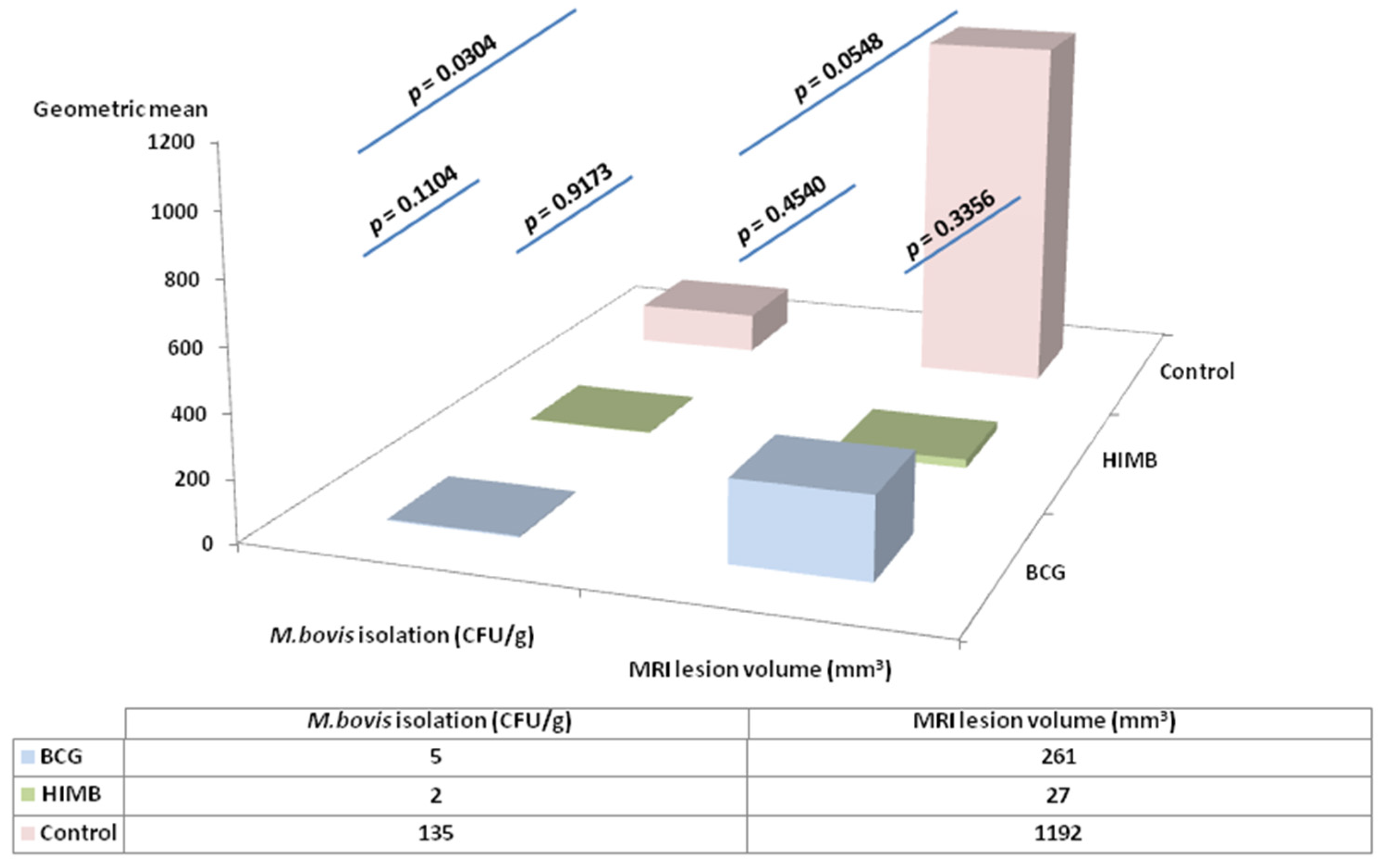
2.4. Statistical Analysis in Relation with Local Response in Lung
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals and Tissue Samples
4.3. Histopathological, Bacteriological and Magnetic Resonance Imaging (MRI) Data
4.4. Immunohistochemistry (IHC)
4.5. Image Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vordermeier, H.M.; Jones, G.J.; Buddle, B.M.; Hewinson, R.G.; Villarreal-Ramos, B. Bovine tuberculosis in Cattle: Vaccines, DIVA Tests and Host Biomarker Discovery. Annu. Rev. Anim. Biosci. 2016, 4, 87–109. [Google Scholar] [CrossRef] [PubMed]
- De Val Pérez, B.; López-Soria, S.; Nofrarías, M.; Martín, M.; Vordermeier, H.M.; Villareal-Ramos, B.; Romera, N.; Escobar, M.; Solanes, D.; Cardona, P.J.; et al. Experimental model of tuberculosis in the domestic goat after endobronchial infection with Mycobacterium caprae. Clin. Vaccine Immunol. 2011, 18, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Mendoza, M.; Romero, B.; del Cerro, A.; Gortázar, C.; García-Marín, J.F.; Menéndez, S.; Moruelo, J.; de Juan, L.; Sáez, J.L.; Delahay, R.J.; et al. Sheep as a potential source of bovine TB: Epidemiology, pathology and evaluation of diagnostic techniques. Transbound. Emerg. Dis. 2015, 63, 635–646. [Google Scholar] [CrossRef]
- Jackson, R.; Cooke, M.M.; Coleman, J.D.; Morris, R.S. Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): I. An epidemiological analysis of lesion distribution. N. Z. Vet. J. 1995, 43, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Renwick, A.R.; White, P.C.; Bengis, R.G. Bovine tuberculosis in southern African wildlife: A multi-species host-pathogen system. Epidemiol. Infect. 2007, 135, 529–540. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, W.L.; Salguero, F.J.; Fernández-Llario, P.; Martínez, R.; Risco, D.; Gough, J.; Ortíz-Peláez, A.; Hermoso-de-Mendoza, J.; Gómez, L. Immunopathology of granulomas produced by Mycobacterium bovis in naturally infected wild boar. Vet. Immunol. Immunopathol. 2013, 156, 54–63. [Google Scholar] [CrossRef]
- García-Jiménez, W.L.; Fernández-Llario, P.; Gómez, L.; Benítez-Medina, J.M.; García-Sánchez, A.; Martínez, R.; Risco, D.; Gough, J.; Ortíz-Peláez, A.; Smith, N.H.; et al. Histological and immunohistochemical characterisation of Mycobacterium bovis induced granulomas in naturally infected fallow deer (Dama dama). Vet. Immunol. Immunopathol. 2012, 149, 66–75. [Google Scholar] [CrossRef]
- Corner, L.A.; Murphy, D.; Gormley, E. Mycobacterium bovis infection in the Eurasian badger (Meles meles): The disease, pathogenesis, epidemiology and control. J. Comp. Pathol. 2011, 144, 1–24. [Google Scholar] [CrossRef]
- MAPA. 2018. Available online: http://www.mapa.gob.es/gl/ganaderia/temas/sanidad-animal-higiene-ganadera/patubes2017_3_tcm37-378321.pdf (accessed on 26 March 2020).
- Corner, L.A.; Clegg, T.A.; More, S.J.; Williams, D.H.; O’Boyle, I.; Costello, E.; Sleeman, D.P.; Griffin, J.M. The effect of varying levels of population control on the prevalence of tuberculosis in badgers in Ireland. Res. Vet. Sci. 2008, 85, 238–249. [Google Scholar] [CrossRef]
- Jenkins, H.E.; Woodroffe, R.; Donnelly, C.A. The duration of the effects of repeated widespread badger culling on cattle tuberculosis following the cessation of culling. PLoS ONE 2010, 5, e9090. [Google Scholar] [CrossRef]
- Gortázar, C.; Delahay, R.J.; McDonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The status of tuberculosis in european wild mammals. Mammal Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Gormley, E.; Collins, J.D. The development of wildlife control strategies for eradication of tuberculosis in cattle in Ireland. Tuber. Lung Dis. 2000, 80, 229–236. [Google Scholar] [CrossRef]
- Gormley, E.; Costello, E. Tuberculosis and badgers: New approaches to diagnosis and control. J. Appl. Microbiol. 2003, 94, 80S–86S. [Google Scholar] [CrossRef]
- Chambers, M.A.; Carter, S.P.; Wilson, G.J.; Jones, G.; Brown, E.; Hewinson, R.G.; Vordermeier, M. Vaccination against tuberculosis in badgers and cattle: An overview of the challenges, developments and current research priorities in Great Britain. Vet. Rec. 2014, 175, 90–96. [Google Scholar] [CrossRef]
- Buddle, B.M.; Vordermeier, H.M.; Chambers, M.A.; de Klerk-Lorist, L.M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Front. Vet. Sci. 2018, 5, 259. [Google Scholar] [CrossRef]
- Nol, P.; Wehtje, M.E.; Bowen, R.A.; Robbe-Austerman, S.; Thacker, T.C.; Lantz, K.; Rhyan, J.C.; Baeten, L.A.; Juste, R.A.; Sevilla, I.A.; et al. Effects of Inactivated Mycobacterium bovis Vaccination on Molokai-Origin Wild Pigs Experimentally Infected with Virulent M. bovis. Pathogens 2020, 9, 199. [Google Scholar] [CrossRef]
- Thomas, J.; Risalde, M.A.; Serrano, M.; Sevilla, I.; Geijo, M.; Ortíz, J.A.; Fuentes, M.; Ruíz-Fons, J.F.; de la Fuente, J.; Domínguez, L.; et al. The response of red deer to oral administration oh heta-inactivated Mycobacterium bovis and challenge with a fiel strain. Vet. Microbiol. 2017, 208, 195–202. [Google Scholar] [CrossRef]
- Díez-Delgado, I.; Sevilla, I.A.; Romero, B.; Tanner, E.; Barasona, J.A.; White, A.R.; Lurz, P.W.W.; Boots, M.; de la Fuente, J.; Domínguez, L.; et al. Impact of piglet oral vaccination against tuberculosis in endemic free-ranging wild boar populations. Prev. Vet. Med. 2018, 155, 11–20. [Google Scholar] [CrossRef]
- Garrido, J.M.; Sevilla, I.A.; Beltrán-Beck, B.; Minguijón, E.; Ballesteros, C.; Galindo, R.C.; Boadella, M.; Lyashchenko, K.P.; Romero, B.; Geijo, M.V.; et al. Protection against tuberculosis in eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS ONE 2011, 6, e24905. [Google Scholar] [CrossRef]
- Beltrán-Beck, B.; Romero, B.; Sevilla, I.A.; Barasona, J.A.; Garrido, J.M.; González-Barrio, D.; Díez-Delgado, I.; Miguijón, E.; Casal, C.; Vicente, J.; et al. Assessment of an oral Mycobacterium bovis BCG vaccine and an inactivated M. bovis preparation for wild boar in terms of adverse reactions, vaccine strain survival, and uptake by nontarget species. Clin. Vaccine Immunol. 2014, 21, 12–20. [Google Scholar] [CrossRef]
- Roy, A.; Risalde, M.A.; Bezos, J.; Casal, C.; Romero, B.; Sevilla, I.; Díez-Guerrier, A.; Rodríguez-Bertos, A.; Domínguez, M.; Garrido, J.; et al. Response of goats to intramuscular vaccination with heat-killed Mycobacterium bovis and natural challenge. Comp. Immunol. Microbiol. Infect. Dis. 2018, 60, 28–34. [Google Scholar] [CrossRef]
- Arrieta-Villegas, C.; Perálvarez, T.; Vidal, E.; Puighibet, Z.; Moll, X.; Canturri, A.; Sevilla, I.A.; Espada, Y.; Juste, R.A.; Domingo, M.; et al. Efficacy of parenteral vaccination against tuberculosis with heat-inactivated Mycobacterium bovis in experimentally challenged goats. PLoS ONE 2018, 13, e0196948. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, A.; Altuzarra, R.; Vidal, E.; Moll, X.; Espada, Y.; Sevilla, I.A.; Domingo, M.; Garrido, J.M.; Juste, R.A.; Prieto, M.; et al. Assessment of BCG and inactivated Mycobacterium bovis vaccines in an experimental tuberculosis infection model in sheep. PLoS ONE 2017, 12, e0180546. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Risalde, M.A.; Contreras, M.; Mateos-Hernández, L.; Vicente, J.; Gortázar, C.; de la Fuente, J. Heat-inactivated Mycobacterium bovis protects zebrafish against mycobacteriosis. J. Fish Dis. 2018, 41, 1515–1528. [Google Scholar] [CrossRef]
- Balseiro, A.; Prieto, J.M.; Álvarez, V.; Lesellier, S.; Davé, D.; Salguero, F.J.; Sevilla, I.A.; Infantes-Lorenzo, J.A.; Garrido, J.M.; Adriaensen, H.; et al. Protective effect of oral BCG and inactivated Mycobacterium bovis vaccines in European badgers (Meles meles) experimentally infected with M. bovis. Front. Vet. Sci. 2020, 7, 41. [Google Scholar] [CrossRef]
- Jones, G.J.; Steinbach, S.; Sevilla, I.A.; Garrido, J.M.; Juste, R.; Vordermeier, H.M. Oral vaccination of cattle with heat inactivated Mycobacterium bovis does not compromise bovine TB diagnostic tests. Vet. Immunol. Immunopathol. 2016, 182, 85–88. [Google Scholar] [CrossRef]
- Manrique, W.G.; Da Silva Claudiano, G.; Pardi de Castro, M.; Petrillo, T.R.; Pereira Figueiredo, M.A.; de Andrade Belo, M.A.; Quiroga Berdeal, M.I.; Rodini de Moraes, J.E.; Ruas de Moraes, F. Expression of cellular components in granulomas inflammatory response in Piaractus mesopotamicus model. PLoS ONE 2015, 10, e0121625. [Google Scholar] [CrossRef]
- Vallejo, R.; García Marín, J.F.; Juste, R.A.; Muñoz-Mendoza, M.; Salguero, F.J.; Balseiro, A. Immunohistochemical characterization of tuberculous lesions in sheep naturally infected with Mycobacterium bovis. BMC Vet. Res. 2018, 14, 154. [Google Scholar] [CrossRef]
- Wangoo, A.; Johnson, L.; Gough, J.; Ackbar, R.; Inglut, S.; Hicks, D.; Spencer, Y.; Hewinson, G.; Vordermeier, M. Advanced granulomatous lesions in Mycobacterium bovis-infected cattel are assiciated with increased expression on type I procollagen, γδ (WC1+) T cells and CD68+ cells. J. Comp. Pathol. 2005, 133, 223–234. [Google Scholar] [CrossRef]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Vet. Pathol. 2007, 44, 863–874. [Google Scholar] [CrossRef]
- Canfield, P.J.; Day, M.J.; Gavier-Widen, D.; Hewinson, R.G.; Chambers, M.A. Immunohistochemical characterization of tuberculous and non-tuberculous lesions in naturally infected European badgers (Meles meles). J. Comp. Pathol. 2002, 126, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Gough, J.; Spencer, Y.; Hewinson, G.; Vordermeier, M.; Wangoo, A. Immunohistochemical markers augment evaluation of vaccine efficacy and disease severity in bacillus Calmette-Guerin (BCG) vaccinated cattle challenged with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2006, 111, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Gibson, S.; García-Jiménez, W.; Gough, J.; Strickland, T.S.; Vordermeier, H.M.; Villarreal-Ramos, B. Differential cell composition and cytokine expression within lymph node granulomas from BCG-vaccinated and non-vaccinated cattle experimentally infected with Mycobacterium bovis. Transbound. Emerg. Dis. 2016, 64, 1734–1749. [Google Scholar] [CrossRef]
- Zuo, Z.; Qi, F.; Yang, J.; Wang, X.; Wu, Y.; Wen, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z.B. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive déficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol. Dis. 2017, 101, 27–39. [Google Scholar] [CrossRef]
- Chung, J.Y.; Lee, E.S.; Lee, W.J.; Kim, H.H.; Min, K.J.; Lee, C. Analysis of the immunologic mechanism of intravesical Bacillus Calmette-Guérin therapy for superficial bladder tumors: Distribution and function of immune cells. J. Korean Med. Sci. 1993, 8, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Schaible, U.E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2013, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J.; Lin, P.L. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.M., Jr.; Rook, G.A.W. Pathogenesis of pulmonary tuberculosis: An interplay of tissue-damaging and macrophages activating immune-response dual mechanisms that control bacillary multiplication. In Tuberculosis Pathogenesis, Protection, and Control; Bloom, B.R., Ed.; American Society for Microbiology Press: Washington, DC, USA, 1994; pp. 459–483. [Google Scholar]
- Soares, A.P.; Scriba, T.J.; Joseph, S.; Harbacheuski, R.; Murray, R.A.; Gelderbloem, S.J.; Hawkridge, A.; Hussey, G.D.; Maecker, H.; Kaplan, G.; et al. Bacille Calmette Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 2010, 180, 3569–3577. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; Van Loenhout, J.; Ramnik, J.X.; Aaby, P.; Van der Meer, J.W.M.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2013, 6, 152–158. [Google Scholar] [CrossRef]
- Kagina, B.M.N.; Abel, B.; Scriba, T.J.; Hughes, E.J.; Keyser, A.; Soares, A.; Gamieldien, H.; Sidibana, M.; Hatherill, M.; Gelderbloem, S.; et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after Bacillus Calmette-Guérin vaccination of newborns. Am. J. Respir. Crit. Care Med. 2010, 182, 1073–1079. [Google Scholar] [CrossRef]
- Forcada Segarra, J.A. Generalidades sobre las vacunas. In Vacuna a Vacuna. Manual de Información Sobre Vacunas on-Line, 4th ed.; Pasquín, M.J.Á., Segarra, J.A.F., Eds.; Amazing books: Zaragoza, Spain, 2019. [Google Scholar]
- Chambers, M.A.; Aldwell, F.; Williams, G.A.; Palmer, S.; Gowtage, S.; Ashford, R.; Dalley, D.J.; Davé, D.; Weyer, U.; Salguero, F.J.; et al. The effect of oral vaccination with Mycobacterium bovis BCG on the development of tuberculosis in captive european badgers (Meles meles). Front. Cell. Infect. Microbiol. 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, S.; Corner, L.; Costello, E.; Sleeman, P.; Lyashchenko, K.; Greenwald, R.; Esfandiari, J.; Singh, M.; Hewinson, R.G.; Chambers, M.; et al. Antigen specific immunological responses of badgers (Meles meles) experimentally infected with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2008, 122, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, S.; Corner, L.; Costello, E.; Lyashchenko, K.; Greenwald, R.; Esfandiari, J.; Singh, M.; Hewinson, R.G.; Chambers, M.; Gormley, E. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 2009, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Krocova, Z.; Plzakova, L.; Pavkova, I.; Kubelkova, K.; Marcela, A.; Ozanic, M.; Marecic, V.; Mihelcic, M.; Santic, M. The role of B cells in an early immune response to Mycobacterium bovis. Microb. Pathog. 2020, 140, 103937. [Google Scholar] [CrossRef]
- Beltrán-Beck, B.; de la Fuente, J.; Garrido, J.M.; Aranaz, A.; Sevilla, I.; Villar, M.; Boadella, M.; Galindo, R.C.; Pérez de la Lastra, M.; Moreno-Cid, J.A.; et al. Oral vaccination with heat inactivated Mycobacterium bovis activates the complement system to protect against tuberculosis. PLoS ONE 2014, 9, e98048. [Google Scholar] [CrossRef]
- Waters, W.R.; Whelan, A.O.; Lyashchenko, K.P.; Greenwald, R.; Palmer, M.V.; Harris, B.N.; Hewinson, R.G.; Vordermeier, H.M. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 2010, 17, 247–252. [Google Scholar] [CrossRef]
- Chambers, M.A.; Lyashchenko, K.P.; Greenwald, R.; Esfandiari, J.; James, E.; Barker, L.; Jones, J.; Watkins, G.; Rolfe, S. Evaluation of a rapid serological test for the determination of Mycobacterium bovis infection in badgers (Meles meles) found dead. Clin. Vaccine Immunol. 2010, 17, 408–411. [Google Scholar] [CrossRef][Green Version]
- Lesellier, S. Immunological responses of European badgers (Meles meles) to infection with Mycobacterium bovis. Comp. Immunol. Microbiol. Infect. Dis. 2018, 61, 9–15. [Google Scholar] [CrossRef]
- Taracón, R.; Domínguez-Andrés, J.; Uranga, S.; Ferreira, A.V.; Groh, L.A.; Domenech, M.; González-Camacho, F.; Riksen, N.P.; Aguilo, N.; Yuste, J.; et al. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog. 2020, 16, e1008404. [Google Scholar]
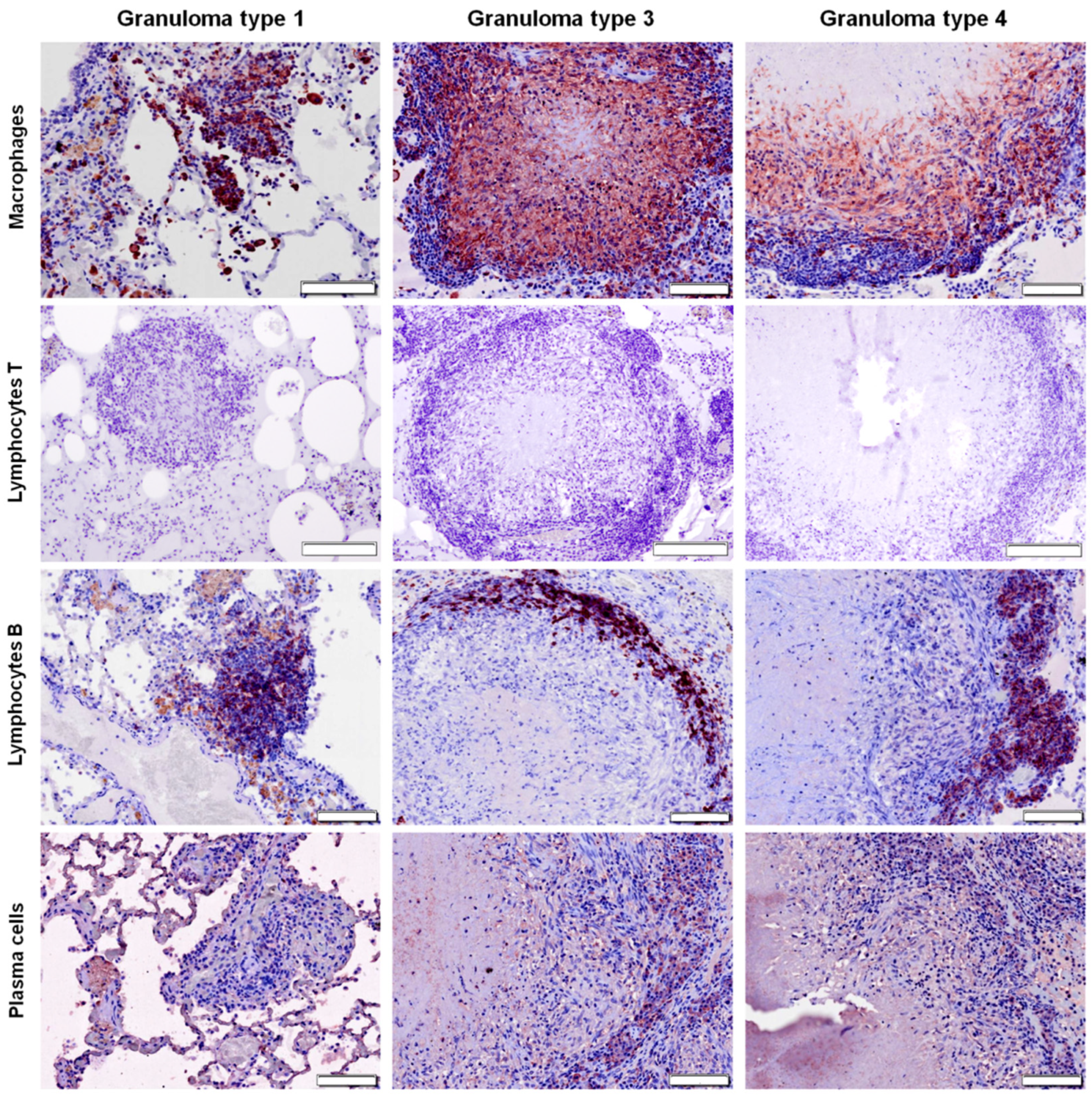
| Treatment Group | Badger ID | Macrophages (%) | T Lymphocytes (%) | B Lymphocytes (%) | Plasma Cells (%) |
|---|---|---|---|---|---|
| Control | 0369 | 21.86 | 0 | 11.242 | 9.612 |
| Control | 0866 | 7.794 | 0 | 7.524 | 6.72 |
| Control | 1464 | 25.628 | 0 | 6.82 | 3.252 |
| Control | 1661 | 3.406 | 0 | 13.48 | 2.678 |
| Control | 2191 | 6.59 | 0 | 27.3 | 2.14 |
| Control | 3959 | 12.146 | 0 | 2.736 | 5.366 |
| Control | 5225 | 8.606 | 0 | 12.962 | 5.852 |
| Control | 5360 | 28.176 | 0 | 15.856 | 6.852 |
| Control | 5338 | 13.286 | 0 | 15.018 | 5.978 |
| Control | 8248 | 7.662 | 0 | 14.362 | 8.146 |
| Control | 9761 | 8.26 | 0 | 8.146 | 6.382 |
| BCG | 2966 | 14.926 | 0 | 29.774 | 4.1125 |
| BCG | 5570 | 12.098 | 0 | 13.8 | 5.09 |
| BCG | 5179 | 25.46 | 0 | 7.966 | 6.17 |
| BCG | 9538 | 26.52 | 0 | 8.726 | 3.22 |
| HIMB | 3902 | 3.524 | 0 | 5.61 | 2.122 |
| HIMB | 4327 | 8.102 | 0 | 7.426 | 7.96 |
| HIMB | 7228 | 5.172 | 0 | 8.084 | 5.814 |
| HIMB | 7603 | 21.208 | 0 | 15.088 | 11.542 |
| HIMB | 9413 | 9.042 | 0 | 7.48 | 4.144 |
| Treatment | ID | Right Middle Lung Lobe Histology Score | Lung (Mediastine) M. bovis Isolation | Lung MRI (mm3) | |
|---|---|---|---|---|---|
| COLGRAM | SCO10 | ||||
| Control | 0369 | 4 | 50.51 | 3.92 | 638 |
| Control | 1464 | 4 | 5314.53 | 8.58 | 573 |
| Control | 2191 | 3 | 0.00 | 0.00 | 464 |
| Control | 5338 | 4 | 1860.96 | 7.53 | 3388 |
| Control | 5360 | 4 | 0.00 | 0.00 | 836 |
| Control | 0866 | 4 | 48.03 | 3.87 | 9653 |
| Control | 1661 | 4 | 110.13 | 4.70 | 9439 |
| Control | 3959 | 4 | 3400.81 | 8.13 | 16,230 |
| Control | 5225 | 4 | 746.27 | 6.62 | 4885 |
| Control | 8248 | 4 | 263.95 | 5.58 | 1982 |
| Control | 9761 | 3 | 151.52 | 5.02 | 0 |
| BCG | 2966 | 2 | 0.00 | 0.00 | 772 |
| BCG | 5179 | 3 | 0.00 | 0.00 | 1503 |
| BCG | 5770 | 3 | 428.57 | 6.06 | 4007 |
| BCG | 9538 | 2 | 0.00 | 0.00 | 0 |
| HIMB | 3902 | 1 | 0.00 | 0.00 | 0 |
| HIMB | 4327 | 3 | 0.00 | 0.00 | 0 |
| HIMB | 7228 | 4 | 53.19 | 3.97 | 0 |
| HIMB | 7603 | 4 | 0.00 | 0.00 | 3721 |
| HIMB | 9413 | 3 | 0.00 | 0.00 | 4125 |
| Primary Antibody | Epitope Demasking | Block Endogenous Peroxidase Activity | Secondary Antibody | |||||
|---|---|---|---|---|---|---|---|---|
| Description | Specificity | Source | Dilution | Description | Source | Dilution | ||
| IBA-1 | Macrophages | Wako Pure Chemical Industries, Osaka, Japan. Catalog number: 019-19741 | 1/1200 | Microwave in citrate pH 6.0 4 × 5 min | Methanol (VWR, Monroeville, PA, USA) with 3% hydrogen peroxide (Sigma-Aldrich, MO, USA) 10 min room temperature | Anti-rabbit biotinilated in goat | Vector Laboratories, Burlingame, CA, USA | 1/200 |
| CD3 | Pan T cell | Novocastra Leica, Newcastle, UK. Catalog number: NCL-L-CD3-565. Clone: LN10 | 1/400 | Microwave in citrate pH 6.0 5 × 5 min | Methanol (VWR, Monroeville, PA, USA) with 3% hydrogen peroxide (Sigma-Aldrich, MO, USA) 10 min room temperature | Anti-mouse biotinilated in horse | Vector Laboratories, Burlingame, CA, USA | 1/200 |
| CD20 | Pan B cell | ThermoFisher, Waltham, MA, USA. Catalog number: PA5-16701 | 1/500 | Microwave in citrate pH 6.0 4 × 5 min | Methanol (VWR, Monroeville, PA, USA) with 3% hydrogen peroxide (Sigma-Aldrich, MO, USA) 10 min room temperature | Anti-rabbit biotinilated in goat | Vector Laboratories, Burlingame, CA, USA | 1/200 |
| Lambda light chain | Plasma cells | Novocastra Leica, Newcastle, UK. Catalog number: NCL-L-LAM-578. Clone: SHL53 | 1/200 | Microwave in citrate pH 6.0 4 × 5 min | Destiled water with 0,5% hydrogen peroxide (Sigma-Aldrich, Saint Louis, MO, USA) 30 min room temperature in obscurity | Anti-rabbit biotinilated in goat | Vector Laboratories, Burlingame, CA, USA | 1/200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco Vázquez, C.; Prieto, M.; Barral, M.; Juste, R.A.; Lesellier, S.; Salguero, F.J.; Davé, D.; Martínez, I.Z.; de Garnica García, M.G.; Casais, R.; et al. Local Lung Immune Response to Mycobacterium bovis Challenge after BCG and M. bovis Heat-Inactivated Vaccination in European Badger (Meles meles). Pathogens 2020, 9, 456. https://doi.org/10.3390/pathogens9060456
Blanco Vázquez C, Prieto M, Barral M, Juste RA, Lesellier S, Salguero FJ, Davé D, Martínez IZ, de Garnica García MG, Casais R, et al. Local Lung Immune Response to Mycobacterium bovis Challenge after BCG and M. bovis Heat-Inactivated Vaccination in European Badger (Meles meles). Pathogens. 2020; 9(6):456. https://doi.org/10.3390/pathogens9060456
Chicago/Turabian StyleBlanco Vázquez, Cristina, Miguel Prieto, Marta Barral, Ramón Antonio Juste, Sandrine Lesellier, Francisco Javier Salguero, Dipesh Davé, Ileana Zorhaya Martínez, María Gracia de Garnica García, Rosa Casais, and et al. 2020. "Local Lung Immune Response to Mycobacterium bovis Challenge after BCG and M. bovis Heat-Inactivated Vaccination in European Badger (Meles meles)" Pathogens 9, no. 6: 456. https://doi.org/10.3390/pathogens9060456
APA StyleBlanco Vázquez, C., Prieto, M., Barral, M., Juste, R. A., Lesellier, S., Salguero, F. J., Davé, D., Martínez, I. Z., de Garnica García, M. G., Casais, R., & Balseiro, A. (2020). Local Lung Immune Response to Mycobacterium bovis Challenge after BCG and M. bovis Heat-Inactivated Vaccination in European Badger (Meles meles). Pathogens, 9(6), 456. https://doi.org/10.3390/pathogens9060456





