Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome
Abstract
1. Introduction
2. Results
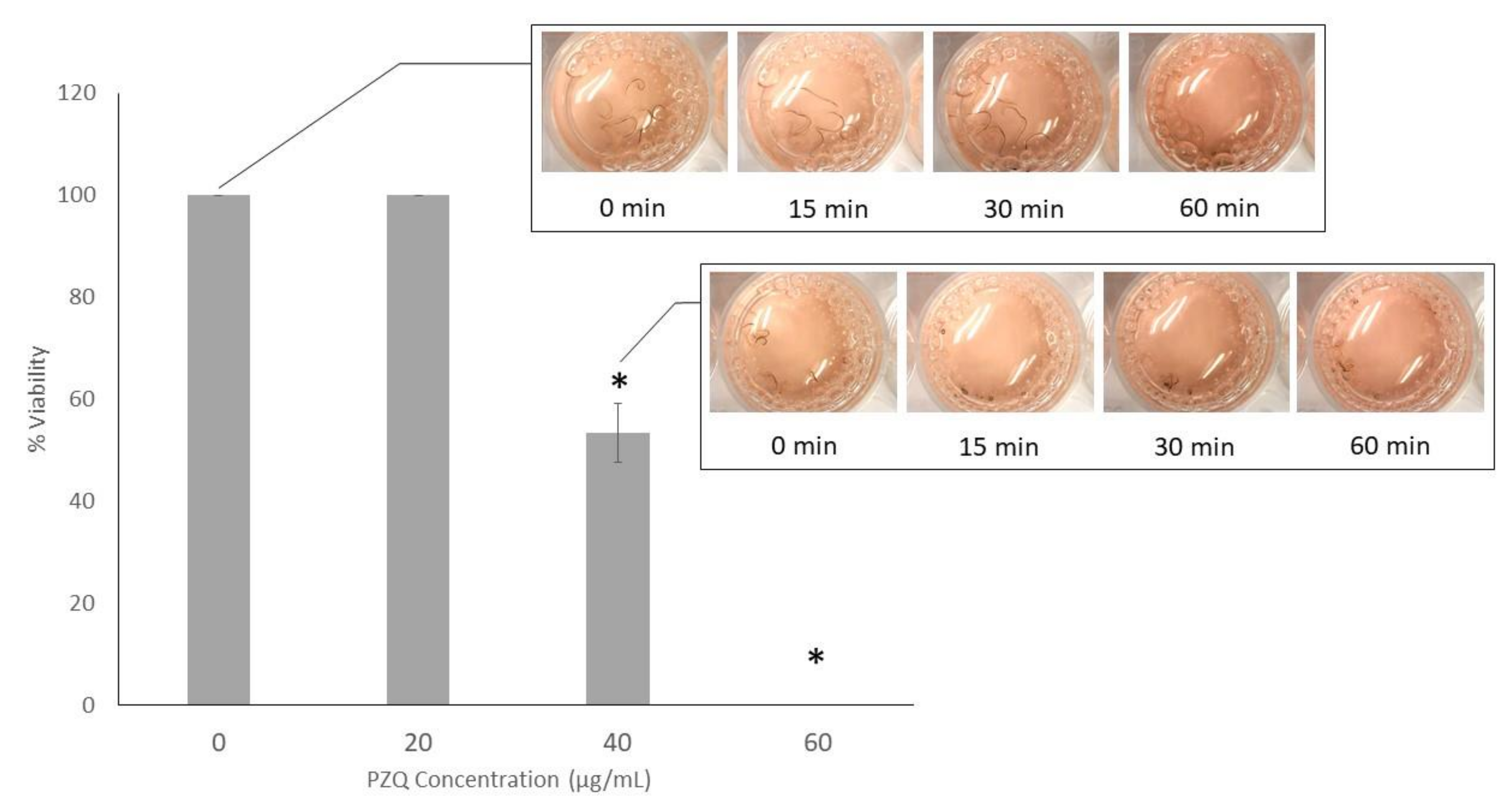
2.1. Anthelmintic Assay of PZQ on S. Mekongi
2.2. Effects of PZQ on S. Mekongi Proteome
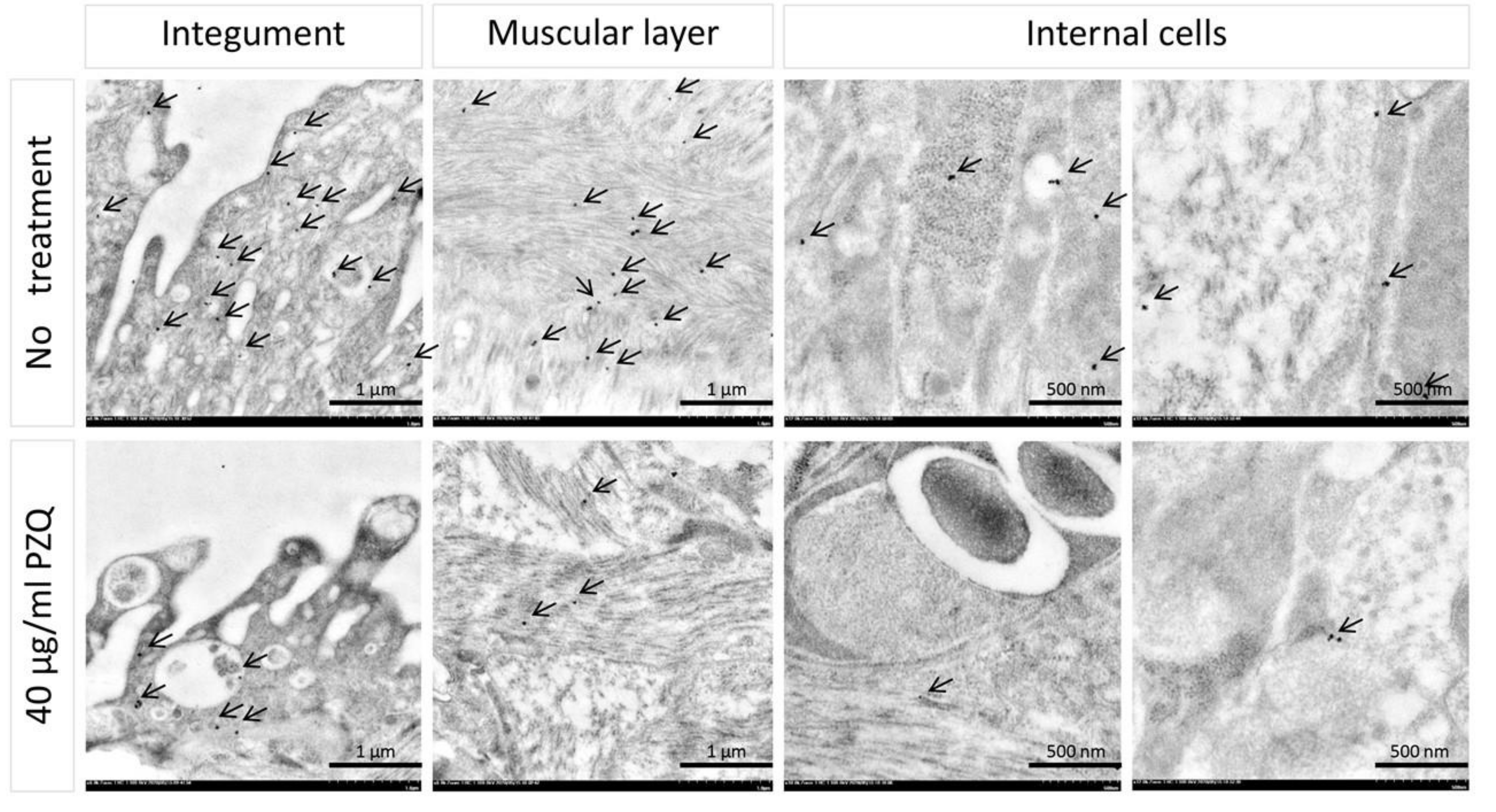
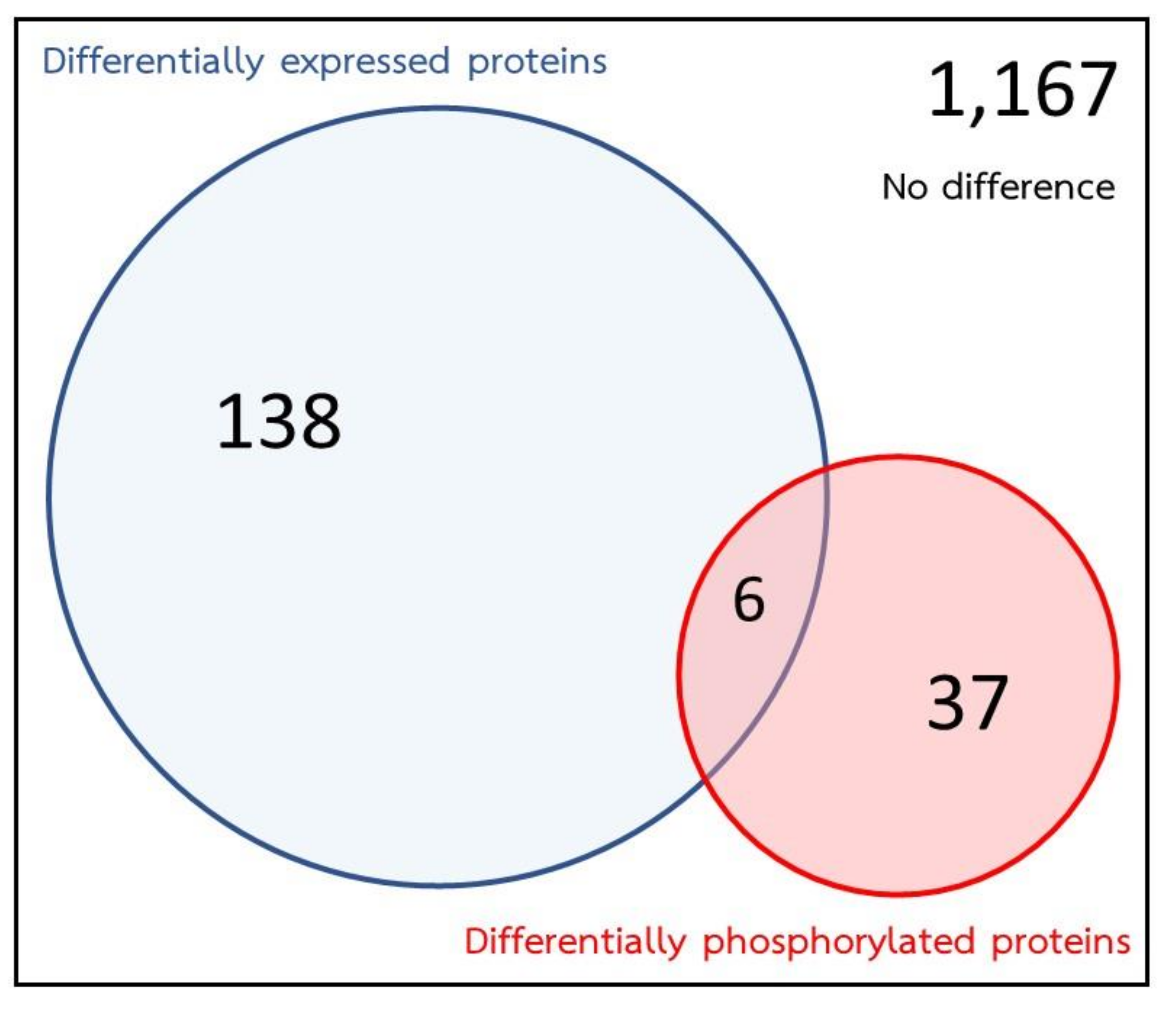
2.3. Effects of PZQ on S. Mekongi Phosphorylation and Phosphoprotein Abundance
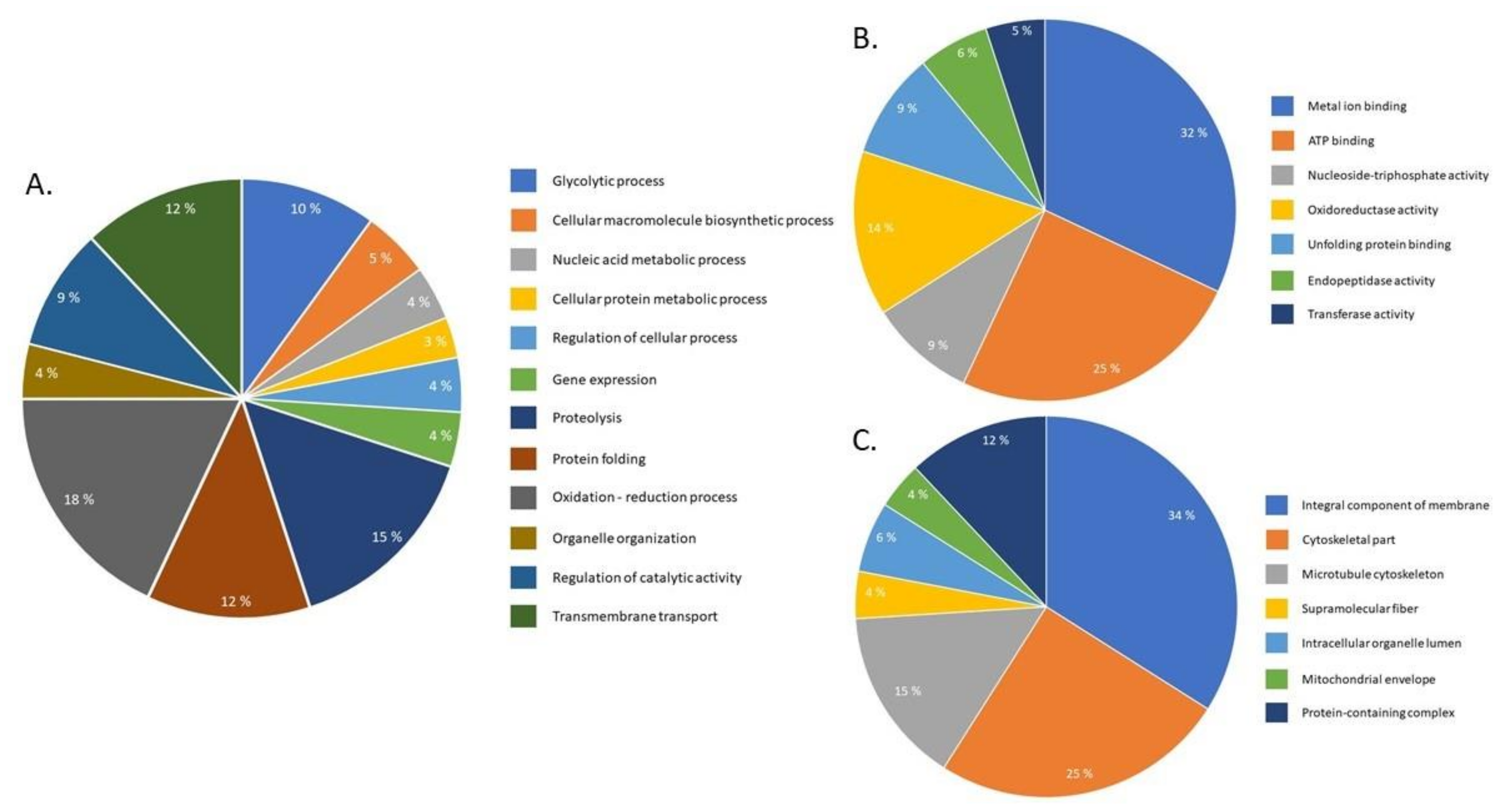
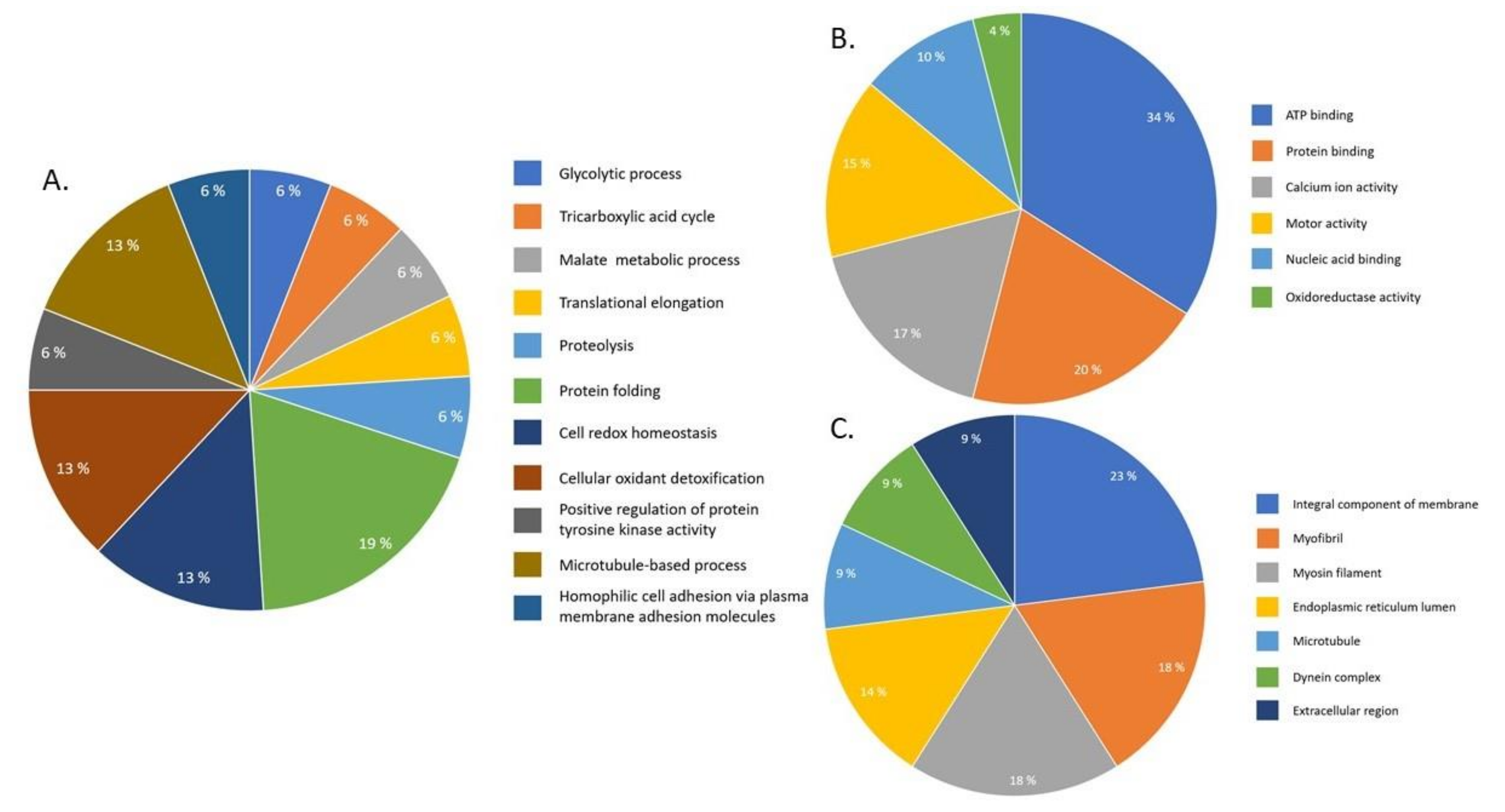
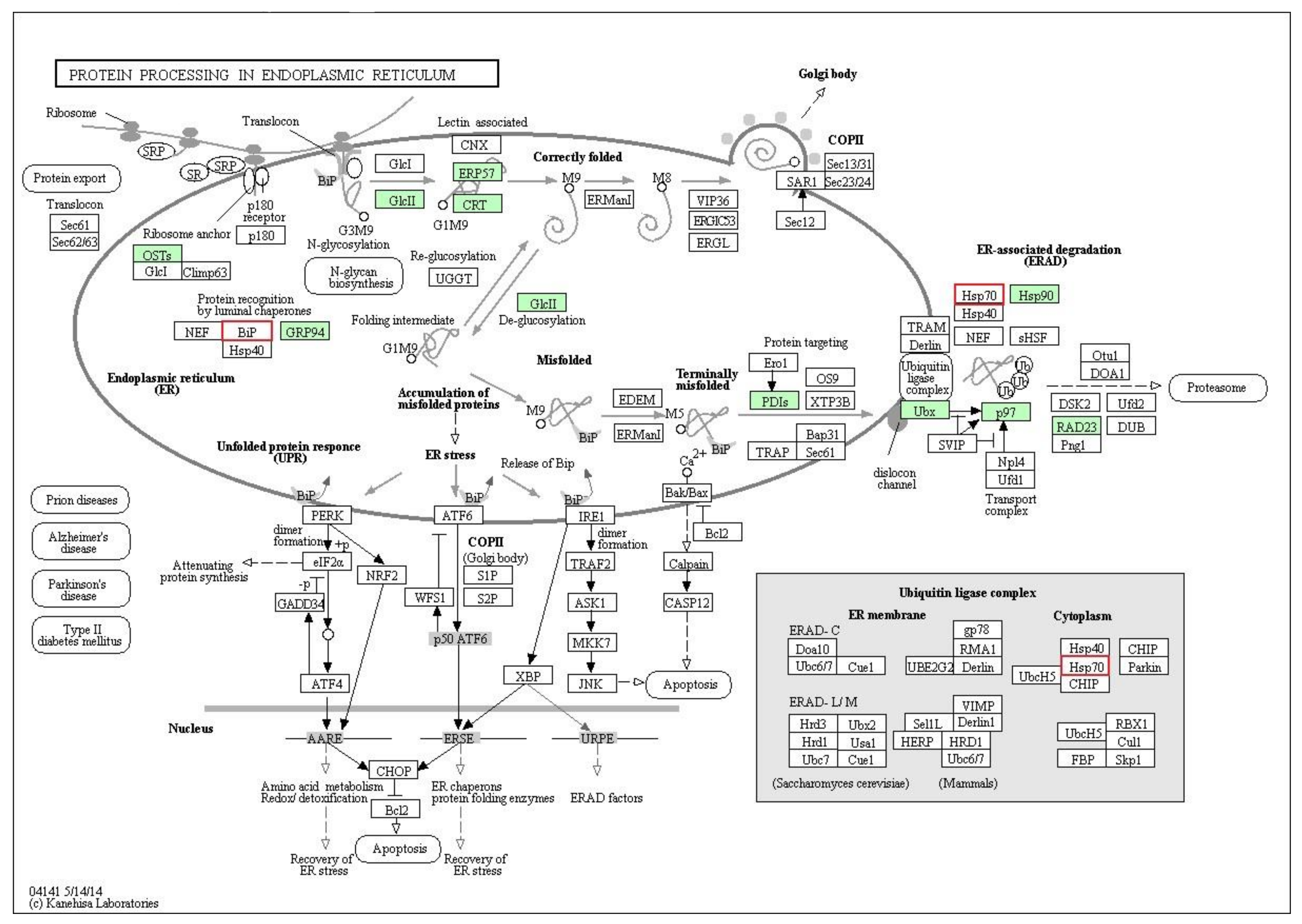
2.4. Pathway Analysis
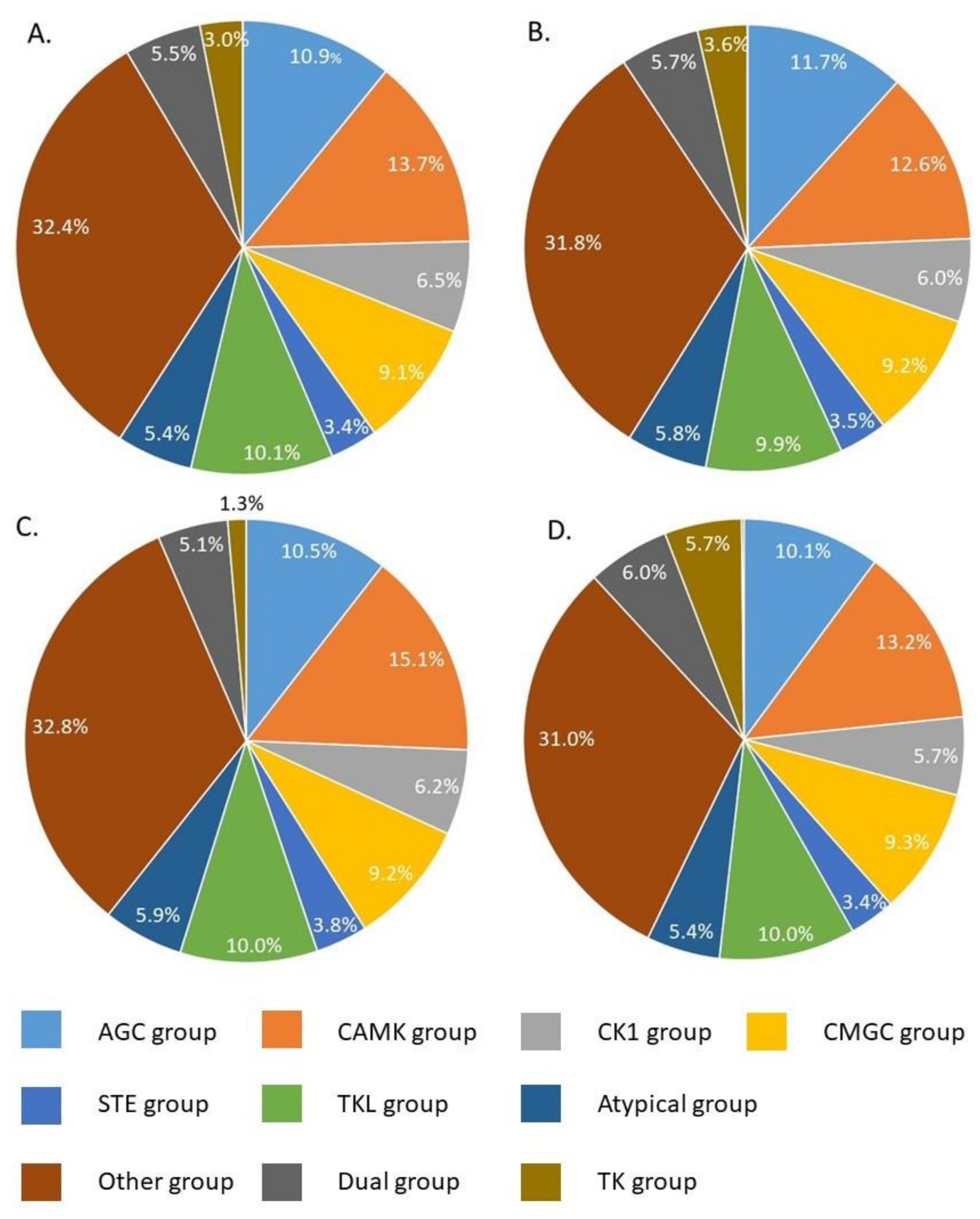
2.5. Prediction of Key Kinases Effected by PZQ
3. Discussion
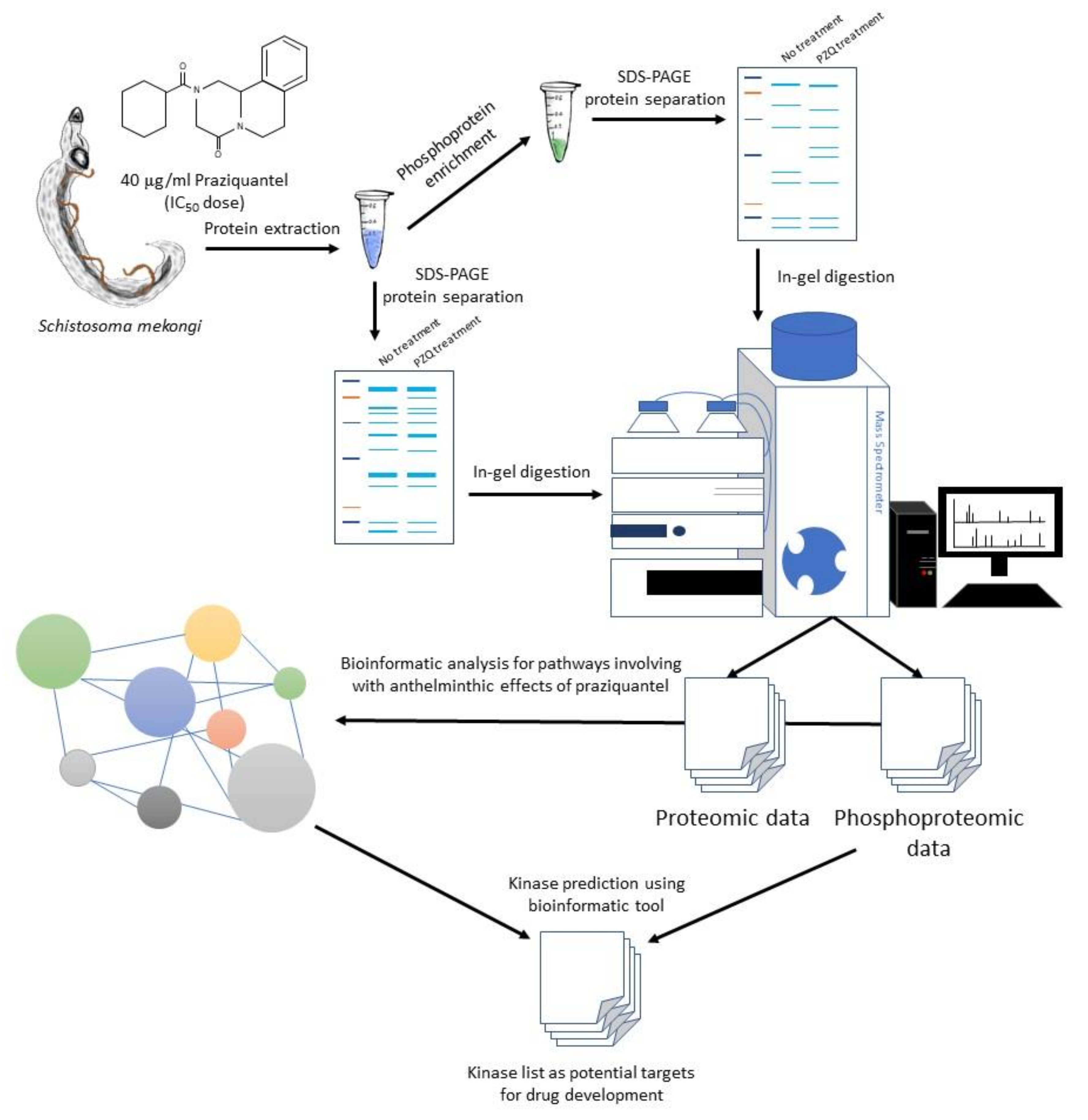
4. Materials and Methods
4.1. S. Mekongi Life Cycle Maintenance, Animal Husbandry and Worm Collection
4.2. PZQ Treatment and Half Maximal Inhibitory Concentration (IC50) Assessment
4.3. Protein Extraction and Phosphoprotein Enrichment
4.4. Protein Separation and In-Gel Digestion
4.5. Protein Identification by MS
4.6. Data Analysis and Pathway Enrichment
4.7. Phosphorylation Level Investigation by Immunogold Labeling and Transmission Electron Microscope (TEM)
4.8. Kinase Prediction for Identified Pathways
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Schistosomiasis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/%20schistosomiasis. (accessed on 18 May 2019).
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Campa, P.; Develoux, M.; Belkadi, G.; Magne, D.; Lame, C.; Carayon, M.J.; Girard, P.M. Chronic Schistosoma mekongi in a traveler—A case report and review of the literature. J. Travel Med. 2014, 21, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H. Infectious diseases of Khmer immigrants in the United States: Review of published reports. J. Ayub Med. Coll. Abbottabad 2011, 23, 149–152. [Google Scholar] [PubMed]
- Leshem, E.; Meltzer, E.; Marva, E.; Schwartz, E. Travel-related schistosomiasis acquired in Laos. Emerg. Infect. Dis. 2009, 15, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, H.; Sinuon, M.; Kirinoki, M.; Matsumoto, J.; Chigusa, Y.; Socheat, D.; Matsuda, H. Schistosomiasis mekongi: From discovery to control. Parasitol. Int. 2004, 53, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Campos, B.; Oliveira, J.F.; Decout, J.L.; Lima, M. Medicinal chemistry of antischistosomal drugs: Praziquantel and oxamniquine. Bioorg. Med. Chem. 2017, 25, 3259–3277. [Google Scholar] [CrossRef]
- Lovis, L.; Mak, T.K.; Phongluxa, K.; Soukhathammavong, P.A.; Vonghachack, Y.; Keiser, J.; Vounatsou, P.; Tanner, M.; Hatz, C.; Utzinger, J.; et al. Efficacy of praziquantel against Schistosoma mekongi and Opisthorchis viverrini: A randomized, single-blinded dose-comparison trial. PLOS Negl. Trop. Dis. 2012, 6, e1726. [Google Scholar] [CrossRef]
- Albonico, M.; Levecke, B.; LoVerde, P.T.; Montresor, A.; Prichard, R.; Vercruysse, J.; Webster, J.P. Monitoring the efficacy of drugs for neglected tropical diseases controlled by preventive chemotherapy. J. Glob. Antimicrob. Resist. 2015, 3, 229–236. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- Greenberg, R.M. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology 2013, 140, 1534–1546. [Google Scholar] [CrossRef]
- Melman, S.D.; Steinauer, M.L.; Cunningham, C.; Kubatko, L.S.; Mwangi, I.N.; Wynn, N.B.; Mutuku, M.W.; Karanja, D.M.S.; Colley, D.G.; Black, C.L.; et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma Mansoni. PLOS Negl. Trop. Dis. 2009, 3, e504. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Botros, S.; Metwally, A.; William, S.; Farghally, A.; Tao, L.F.; Bennett, J.L.; Farghally, A.; Metwally, A. Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999, 60, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.Y.W.; Wong, B.K.; Lu, D.; Zhong, B. Human Schistosomiasis resistance to praziquantel in China: Should we be worried? Am. J. Trop. Med. Hyg. 2011, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Muchiri, E.M.; Ouma, J.H. Evidence against rapid emergence of praziquantel resistance in Schistosoma haematobium, Kenya. Emerg. Infect. Dis. 2000, 6, 585–594. [Google Scholar] [CrossRef]
- Chelladurai, J.J.; Kifleyohannes, T.; Scott, J.; Brewer, M.T. Praziquantel resistance in the zoonotic cestode Dipylidium caninum. Am. J. Trop. Med. Hyg. 2018, 99, 1201–1205. [Google Scholar] [CrossRef]
- Lateef, M.; Zargar, S.A.; Khan, A.R.; Nazir, M.; Shoukat, A. Successful treatment of niclosamide- and praziquantel-resistant beef tapeworm infection with nitazoxanide. Int. J. Infect. Dis. 2008, 12, 80–82. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4. [Google Scholar] [CrossRef]
- Siqueira, L.P.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelosa, M.A.; Silva, L.; De Melo, C.G.; Rolim, L.A.; Da Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs used and treatment strategies. Acta. Trop. 2017, 176, 179–186. [Google Scholar] [CrossRef]
- Chai, J.Y. Praziquantel treatment in trematode and cestode infections: An update. Infect. Chemother. 2013, 45, 32–43. [Google Scholar] [CrossRef]
- Chan, J.D.; Zarowiecki, M.; Marchant, J.S. Ca2+ channels and Praziquantel: A view from the free world. Parasitol. Int. 2013, 62, 619–628. [Google Scholar] [CrossRef]
- Andrew, P. Praziquantel: Mechanism of anti-schistosomal activity. Pharm. Ther. 1985, 29, 129–156. [Google Scholar] [CrossRef]
- Brindley, P.J.; Strand, M.; Norden, A.P.; Sher, A. Role of host antibody in the chemotherapeutic action of praziquantel against Schistosoma mansoni: Identification of target antigens. Mol. Biochem. Parasitol. 1989, 34, 99–108. [Google Scholar] [CrossRef]
- Aragon, A.D.; Imani, R.A.; Blackburn, V.R.; Cupit, P.M.; Melman, S.D.; Goronga, T.; Webb, T.; Loker, E.S.; Cunningham, C. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 2009, 164, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Dissous, C.; Ahier, A.; Khayath, N. Protein tyrosine kinases as new potential targets against human schistosomiasis. BioEssays 2007, 29, 1281–1288. [Google Scholar] [CrossRef]
- Ressurreicao, M.; Saram, P.; Kirk, R.S.; Rollinson, D.; Emery, R.M.; Page, N.M.; Davies, A.J.; Walker, A.J. Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2924. [Google Scholar] [CrossRef]
- You, H.; McManus, D.P.; Hu, W.; Smout, M.J.; Brindley, P.J.; Gobert, G.N. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 2013, 9, e1003254. [Google Scholar] [CrossRef]
- Duan, O.; Zhao, H.; Zhang, Z.; Li, H.; Wu, H.; Shen, Q.; Wang, C.; Yin, T. Mechanistic evaluation and translational signature of gemcitabine-induced chemoresistance by quantitative phosphoproteomics analysis with iTRAQ labeling mass spectrometry. Sci. Rep. 2017, 7, 12891. [Google Scholar] [CrossRef]
- Ctortecka, C.; Palve, V.; Kuenzi, B.M.; Fang, B.; Sumi, N.J.; Izumi, V.; Novakova, S.; Kinose, F.; Rix, L.L.R.; Haura, E.B.; et al. Functional proteomics and deep network interrogation reveal a complex mechanism of action of midostaurin in lung cancer cells. Mol. Cell Proteom. 2018, 17, 2434–2447. [Google Scholar] [CrossRef]
- Bernardini, G.; Laschi, M.; Serchi, T.; Spreafico, B.; Botta, M.; Schenone, S. Proteomics and phosphoproteomics provide insights into the mechanism of action of a novel pyrazolo [3,4-d] pyrimidine Src inhibitor in human osteosarcoma. Mol. BioSyst. 2014, 10, 1305–1312. [Google Scholar] [CrossRef]
- Hirst, N.L.; Nebel, J.C.; Lawton, S.P.; Walker, A.J. Deep phosphoproteome analysis of Schistosoma mansoni leads development of a kinomic array that highlights sex-biased differences in adult worm protein phosphorylation. PLoS Negl. Trop. Dis. 2020, 14, e0008115. [Google Scholar] [CrossRef]
- Corrêa, S.; Oliveira, R.N.; Mendes, T.M.F.; Santos, K.R.; Boaventura, S.B., Jr.; Garcia, V.L.; Jeraldo, V.D.L.S.; Allegretti, S.M. In vitro and in vivo evaluation of six artemisinin derivatives against Schistosoma mansoni. Parasitol. Res. 2019, 118, 505–516. [Google Scholar] [PubMed]
- Kovač, J.; Vargas, M.; Keiser, J. In vitro and in vivo activity of R- and S-praziquantel enantiomers and the main human metabolite trans-4-hydroxypraziquantel against Schistosoma haematobium. Parasit. Vectors 2017, 10. [Google Scholar] [CrossRef]
- Pica-Mattoccia, L.; Cioli, D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004, 34, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Naga, I.F.; Amer, E.I.; Boulos, L.M.; El-Faham, M.H.; Abou Seada, N.M.; Younis, S.S. Biological and proteomic studies of Schistosoma mansoni with decreased sensitivity to praziquantel. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101341. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Cupit, P.M.; Bu, L.; Cunningham, C. Transcriptomic analysis of reduced sensitivity to praziquantel in Schistosoma Mansoni. Mol. Biochem. Parasitol. 2019, 228, 6–15. [Google Scholar] [CrossRef]
- Cupit, P.M.; Cunningham, C. What is the mechanism of action of praziquantel and how might resistance strike? Future Med. Chem. 2015, 7, 701–705. [Google Scholar] [CrossRef]
- Xiao, S.; Sun, J.; Chen, M. Pharmacological and immunological effects of praziquantel against Schistosoma japonicum: A scoping review of experimental studies. Infect. Dis. Poverty 2018, 7, 9. [Google Scholar] [CrossRef]
- Kohn, A.B.; Roberts-Misterly, J.M.; Anderson, P.A.V.; Greenberg, R.M. Creation by mutagenesis of a mammalian Ca2+ channel b subunit that confers praziquantel sensitivity to a mammalian Ca2+ channel. Int. J. Parasitol. 2003, 33, 1303–1308. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De, S.; Meir, A. The mitochondrial voltage-dependent anion channel 1, Ca2+ transport, apoptosis and their regulation. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef]
- Ben-Hail, D.; Palty, R.; Shoshan-Barmatz, V. Measurement of mitochondrial Ca2+ transport mediated by three transport proteins: VDAC1, the Na+/Ca2+ exchanger and the Ca2+ uniporter. Cold Spring Harb. Protoc. 2014, 2, 161–166. [Google Scholar] [CrossRef]
- Doenhoff, M.J.; Sabah, A.A.; Fletcher, C.; Webbe, G.; Bain, J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 947–951. [Google Scholar] [CrossRef]
- Fallon, P.G.; Doenhoff, M.J. Active immunization of mice with Schistosoma mansoni worm membrane antigens enhances efficacy of praziquantel. Parasite Immunol. 1995, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Roy, B. Praziquantel induced oxidative stress and apoptosis-like cell death in Raillietina echinobothrida. Acta. Trop. 2016, 159, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, F.; Cui, S.; Xu, B.; Zhou, X.; Hu, W. Proteomic analysis of adult Schistosoma japonicum treated with praziquantel. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2008, 26, 258–262. [Google Scholar] [PubMed]
- Manna, P.T.; Boehm, C.; Leung, K.F.; Natesan, S.K.; Field, M.C. Life and times: Synthesis, trafficking, and evolution of VSG. Trends Parasitol. 2014, 30, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, C.J.; Brodsky, J.L. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol. Rev. 2012, 92, 537–576. [Google Scholar] [CrossRef] [PubMed]
- Printsev, I.; Curiel, D.; Carraway III, K.L. Membrane protein quantity control at the endoplasmic reticulum. J. Membr. Biol. 2017, 250, 379–392. [Google Scholar] [CrossRef]
- Tiengwe, C.; Muratore, K.A.; Bangs, J.D. Variant Surface Glycoprotein, Transferrin Receptor, and ERAD in Trypanos. Brucei. Cell Microbiol. 2016, 18, 1673–1688. [Google Scholar] [CrossRef]
- Chung, D.D.; Ponts, N.; Prudhomme, J.; Rodrigues, E.M.; Roch, K.G. Characterization of the ubiquitylating components of the human malaria parasite’s protein degradation pathway. PLoS ONE 2012, 7, e43477. [Google Scholar] [CrossRef]
- Harbut, M.B.; Patel, B.A.; Yeung, B.K.S.; McNamara, C.W.; Bright, A.T.; Ballard, J.; Supek, F.; Golde, T.E.; Winzeler, E.A.; Diagana, T.T.; et al. Targeting the ERAD pathway via inhibition of signal peptide peptidase for antiparasitic therapeutic design. Proc. Natl. Acad. Sci. USA 2012, 109, 21486–21491. [Google Scholar] [CrossRef]
- Hull, R.; Dlamini, Z. The role played by alternative splicing in antigenic variability in human endo-parasites. Parasit Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Marco, R.; Mathieson, W.; Manuel, S.J.; Dillon, G.P.; Curwen, R.S.; Ashton, P.; Ivens, A.; Berriman, M.; Verjovski-Almeida, S.; Wilson, R.A. Protein variation in blood-dwelling schistosome worms generated by differential splicing of micro-exon gene transcripts. Genome. Res. 2010, 20, 1112–1121. [Google Scholar]
- Liu, S.; Cai, P.; Piao, X.; Hou, N.; Zhou, X.; Wu, C.; Wang, H.; Chen, Q. Expression profile of the Schistosoma japonicum degradome reveals differential protease expression patterns and potential anti-schistosomal intervention targets. PLoS Comput. Biol. 2014, 10, e1003856. [Google Scholar] [CrossRef] [PubMed]
- Nawaratna, S.S.K.; You, H.; Jones, M.K.; McManus, D.P.; Gobert, G.N. Calcium and Ca2+/Calmodulin-dependent kinase II as targets for helminth parasite control. Biochem. Soc. Trans. 2018, 46, 1743–1751. [Google Scholar] [CrossRef]
- Walker, A.J.; Ressurreição, M.; Rothermel, R. Exploring the function of protein kinases in schistosomes: Perspectives from the laboratory and from comparative genomics. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Avelar, L.G.A.; Nahum, L.A.; Andrade, L.F.; Oliveira, G. Functional diversity of the Schistosoma mansoni tyrosine kinases. J. Signal Transduct. 2011, 2011, 603290. [Google Scholar] [CrossRef]
- Knobloch, J.; Kunz, W.; Grevelding, C.G. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int. J. Parasitol. 2006, 36, 1261–1272. [Google Scholar] [CrossRef]
- Phuphisut, O.; Ajawatanawong, P.; Limpanont, Y.; Reamtong, O.; Nuamtanong, S.; Ampawong, S.; Chaimon, S.; Dekumyoy, P.; Watthanakulpanich, D.; Swierczewski, B.; et al. Transcriptomic analysis of male and female Schistosoma mekongi adult worms. Parasit. Vectors 2018, 11. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Z.; Cao, J.; Ma, Q.; Gao, X.; Wang, Q.; Jin, C.; Zhou, Y.; Wen, L.; Ren, J. GPS 2.1: Enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein. Eng. Des. Sel. 2011, 24, 255–260. [Google Scholar] [CrossRef] [PubMed]
| No. | Accession No. Uniprot | Protein Name | MW | pI | Protein Score | Sequence Coverage | Average Fold-Change |
|---|---|---|---|---|---|---|---|
| Structural protein | |||||||
| 1 | Q9Y1U7 | Myosin light chain | 18.3 | 4.5 | 37 | 31.3 | 2 |
| 2 | Q26507 | Paramyosin, partial | 51.6 | 5.03 | 522 | 46 | 5.96 |
| 3 | Q26595 | Alpha tubulin | 49.9 | 4.97 | 104 | 17.5 | - 1 |
| Energy | |||||||
| 4 | A0A094ZC89 | L-lactate dehydrogenase A chain | 32.7 | 6.75 | 97 | 19.6 | 2 |
| 5 | G4VP51 | Putative ADP,ATP carrier protein | 29.9 | 9.47 | 518 | 48.2 | 2.29 |
| 6 | C1LV81 | Aldolase | 39.5 | 6.56 | 447 | 53.4 | 3.27 |
| 7 | A0A095AJN4 | Enolase | 46.6 | 6.34 | 130 | 27.9 | 2.56 |
| Protease | |||||||
| 8 | C1L5C5 | Putative aminopeptidase W07G4.4 | 56.2 | 7.14 | 360 | 25.7 | 2.31 |
| 9 | A0A094ZYF3 | Putative aminopeptidase W07G4.4 | 57.6 | 7.55 | 169 | 25.3 | 2.16 |
| 10 | P43157 | Cathepsin B | 38.7 | 7.14 | 522 | 30.4 | 2.33 |
| 11 | A0A095B296 | Cathepsin B-like cysteine proteinase | 38.6 | 7.51 | 43 | 38.8 | 2.05 |
| Antioxidant | |||||||
| 12 | Q26513 | Glutathione-S-transferase | 23.8 | 6.72 | 76 | 19.4 | - 1 |
| Transcription and translation | |||||||
| 13 | C1LWP5 | Eukaryotic translation elongation factor 1 alpha 2 | 25.7 | 7.67 | 149 | 36.5 | 3.69 |
| Immune system | |||||||
| 14 | C1L7Y4 | Annexin A13 (Annexin XIII) | 39.5 | 5.1 | 242 | 24.9 | 2.48 |
| 15 | Q86DV3 | Annexin | 36.7 | 6.13 | 225 | 36.4 | 3.05 |
| 16 | C1LKB8 | Prohibitin-2 (B-cell receptor-associated protein BAP37) | 28.8 | 9.72 | 226 | 38.5 | 3.29 |
| Antigen | |||||||
| 17 | 55kD antigen | 45.6 | 4.59 | 40 | 20.2 | - 1 | |
| Unknown | |||||||
| 18 | Q5DE25 | SJCHGC06488 protein | 30.2 | 5.54 | 232 | 25.9 | 3.54 |
| 19 | Q5DGY1 | Unknown | 38.7 | 7.86 | 123 | 21.3 | 3.22 |
| 20 | Q5DCK2 | SJCHGC06304 protein | 60.2 | 7.59 | 46 | 11.6 | 2.4 |
| No. | Accession No.Uniprot | Protein Name | MW | pI | Protein Score | Sequence Coverage | AverageFold–Change |
|---|---|---|---|---|---|---|---|
| Structural protein | |||||||
| 1 | G4VD36 | Tropomyosin, partial | 32.9 | 4.62 | 610 | 63.7 | 0 1 |
| 2 | Q02456 | Myosin heavy chain | 222.2 | 5.55 | 1003 | 27.8 | 0 1 |
| 3 | G4M0G1 | Myosin heavy chain, putative | 182.4 | 5.6 | 873 | 28.2 | −2.0 |
| Energy | |||||||
| 4 | B2LXU1 | Enolase | 46.7 | 6.77 | 130 | 24.7 | −3.12 |
| 5 | C1LFP4 | Putative aldehyde dehydrogenase 1B1 precursor | 53.5 | 6.06 | 379 | 28.9 | −4.0 |
| 6 | G4LWI3 | Aldehyde dehydrogenase, putative | 53.7 | 5.76 | 113 | 19.8 | −2.86 |
| 7 | B2LXU3 | Glyceraldehyde 3-phosphate dehydrogenase | 36.5 | 7.68 | 152 | 33.4 | −2.63 |
| 8 | C7TRL1 | Glyceraldehyde 3-phosphate dehydrogenase | 36.5 | 8.4 | 340 | 33.7 | −2.63 |
| 9 | G4M130 | Sm14 fatty acid-binding protein delta E3 variant | 10.9 | 8.82 | 85 | 42.9 | 01 |
| Stress response | |||||||
| 10 | G4VJ99 | Putative heat shock protein | 80.7 | 5.91 | 321 | 12.8 | −2.27 |
| Signaling | |||||||
| 11 | A0A095B084 | Pyruvate kinase PKM | 62 | 6.63 | 136 | 19.3 | −2.38 |
| 12 | C1LEA0 | TNF receptor-associated protein 1 | 80.2 | 6.24 | 321 | 9.8 | −2.27 |
| 13 | C1LFZ8 | Arginine kinase | 80.1 | 8.46 | 56 | 12.8 | −2.27 |
| 14 | A0A095ASB2 | Inositol hexakisphosphate and diphosphoinositol–pentakisphosphate kinase 2 | 178 | 8.23 | 34 | 8.3 | −2.0 |
| Unknown | |||||||
| 15 | Q5DAM7 | SJCHGC06305 protein | 61.1 | 6.48 | 278 | 28 | −3.33 |
| 16 | B3GUT7 | Unknown | 16 | 6.9 | 33 | 18.2 | 0 1 |
| 17 | Q5DBJ9 | Unknown | 38.8 | 8.44 | 69 | 22.2 | −2.0 |
| 18 | Q5C296 | SJCHGC01885 protein, partial | 111.8 | 5.26 | 924 | 37.3 | −2.04 |
| 19 | Q5DEG6 | SJCHGC00214 protein | 53.4 | 8.56 | 306 | 25.1 | −2.22 |
| 20 | Q5DGD4 | SJCHGC06677 protein | 90.6 | 5.08 | 61 | 12.5 | −2.38 |
| No. | Accession No. Uniprot | Protein Name | Phosphorylation Site | Average Fold–Change | |
|---|---|---|---|---|---|
| Peptide Sequence | Number | ||||
| Structural protein | |||||
| 1 | A5A6F8 | Paramyosin | QASEDRATR | 1 (ST) | 2 |
| GVSPSTTRLESR | 1 (ST) | ||||
| MMNHDTESHVKISR | 1 (ST) | ||||
| TIYRGVSPSTTR | 5 (ST), 1 (Y) | ||||
| GMRATSMM | 2 (ST) | ||||
| ADMAERTVTVR | 1 (ST) | ||||
| AKSSLESQVDDLK | 1 (ST) | ||||
| LKAVSLEK | 1 (ST) | ||||
| GVSPSTTR | 3 (ST) | ||||
| QLTELNNAK | 1 (ST) | ||||
| LEGLDSQLNR | 1 (ST) | ||||
| LDEAGGSTTQTQELLKR | 3 (ST) | ||||
| ISRTIYR | 2 (ST) | ||||
| ATRLNNEVLR | 1 (ST) | ||||
| ELSVTSNRGMR | 2 (ST) | ||||
| KHAETELEEAQSR | 1 (ST) | ||||
| 2 | Q05870 | Paramyosin | GVSPSTTRLESR | 1 (ST) | 2.47 |
| MMNHDTESHVKISR | 1 (ST) | ||||
| TIYRGVSPSTTR | 5 (ST), 1 (Y) | ||||
| AERHAADLSYQVDALSER | 1 (ST) | ||||
| GMRATSMM | 1 (ST) | ||||
| ADMAERTVTVR | 1 (ST) | ||||
| AKSSLESQVDDLK | 1 (ST) | ||||
| VSELTIQVNTLSNDK | 1 (ST) | ||||
| LKAVSLEK | 1 (ST) | ||||
| GVSPSTTR | 3 (ST) | ||||
| QLTELNNAK | 1 (ST) | ||||
| LEGLDSQLNR | 1 (ST) | ||||
| LDEAGGSTTQTQELLKR | 3 (ST) | ||||
| 3 | Q26507 | Paramyosin, partial | ATRLNNEVLR | 1 (ST) | - 1 |
| 4 | A0A095BXK4 | Actin-1 | GTAASSSALEK | 4 (ST) | - 1 |
| MQKEISALAPSTMK | 1 (ST) | ||||
| QEYDESGPSIVHRK | 1 (ST), 1 (Y) | ||||
| EISALAPSTMK | 1 (ST) | ||||
| DLYSNTVLSGGSTMYPGIADR | 1 (ST), 1 (Y) | ||||
| 5 | A0A094ZID2 | Actin-1 | EICNLAPTTMKIK | 2 (ST) | - 1 |
| KDLYSNIVLSGGSTMFPGISDR | 2 (ST) | ||||
| Energy | |||||
| 6 | G4VBJ0 | Putative malate dehydrogenase | RIQEAGTEVVEAK | 1 (ST) | 2.25 |
| AGAGSATLSMAYAGVR | 2 (ST), 1 (Y) | ||||
| FGISFRSFLTSSK | 5 (ST) | ||||
| LFGVTTLDVVR | 1 (ST) | ||||
| SFLTSSKHSPK | 4 (ST) | ||||
| AMICIITNPVNSTVPIAAEILK | 2 (ST) | ||||
| 7 | A0A095CGL8 | 78 kDa glucose-regulated protein, partial | FDESTVQNDITHYPFSVVNKK | 3 (ST), 1 (Y) | - 1 |
| DIKTNK | 1 (ST) | ||||
| EFAESYLGK | 1 (ST) | ||||
| FDESTVQNDITHYPFSVVNKK | 3 (ST), 1 (Y) | ||||
| Protease | |||||
| 8 | P43157 | Cathepsin B | DIMMYGPVEAAFDVYEDFLNYK | 1 (Y) | - 1 |
| IAVYIVSLFTFLEAHVTTR | 3 (ST) | ||||
| MATLGTGMR | 2 (ST) | ||||
| AEISMLEGAVLDIRYGVSR | 1 (ST), 1 (Y) | ||||
| IDDEINTLMTGALENPNEEITATMDK | 3 (ST) | ||||
| YGVSRIAYSK | 2 (ST), 1 (Y) | ||||
| MATLGTGMRCLK | 1 (ST) | ||||
| Antigen | |||||
| 9 | Q03528 | 22.6kd tegumental associated antigen | MATTEYRLSLMEQFIR | 1 (ST) | - 1 |
| ATTEYR | 1 (ST), 1 (Y) | ||||
| ATTEYRLSLMEQFIR | 1 (ST) | ||||
| 10 | P08418 | 70,000 mol wt antigen/hsp70 homologue (619 AA), partial | TTPSYVAFTDSER | 3 (ST), 1 (Y) | - 1 |
| MFSPEEISSMVLTKMK | 1 (ST) | ||||
| 11 | C1L7Y4 | Annexin A13 (Annexin XIII) | IIGILGYR | 1 (Y) | - 1 |
| MLLTDTDKMNAR | 1 (ST) | ||||
| Transcription and translation | |||||
| 12 | G4VAD2 | Putative elongation factor 1-alpha (EF-1-alpha) | TEDNPKCIK | 1 (ST) | - 1 |
| ESGEMGKGSFK | 1 (ST) | ||||
| KESAAK | 1 (ST) | ||||
| MTDKPLR | 1 (ST) | ||||
| MDCTEPPFSEDR | 1 (ST) | ||||
| 13 | A0A094ZN49 | S phase cyclin A-associated protein in the endoplasmic reticulum | TASNTRSGSLISTNSR | 2 (ST) | - 1 |
| SGSLISTNSR | 2 (ST) | ||||
| 14 | G4M170 | Meiotic checkpoint regulator cut4, putative | GISSSSR | 2 (ST) | - 1 |
| HNSINMTTLR | 1 (ST) | ||||
| GASFSSSTFR | 4 (ST) | ||||
| CVDSLDSNKFLR | 1 (ST) | ||||
| YTGNIVSSECQNNVGHIFQSSKR | 1 (ST) | ||||
| GMLTFGILTGSK | 1 (ST) | ||||
| SSGSGLFYECSRK | 3 (ST), 1 (Y) | ||||
| WYIISNAPGTKSSYK | 1 (ST) | ||||
| CPDQTFTDSLYHAYGR | 2 (ST) | ||||
| VWASVIGRGMLTFGILTGSK | 1 (ST) | ||||
| Immune system | |||||
| 15 | G4VL68 | Putative annexin | STSRLVSR | 2 (ST) | - 1 |
| CEVDMNTLKSMYR | 1 (ST), 1 (Y) | ||||
| GDSDTFIK | 2 (ST) | ||||
| EDTSGDFR | 2 (ST) | ||||
| CEVDMNTLK | 1 (ST) | ||||
| GLSTDEETITK | 2 (ST) | ||||
| NVVITVPAYFNDSQR | 1 (ST) | ||||
| Stress response | |||||
| 16 | A0A094ZG26 | Heat shock 70 kDa protein-like protein, partial | RTLSSST | 4 (ST) | - 1 |
| LFSAEEISSMVLTK | 1 (ST) | ||||
| 17 | Q8MXA4 | Heat shock protein HSP60, partial | EEMEASNSEYEKEK | 1 (ST), 1 (Y) | - 1 |
| DMAVGTGGIVFGDEADMYK | 1 (ST), 1 (Y) | ||||
| SAMLVGVDILADADAVTMGPKGR | 1 (ST) | ||||
| 18 | O45038 | HSP70 | MKSSAESYLGK | 2 (ST) | - 1 |
| 19 | G4LWI2 | Heat shock protein HSP60, putative | EEMEASNSEYEKEK | 1 (ST), 1 (Y) | - 1 |
| VGNDGTITVKMK | 1 (ST) | ||||
| Transporter | |||||
| 20 | A0A094ZQ18 | Putative sodium-coupled neutral amino acid transporter 10 | SFQPTLSNMR | 3 (ST) | - 1 |
| NPPEPIDLTKIQSQMNVNNK | 1 (ST) | ||||
| Antioxidant | |||||
| 21 | A0A095A0V3 | Peroxiredoxin-2 | - 1 | ||
| 22 | Glutathione S-transferase | GIPGNSSMASK | 2 (ST) | - 1 | |
| AEISMLEGAVLDIRYGVSR | 1 (ST), 1 (Y) | ||||
| YGVSRIAYSK | 2 (ST), 1 (Y) | ||||
| 23 | G4LXF8 | 26 Kd glutathione-S-transferase, Sm26, putative | - 1 | ||
| Unknown | |||||
| 24 | Q5DBM4 | SJCHGC01909 protein | MNHDSESHVK | 2 (ST) | 6.13 |
| GVSPSTTRLESR | 1 (ST) | ||||
| TIYRGVSPSTTR | 5 (ST), 1 (Y) | ||||
| AERHAADLSYQVDALSER | 1 (ST) | ||||
| GVSPSTTR | 3 (ST) | ||||
| QLTELNNAK | 1 (ST) | ||||
| LEGLDSQLNR | 1 (ST) | ||||
| MNHDSESHVK | 2 (ST) | ||||
| LDEAGGSTTQTQELLKR | 3 (ST) | ||||
| ISRTIYR | 2 (ST) | ||||
| 25 | G4VM49 | Hypothetical protein Smp_179350 | WSSSSSNK | 1 (ST) | - 1 |
| FSSTSSTR | 3 (ST) | ||||
| SINDNSSIEK | 2 (ST) | ||||
| TMRSLDSCIVPR | 1 (ST) | ||||
| ISGLTPVSTIFTGASNTTSTSTACGAVK | 4 (ST) | ||||
| LNTSR | 1 (ST) | ||||
| KQHSQSHDNLECSK | 1 (ST) | ||||
| 26 | B3GUU7 | Unknown | - 1 | ||
| 27 | Q5DGY9 | SJCHGC06312 protein | MAGESGTKTESQSDESTK | 2 (ST) | - 1 |
| TESQSDESTK | 1 (ST) | ||||
| DSETAESIK | 3 (ST) | ||||
| 28 | G4M072 | Hypothetical protein Smp_153120 | LSHSGVDDK | 1 (ST) | - 1 |
| QCLNLTSYSK | 1 (ST) | ||||
| TPSSIKVYDLK | 1 (ST) | ||||
| SKYGWAPNSLLLGK | 1 (Y) | ||||
| MNDVTLSMVCNIR | 1 (ST) | ||||
| TSASNTVHTIHDITVKR | 4 (ST) | ||||
| No. | Accession No. Uniprot | Protein Name | Phosphorylation Site | Average Fold–Change | |
|---|---|---|---|---|---|
| Peptide Sequence | Number | ||||
| Structural protein | |||||
| 1 | G4VLW1 | Putative actin | GYSFTTTAER | 2 (ST) | 0 1 |
| DSYVGDEAQSK | 2 (ST) | ||||
| GDEDVAALVIDNGSGMCK | 1 (ST) | ||||
| YSVWIGGSILASLSTFQQMWISK | 1 (ST), 1 (Y) | ||||
| 2 | G4VD37 | Putative tropomyosin | EKAEAEVAAMTR | 1 (ST) | 0 1 |
| EESYEETIRDLTER | 3 (ST), 1 (Y) | ||||
| LLEEDLEVSSSRLTETLTK | 2 (ST) | ||||
| Energy | |||||
| 3 | C1LV81 | Aldolase | HNMVTAGQACK | 1 (ST) | −2.38 |
| TLPTLLAERHIIPGIK | 1 (ST) | ||||
| TLPTLLAER | 1 (ST) | ||||
| Antigen | |||||
| 4 | C1L9U2 | Tegument antigen (I(H)A) | EGKVSTLPLVIQIIAATMSK | 2 (ST) | 0 1 |
| Scaffold | |||||
| 5 | G4VM27 | Putative zinc finger protein | DNQSSNLEESFKHECDSDK | 1 (ST) | 0 1 |
| YIQRSGLNR | 1 (Y) | ||||
| TFTTKSHLNK | 2 (ST) | ||||
| IKYSCVQCYK | 1 (Y) | ||||
| DNQSSNLEESFKHECDSDK | 1 (ST) | ||||
| Antioxidant | |||||
| 6 | O97161 | Thioredoxin peroxidase | KSGGLGHMK | 1 (ST) | 0 1 |
| Transcription and translation | |||||
| 7 | A0A094ZK92 | Putative ATP-dependent RNA helicase DDX56 | LSEVDGFRYR | 1 (ST) | 0 1 |
| CRDVEYGVSR | 1 (Y) | ||||
| ELCSQVASNIKCLCK | 1 (ST) | ||||
| 8 | G4VDN8 | Putative dead box ATP-dependent RNA helicase | LSEVDGFRYR | 1 (ST) | 0 1 |
| CRDVEYGVSR | 1 (Y) | ||||
| ELCSQVASNIKCLCK | 1 (ST) | ||||
| Stress response | |||||
| 9 | A0A094ZZQ1 | 60 kDa heat shock protein, mitochondrial | SPKITK | 1 (ST) | 0 1 |
| GTLAPAR | 1 (ST) | ||||
| GSKTDVDK | 2 (ST) | ||||
| DGVTVAKGIELK | 1 (ST) | ||||
| EESGANVAGMGGMGGMGGMGGMM | 1 (ST) | ||||
| Signaling | |||||
| 10 | A0A095A3D3 | 20 kDa calcium-binding protein | FNNDSYK | 1 (Y) | 0 1 |
| QCLTNHSNQSIR | 1 (ST) | ||||
| ENSSTMR | 2 (ST) | ||||
| SVRLSIIPLNPIYK | 1 (ST), 1 (Y) | ||||
| 11 | G4LZ30 | Calcium-binding protein, putative | LVSKENPSTMR | 3 (ST) | 0 1 |
| SVRLSIIPLNPIYK | 1 (ST), 1(Y) | ||||
| Unknown | |||||
| 12 | A0A095CG55 | Hypothetical protein MS3_10497, partial | AQAVISQR | 1 (ST) | 0 1 |
| SLLSALSTVYSHR | 3 (ST), 1 (Y) | ||||
| 13 | G4VH32 | Hypothetical protein Smp_179900 | SQISNSEASNFDNSK | 1 (ST) | 0 1 |
| VVVHSTVSMPFTPSGLSTGNR | 2 (ST) | ||||
| 14 | C7TYF2 | Hypothetical protein | TVINEMSLIPSINK | 1 (ST) | 0 1 |
| 15 | G4V5K0 | Hypothetical protein Smp_057140 | QSSSFFMR | 1 (ST) | 0 1 |
| YIIASARQR | 1 (ST) | ||||
| SLLSALSTVYSHR | 3 (ST), 1 (Y) | ||||
| HSSGSDSVVGAITTR | 4 (ST) | ||||
| QSCSLQYKYIIASAR | 1 (Y) | ||||
| No. | Accession No. Uniprot | Protein Name | Status | |
|---|---|---|---|---|
| Expression | Phosphorylation | |||
| Proteins involving with calcium binding | ||||
| 1 | C1L7Y4 | Annexin A13 (Annexin XIII) | Up | – |
| 2 | C1LNX4 | Voltage-dependent anion-selective channel protein 2 | Down | – |
| 3 | G4M1U8 | Voltage-dependent anion-selective channel, putative | Down | – |
| 4 | G4VL68 | Putative annexin | Down | Up |
| 5 | Q03528 | 22.6kd tegumental associated antigen | – | Up |
| 6 | G4LZ30 | Calcium-binding protein, putative | – | Down |
| 7 | A0A095A3D3 | 20 kDa calcium-binding protein | – | Down |
| 8 | C1L9U2 | Tegument antigen (I(H)A) | – | Down |
| 9 | G4VH32 | Hypothetical protein Smp_179900 | – | Down |
| Proteins involving with worm antigens | ||||
| 10 | Q26595 | Alpha tubulin | Up | – |
| 11 | Q86DV9 | Similar to GenBank Accession Number Z29075 myophilin antigen in Echinococcus granulosus | Up | – |
| 12 | A0A094ZPJ2 | Putative citrate synthase 2, mitochondrial | Up | – |
| 13 | 55kD antigen | Up | – | |
| 14 | Q06814 | Antigen | Down | – |
| 15 | Q5D9C5 | SJCHGC09453 protein | Down | – |
| 16 | P08418 | 70,000 mol wt antigen/hsp70 homolog (619 AA), partial | – | Up |
| 17 | Q03528 | 22.6kd tegumental associated antigen | – | Up |
| 18 | C1L9U2 | Tegument antigen (I(H)A) | – | Down |
| 19 | A0A094ZZQ1 | 60 kDa heat shock protein, mitochondrial | – | Down |
| Proteins involving with oxidative stress | ||||
| 20 | C1LV40 | Tryparedoxin peroxidase | Up | – |
| 21 | Q26513 | Glutathione-S-transferase | Up | – |
| 22 | A0A094ZC89 | L-lactate dehydrogenase A chain | Up | – |
| 23 | P37227 | Malate dehydrogenase, partial | Up | – |
| 24 | C1LD38 | Glyceraldehyde 3-phosphate dehydrogenase | Up | – |
| 25 | A0A095AJN4 | Enolase | Up | – |
| 26 | Q7Z1I3 | Lactate dehydrogenase-like protein | Up | – |
| 27 | A0A094ZPJ2 | Putative citrate synthase 2, mitochondrial | Up | – |
| 28 | Q8WT66 | Phosphoglycerate mutase | Up | – |
| 29 | C1LV81 | Aldolase | Up | – |
| 30 | C1LFZ8 | Arginine kinase | Down | – |
| 31 | A0A095B084 | Pyruvate kinase PKM | Down | – |
| 32 | C7TRL1 | Glyceraldehyde 3-phosphate dehydrogenase | Down | – |
| 33 | C1LD30 | Glyceraldehyde 3-phosphate dehydrogenase | Down | – |
| 34 | B2LXU3 | Glyceraldehyde 3-phosphate dehydrogenase | Down | – |
| 35 | B2LXU1 | Enolase | Down | – |
| 36 | C1LFP4 | Putative aldehyde dehydrogenase 1B1 precursor | Down | – |
| 37 | G4LWI3 | Aldehyde dehydrogenase, putative | Down | – |
| 38 | A0A095A4B6 | Aldehyde dehydrogenase X, mitochondrial | Down | – |
| 39 | Q5DAK2 | SJCHGC06828 protein | Down | – |
| 40 | Q5BXP5 | SJCHGC05927 protein, partial | Down | – |
| 41 | Q5DAM7 | SJCHGC06305 protein | Down | – |
| 42 | Q5DFZ8 | SJCHGC00411 protein | Down | – |
| 43 | C1L6T7 | Hypothetical protein | Down | – |
| 44 | G4VBJ0 | Putative malate dehydrogenase | – | Up |
| 45 | A0A095A0 V3 | Peroxiredoxin-2 | – | Up |
| 46 | G4LXF8 | 26 Kd glutathione-S-transferase, Sm26, putative | – | Up |
| 47 | O97161 | Thioredoxin peroxidase | – | Down |
| Proteins involving with protein folding and proteolysis | ||||
| 48 | P43157 | Cathepsin B | Up | Up |
| 49 | C1L5C5 | Putative aminopeptidase W07G4.4 | Up | – |
| 50 | A0A094ZYF3 | Putative aminopeptidase W07G4.4 | Up | – |
| 51 | A0A095B296 | Cathepsin B-like cysteine proteinase | Up | – |
| 52 | C1LA34 | Cathepsin B-like cysteine proteinase precursor | Up | – |
| 53 | C1L8N7 | Putative Lysosomal Pro-X carboxypeptidase precursor | Up | – |
| 54 | G4LYP1 | Mitochondrial processing peptidase beta-subunit (M16 family) | Up | – |
| 55 | G4VPS5 | Putative chaperonin containing t-complex protein 1, theta subunit, tcpq | Up | – |
| 56 | A0A095AUU9 | T-complex protein 1 subunit zeta | Up | – |
| 57 | A0A095CBQ7 | T-complex protein 1 subunit theta | Up | – |
| 58 | A9CBJ4 | DNA repair protein | Up | – |
| 59 | Q26551 | Cyclophilin B | Up | – |
| 60 | Q5DC69 | SJCHGC01960 protein, partial | Up | – |
| 61 | Q5DDG6 | SJCHGC02536 protein | Up | – |
| 62 | Q5DE64 | Unknown | Up | – |
| 63 | Q5C3A0 | Unknown | Up | – |
| 64 | Q5DGY1 | Unknown | Up | – |
| 65 | C1L6Z6 | Mitochondrial processing peptidase | Down | – |
| 66 | C1LNT2 | Serine protease inhibitor serpin | Down | – |
| 67 | O45039 | HSP70, partial | Down | – |
| 68 | C7TZI9 | Heat shock protein 90kDa alpha, partial | Down | – |
| 69 | G4VJ99 | Putative heat shock protein | Down | – |
| 70 | C1LEA0 | TNF receptor-associated protein 1 | Down | – |
| 71 | Q06814 | Calreticulin | Down | – |
| 72 | Q5BYY8 | SJCHGC04813 protein, partial | Down | – |
| 73 | Q5C3 V3 | SJCHGC05011 protein, partial | Down | – |
| 74 | Q5DBB0 | SJCHGC02058 protein | Down | – |
| 75 | Q5DGD4 | SJCHGC06677 protein | Down | – |
| 76 | Q5D9K8 | Unknown | Down | – |
| 77 | Q5DBJ9 | Unknown | Down | – |
| 78 | G4LWI2 | Heat shock protein HSP60, putative | – | Up |
| 79 | Q8MXA4 | Heat shock protein HSP60, partial | – | Up |
| 80 | Q5DGY9 | SJCHGC06312 protein | – | Up |
| 81 | A0A094ZZQ1 | 60 kDa heat shock protein, mitochondrial | – | Down |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chienwichai, P.; Ampawong, S.; Adisakwattana, P.; Thiangtrongjit, T.; Limpanont, Y.; Chusongsang, P.; Chusongsang, Y.; Reamtong, O. Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome. Pathogens 2020, 9, 417. https://doi.org/10.3390/pathogens9060417
Chienwichai P, Ampawong S, Adisakwattana P, Thiangtrongjit T, Limpanont Y, Chusongsang P, Chusongsang Y, Reamtong O. Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome. Pathogens. 2020; 9(6):417. https://doi.org/10.3390/pathogens9060417
Chicago/Turabian StyleChienwichai, Peerut, Sumate Ampawong, Poom Adisakwattana, Tipparat Thiangtrongjit, Yanin Limpanont, Phiraphol Chusongsang, Yupa Chusongsang, and Onrapak Reamtong. 2020. "Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome" Pathogens 9, no. 6: 417. https://doi.org/10.3390/pathogens9060417
APA StyleChienwichai, P., Ampawong, S., Adisakwattana, P., Thiangtrongjit, T., Limpanont, Y., Chusongsang, P., Chusongsang, Y., & Reamtong, O. (2020). Effect of Praziquantel on Schistosoma mekongi Proteome and Phosphoproteome. Pathogens, 9(6), 417. https://doi.org/10.3390/pathogens9060417






