Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review
Abstract
1. Introduction
1.1. Human Papillomaviruses (HPVs) and Their Role in Colorectal Cancer
1.2. Epstein Barr Virus and its Role in Human Colorectal Cancer
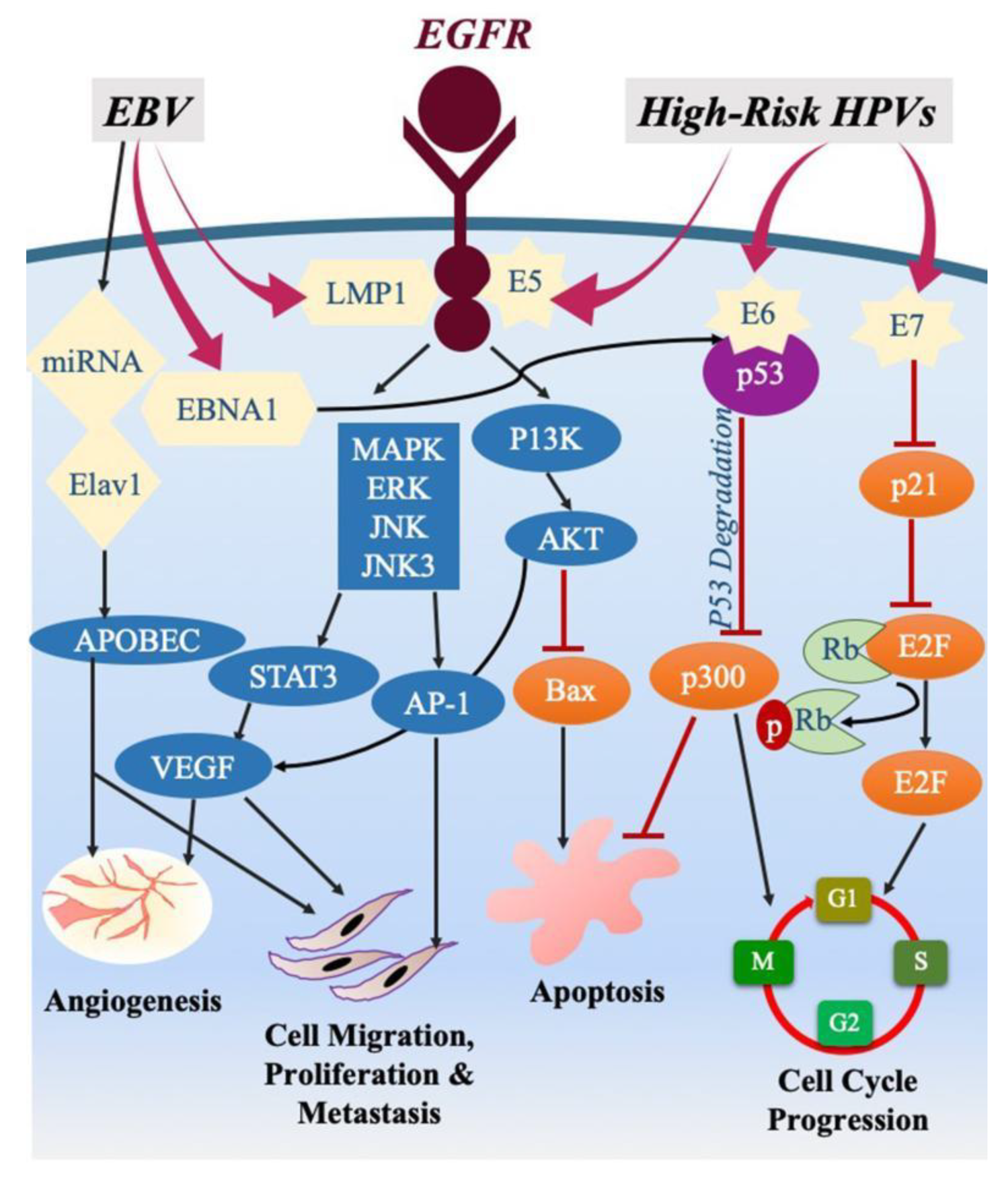
1.3. The Interaction of High-Risk HPVs and EBV in Human Cancers
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, R.G.; Faria, F.F.; Lima Junior, A.C.B.; Rodrigues, F.G.; Andrade, M.M.D.A.; Gomes, D.M.B.M.; Neves, P.M.; Constantino, J.R.M.; Braga, Á.C.G.; Ferreira, R.M.S.; et al. Surgery in colorectal cancer: Surgical approach of 74 patients from the Brazilian National Health System with colorectal cancer in a postgraduate program (residency) in coloproctology. Rev. Bras. Cancerol. 2011, 31, 44–57. [Google Scholar]
- Souto, R.; Falhari, J.P.B.; Cruz, A.D. Aparecido Divino da Cruz. O Papilomavírus Humano: Um fator relacionado com a formação de neoplasias. Rev. Bras. Cancerol. 2005, 51, 155–160. [Google Scholar]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon. Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Shrubsole, M.J.; Smalley, W.E.; Wu, H.; Chen, Z.; Shyr, Y.; Ness, R.M.; Zheng, W. Lifestyle factors and their combined impact on the risk of colorectal polyps. Am. J. Epidemiol. 2012, 176, 766–776. [Google Scholar] [CrossRef]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zheng, B.; He, Z.; Winberg, G.; Ernberg, I. An update on viral association of human cancers. Arch. Virol. 2013, 158, 1433–1443. [Google Scholar] [CrossRef]
- Aran, V.; Victorino, A.P.; Thuler, L.C.; Ferreira, C.G. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin. Colorectal Cancer 2016, 15, 195–203. [Google Scholar] [CrossRef]
- Leila, Z.; Arabzadeh, S.A.; Afshar, R.M.; Afshar, A.A.; Mollaei, H.R. Detection of Epstein-Barr Virus and Cytomegalovirus in Gastric Cancers in Kerman, Iran. Asian Pac. J. Cancer Prev. 2016, 17, 2423–2428. [Google Scholar]
- Al Moustafa, A.-E.; Al-Awadhi, R.; Missaoui, N.; Adam, I.; Durusoy, R.; Ghabreau, L.; Akil, N.; Ahmed, H.G.; Yasmeen, A.; Alsbeih, G. Human papillomaviruses-related cancers. Presence and prevention strategies in the Middle east and north African regions. Hum. Vaccines Immunother. 2014, 10, 1812–1821. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E.; Al-Antary, N.; Aboulkassim, T.; Akil, N.; Batist, G.; Yasmeen, A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum. Vaccines Immunother. 2016, 12, 1936–1939. [Google Scholar] [CrossRef]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U. The clinical importance of the nomenclature, evolution and taxonomy of human papillomaviruses. J. Clin. Virol. 2005, 32, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, E.; Bianchi, S.; Frati, E.R.; Amicizia, D.; Martinelli, M.; Bragazzi, N.L.; Brisigotti, M.P.; Colzani, D.; Fasoli, E.; Zehender, G.; et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J. Gen. Virol. 2015, 96, 206–209. [Google Scholar] [CrossRef][Green Version]
- Heng, B.; Glenn, W.K.; Ye, Y.; Tran, B.; Delprado, W.; Lutze-Mann, L.; Whitaker, N.J.; Lawson, J.S. Human papilloma virus is associated with breast cancer. Br. J. Cancer 2009, 101, 1345–1350. [Google Scholar] [CrossRef]
- Barghi, M.R.; Rahjoo, T.; Borghei, M.; Hosseini-Moghaddam, S.M.; Amani, D.; Farrokhi, B. Association between the evidence of human papilloma virus infection in bladder transitional cell carcinoma in men and cervical dysplasia in their spouses. Arch. Iran Med. 2012, 15, 572–574. [Google Scholar]
- Guo, F.; Liu, Y.; Wang, X.; He, Z.; Weiss, N.S.; Madeleine, M.M.; Liu, F.; Tian, X.; Song, Y.; Pan, Y.; et al. Human papillomavirus infection and esophageal squamous cell carcinoma: A case-control study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 780–785. [Google Scholar] [CrossRef]
- Stanley, M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef]
- Gornick, M.C.; Castellsague, X.; Sanchez, G.; Giordano, T.J.; Vinco, M.; Greenson, J.K.; Capella, G.; Raskin, L.; Rennert, G.; Gruber, S.B.; et al. Human papillomavirus is not associated with colorectal cancer in a large international study. Cancer Causes Control 2010, 21, 737–743. [Google Scholar] [CrossRef]
- Lorenzon, L.; Mazzetta, F.; Pilozzi, E.; Uggeri, G.; Torrisi, M.R.; Ferri, M.; Ziparo, V.; French, D. Human papillomavirus does not have a causal role in colorectal carcinogenesis. World J. Gastroenterol. 2015, 21, 342–350. [Google Scholar] [CrossRef]
- Damin, D.C.; Caetano, M.B.; Rosito, M.A.; Schwartsmann, G.; Damin, A.S.; Frazzon, A.P.; Ruppenthal, R.D.; Alexandre, C.O.P. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur. J. Surg. Oncol. EJSO 2007, 33, 569–574. [Google Scholar] [CrossRef]
- Ragin, C.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef]
- Bernabe-Dones, R.D.; Gonzalez-Pons, M.; Villar-Prados, A.; Lacourt-Ventura, M.; Rodríguez-Arroyo, H.; Fonseca-Williams, S.; Velazquez, F.E.; Diaz-Algorri, Y.; Lopez-Diaz, S.M.; Rodríguez, N.; et al. High Prevalence of Human Papillomavirus in Colorectal Cancer in Hispanics: A Case-Control Study. Gastroenterol. Res. Pract. 2016, 2016, 7896716. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, S.; Yamanegi, K.; Xiao, S.-Y.; Da Costa, M.; Palefsky, J.M.; Zheng, Z.-M. Colorectal papillomavirus infection in patients with colorectal cancer. Clin. Cancer Res. 2005, 11, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.B.G.; Rizzo, C.; Mafera, B.; Rahimi, S.; Vigili, M. Presence of HPV in head and neck tumours: High prevalence in tonsillar localization. J. Exp. Clin. Cancer Res. 2004, 23, 561–566. [Google Scholar] [PubMed]
- Bedri, S.; Sultan, A.A.; Alkhalaf, M.; Al Moustafa, A.-E.; Vranic, S. Epstein-Barr virus (EBV) status in colorectal cancer: A mini review. Hum. Vaccines Immunother. 2019, 15, 603–610. [Google Scholar] [CrossRef]
- Mui, U.N.; Haley, C.T.; Tyring, S.K. Viral Oncology: Molecular Biology and Pathogenesis. J. Clin. Med. 2017, 6, 111. [Google Scholar] [CrossRef]
- De Lima, M.A.P.; Neto, P.J.N.; Lima, L.P.M.; Gonçalves Júnior, J.; Teixeira Junior, A.G.; Teodoro, I.P.P.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr virus (EBV) and cervical carcinoma: A meta-analysis. Gynecol. Oncol. 2018, 148, 317–328. [Google Scholar] [CrossRef]
- Vedham, V.; Verma, M.; Mahabir, S. Early-life exposures to infectious agents and later cancer development. Cancer Med. 2015, 4, 1908–1922. [Google Scholar] [CrossRef]
- Vockerodt, M.; Yap, L.F.; Shannon-Lowe, C.; Curley, H.; Wei, W.; Vrzalikova, K.; Murray, P.G. The Epstein-Barr virus and the pathogenesis of lymphoma. J. Pathol. 2015, 235, 312–322. [Google Scholar] [CrossRef]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, N.J.; Glenn, W.K.; Sahrudin, A.; Orde, M.M.; Delprado, W.; Lawson, J.S. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate 2013, 73, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.R.; Ramezani, M.; Janbakhsh, A.; Sadeghi, M. Malignant Salivary Gland Tumors and Epstein-Barr Virus (EBV) Infection: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2017, 18, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, H.; Ghabreau, L.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.-E. Co-Incidence of Epstein-Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian Women. Front. Oncol. 2018, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000, 92, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Lowy, D.R.; Frazer, I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine 2006, 24, 106–113. [Google Scholar] [CrossRef]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef]
- Pinidis, P.; Tsikouras, P.; Iatrakis, G.; Zervoudis, S.; Koukouli, Z.; Bothou, A.; Galazios, G.; Vladareanu, S. Human Papilloma Virus’ Life Cycle and Carcinogenesis. Maedica 2016, 11, 48–54. [Google Scholar]
- Psyrri, A.; DiMaio, D. Human papillomavirus in cervical and head-and-neck cancer. Nat. Clin. Pract. Oncol. 2008, 5, 24–31. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Munger, K. Viruses associated with human cancer. Biochim. Biophys. Acta 2008, 1782, 127–150. [Google Scholar] [CrossRef]
- Korzeniewski, N.; Spardy, N.; Duensing, A.; Duensing, S. Genomic instability and cancer: Lessons learned from human papillomaviruses. Cancer Lett. 2011, 305, 113–122. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef]
- Ghabreau, L.; Segal, E.; Yasmeen, A.; Kassab, A.; Akil, N.; Al Moustafa, A.-E. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: A tissue microarray study. Clin. Cancer Investig. J. 2012, 1, 26–30. [Google Scholar] [CrossRef]
- Salepci, T.; Yazici, H.; Dane, F.; Topuz, E.; Dalay, N.; Onat, H.; Aykan, F.; Seker, M.; Aydiner, A. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. J. BUON 2009, 14, 495–499. [Google Scholar] [PubMed]
- Buyru, N.; Tezol, A.; Dalay, N. Coexistence of K-ras mutations and HPV infection in colon cancer. BMC Cancer 2006, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Yavuzer, D.; Karadayi, N.; Salepci, T.; Baloglu, H.; Dabak, R.; Bayramicli, O.U. Investigation of human papillomavirus DNA in colorectal carcinomas and adenomas. Med. Oncol. 2011, 28, 127–132. [Google Scholar] [CrossRef]
- Damin, D.C.; Ziegelmann, P.K.; Damin, A.P. Human papillomavirus infection and colorectal cancer risk: A meta-analysis. Colorectal Dis. 2013, 15, e420–e428. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.F.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int.J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E. Role of high-risk human papillomaviruses in breast carcinogenesis. In Breast Carcinogenesis; Oncoviruses and Their Inhibitors; Gupta, S., Ed.; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2014; pp. 245–262. [Google Scholar]
- Malekpour Afshar, R.; Deldar, Z.; Mollaei, H.R.; Arabzadeh, S.A.; Iranpour, M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pac. J. Cancer Prev. 2018, 19, 193–198. [Google Scholar] [CrossRef]
- Mahmoudvand, S.; Safaei, A.; Erfani, N.; Sarvari, J. Presence of Human Papillomavirus DNA in Colorectal Cancer Tissues in Shiraz, Southwest Iran. Asian Pac. J. Cancer Prev. 2015, 16, 7883–7887. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Saberfar, E.; Shamsaie, A.; Ghasemian, E. The aetiological role of human papillomavirus in colorectal carcinoma: An Iranian population- based case control study. Asian Pac. J. Cancer Prev. 2014, 15, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, A.; Azadeh, P.; Hashemi, M.; Molaei, M.; MajidSheibani, K.; Alidoosti, A.; Fazlalizadeh, A.; Shafaghi, B.; Fudazi, M.; Valaei, N.; et al. Human Papillomavirus Infection, p53 Overexpression and Histopathologic Characteristics in Colorectal Cancer. Govaresh 2012, 12, 8. [Google Scholar]
- Kirgan, D.; Manalo, P.; McGregor, B. Immunohistochemical demonstration of human papilloma virus antigen in human colon neoplasms. J. Surg. Res. 1990, 48, 397–402. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Sheu, L.F.; Meng, C.L.; Lee, W.H.; Lin, J.C. Detection of human papillomavirus DNA in colorectal carcinomas by polymerase chain reaction. Gut 1995, 37, 87–90. [Google Scholar] [CrossRef][Green Version]
- McGregor, B.; Byrne, P.; Kirgan, D.; Albright, J.; Manalo, P.; Hall, M. Confirmation of the association of human papillomavirus with human colon cancer. Am. J. Surg. 1993, 166, 738–740. [Google Scholar] [CrossRef]
- Soto, Y.; Limia, C.M.; Gonzalez, L.; Gra, B.; Hano, O.M.; Martinez, P.A.; Kouri, V. Molecular evidence of high-risk human papillomavirus infection in colorectal tumours from Cuban patients. Mem. Inst. Oswaldo Cruz 2016, 111, 731–736. [Google Scholar] [CrossRef]
- Ibragimova, M.K.; Tsyganov, M.M.; Litviakov, N.V. Human papillomavirus and colorectal cancer. Med. Oncol. 2018, 35, 140. [Google Scholar] [CrossRef]
- Chakrabarti, O.; Krishna, S. Molecular interactions of ‘high risk’ human papillomaviruses E6 and E7 oncoproteins: Implications for tumour progression. J. Biosci. 2003, 28, 337–348. [Google Scholar] [CrossRef]
- Pett, M.R.; Alazawi, W.O.; Roberts, I.; Dowen, S.; Smith, D.I.; Stanley, M.A.; Coleman, N. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004, 64, 1359–1368. [Google Scholar] [CrossRef]
- Badaracco, G.; Venuti, A.; Sedati, A.; Marcante, M.L. HPV16 and HPV18 in genital tumors: Significantly different levels of viral integration and correlation to tumor invasiveness. J. Med. Virol. 2002, 67, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A.; Bismar, T.A.; Kandouz, M.; Foulkes, W.D.; Desprez, P.-Y.; Al Moustafa, A.-E. E6/E7 of HPV Type 16 Promotes Cell Invasion and Metastasis of Human Breast Cancer Cells. Cell Cycle 2007, 6, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A.; Hosein, A.N.; Yu, Q.; Al Moustafa, A.-E. Critical role for D-type cyclins in cellular transformation induced by E6/E7 of human papillomavirus type 16 and E6/E7/ErbB-2 cooperation. Cancer Sci. 2007, 98, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Foulkes, W.D.; Wong, A.; Jallal, H.; Batist, G.; Yu, Q.; Herlyn, M.; Sicinski, P.; Alaoui-Jamali, M.A. Cyclin D1 is essential for neoplastic transformation induced by both E6/E7 and E6/E7/ErbB-2 cooperation in normal cells. Oncogene 2004, 23, 5252–5256. [Google Scholar] [CrossRef][Green Version]
- Yasmeen, A.; Alachkar, A.; Dekhil, H.; Gambacorti-Passerini, C.; Al Moustafa, A.-E. Locking Src/Abl Tyrosine Kinase Activities Regulate Cell Differentiation and Invasion of Human Cervical Cancer Cells Expressing E6/E7 Oncoproteins of High-Risk HPV. J. Oncol. 2010. [Google Scholar] [CrossRef]
- Coluccia, A.M.L.; Benati, D.; Dekhil, H.; De Filippo, A.; Lan, C.; Gambacorti-Passerini, C. SKI-606 Decreases Growth and Motility of Colorectal Cancer Cells by Preventing pp60(c-Src)–Dependent Tyrosine Phosphorylation of β-Catenin and Its Nuclear Signaling. Cancer Res. 2006, 66, 2279–2286. [Google Scholar] [CrossRef]
- Al Moustafa, A.; Kassab, A.; Darnel, A.; Yasmeen, A. High-risk HPV/ErbB-2 interaction on E-cadherin/catenin regulation in human carcinogenesis. Curr. Pharm. Des. 2008, 14, 2159–2172. [Google Scholar] [CrossRef]
- Ricciardi, R.; Ghabreau, L.; Yasmeen, A.; Darnel, A.D.; Akil, N.; Al Moustafa, A.E. Role of E6/E7 onco-proteins of high-risk human papillomaviruses in human colorectal carcinogenesis. Cell Cycle 2009, 8, 1964–1965. [Google Scholar] [CrossRef]
- Kim, S.H.; Juhnn, Y.S.; Kang, S.; Park, S.W.; Sung, M.W.; Bang, Y.J.; Song, Y.S. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell. Mol. Life Sci. CMLS 2006, 63, 930–938. [Google Scholar] [CrossRef]
- Suprynowicz, F.A.; Disbrow, G.L.; Krawczyk, E.; Simic, V.; Lantzky, K.; Schlegel, R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene 2008, 27, 1071–1078. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kim, S.-H.; Cho, E.-A.; Song, Y.-S.; Kim, W.-H.; Juhnn, Y.-S. Human papillomavirus type 16 E5 protein inhibits hydrogen peroxide-induced apoptosis by stimulating ubiquitin–proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis 2009, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, Y.B.; Suh, K.W.; Paek, O.J.; Moon, H.Y. Prognostic Impact of Fascin-1 Expression is More Significant in Advanced Colorectal Cancer. J. Sur. Res. 2012, 172, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-T.; Wang, X.; Zhang, X.; Wong, Y.-C. The multiple roles of Id-1 in cancer progression. Differentiation 2006, 74, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Van Marck, V.; Stove, C.; Jacobs, K.; Van den Eynden, G.; Bracke, M. P-cadherin in adhesion and invasion: Opposite roles in colon and bladder carcinoma. Int. J. Cancer 2011, 128, 1031–1044. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh. Migr. 2015, 9, 392–393. [Google Scholar] [CrossRef]
- Ferber, M.J.; Thorland, E.C.; Brink, A.A.; Rapp, A.K.; Phillips, L.A.; McGovern, R.; Gostout, B.S.; Cheung, T.H.; Chung, T.K.; Fu, W.Y.; et al. Preferential integration of human papillomavirus type 18 near the c-myc locus in cervical carcinoma. Oncogene 2003, 22, 7233–7242. [Google Scholar] [CrossRef]
- Thorland, E.C.; Myers, S.L.; Gostout, B.S.; Smith, D.I. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene 2003, 22, 1225–1237. [Google Scholar] [CrossRef]
- Ryan, K.M.; Birnie, G.D. Myc oncogenes: The enigmatic family. Biochem. J. 1996, 314, 713–721. [Google Scholar] [CrossRef][Green Version]
- Augenlicht, L.H.; Wadler, S.; Corner, G.; Richards, C.; Ryan, L.; Multani, A.S.; Pathak, S.; Benson, A.; Haller, D.; Heerdt, B.G. Low-level c-myc amplification in human colonic carcinoma cell lines and tumors: A frequent, p53-independent mutation associated with improved outcome in a randomized multi-institutional trial. Cancer Res. 1997, 57, 1769–1775. [Google Scholar]
- Obara, K.; Yokoyama, M.; Asano, G.; Tanaka, S. Evaluation of myc and chromosome 8 copy number in colorectal cancer using interphase cytogenetics. Int. J. Oncol. 2001, 18, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, B.; Simon, R.; Ruiz, C.; Oeggerli, M.; Mihatsch, M.J.; Gasser, T.; Sauter, G.; Toncheva, D. High-throughput tissue microarray analysis of CMYC amplificationin urinary bladder cancer. Int. J. Cancer 2005, 117, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar] [PubMed]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Immune escape of tumors: Apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002, 71, 907–920. [Google Scholar]
- Jiang, P.; Yue, Y. Human papillomavirus oncoproteins and apoptosis (Review). Exp. Ther. Med. 2014, 7, 3–7. [Google Scholar] [CrossRef]
- Kabsch, K.; Alonso, A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J. Virol. 2002, 76, 12162–12172. [Google Scholar] [CrossRef]
- Karbasi, A.; Borhani, N.; Daliri, K.; Kazemi, B.; Manoochehri, M. Downregulation of external death receptor genes FAS and DR5 in colorectal cancer samples positive for human papillomavirus infection. Pathol. Res. Pract. 2015, 211, 444–448. [Google Scholar] [CrossRef]
- Burnett-Hartman, A.N.; Feng, Q.; Popov, V.; Kalidindi, A.; Newcomb, P.A. Human papillomavirus DNA is rarely detected in colorectal carcinomas and not associated with microsatellite instability: The Seattle colon cancer family registry. Cancer Epidemiol. Biomark. Prev. 2013, 22, 317–319. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G. Various forms of Epstein-Barr virus infection in man: Established facts and a general concept. Lancet 1973, 2, 836–839. [Google Scholar] [CrossRef]
- Epstein, M.A.; Henle, G.; Achong, B.G.; Barr, Y.M. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt’s lymphoma. J. Exp. Med. 1965, 121, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Kieff, E.; Given, D.; Powell, A.L.; King, W.; Dambaugh, T.; Raab-Traub, N. Epstein-Barr virus: Structure of the viral DNA and analysis of viral RNA in infected cells. Biochim. Biophys. Acta 1979, 560, 355–373. [Google Scholar] [CrossRef]
- Evans, A.S. The spectrum of infections with Epstein-Barr virus: A hypothesis. J. Infect. Dis. 1971, 124, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Crawford, D.H. Epstein-Barr virus: The impact of scientific advances on clinical practice. Blood 2006, 107, 862–869. [Google Scholar] [CrossRef]
- Borza, C.M.; Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 2002, 8, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Tsao, S.W. The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol. Sin. 2015, 30, 107–121. [Google Scholar] [CrossRef]
- Fernandes, Q.; Merhi, M.; Raza, A.; Inchakalody, V.P.; Abdelouahab, N.; Zar Gul, A.R.; Uddin, S.; Dermime, S. Role of Epstein-Barr Virus in the Pathogenesis of Head and Neck Cancers and Its Potential as an Immunotherapeutic Target. Front. Oncol. 2018, 8, 257. [Google Scholar] [CrossRef]
- Chen, X.Z.; Chen, H.; Castro, F.A.; Hu, J.K.; Brenner, H. Epstein-Barr virus infection and gastric cancer: A systematic review. Medicine 2015, 94, e792. [Google Scholar] [CrossRef]
- Longnecker, R.M.; Kieff, E.; Cohen, J.I. Epstein-Barr virus. In Fields Virology, 6th ed.; Wolters Kluwer Health Adis (ESP): Alphen aan den Rijn, The Netherlands, 2013; Volume 1. [Google Scholar]
- Rickinson, A. Epstein-Barr virus. Virus. Res. 2002, 82, 109–113. [Google Scholar] [CrossRef]
- Ambinder, R.F.; Browning, P.J.; Lorenzana, I.; Leventhal, B.G.; Cosenza, H.; Mann, R.B.; MacMahon, E.M.; Medina, R.; Cardona, V.; Grufferman, S. Epstein-Barr virus and childhood Hodgkin’s disease in Honduras and the United States. Blood 1993, 81, 462–467. [Google Scholar] [CrossRef]
- Fox, R.I.; Luppi, M.; Pisa, P.; Kang, H.I. Potential role of Epstein-Barr virus in Sjögren’s syndrome and rheumatoid arthritis. J. Rheumatol. Suppl. 1992, 32, 18–24. [Google Scholar] [PubMed]
- Evans, A.S. The history of infectious mononucleosis. Am. J. Med. Sci. 1974, 267, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Rickinson, A.B. Co-infections, inflammation and oncogenesis: Future directions for EBV research. Semin. Cancer Biol. 2014, 26, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Tafvizi, F.; Fard, Z.T.; Assareh, R. Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Pol. J. Pathol. 2015, 66, 154–160. [Google Scholar] [CrossRef]
- Liu, H.X.; Ding, Y.Q.; Li, X.; Yao, K.T. Investigation of Epstein-Barr virus in Chinese colorectal tumors. World J. Gastroenterol. 2003, 9, 2464–2468. [Google Scholar] [CrossRef]
- Sarvari, J.; Mahmoudvand, S.; Pirbonyeh, N.; Safaei, A.; Hosseini, S.Y. The Very Low Frequency of Epstein-Barr JC and BK Viruses DNA in Colorectal Cancer Tissues in Shiraz, Southwest Iran. Pol. J. Microbiol. 2018, 67, 73–79. [Google Scholar] [CrossRef]
- Gupta, I.; Jabeen, A.; Skenderi, F.; Malki, M.I.; Al-Thawadi, H.; Al Moustafa, A.E.; Vranic, S. High-risk Human Papillomaviruses (HPV) and Epstein—Barr virus (EBV) are Commonly Present in Rectal Cancer. Mod. Pathol. 2020, 33, 676. [Google Scholar]
- Malki, M.I.; Gupta, I.; Fernandes, Q.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.E.; Al-Thawadi, H.A. Co-presence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum. Vaccins Immunother. 2020, 1–5. [Google Scholar] [CrossRef]
- Martins, S.F.; Mariano, V.; Rodrigues, M.; Longatto-Filho, A. Human papillomavirus (HPV) 16 infection is not detected in rectal carcinoma. Infect. Agents Cancer 2020, 15, 17. [Google Scholar] [CrossRef]
- Jarzyński, A.; Zając, P.; Żebrowski, R.; Boguszewska, A.; Polz-Dacewicz, M. Occurrence of BK Virus and Human Papilloma Virus in colorectal cancer. Ann. Agric. Environ. Med. 2017, 24, 440–445. [Google Scholar] [CrossRef]
- Pelizzer, T.; Dias, C.P.; Poeta, J.; Torriani, T.; Roncada, C. Colorectal cancer prevalence linked to human papillomavirus: A systematic review with meta-analysis. Braz. J. Epidemiol. 2016, 19, 791–802. [Google Scholar] [CrossRef]
- Pérez, L.O.; Barbisan, G.; Ottino, A.; Pianzola, H.; Golijow, C.D. Human papillomavirus DNA and oncogene alterations in colorectal tumors. Pathol. Oncol. Res. 2010, 16, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; Ronci, C.; Bonifacio, D.; Di Bonito, L.; Favalli, C.; Perno, C.F.; Syrjänen, K.; Ciotti, M. Detection of oncogenic DNA viruses in colorectal cancer. Anticancer Res. 2008, 28, 1405–1410. [Google Scholar] [PubMed]
- Bodaghi, S.; Wood, L.V.; Roby, G.; Ryder, C.; Steinberg, S.M.; Zheng, Z.-M. Could human papillomaviruses be spread through blood? J. Clin. Microbiol. 2005, 43, 5428–5434. [Google Scholar] [CrossRef]
- Pérez, L.O.; Abba, M.C.; Laguens, R.M.; Golijow, C.D. Analysis of adenocarcinoma of the colon and rectum: Detection of human papillomavirus (HPV) DNA by polymerase chain reaction. Colorectal Dis. 2005, 7, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.V.; Daniel, R.W.; Simons, J.W.; Vogelstein, B. Investigation of colon cancers for human papillomavirus genomic sequences by polymerase chain reaction. J. Surg. Oncol. 1992, 51, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Samaha, S.; Tawfik, O.; Horvat, R.; Bhatia, P. Lymphoepithelioma-like carcinoma of the colon: Report of a case with histologic, immunohistochemical, and molecular studies for Epstein-Barr virus. Dis. Colon. Rectum 1998, 41, 925–928. [Google Scholar] [CrossRef]
- Young, L.S.; Dawson, C.W. Epstein-Barr virus and nasopharyngeal carcinoma. Chin. J. Cancer 2014, 33, 581–590. [Google Scholar] [CrossRef]
- Terada, T. Epstein-Barr virus associated lymphoepithelial carcinoma of the esophagus. Int. J. Clin. Exp. Med. 2013, 6, 219–226. [Google Scholar]
- Liu, C.-Y.; Huang, S.-H. EBV-associated lymphoepithelioma-like thyroid carcinoma with favorable outcome: Case report with cytopathologic and histopathologic study. Diagn. Pathol. 2018, 13, 39. [Google Scholar] [CrossRef]
- Hong, S.; Liu, D.; Luo, S.; Fang, W.; Zhan, J.; Fu, S.; Zhang, Y.; Wu, X.; Zhou, H.; Chen, X.; et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat. Commun. 2019, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
- Elawabdeh, N.; Cone, B.M.; Abramowsky, C.R.; Wrubel, D.M.; Grossniklaus, H.; Walrath, J.; Bashir, M.Z.; Shehata, B.M. Epstein-Barr virus associated smooth muscle tumors in post transplant pediatric patients two cases of rare locations, and review of the literature. Fetal Pediatr. Pathol. 2013, 32, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.B.; Bonatti, H.; Stauffer, J.; Dickson, R.C.; Nguyen, J.; Harnois, D.; Jeanpierre, C.; Hinder, R.; Steers, J.; Chua, H.; et al. Colorectal and anal neoplasms following liver transplantation. Colorectal Dis. 2010, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Locker, J.; Nalesnik, M.; Reyes, J.; Jaffe, R.; Alashari, M.; Nour, B.; Tzakis, A.; Dickman, P.S. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N. Engl. J. Med. 1995, 332, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Choi, M.-G.; Kim, S.W.; Chung, I.-S.; Yang, C.W.; Kim, Y.S.; Jung, C.K.; Lee, K.Y.; Kang, J.-H. Increased Incidence of Colorectal Malignancies in Renal Transplant Recipients: A Case Control Study. Am. J. Transplant. 2010, 10, 2043–2050. [Google Scholar] [CrossRef]
- Medlicott, S.A.; Devlin, S.; Helmersen, D.S.; Yilmaz, A.; Mansoor, A. Early post-transplant smooth muscle neoplasia of the colon presenting as diminutive polyps: A case complicating post-transplant lymphoproliferative disorder. Int. J. Surg. Pathol. 2006, 14, 155–161. [Google Scholar] [CrossRef]
- Song, L.B.; Zhang, X.; Zhang, C.Q.; Zhang, Y.; Pan, Z.Z.; Liao, W.T.; Li, M.Z.; Zeng, M.S. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Chin. J. Cancer 2006, 25, 1356–1360. [Google Scholar]
- Fiorina, L.; Ricotti, M.; Vanoli, A.; Luinetti, O.; Dallera, E.; Riboni, R.; Paolucci, S.; Brugnatelli, S.; Paulli, M.; Pedrazzoli, P.; et al. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect. Agents Cancer 2014, 9, 18. [Google Scholar] [CrossRef]
- Salyakina, D.; Tsinoremas, N.F. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum. Genom. 2013, 7, 23. [Google Scholar] [CrossRef]
- Guan, X.; Yi, Y.; Huang, Y.; Hu, Y.; Li, X.; Wang, X.; Fan, H.; Wang, G.; Wang, D. Revealing potential molecular targets bridging colitis and colorectal cancer based on multidimensional integration strategy. Oncotarget 2015, 6, 37600–37612. [Google Scholar] [CrossRef]
- Al-Antary, N.; Farghaly, H.; Aboulkassim, T.; Yasmeen, A.; Akil, N.; Al Moustafa, A.-E. Epstein-Barr virus and its association with Fascin expression in colorectal cancers in the Syrian population: A tissue microarray study. Hum. Vaccines Immunother. 2017, 13, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, P.; Myszka, A.; Ramsey, D.; Kielan, W.; Sasiadek, M.M. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol. 2011, 32, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani-Khasraghi, S.; Ameli, M.; Khalily, F. Demonstration of Herpes Simplex Virus, Cytomegalovirus, and Epstein-Barr Virus in Colorectal Cancer. Iran Biomed. J. 2016, 20, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Sole, C.V.; Calvo, F.A.; Ferrer, C.; Alvarez, E.; Carreras, J.L.; Ochoa, E. Human cytomegalovirus and Epstein-Barr virus infection impact on (18)F-FDG PET/CT SUVmax, CT volumetric and KRAS-based parameters of patients with locally advanced rectal cancer treated with neoadjuvant therapy. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 186–196. [Google Scholar] [CrossRef]
- Nishigami, T.; Kataoka, T.R.; Torii, I.; Sato, A.; Tamura, K.; Hirano, H.; Hida, N.; Ikeuchi, H.; Tsujimura, T. Concomitant adenocarcinoma and colonic non-Hodgkin’s lymphoma in a patient with ulcerative colitis: A case report and molecular analysis. Pathol. Res. Pract. 2010, 206, 846–850. [Google Scholar] [CrossRef]
- Militello, V.; Trevisan, M.; Squarzon, L.; Biasolo, M.A.; Rugge, M.; Militello, C.; Palù, G.; Barzon, L. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int. J. Cancer 2009, 124, 2501–2503. [Google Scholar] [CrossRef]
- Wong, N.A.; Herbst, H.; Herrmann, K.; Kirchner, T.; Krajewski, A.S.; Moorghen, M.; Niedobitek, F.; Rooney, N.; Shepherd, N.A.; Niedobitek, G. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: Detection of the virus in lymphomas but not in adenocarcinomas. J. Pathol. 2003, 201, 312–318. [Google Scholar] [CrossRef]
- Grinstein, S.; Preciado, M.V.; Gattuso, P.; Chabay, P.A.; Warren, W.H.; De Matteo, E.; Gould, V.E. Demonstration of Epstein-Barr Virus in Carcinomas of Various Sites. Cancer Res. 2002, 62, 4876–4878. [Google Scholar]
- Kijima, Y.; Hokita, S.; Takao, S.; Baba, M.; Natsugoe, S.; Yoshinaka, H.; Aridome, K.; Otsuji, T.; Itoh, T.; Tokunaga, M.; et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J. Med. Virol. 2001, 64, 513–518. [Google Scholar] [CrossRef]
- Cho, Y.J.; Chang, M.S.; Park, S.H.; Kim, H.S.; Kim, W.H. In situ hybridization of Epstein-Barr virus in tumor cells and tumor-infiltrating lymphocytes of the gastrointestinal tract. Hum. Pathol. 2001, 32, 297–301. [Google Scholar] [CrossRef]
- Yuen, S.T.; Chung, L.P.; Leung, S.Y.; Luk, I.S.; Chan, S.Y.; Ho, J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am. J. Surg. Pathol. 1994, 18, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Boguszaková, L.; Hirsch, I.; Brichácek, B.; Faltýn, J.; Fric, P.; Dvoráková, H.; Vonka, V. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol. 1988, 32, 303–308. [Google Scholar] [PubMed]
- Morewaya, J.; Koriyama, C.; Akiba, S.; Shan, D.; Itoh, T.; Eizuru, Y. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncol. Rep. 2004, 12, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kan, J.; Yuen, S.T.; Shi, S.T.; Chu, K.M.; Law, S.; Chan, T.L.; Kan, Z.; Chan, A.S.; Tsui, W.Y.; et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat. Genet. 2011, 43, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.J.; Cutcutache, I.; Poon, S.L.; Zhang, S.L.; McPherson, J.R.; Tao, J.; Rajasegaran, V.; Heng, H.L.; Deng, N.; Gan, A.; et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat. Genet. 2012, 44, 570–574. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Analysis Working Group. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Chen, K.; Yang, D.; Li, X.; Sun, B.; Song, F.; Cao, W.; Brat, D.J.; Gao, Z.; Li, H.; Liang, H.; et al. Mutational landscape of gastric adenocarcinoma in Chinese: Implications for prognosis and therapy. Proc. Natl. Acad. Sci. USA 2015, 112, 1107–1112. [Google Scholar] [CrossRef]
- Kim, T.M.; Jung, S.H.; Kim, M.S.; Baek, I.P.; Park, S.W.; Lee, S.H.; Lee, H.H.; Kim, S.S.; Chung, Y.J. The mutational burdens and evolutionary ages of early gastric cancers are comparable to those of advanced gastric cancers. J. Pathol. 2014, 234, 365–374. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Cajuso, T.; Hanninen, U.A.; Kondelin, J.; Gylfe, A.E.; Tanskanen, T.; Katainen, R.; Pitkanen, E.; Ristolainen, H.; Kaasinen, E.; Taipale, M.; et al. Exome sequencing reveals frequent inactivating mutations in ARID1A, ARID1B, ARID2 and ARID4A in microsatellite unstable colorectal cancer. Int. J. Cancer 2014, 135, 611–623. [Google Scholar] [CrossRef]
- Jones, S.; Li, M.; Parsons, D.W.; Zhang, X.; Wesseling, J.; Kristel, P.; Schmidt, M.K.; Markowitz, S.; Yan, H.; Bigner, D.; et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum. Mutat. 2012, 33, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Chong, I.Y.; Cunningham, D.; Barber, L.J.; Campbell, J.; Chen, L.; Kozarewa, I.; Fenwick, K.; Assiotis, I.; Guettler, S.; Garcia-Murillas, I.; et al. The genomic landscape of oesophagogastric junctional adenocarcinoma. J. Pathol. 2013, 231, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Schwaederle, M.; Daniels, G.A.; Piccioni, D.; Kesari, S.; Bazhenova, L.; Shimabukuro, K.; Parker, B.A.; Fanta, P.; Kurzrock, R. Cyclin-dependent kinase pathway aberrations in diverse malignancies: Clinical and molecular characteristics. Cell Cycle 2015, 14, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Wang, L.; Wang, Z.; Xu, L.; Sun, L.; Yang, H.; Li, W.D.; Wang, K. A pathway-centric survey of somatic mutations in Chinese patients with colorectal carcinomas. PLoS ONE 2015, 10, e0116753. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.; Glaros, S.; Thompson, E.A. The SWI/SNF complex and cancer. Oncogene 2009, 28, 1653–1668. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, H.; Choi, J.W.; Oh, H.E.; Lee, J.H. Unique characteristics of ARID1A mutation and protein level in gastric and colorectal cancer: A meta-analysis. Saudi J. Gastroenterol. 2017, 23, 268–274. [Google Scholar] [CrossRef]
- Alam, H.; Bhate, A.V.; Gangadaran, P.; Sawant, S.S.; Salot, S.; Sehgal, L.; Dange, P.P.; Chaukar, D.A.; D’cruz, A.K.; Kannanl, S.; et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer 2012, 12, 32. [Google Scholar] [CrossRef]
- Papaspyrou, K.; Brochhausen, C.; Schmidtmann, I.; Fruth, K.; Gouveris, H.; Kirckpatrick, J.; Mann, W.; Brieger, J. Fascin upregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. Oncol. Lett. 2014, 7, 2041–2046. [Google Scholar] [CrossRef]
- Qualtrough, D.; Singh, K.; Banu, N.; Paraskeva, C.; Pignatelli, M. The actin-bundling protein fascin is overexpressed in colorectal adenomas and promotes motility in adenoma cells in vitro. Br. J. Cancer 2009, 101, 1124–1129. [Google Scholar] [CrossRef][Green Version]
- Yao, J.; Qian, C.-J.; Ye, B.; Zhao, Z.-Q.; Wei, J.; Liang, Y.; Zhang, X. Signal transducer and activator of transcription 3 signaling upregulates fascin via nuclear factor-κB in gastric cancer: Implications in cell invasion and migration. Oncol. Lett. 2014, 7, 902–908. [Google Scholar] [CrossRef]
- Omran, O.M.; Al Sheeha, M. Cytoskeletal Focal Adhesion Proteins Fascin-1 and Paxillin Are Predictors of Malignant Progression and Poor Prognosis in Human Breast Cancer. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.Y.; Lewis, S.J.; Adams, J.C.; Martin, R.M. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: A systematic review and meta-analysis. BMC Med. 2013, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.-L.; Hu, L.-J.; Cao, J.-Y.; Huang, Y.-J.; Xu, L.-H.; Liang, Y.; Xiong, D.; Guan, S.; Guo, B.-H.; Mai, H.-Q.; et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010, 6, e1000940. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Yoshizaki, T.; Kondo, S.; Furukawa, M.; Kaizaki, Y.; Pagano, J.S. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br. J. Cancer 2011, 104, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, W.-D.; Xu, X.; Nie, B.; Lu, J.; Liu, X.; Zhang, B.; Dong, Q.; Sunwoo, J.B.; Li, G.; et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer 2014, 120, 363–372. [Google Scholar] [CrossRef]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef]
- Raab-Traub, N. Novel mechanisms of EBV-induced oncogenesis. Curr. Opin. Virol. 2012, 2, 453–458. [Google Scholar] [CrossRef]
- QingLing, Z.; LiNa, Y.; Li, L.; Shuang, W.; YuFang, Y.; Yi, D.; Divakaran, J.; Xin, L.; YanQing, D. LMP1 antagonizes WNT/β-catenin signalling through inhibition of WTX and promotes nasopharyngeal dysplasia but not tumourigenesis in LMP1B95-8 transgenic mice. J. Pathol. 2011, 223, 574–583. [Google Scholar] [CrossRef]
- Al Moustafa, A.; Foulkes, W.D.; Benlimame, N.; Wong, A.; Yen, L.; Bergeron, J.; Batist, G.; Alpert, L.; Alaoui-Jamali, M.A. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene 2004, 23, 350–358. [Google Scholar] [CrossRef]
- Glenn, W.K.; Heng, B.; Delprado, W.; Iacopetta, B.; Whitaker, N.J.; Lawson, J.S. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS ONE 2012, 7, e48788. [Google Scholar] [CrossRef]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.-F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.T.; Scott, R.S. The interaction between human papillomavirus and other viruses. Virus Res. 2017, 231, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Chen, D.; Ghabreau, L.; Akil, N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med. Hypotheses 2009, 73, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Salmons, B.; Glenn, W.K. Oncogenic Viruses and Breast Cancer: Mouse Mammary Tumor Virus (MMTV), Bovine Leukemia Virus (BLV), Human Papilloma Virus (HPV), and Epstein-Barr Virus (EBV). Front. Oncol. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Goudarzi, H.; Eslami, G.; Faghihloo, E. Role of viruses in gastrointestinal cancer. J. Cell. Physiol. 2018, 233, 4000–4014. [Google Scholar] [CrossRef]
- Polz-Gruszka, D.; Stec, A.; Dworzański, J.; Polz-Dacewicz, M. EBV, HSV, CMV and HPV in Laryngeal and Oropharyngeal Carcinoma in Polish Patients. Anticancer Res. 2015, 35, 1657–1661. [Google Scholar]
- Cyprian, F.S.; Al-Farsi, H.F.; Vranic, S.; Akhtar, S.; Al Moustafa, A.-E. Epstein-Barr Virus and Human Papillomaviruses Interactions and Their Roles in the Initiation of Epithelial-Mesenchymal Transition and Cancer Progression. Front. Oncol. 2018, 8, 111. [Google Scholar] [CrossRef]
- Al-Daraji, W.I.; Smith, J.H. Infection and cervical neoplasia: Facts and fiction. Int. J. Clin. Exp. Pathol. 2009, 2, 48–64. [Google Scholar]
- Polz-Dacewicz, M.; Strycharz-Dudziak, M.; Dworzanski, J.; Stec, A.; Kocot, J. Salivary and serum IL-10, TNF-alpha, TGF-beta, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect. Agent Cancer 2016, 11, 45. [Google Scholar] [CrossRef]
- Meckes, D.G.; Gunawardena, H.P.; Dekroon, R.M.; Heaton, P.R.; Edwards, R.H.; Ozgur, S.; Griffith, J.D.; Damania, B.; Raab-Traub, N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc. Natl. Acad. Sci. USA 2013, 110, E2925–E2933. [Google Scholar] [CrossRef]
- Pegtel, D.M.; van de Garde, M.D.; Middeldorp, J.M. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochim. Biophys. Acta 2011, 1809, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kahla, S.; Oueslati, S.; Achour, M.; Kochbati, L.; Chanoufi, M.B.; Maalej, M.; Oueslati, R. Correlation between ebv co-infection and HPV16 genome integrity in Tunisian cervical cancer patients. Braz. J. Microbiol. 2012, 43, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Szostek, S.; Zawilinska, B.; Kopec, J.; Kosz-Vnenchak, M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 2009, 56, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host. Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- Nikitin, P.A.; Yan, C.M.; Forte, E.; Bocedi, A.; Tourigny, J.P.; White, R.E.; Allday, M.J.; Patel, A.; Dave, S.S.; Kim, W.; et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe 2010, 8, 510–522. [Google Scholar] [CrossRef]
- Harris, R.S.; Dudley, J.P. APOBECs and virus restriction. Virology 2015, 479, 131–145. [Google Scholar] [CrossRef]
- Cullen, B.R. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J. Virol. 2006, 80, 1067–1076. [Google Scholar] [CrossRef]
- Suspène, R.; Aynaud, M.-M.; Koch, S.; Pasdeloup, D.; Labetoulle, M.; Gaertner, B.; Vartanian, J.-P.; Meyerhans, A.; Wain-Hobson, S. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J. Virol. 2011, 85, 7594–7602. [Google Scholar] [CrossRef]
- Ohba, K.; Ichiyama, K.; Yajima, M.; Gemma, N.; Nikaido, M.; Wu, Q.; Chong, P.; Mori, S.; Yamamoto, R.; Wong, J.E.L.; et al. In vivo and in vitro studies suggest a possible involvement of HPV infection in the early stage of breast carcinogenesis via APOBEC3B induction. PLoS ONE 2014, 9, e97787. [Google Scholar] [CrossRef]
- Wang, Z.; Wakae, K.; Kitamura, K.; Aoyama, S.; Liu, G.; Koura, M.; Monjurul, A.M.; Kukimoto, I.; Muramatsu, M. APOBEC3 deaminases induce hypermutation in human papillomavirus 16 DNA upon beta interferon stimulation. J. Virol. 2014, 88, 1308–1317. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, W.; Xu, J.; Kaufmann, A.M.; Albers, A.E. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int. J. Oncol. 2015, 47, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.; Khan, N.; Koriyama, C.; González, C.; Ampuero, S.; Padilla, O.; Solís, L.; Eizuru, Y.; Corvalán, A.; Akiba, S. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect. Agents Cancer 2011, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Naushad, W.; Surriya, O.; Sadia, H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: A possible etiological role of viruses in breast cancer. Infect. Genet. Evol. 2017, 54, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.A.; Sand, L. Human Papilloma Virus, Herpes Simplex Virus and Epstein Barr Virus in Oral Squamous Cell Carcinoma from Eight Different Countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar] [PubMed]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein–Barr virus coinfection and oral carcinogenesis. J.Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef]
- Vranic, S.; Cyprian, F.S.; Akhtar, S.; Al Moustafa, A.-E. The Role of Epstein-Barr Virus in Cervical Cancer: A Brief Update. Front. Oncol. 2018, 8, 113. [Google Scholar] [CrossRef]
- Ammatuna, P.; Giovannelli, L.; Giambelluca, D.; Mancuso, S.; Rubino, E.; Colletti, P.; Mazzola, G.; Belfiore, P.; Lima, R. Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J. Med. Virol. 2000, 62, 410–415. [Google Scholar] [CrossRef]
- Gunasekharan, V.; Laimins, L.A. Human Papillomaviruses Modulate MicroRNA 145 Expression To Directly Control Genome Amplification. J. Virol. 2013, 87, 6037–6043. [Google Scholar] [CrossRef]
- Nawandar, D.M.; Wang, A.; Makielski, K.; Lee, D.; Ma, S.; Barlow, E.; Reusch, J.; Jiang, R.; Wille, C.K.; Greenspan, D.; et al. Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells. PLoS Pathog. 2015, 11, e1005195. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Maruo, S.; Takada, K. EBV lytic infection enhances transformation of B-lymphocytes infected with EBV in the presence of T-lymphocytes. J. Med. Virol. 2012, 84, 504–510. [Google Scholar] [CrossRef]
- Ma, S.-D.; Hegde, S.; Young, K.H.; Sullivan, R.; Rajesh, D.; Zhou, Y.; Jankowska-Gan, E.; Burlingham, W.J.; Sun, X.; Gulley, M.L.; et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 2011, 85, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Meusel, T.R.; Imani, F. Viral Induction of Inflammatory Cytokines in Human Epithelial Cells Follows a p38 Mitogen-Activated Protein Kinase-Dependent but NF-κB-Independent Pathway. J. Immunol. 2003, 171, 3768–3774. [Google Scholar] [CrossRef] [PubMed]
- Arvey, A.; Tempera, I.; Tsai, K.; Chen, H.-S.; Tikhmyanova, N.; Klichinsky, M.; Leslie, C.; Lieberman, P.M. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 2012, 12, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Arvey, A.; Tempera, I.; Lieberman, P.M. Interpreting the Epstein-Barr Virus (EBV) epigenome using high-throughput data. Viruses 2013, 5, 1042–1054. [Google Scholar] [CrossRef]
| Population (Year) | Number of Samples | HPV Status (%) | Assay (Detection Method) | References |
|---|---|---|---|---|
| Bosnian (2020) | 106 | Positive (50%) | PCR and IHC | [109] |
| Syrian (2020) | 102 | Positive (37%) | PCR and IHC | [110] |
| Portuguese (2020) | 144 | Negative | RT-PCR | [111] |
| Polish (2017) | 50 | Positive (20%) | PCR | [112] |
| Puerto Rican (2016) | 45 | Positive (42%) | PCR | [23] |
| Brazilian (2016) | 1,549 | Positive (52%) | Meta-analysis | [113] |
| Syrian (2012) | 78 | Positive (54%) | PCR and IHC | [44] |
| Turkish (2011) | 106 | Negative | PCR | [47] |
| Argentinian (2010) | 75 | Positive (44%) | PCR | [114] |
| Israeli (2010) | 106 | Negative | RLB and LiPA | [19] |
| USA (2010) | 73 | Negative | RLB and LiPA | [19] |
| Spain (2010) | 100 | Negative | RLB and LiPA | [19] |
| Turkish (2009) | 56 | Positive (82%) | PCR and southern blot hybridization | [45] |
| Italian (2008) | 66 | Positive (33%) | PCR | [115] |
| Brazilian (2007) | 72 | Positive (83%) | PCR | [21] |
| Turkish (2006) | 53 | Positive (81%) | PCR | [46] |
| USA (2005) | 55 | Positive (51%) | PCR | [116] |
| Argentinian (2005) | 27 | Positive (74%) | PCR | [117] |
| USA (1992) | 50 | Negative | PCR | [118] |
| Population (Year) | Number of Samples | EBV Status (%) | Assay (Detection Method) | References |
|---|---|---|---|---|
| Bosnian (2019) | 108 | Positive (25%) | PCR and IHC | [92] |
| Iranian (2018) | 210 | Positive (1.4%) | PCR | [118] |
| Syrian (2017) | 102 | Positive (36%) | PCR and IHC | [133] |
| Iranian (2016) | 35 | Negative | PCR | [135] |
| Iranian (2015) | 50 | Positive (38%) | PCR | [116] |
| Chile (2015) | 37 | Positive (46%) | PCR | [136] |
| Italian (2014) | 44 | Negative | RT-PCR and IHC | [130] |
| North America (2013) | 117 | Positive (21%) | PCR | [131] |
| Polish (2011) | 186 | Positive (19%) | PCR | [134] |
| South Korean (2010) | 72 | Positive (30.6%) | IHC and ISH | [127] |
| Japanese (2010) | 1 | Negative | IHC | [137] |
| Italian (2009) | 100 | Positive (2.8–39%) | RT-PCR and sequencing | [138] |
| Chinese (2006) | 90 | Positive (30%) | IHC and ISH | [129] |
| Chinese (2003) | 130 | Positive (5–8%) | IHC, ISH and PCR | [117] |
| Scotland (2003) | 26 | Negative | ISH | [139] |
| Argentina (2002) | 19 | Positive (5%) | ISH | [140] |
| Japanese (2001) | 102 | Negative | ISH | [141] |
| South Korean (2001) | 274 | Negative | ISH | [142] |
| Chinese (1994) | 36 | Negative | ISH | [143] |
| Czechoslovakia (1988) | 13 | Negative | PCR | [144] |
| New Guinean (2004) | 46 | Positive (46%) | ISH | [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, Q.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review. Pathogens 2020, 9, 300. https://doi.org/10.3390/pathogens9040300
Fernandes Q, Gupta I, Vranic S, Al Moustafa A-E. Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review. Pathogens. 2020; 9(4):300. https://doi.org/10.3390/pathogens9040300
Chicago/Turabian StyleFernandes, Queenie, Ishita Gupta, Semir Vranic, and Ala-Eddin Al Moustafa. 2020. "Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review" Pathogens 9, no. 4: 300. https://doi.org/10.3390/pathogens9040300
APA StyleFernandes, Q., Gupta, I., Vranic, S., & Al Moustafa, A.-E. (2020). Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review. Pathogens, 9(4), 300. https://doi.org/10.3390/pathogens9040300







